Abstract
Aim
Anxiety has a negative impact on daily functioning and quality of life in patients with Parkinson’s disease (PD). This study aims at assessing which sociodemographic and clinical characteristics predict the course of anxiety in early PD.
Methods
The participants of this two-year prospective cohort study were recently diagnosed PD patients not receiving psychiatric medications or dopamine replacement therapy at baseline. Assessments were performed annually after baseline. The primary outcome measure was anxiety, as measured with the State-Trait Anxiety Inventory (STAI). Covariates were age, gender, family history, striatal dopamine transporter binding ratios, and severity of motor and non-motor features of PD at baseline. Data were analyzed using a mixed model analysis.
Results
Inclusion criteria were met by 306 subjects. An increase in STAI total score was predicted by older age, lower score on the Montreal Cognitive Assessment, and the presence of a probable REM-sleep behavior disorder (RBD) at baseline. A decrease in STAI total score over time was predicted by a higher baseline score on the 15-item Geriatric Depression Scale, compulsive behavior at baseline and a family history of PD.
Conclusions
More severe baseline anxiety was associated with compulsive behavior and depressive symptoms. These symptoms had a parallel course, showing a decrease over time. An increase in anxiety was predicted by older age, worse cognitive functioning and the presence of RBD. Our findings, when replicated in a sample of PD patients in a more advanced disease stage, could provide starting points for prevention of anxiety in PD patients.
Keywords: Parkinson’s disease, Neuropsychiatry, Anxiety, Risk factor, Longitudinal
1. Introduction
About 25% of PD patients experience clinically relevant symptoms of anxiety, and approximately one-third suffers from an anxiety disorder as specified by the Diagnostic and Statistical Manual of Mental Disorders (DSM) [1]. Anxiety frequently predates the development of motor symptoms in PD, and might be considered as one of the earliest manifestations of PD. Once motor symptoms develop, anxiety remains more frequent in PD patients than in controls [2]. It can be socially disruptive, constitutes a source of disability [3], and has a negative impact on health-related quality of life [4]. However, in neurological practice, anxiety is often under-recognized [5], and anxiety in PD has received little scientific attention.
The pathophysiology of anxiety in PD patients still has to be elucidated. Previous research suggests that anxiety in PD results from an interplay of psychological, social and neurobiological factors. Anxiety in PD may be partially explained as a psychological reaction to the development of disabling motor and non-motor symptoms. In addition, there is increasing evidence that anxiety disorders are directly related to the neurochemical changes in PD. Patients with response fluctuations may report anxiety when the effect of dopaminergic medication wears off [6], and some studies demonstrate a positive effect of dopaminergic treatment on anxiety (e.g., Stacy et al. (2010)) [7]. These clinical findings suggest that the dopaminergic system is involved not only in the etiology of motor symptoms, but also in the development of anxiety. Dopamine transporter (DAT) single-photon emission computed tomography (SPECT) studies demonstrate a negative correlation between striatal DAT availability and symptoms of anxiety and depression in PD patients [8,9]. Besides the dopaminergic system, the noradrenergic and serotonergic systems are thought to be involved in the neurobiology of anxiety in PD, as well [9].
A better knowledge of risk factors for anxiety in PD might provide new starting points for both fundamental research on the pathophysiology of anxiety in PD, and for clinical studies on screening, prevention and treatment. In previous studies, anxiety in PD patients was associated with female sex, a history of anxiety disorders, a current depressive or impulse-control disorder, the severity of motor symptoms, striatal dopamine transporter (DAT) availability and autonomic failure [4,6,8,10–12]. An important limitation of most previous research on risk factors for anxiety in PD is the cross-sectional nature of the design. One longitudinal study on the course of neuropsychiatric symptoms has been performed [13], but this study only focused on the initiation of dopamine replacement therapy (DRT) as a risk factor for anxiety. The current study therefore aims at assessing which factors predict the course of anxiety in patients with early-stage PD, with a primary focus on clinical and sociodemographic predictors, and an assessment of striatal dopamine DAT availability.
2. Methods
2.1. Study design
For this study, we used data from the Parkinson’s Progression Markers Initiative (PPMI), an ongoing multi-center cohort study designed to identify PD progression biomarkers [14]. Follow-up assessments took place one (T1) and two years (T2) after baseline assessment (T0). The study received ethical approval from the institutional board at each site. For a complete overview of study procedures we refer to Marek et al. (2011) [14]. We used data that were collected between June 2010 and November 2014.
2.2. Study population
Subjects with a recent diagnosis of idiopathic PD were eligible for inclusion. None of the subjects had yet started DRT at T0. All subjects provided written informed consent. We used the screening criteria for the PPMI study, as stated in the study protocol on the PPMI website (http://www.ppmi-info.org). In order to obtain proper assessments of the outcome measure and covariates of this sub-study, we also excluded subjects aged under 30 years at PD onset and subjects with a bipolar disorder, psychotic disorder or current substance abuse. Since psychiatric medications, especially antidepressants and anxiolytic/hypnotic medications can decrease the severity of anxiety symptoms and stabilize the affective status, we also excluded subjects that were using these medications already at baseline. The start of psychiatric medications during follow-up was allowed. We assessed the effect of the start of anti-depressants and anxiolytic/hypnotic medications during follow-up on the association between anxiety and the predictors with a post hoc analysis.
