Abstract
We report the case of a 14-year-old girl with a wide non-compressive pineal cyst, associated with the inability to control her sleep-wake schedule. Actigraphic monitoring showed a 24-hour free-running disorder (tau 26.96 hours). A 24-hour serum melatonin curve assay, with concomitant video-polysomnographic and body-core temperature monitoring, was performed. Melatonin curve showed a blunted nocturnal peak, lower total quantity of melatonin, and prolonged melatonin secretion in the morning, with normal temperature profile and sleep parameters. Treatment with melatonin up to 14 mg at bedtime was initiated with complete realignment of the sleep-wake rhythm (tau 23.93 hours). The role of the pineal cyst in the aforementioned alteration of melatonin secretion and free-running disorder remains controversial, but our case supports the utility of monitoring sleep/wake, temperature, and melatonin rhythms in the diagnostic work-up of pineal cysts associated with free-running disorder.
Citation:
Ferri L, Filardi M, Moresco M, Pizza F, Vandi S, Antelmi E, Toni F, Zucchelli M, Pierangeli G, Plazzi G. Non-24-hour sleep-wake rhythm disorder and melatonin secretion impairment in a patient with pineal cyst. J Clin Sleep Med. 2017;13(11):1355–1357.
Keywords: actigraphy, body-core temperature, free-running disorder, melatonin, pineal cyst
INTRODUCTION
Non-24-hour sleep-wake rhythm disorder, also known as free-running disorder (FRD), is a circadian rhythm sleep-wake disorder (CRSWD) characterized by the inability to maintain an entrained circadian sleep-wake rhythm with dissociation between the endogenous circadian phase and the external clock time, leading to insomnia and excessive daytime sleepiness.1 Lack of concentration and social dysfunction are also part of the disorder.1,2
FRD affects a significant proportion of blind subjects and is rarely reported in sighted persons, with a higher prevalence in young males with comorbid psychiatric disorders.1 In sighted subjects, most cases remains idiopathic, but few studies reported CRSWD in patients with hypothalamic lesions, namely tumors, and postsurgical or actinic lesions.2–4
The role of pineal gland lesions in causing FRD is controversial.5,6
FRD is not listed among the pineal cyst-related symptoms, which encompass instead headache, visual disturbances, vertigo, vomiting, and other sleep-related complaints.7 Here we report a case of CRSWD associated with a voluminous pineal cyst with documented altered melatonin secretion.
REPORT OF CASE
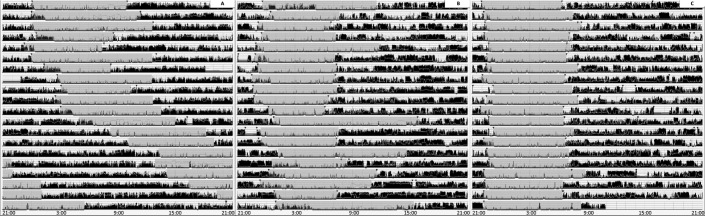
A 14-year-old girl presented with difficulty initiating sleep and inability to control her sleep-wake schedule. Since she was 11 years old she had complained about a progressive delay in sleep onset, difficulty waking up in the morning, remarkable sleep inertia, and need for long-lasting naps in the afternoon. She also had headache, photophobia, and phonophobia. Clinical and neurological examinations were unremarkable. The patient subjectively reported a poor sleep quality (Pittsburgh Sleep Quality Index [PSQI] = 12/21), and Morningness-Eveningness questionnaire for children and adolescents disclosed an evening chronotype. Brain magnetic resonance imaging showed a wide pineal cyst (maximum diameter 20 mm) without evidence of aqueductal or mesencephalic compression (see Figure S1 in the supplemental material). The patient underwent 22 days of actigraphic monitoring (Micro Motionlogger Watch actigraph initialized to store data in 1-minute epochs) that showed a 24-hour profile characterized by increased sleep onset latency (mean = 53.07 minutes, range 13–115 minutes) and a free-running sleep-wake rhythm with a phase length (tau) of 26.96 hours (Figure 1A). The patient underwent in-hospital, 96-hour, continuous video-polysomnography that showed a progressive delay in bedtime, from 9:50 PM to 11:22 PM, mean sleep duration of 7 hours with sleep efficiency of 92.67%, REM sleep latency of 79 minutes, periodic limb movement index of 0.6, and occasional diurnal naps.
Figure 1. Prolonged home actigraphy monitoring.
(A) From the sixth day administration of 7 mg of melatonin was discontinued and the major sleep (ie, inactivity) period progressively shifted forward. (B) Treatment with 7 mg of melatonin determined a partial alignment of the major sleep period, with persistent sleep onset insomnia and tendency to sleep in the early morning. (C) Restoration of wake-sleep rhythm coupled with the day/night cycle after treatment with 14 mg of melatonin.