2.3. Outcome and covariates
The primary outcome measure of this study was the total score on the State-Trait Anxiety Inventory (STAI) [15], measured at T0, T1 and T2. The STAI has a high validity and acceptable internal consistency and test-retest reliability in non-PD samples [16]. While the STAI has not been validated in a sample of PD patients, the Movement Disorders Society task force on rating scales for PD suggests that it is a suitable outcome in PD subjects, based on experience with the STAI in previous studies in PD populations [17]. The inventory comprises two subscales: a State and Trait subscale. The STAI has not been validated as an outcome measure in longitudinal studies. However, the State subscale was designed to measure a temporary state of anxiety, while the Trait subscale measures a more enduring pattern of anxiety. The combined STAI total score is therefore expected to be sensitive to change. Clinically relevant anxiety is commonly defined as a STAI State score of ≥39 [18]. For secondary research outcomes, we used the scores on the State and Trait subscales.
Age, sex, a self-reported family history of PD, and the severity of PD symptoms at baseline were included as covariates in the prediction model. The Movement Disorders Society - Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) was used to assess motor and non-motor symptoms [19]. Since item 1.4 inquires about an anxious mood, we omitted this item from the MDS-UPDRS part 1a and total score. Part IV covers motor complications and was not used in the analysis because these are uncommon in early, unmedicated PD. Other covariates were the total score on the Scales for Outcomes in Parkinson’s disease – Autonomic (SCOPA-AUT), the Montreal Cognitive Assessment (MoCA), and the 15-item Geriatric Depression Scale (GDS-15) [20–22]. Depression in PD is associated with the presence of a rapid eye movement (REM)-sleep behavior disorder (RBD) and excessive daytime sleepiness (EDS) [23,24]. Given the frequent co-occurrence of anxiety and depression in PD [25], RBD and EDS might also be markers for developing anxiety in PD. In this study, the presence of a probable RBD was defined as a score ≥6 on the REM-sleep Behavior Disorder Screening Questionnaire (RBDSQ) [26]. The presence of a probable impulse control disorder (ICD) or compulsive behavior was determined with the cut-off scores on the abbreviated version of the Questionnaire for Impulsive-Compulsive Disorders in Parkinson’s Disease (QUIP) as described in the validation study by Weintraub et al. [27]. Probable EDS was defined as a score ≥10 on the Epworth Sleepiness Scale (ESS) [28,29].
Finally, we used the SPECT DAT binding ratios of the left and right caudate nucleus and putamen separately. For the procedures involving DAT scanning and calculation of binding ratios, we refer to the SPECT manual on the PPMI website.
2.4. Statistical analysis
Percentages or mean scores with standard deviations were calculated for demographics and clinical characteristics of the study population.
A linear mixed model analysis was used to determine which covariates at baseline predicted a change in STAI scores over the course of two years. Mixed models analysis is considered to be particularly suitable for analyzing longitudinal data. This technique has the advantage that it handles missing data by placing the data in long format, where the available data of each measurement are nested within persons. Therefore, imputation of missing values is not necessary.
The STAI total, State and Trait subscale score were the outcomes used in these analyses. For the selection of covariates with a significant contribution to the model, i.e. the predictors of anxiety, we used a stepwise forward selection procedure. Alpha was set at p < 0.10, which is common in a selection procedure for a prediction model [30]. We assessed whether the change in STAI scores over time was influenced by the baseline values of the covariates, by adding interaction terms with time (covariate by time) to the model. Random intercepts were included for study site and patient within study site. We calculated the regression coefficient with standard error (SE), Wald-statistic and 95% confidence interval for the interactions between covariate and time with a significant contribution to the regression model. Assumptions for mixed model analyses were checked.
For the mixed model analysis, we used MLwiN v2.32. All other analyses were performed with IBM SPSS Statistics 20.
3. Results
3.1. Subjects
At the time of analysis, PPMI had enrolled 423 subjects, of which 383 subjects completed the T1 and 229 subjects the T2 assessments. We excluded 114 subjects using psychiatric medications. Three additional subjects were excluded due to missing data for all three assessments of the STAI, resulting in a total sample size of 306 subjects. At the time of analysis, T1 assessment was completed by a total of 257 subjects, and T2 assessment by 171. A flow chart of this sub-study can be found in Fig. 1.
Fig. 1.
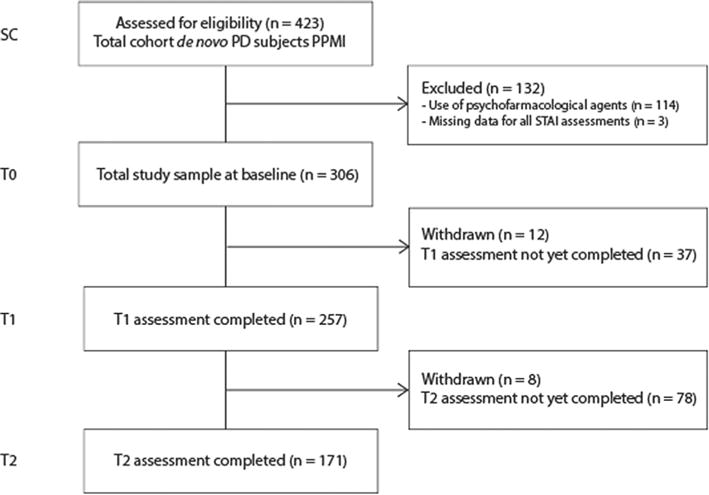
Flow chart.
As a sensitivity analysis, we compared the mean STAI total score for subjects that were included and excluded with an independent samples t-test. Subjects that were excluded had a higher mean STAI total score at baseline (72.5 vs. 62.9, t = −4.30, df = 160.4, p < 0.001), which was still significantly higher at T1 (69.1 vs. 63.6, t = −2.56, df = 356.0, p < 0.05), but not at T2 (68.4 vs. 63.8, t = −4.68, df = 237.0, p = 0.06).
Demographic and clinical characteristics of the study sample are presented in Table 1.
Table 1.
Demographic and clinical characteristics of the study population (n = 306).
| % | Mean (SD) | Range | |
|---|---|---|---|
| Female | 32.0% | ||
| Age (yrs) | 61.5 (10.1) | 34–84 | |
| Family history of PD | 24.3% | ||
| H&Y stage | |||
| Stage I | 46.1% | ||
| Stage II | 53.3% | ||
| Stage III | 0.7% | ||
| MDS-UPDRS totaladjusteda | 30.2 (12.5) | 7–71 | |
| MDS-UPDRS part IAadjusteda | 0.7 (1.0) | 0–5 | |
| MDS-UPDRS part IB | 3.9 (2.9) | 0–14 | |
| MDS-UPDRS part II | 5.5 (4.0) | 0–22 | |
| MDS UPDRS part III | 20.1 (8.8) | 4–51 | |
| SCOPA-AUT total score | 8.9 (5.8) | 0–39 | |
| MoCA total score | 26.9 (2.4) | 17–30 | |
| GDS-15 total score | 2.0 (2.3) | 0–14 | |
| RBDSQ total score | 3.9 (2.6) | 0–12 | |
| Probable RBD | 22.9% | ||
| QUIP total score | 0.3 (0.8) | 0–8 | |
| Probable ICD | 9.2% | ||
| Compulsive behavior | 11.1% | ||
| ESS total score | 5.8 (3.4) | 0–20 | |
| Probable EDS | 15.7% | ||
| DAT binding ratio | |||
| right caudate nucleus | 2.04 (0.59) | 0.68–3.98 | |
| left caudate nucleus | 2.03 (0.57) | 0.57–3.72 | |
| right putamen | 0.87 (0.35) | 0.14–2.35 | |
| left putamen | 0.82 (0.33) | 0.27–2.32 |
H&Y = Hoehn & Yahr; MDS-UPDRS = Movement Disorders Society - Unified Parkinson’s Disease Rating Scale; SCOPA-AUT = Scales for Outcomes in Parkinson’s disease — Autonomic; MoCA = Montreal Cognitive Assessment; GDS-15 = 15-item Geriatric Depression Scale; RBDSQ = REM-sleep behavior disorder Screening Questionnaire; RBD = REM-sleep behavior disorder; QUIP = Questionnaire for impulsive-compulsive disorders in Parkinson’s disease; ICD = impulse control disorder; ESS = Epworth Sleepiness Scale; EDS = Excessive Daytime Sleepiness; DBR = DAT-binding ratio.
Score on item 1.4 of the MDS-UPDRS was not included in the sum score.
3.2. Occurrence and course of anxiety
The mean STAI total score at baseline was 62.9 (SD ± 16.5) and, at group level, changed little over time. Across all visits, approximately 20% of subjects had clinically relevant symptoms of anxiety (see Supplementary Material).
3.3. Predictors of the course of anxiety
For an overview of the univariate analyses, we refer to the Supplementary Material. Final models resulting from the multivariate mixed models analyses are presented in Table 2. To facilitate interpretation, the influence of the predictors on the STAI total scores are illustrated in Fig. 2a–e. Note that in these figures, it is assumed that the values of all other predictors are zero.
Table 2.