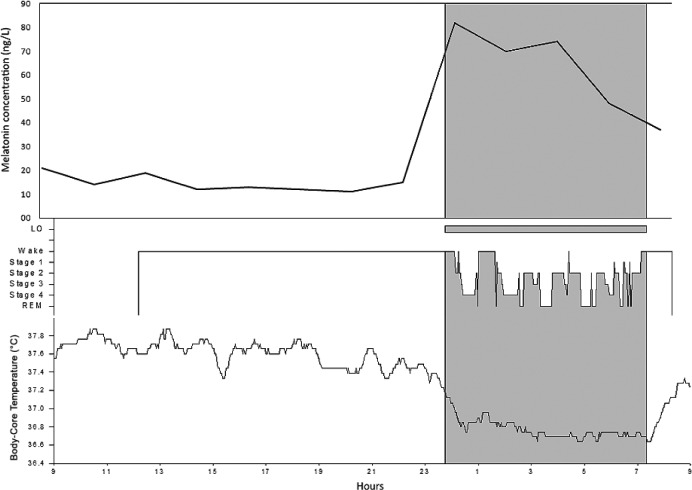
We performed a 24-hour serum melatonin assay in a room with controlled luminance levels and concomitant video-polysomnographic and body-core temperature (BcT) monitoring. During the daytime room lighting was maintained between 100–120 lux, from 11:00 PM to 8:00 AM lights were off and room lighting was maintained at less than 5 lux.8 Blood samples were collected every 2 hours from morning awakening (at 8:30 AM). Melatonin levels were measured by radioimmunoassay (RIA-3972; DRG International, Diagnostic GmbH, New Jersey, United States). BcT rhythm was within the normal range (Figure 2, lower panel) whereas the melatonin curve showed dim light melatonin onset at 10:30 PM, a blunted nocturnal peak (82 ng/L; median maximum peak reference values = 100 ng/L, range 81–143 ng/L), a total quantity of melatonin within the lower normal range (area under the curve 798 ng*h/L; median area under the curve reference values = 1249 ng*h/L, range 657–1321), and higher melatonin levels in the morning of the second day (37 ng/L versus 21 ng/L) (Figure 2, upper panel).9 Based on actigraphic findings, FRD was diagnosed and a treatment with melatonin up to 7 mg/d was started (administration time at 9:30 PM). After 6 months, the patient reported amelioration of sleep quality (PSQI = 7/21), with partial normalization of sleep-wake rhythm (Figure 1B) and occasional diurnal naps (2/wk). Brain MRI (performed after 19 months) showed that the cyst volume was stable. Morning light therapy and administration of modafinil up to 300 mg did not achieve any improvement in symptoms and therefore were discontinued, whereas melatonin was titrated up to 14 mg/d. After 6 months, the patient showed further improvement in sleep quality (PSQI = 4/12), the need for daytime sleep had disappeared, and she was able to restore her social functions. Actigraphy showed a complete realignment of the sleep-wake rhythm (tau = 23.93 hours; Figure 1C).
Figure 2. Melatonin curve plotting (upper panel) together with hypnogram and body-core temperature curve (lower panel).
Maximum peak = 82 ng/L; mean concentration during nighttime period = 68.5 ng/L, area under the curve 798 ng*h/L. Note the dim light melatonin onset occurs 1 hour before sleep onset, and melatonin peak is anticipated and lower than normal range. The parallel visualization of sleep, body-core temperature and melatonin concentration highlight a dissociation/desynchronization between melatonin peak and body-core temperature nadir.
DISCUSSION
The current study reports the restoration of a normal sleep-wake cycle after high-dose melatonin administration in a girl with a voluminous pineal cyst. Although data about the indication and effectiveness of melatonin treatment in free-running sighted subjects arise from few case reports without sufficient level of evidence,10 based on the altered melatonin curve profile we decided to use it to treat our patient. Melatonin titration up to high doses (14 mg/d) was empirically guided based on clinical and actigraphic follow-up evaluation. The restoration of a normal sleep-wake cycle after exogenous administration of melatonin point to a possible role of altered melatonin secretion in the FRD observed in our patient.11
Indeed, in our patient the melatonin curve profile showed subtle alterations (advanced dim light melatonin onset, blunted nocturnal peak, higher levels at morning) consistent with an altered secretion, whereas BcT rhythm did not show any alteration.