Results of the mixed models analysis of the STAI total, State and Trait subscale scores. Regression coefficients (B), standard error (SE), Wald-statistic and 95% confidence interval (95% CI) for the interaction terms of covariate X time that have a significant contribution to the model are printed in bold font.
| STAI total score
|
STAI State score
|
STAI Trait score
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| B (SE) | Wald | 95% CI | B (SE) | Wald | 95% CI | B (SE) | Wald | 95% CI | |
| Compulsive behavior | 5.89 (2.51) | 5.49 | [0.96, 10.81] | 5.13 (1.60) | 10.28 | [2.00, 8.27] | 3.11 (1.31) | 5.69 | [0.55, 5.67] |
| Compulsive behavior X Time | −3.13 (1.47) | 4.54 | [−6.00, −0.25] | −2.10 (0.89) | 5.64 | [−3.84, −0.37] | −1.60 (0.75) | 4.62 | [−3.07, −0.14] |
| Age | −0.14(0.08) | 3.04 | [−0.29, −0.02] | – | – | – | −0.11 (0.04) | 7.33 | [−0.19, −0.03] |
| Age X time | 0.11 (0.05) | 5.31 | [0.02, 0.20] | – | – | – | 0.07 (0.02) | 7.34 | [0.02, 0.11] |
| Family history of PD | 0.31(1.82) | 0.03 | [−3.25, 3.87] | 0.58(1.15) | 0.25 | [−1.69, 2.83] | – | – | – |
| Family history of PD X Time | −2.03 (1.10) | 3.67 | [−4.10, 0.05] | −1.25 (0.64) | 3.80 | [−2.51, 0.01] | – | – | – |
| Probable RBD | 0.86 (1.87) | 0.21 | [−2.81, 4.53] | – | – | – | 0.32 (0.97) | 0.11 | [−1.58, 2.23] |
| Probable RBD X time | 2.05 (1.09) | 5.35 | [0.38, 4.64] | – | – | – | 1.19 (0.55) | 4.72 | [0.12, 2.26] |
| GDS-15 | 4.40(0.36) | 148.22 | [3.69, 5.10] | – | – | – | 2.38 (0.19) | 165.23 | [2.02, 2.74] |
| GDS-15 X time | −0.42 (0.21) | 4.06 | [−0.82, −0.01] | – | – | – | −0.21 (0.11) | 4.04 | [−0.42, −0.01] |
| MoCA | – | – | – | −0.19 (0.20) | 0.88 | [−0.58, 0.21] | – | – | – |
| MoCA X Time | – | – | – | −0.25 (0.12) | 4.65 | [−0.47, −0.02] | – | – | – |
STAI = State-Trait Anxiety Inventory; RBD = REM-sleep behavior disorder; GDS-15 = 15-item Geriatric Depression Scale; MoCA = Montreal Cognitive Assessment.
Fig. 2.
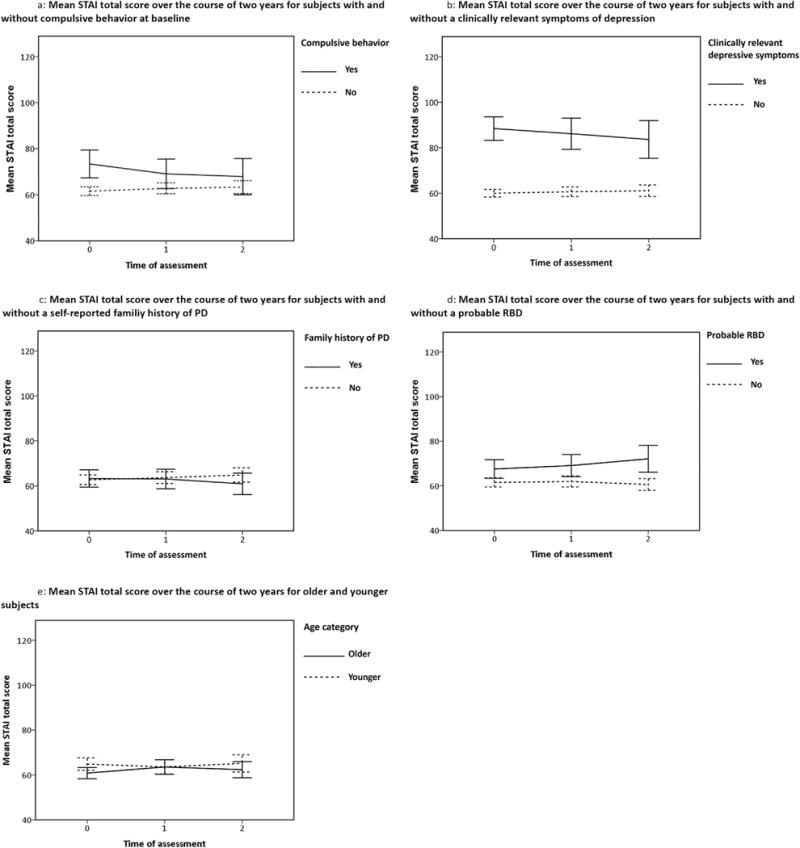
Course of the mean STAI total score over two years for different subgroups.
3.3.1. Predictors of STAI total score
The first column of Table 2 displays details of the interactions between covariate and time with a significant contribution to the prediction model for the STAI total score. The value of the regression coefficient of the covariate indicates whether the association between the predictor at baseline and the STAI total score is positive or negative. The value of the regression coefficient of the interaction of the covariate with time indicates in which direction the association changes over time. E.g., for the subjects with compulsive behavior, there was a positive association with STAI total score at baseline, while the negative interaction with time (p < 0.05) indicates that the difference in mean STAI total score between the subjects with and without compulsive behavior decreased over time (and at some point might even reverse). The course of anxiety in these subgroups is demonstrated in Fig. 2a.
A decrease in STAI total score over time was also predicted by a higher GDS-15 score at baseline (p < 0.05). In Fig. 2b, subjects were divided into two groups based on the cut-off GDS-15 score ≥5, indicative of clinically relevant symptoms of depression [31]. Subjects with clinically relevant symptoms of depression at baseline had a higher mean STAI total score at baseline that decreased over time, whereas the mean STAI total score in the subgroup without depression was lower at baseline and increased during follow-up. A positive family history of PD also predicted a decrease in STAI total score (see Fig. 2c), but this association was less strong (0.05 > p < 0.10).
An increase in STAI total score over time was predicted by older age (p < 0.05) and the presence of probable RBD at baseline (p < 0.05). Fig. 2d demonstrates that subjects with probable RBD had higher mean STAI scores at baseline, which increased over time, while the subjects without RBD had a lower mean STAI baseline score, which decreased further during follow-up. In the subgroups for age, divided using a median split for age, the mean STAI total scores at baseline differed little, and showed a fluctuating course over time (see Fig. 2e).
3.3.2. Predictors of STAI State and Trait subscale scores
Details of the interactions between covariate and time with a significant contribution to the prediction models for the STAI State and Trait score are displayed in Table 2. For an illustration of the course of the STAI subscale scores for these predictors, we refer to the Supplementary Material.
A decrease in STAI State score over time was predicted by compulsive behavior at baseline (p < 0.05). The negative association between STAI State score and the interaction of a family history of PD with time was less strong (0.05 > p < 0.10). A lower baseline MoCA score predicted an increase in STAI State score (p < 0.05). Supplementary Fig. S1c presents the differences between subjects with and without probable cognitive impairment, based on a MoCA cut-off score ≤26 [20]. Subjects with probable cognitive impairment at baseline showed an increase in mean STAI State score over time, while the STAI State score remained stable in subjects without cognitive impairment.
A decrease in STAI Trait score was predicted by compulsive behavior (p < 0.05) and a higher GDS-15 score (p < 0.05) at baseline. An increase in STAI Trait score was predicted by older age (p < 0.01) and the presence of probable RBD (p < 0.05) at baseline.
As a post hoc analysis, we assessed the influence of the initiation of antiparkinsonian agents (DRT, amantadine or parasympaticolytics), antidepressants or anxiolytics on the prediction models of the STAI total, State and Trait scores. There was no confounding effect of these medications during follow-up.
4. Discussion
This is the first study to identify predictors of the course of symptoms of anxiety in early-stage PD patients. The longitudinal course of neuropsychiatric symptoms in PD was only studied previously by de la Riva et al. (2014), in the complete PPMI cohort, with a specific focus on the initiation of DRT as risk factor for anxiety [13]. The start of DRT was not associated with new-onset anxiety, which is in line with the results of our post hoc analysis on the effect of DRT. Another post hoc analysis demonstrated that the start of antidepressants or anxiolytics also had no significant influence on the relation between the predictors and anxiety in our study population.
In a previous cross-sectional study, there was a positive association between higher anxiety scores and a younger age at PD onset [4]. In our study, younger subjects also had higher anxiety scores at baseline compared to older subjects, but an older age predicted an increase in anxiety during follow-up. Of note, age had only a small effect on anxiety.
A decrease in STAI scores over time was predicted by higher GDS-15 baseline score, compulsive behavior at baseline, and a family history of PD. Since a family history of PD was not a strong predictor (0.05 > p < 0.10), we will not discuss this finding in detail. Our findings are in contrast with previous studies demonstrating that a history of mental illness is associated with a higher prevalence of anxiety disorders in PD [4,6]. Fig. 2a and b suggest that these results are accounted for by the phenomenon of ‘regression to the mean’ [32]. Subjects with compulsive behavior and a higher depression score also have higher levels of anxiety at baseline. Due to regression to the mean, subjects in the higher part of the distribution of STAI scores at baseline are less likely to have high STAI scores at follow-up assessments [32]. An alternative explanation is that symptoms of anxiety, depression and compulsive behavior in our sample can be regarded at least partly as a reaction to the recent diagnosis of PD. The decrease in these symptoms might reflect a psychological adjustment over time. Unfortunately, subjects participating in PPMI were not asked whether they had started non-pharmacological treatment for symptoms of anxiety, such as psychotherapy. Therefore, we were unable to control for this factor.