We could speculate that the altered melatonin secretion could be related to the parenchymal changes of the pineal gland due to the cyst. Nonetheless, given the non-compressive nature of the cyst, the role of the pineal cyst in alteration of melatonin secretion is uncertain; however, a correlation between solid pi-neal parenchyma and maximum melatonin concentration over 24 hours (higher slope of melatonin secretion in solid gland compared to cystic gland) has been previously reported.11
Desynchronization between melatonin and BcT rhythms could instead be due to the fact that BcT rhythm is mainly driven by the homeostatic process and secondarily by the circadian system and therefore, it may be inferred that altered melatonin secretion is strong enough to impair the sleep-wake rhythm, but not enough strong to influence BcT profile.12
However, the issue remains controversial, as 24-hour melatonin levels vary widely among healthy subjects, making the distinction between normal and altered values difficult and a recent study did not find any alteration of the sleep parameters after pineal gland resection other than a depletion of salivary melatonin below the threshold of 3 pg/mL.6 The current case highlights that patients presenting with a vo-luminous pineal cysts should be referred to sleep clinicians in order to recognize, and eventually characterize (through assessments of temperature, melatonin and sleep-wake rhythm), a possible CRSWD.
DISCLOSURE STATEMENT
Work for this study was performed at DIBINEM – Department of Biomedical and Neuromotor Sciences, University of Bologna, Bologna, Italy and IRCCS – Istituto delle Scienze Neurologiche, AUSL di Bologna, Italy. Lorenzo Ferri, Marco Filardi, Monica Moresco, Fabio Pizza, Stefano Vandi, Elena Antelmi, Francesco Toni, Mino Zucchelli, and Giulia Pierangeli have no potential conflicts of interest and no financial relationships relevant to this article to disclose; Giuseppe Plazzi participated in an advisory board for UCB Pharma, Jazz Pharmaceuticals, and Bioproject. All authors have read and approved the final version of the manuscript.
REFERENCES
- 1.American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 2.Garbazza C, Bromundt V, Eckert A, et al. Non-24-hour sleep-wake disorder revisited - a case study. Front Neurol. 2016;7:17. doi: 10.3389/fneur.2016.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pickering L, Jennum P, Gammeltoft S, Poulsgaard L, Feldt-Rasmussen U, Klose M. Sleep-wake and melatonin pattern in craniopharyngioma patients. Eur J Endocrinol. 2014;170(6):873–884. doi: 10.1530/EJE-13-1025. [DOI] [PubMed] [Google Scholar]
- 4.Joustra SD, Thijs RD, van den Berg R, et al. Alterations in diurnal rhythmicity in patients treated for nonfunctioning pituitary macroadenoma: a controlled study and literature review. Eur J Endocrinol. 2014;171(2):217–228. doi: 10.1530/EJE-14-0172. [DOI] [PubMed] [Google Scholar]
- 5.Quera-Salva MA, Hartley S, Claustrat B, Brugiéres L. Circadian rhythm disturbances associated with psychiatric symptoms in a patient with a pineal region tumor. Am J Psychiatry. 2011;168(1):99–100. doi: 10.1176/appi.ajp.2010.10101440. [DOI] [PubMed] [Google Scholar]
- 6.Slawik H, Stoffel M, Riedl L, et al. Prospective study on salivary evening melatonin and sleep before and after pinealectomy in humans. J Biol Rhythms. 2016;31(1):82–93. doi: 10.1177/0748730415616678. [DOI] [PubMed] [Google Scholar]
- 7.Májovský M, Netuka D, Beneš V. Clinical management of pineal cysts: a worldwide online survey. Acta Neurochir (Wien) 2016;158(4):663–669. doi: 10.1007/s00701-016-2726-3. [DOI] [PubMed] [Google Scholar]
- 8.La Morgia C, Ross-Cisneros FN, Sadun AA, et al. Melanopsin retinal ganglion cells are resistant to neurodegeneration in mitochondrial optic neuropathies. Brain. 2010;133(Pt 8):2426–2438. doi: 10.1093/brain/awq155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brodner W, Krepler P, Nicolakis M, et al. Melatonin and adolescent idiopathic scoliosis. J Bone Joint Surg Br. 2000;82(3):399–403. doi: 10.1302/0301-620x.82b3.10208. [DOI] [PubMed] [Google Scholar]
- 10.Auger RR, Burgess HJ, Emens JS, Deriy LV, Thomas SM, Sharkey KM. Clinical practice guideline for the treatment of intrinsic circadian rhythm sleep-wake disorders: advanced sleep-wake phase disorder (ASWPD), delayed sleep-wake phase disorder (DSWPD), non-24-hour sleep-wake rhythm disorder (N24SWD), and irregular sleep-wake rhythm disorder (ISWRD). An update for 2015: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2015;11(10):1199–1236. doi: 10.5664/jcsm.5100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nölte I, Lütkhoff AT, Stuck BA, et al. Pineal volume and circadian melatonin profile in healthy volunteers: an interdisciplinary approach. J Magn Reson Imaging. 2009;30(3):499–505. doi: 10.1002/jmri.21872. [DOI] [PubMed] [Google Scholar]
- 12.Kräuchi K. The thermophysiological cascade leading to sleep initiation in relation to phase of entrainment. Sleep Med Rev. 2007;11(6):439–451. doi: 10.1016/j.smrv.2007.07.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.