Worse cognitive functioning at baseline was associated with an increase in STAI State score over time. This may be a reflection of the underlying neurodegenerative process: neuropathological research demonstrates involvement of the limbic system in PD subjects with MCI, which might explain co-occurrence of cognitive dysfunction and anxiety [33]. Alternatively, it may be a psychological reaction to the experienced cognitive changes. Disturbances of executive functions, which constitute the core feature of neuropsychological deficits in PD patients, reduce the capacity to control cognitive, emotional and behavioral responses to challenging environmental situations [34]. In clinical practice, PD patients report that they can get anxious when confronted with unexpected events, or when they feel flooded by an excess of stimuli. Moreover, the subjective experience of cognitive deterioration can elicit anticipatory anxiety for disease progression and future disability. In PD patients with early cognitive dysfunctions, improving executive control using cognitive rehabilitation strategies might increase the resilience to the development of anxiety. Moreover, future research on the effects of cholinesterase inhibitors should include the effect on symptoms of anxiety in PD patients with more severe cognitive dysfunctions.
Subjects with probable RBD had more symptoms of anxiety at baseline that increased over time, which is in line with a previous study [24]. A potential explanation for this finding is a more diffuse neurodegenerative process in PD subjects with RBD, which is expressed in a different phenotype. Indeed, the presence of RBD in PD is associated with a higher risk of developing dementia, and more autonomic and psychiatric symptoms [35]. Alternatively, the reduced quality of sleep caused by RBD leads to anxiety. Patients with PD and RBD display instability in the wake–sleep and non-REM—REM sleep transitions [36], that may result in a lower sleep quality. Sleep plays an important role in maintaining adaptive emotional regulation and reactivity [37] and sleep disturbances increase the risk of developing an anxiety disorder [38]. Further research is necessary to assess whether pharmacological treatment of RBD in PD patients decreases or prevents symptoms of anxiety in this population.
In contrast to previous studies [8,9], striatal DAT-binding ratio was not found to be significantly associated with anxiety. This is probably due to the fact that we investigated a study sample of patients that recently entered the symptomatic phase of PD, while the subjects in the abovementioned studies had a longer disease duration and therefore probably a more advanced stage of dopaminergic neurodegeneration.
This brings us to the limitations of our study. Throughout the study, STAI scores were relatively low and changed little over time. Approximately 20% of our subjects suffered from clinically relevant symptoms of anxiety, which is substantially lower than in other studies in PD patients [1]. Although this may be partially explained by the use of different instruments to measure anxiety across studies, it is probably due to a selection bias. All subjects in our study are early-stage, medication-naïve PD patients, and we excluded subjects using psychiatric medications at baseline. Sensitivity analysis showed that the excluded subjects demonstrated higher mean STAI total scores during follow-up. With our in- and exclusion criteria, we thus selected a study sample that is presumably mentally healthier than the general PD population. This limits the generalizability of our results. Conversely, the exclusion of subjects taking psychiatric medications or dopaminergic agents at baseline, ensured that there was no influence of these pharmacological agents on the assessment of anxiety at, or shortly after, baseline assessment. Moreover, our study sample comprised PD patients in an early stage of their disease. These characteristics of our study sample are unique compared to other studies on anxiety in PD.
As follow-up of the PPMI cohort continues, we expect to find larger changes in both motor and non-motor symptoms within subjects. Therefore, it will be informative to repeat this study when the subjects in the PPMI cohort have progressed to a more severe disease stage. We did, however, identify potential risk factors for anxiety in PD that warrant future research and might be relevant for clinical practice. Of note, we realize that the clinical relevance of the observed changes in STAI scores is questionable, and therefore we want to emphasize that we present the implications of our findings for clinical practice with reserve.
In conclusion, we found that the course of anxiety over two years in a sample of early-stage PD patients was predicted by age, cognitive functioning, the severity of depressive symptoms, and the presence of a RBD and compulsive behavior at baseline. These findings may provide new starting points for research on the pathophysiology, prevention and treatment of anxiety disorders in PD.
Supplementary Material
Acknowledgments
Data used in the preparation of this article were obtained from the Parkinson’s Progression Markers Initiative (PPMI) database (www.ppmi-info.org/data). For up-to-date information on the study, visit www.ppmi-info.org.
Funding
PPMI – a public-private partnership – is funded by the Michael J. Fox Foundation for Parkinson’s Research and funding partners, including Abbvie, Avid Radiopharmaceuticals, Biogen, Bristol-Myers Squibb, Covance, GE Healthcare, Genentech, GlaxoSmithKline, Lilly, Lundbeck, Merck, Meso Scale Discovery, Pfizer, Piramidal, Roche, Servier, UCB and Golub Capital.
For this sub-study, the researchers did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.parkreldis.2017.06.024.
Footnotes
Conflicts of interest
None.
Authors’ contributions
All authors made substantial contributions the conception and design of the study, revision of the manuscript critically for important intellectual content, and final approval of the version to be submitted. SR was responsible for analysis and interpretation of the data and drafting the article. PvdV made substantial contribution to analysis an interpretation of the data.
References
- 1.Broen MP, Narayen NE, Kuijf ML, Dissanayaka NN, Leentjens AF. Prevalence of anxiety in Parkinson’s disease: a systematic review and meta-analysis. Mov Disord. 2016;31(8):1125–1133. doi: 10.1002/mds.26643. [DOI] [PubMed] [Google Scholar]
- 2.Richard IH. Anxiety disorders in Parkinson’s disease. Adv Neurol. 2005;96:42–55. [PubMed] [Google Scholar]
- 3.Siemers ER, Shekhar A, Quaid K, Dickson H. Anxiety and motor performance in Parkinson’s disease. Mov Disord. 1993;8(4):501–506. doi: 10.1002/mds.870080415. [DOI] [PubMed] [Google Scholar]
- 4.Dissanayaka NN, Sellbach A, Matheson S, O’Sullivan JD, Silburn PA, Byrne GJ, et al. Anxiety disorders in Parkinson’s disease: prevalence and risk factors. Mov Disord. 2010;25(7):838–845. doi: 10.1002/mds.22833. [DOI] [PubMed] [Google Scholar]
- 5.Shulman LM, Taback RL, Rabinstein AA, Weiner WJ. Non-recognition of depression and other non-motor symptoms in Parkinson’s disease. Park Relat Disord. 2002:193–197. doi: 10.1016/s1353-8020(01)00015-3. [DOI] [PubMed] [Google Scholar]
- 6.Leentjens AF, Dujardin K, Marsh L, Martinez-Martin P, Richard IH, Starkstein SE. Symptomatology and markers of anxiety disorders in Parkinson’s disease: a cross-sectional study. Mov Disord. 2011;26(3):484–492. doi: 10.1002/mds.23528. [DOI] [PubMed] [Google Scholar]
- 7.Stacy M, Murck H, Kroenke K. Responsiveness of motor and nonmotor symptoms of Parkinson’s disease to dopaminergic therapy, Prog. Neuropsychopharmacol. Biol Psychiatr. 2010;34:57–61. doi: 10.1016/j.pnpbp.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 8.Weintraub D, Newberg AB, Cary MS, Siderowf AD, Moberg PJ, Kleiner-Fisman G, et al. Striatal dopamine transporter imaging correlates with anxiety and depression symptoms in Parkinson’s disease. J Nucl Med. 2005;46(2):227–232. [PubMed] [Google Scholar]
- 9.Remy P, Doder M, Lees A, Turjanski N, Brooks D. Depression in Parkinson’s disease: loss of dopamine and noradrenaline innervation in the limbic system. Brain. 2005;128:1314–1322. doi: 10.1093/brain/awh445. [DOI] [PubMed] [Google Scholar]
- 10.Berrios GE, Campbell C, Politynska BE. Autonomic failure, depression and anxiety in Parkinson’s disease. Br J Psychiatr. 1995;166(6):789–792. doi: 10.1192/bjp.166.6.789. [DOI] [PubMed] [Google Scholar]
- 11.Erro R, Pappata S, Amboni M, Vicidomini C, Longo K, Santangelo G, et al. Anxiety is associated with striatal dopamine transporter availability in newly diagnosed untreated Parkinson’s disease patients. Park Relat Disord. 2012;18(9):1034–1038. doi: 10.1016/j.parkreldis.2012.05.022. [DOI] [PubMed] [Google Scholar]
- 12.Voon V, Sohr M, Lang A, Potenza M, Siderowf A, Whetteckey J, et al. Impulse control disorders in Parkinson’s disease - a multicenter case-control study. Ann Neurol. 2011;69:986–996. doi: 10.1002/ana.22356. [DOI] [PubMed] [Google Scholar]
- 13.de la Riva P, Smith K, Xie S, Weintraub D. Course of psychiatric symptoms and global cognition in early Parkinson’s disease. Neurology. 2014;83:1096–1103. doi: 10.1212/WNL.0000000000000801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marek K, Jennings D, Lasch S, Siderowf A, Tanner C, Simuni T, et al. The Parkinson progression marker initiative (PPMI) Prog Neurobiol. 2011;95:629–635. doi: 10.1016/j.pneurobio.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spielberger CD. Manual for the State-trait Anxiety Inventory STAI (Form Y) Mind Garden; Palo Alto, CA: 1983. [Google Scholar]
- 16.Barnes L, Harp D, Jung W. Reliability generalization of scores on the spielberger state-trait anxiety inventory. Educ Psychol Meas. 2002;62(2):603–618. [Google Scholar]
- 17.Leentjens AF, Dujardin K, Marsh L, Richard IH, Starkstein SE, Martinez-Martin P. Anxiety rating scales in Parkinson’s disease: a validation study of the Hamilton anxiety rating scale, the Beck anxiety inventory, and the hospital anxiety and depression scale. Mov Disord. 2008;26(3):407–415. doi: 10.1002/mds.23184. [DOI] [PubMed] [Google Scholar]
- 18.Knight RG, Waal-Manning HJ, Spears GF. Some norms and reliability data for the state–trait anxiety inventory and the zung self-rating depression scale. Br J Clin Psychol. 1983;22:245–249. doi: 10.1111/j.2044-8260.1983.tb00610.x. [DOI] [PubMed] [Google Scholar]
- 19.Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, et al. Movement disorder society-sponsored revision of the unified Parkinson’s disease rating scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23(15):2129–2170. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- 20.Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 21.Visser M, Marinus J, Stiggelbout AM, Van Hilten JJ. Assessment of autonomic dysfunction in Parkinson’s disease: the SCOPA-AUT. Mov Disord. 2004;19(11):1306–1312. doi: 10.1002/mds.20153. [DOI] [PubMed] [Google Scholar]
- 22.Yesavage J, Brink T, Rose T, Lum O, Huang V, Adey M, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Sychiatr Res. 1983;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 23.Fantini ML, Macedo L, Zibetti M, Sarchioto M, Vidal T, Pereira B, et al. Increased risk of impulse control symptoms in Parkinson’s disease with REM sleep behaviour disorder. J Neurol Neurosurg Psychiatr. 2015;86(2):174–179. doi: 10.1136/jnnp-2014-307904. [DOI] [PubMed] [Google Scholar]
- 24.Rolinski M, Szewczyk-Krolikowski K, Tomlinson PR, Nithi K, Talbot K, Ben-Shlomo Y, et al. REM sleep behaviour disorder is associated with worse quality of life and other non-motor features in early Parkinson’s disease. J Neurol Neurosurg Psychiatr. 2014;85(5):560–566. doi: 10.1136/jnnp-2013-306104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamanishi T, Tachibana H, Oguru M, Matsui K, Toda K, Okuda B, et al. Anxiety and depression in patients with Parkinson’s disease. Int Med (Tokyo, Japan) 2013;52(5):539–545. doi: 10.2169/internalmedicine.52.8617. [DOI] [PubMed] [Google Scholar]
- 26.Nomura T, Inoue Y, Kagimura T, Uemura Y, Nakashima K. Utility of the REM sleep behavior disorder screening questionnaire (RBDSQ) in Parkinson’s disease patients. Sleep Med. 2011;12(7):711–713. doi: 10.1016/j.sleep.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 27.Weintraub D, Hoops S, Shea JA, Lyons KE, Pahwa R, Driver-Dunckley ED, et al. Validation of the questionnaire for impulsive-compulsive disorders in Parkinson’s disease. Mov Disord. 2009;24(10):1461–1467. doi: 10.1002/mds.22571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 29.Hogl B, Arnulf I, Comella C, Ferreira J, Iranzo A, Tilley B, et al. Scales to assess sleep impairment in Parkinson’s disease: critique and recommendations. Mov Disord. 2010;25(16):2704–2716. doi: 10.1002/mds.23190. [DOI] [PubMed] [Google Scholar]
- 30.Twisk JWR. Inleiding in de toegepaste biostatistiek. Reed Business; Amsterdam: 2010. [Google Scholar]
- 31.Baillon S, Dennis M, Lo N, Lindesay J. Screening for depression in Parkinson’s disease: the performance of two screening questions. Age Ageing. 2014;43:200–205. doi: 10.1093/ageing/aft152. [DOI] [PubMed] [Google Scholar]
- 32.Galton F. Regression towards mediocrity in hereditary stature. J R Anthropol Inst. 1886;15:246–263. [Google Scholar]
- 33.Halliday G, JB L, Schneider J, Adler C. The neurobiological basis of cognitive impairment in Parkinson’s disease. Mov Disord. 2014;29(5):634–650. doi: 10.1002/mds.25857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caballol N, Marti MJ, Tolosa E. Cognitive dysfunction and dementia in Parkinson disease. Mov Disord. 2007;22(Suppl. 17):S358–S366. doi: 10.1002/mds.21677. [DOI] [PubMed] [Google Scholar]
- 35.Arnulf I. REM sleep behavior disorder: motor manifestations and pathophysiology. Mov Disord. 2012;27(6):677–689. doi: 10.1002/mds.24957. [DOI] [PubMed] [Google Scholar]
- 36.Christensen JAE, Jennum P, Koch H, Frandsen R, Zoetmulder M, Arvastson L, et al. Sleep stability and transitions in patients with idiopathic REM sleep behavior disorder and patients with Parkinson’s disease. Clin Neurophysiol. 2015;127(1):537–543. doi: 10.1016/j.clinph.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 37.van der Helm E, Walker MP. Sleep and emotional memory processing. Sleep Med Clin. 2011;6(1):31–43. doi: 10.1016/j.jsmc.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Uhde TW, Cortese BM, Vedeniapin A. Anxiety and sleep problems: emerging concepts and theoretical treatment implications. Curr Psychiatr Rep. 2009;11(4):269–276. doi: 10.1007/s11920-009-0039-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


