Abstract
From a biological perspective, a natural product can be defined as a compound evolved by an organism for chemical interactions with another organism including prey, predator, competitor, pathogen, symbiont or host. Natural products hold tremendous potential as drug leads and have been extensively studied by chemists and biochemists in the pharmaceutical industry. However, the biological purpose for which a natural product evolved is rarely addressed. By focusing on a well-studied group of natural products – venom components from predatory marine cone snails – this review provides a rationale for why a better understanding of the evolution, biology and biochemistry of natural products will facilitate both neuroscience and the potential for drug leads. The larger goal is to establish a new sub-discipline in the broader field of neuroethology that we refer to as “Chemical Neuroethology”, linking the substantial work carried out by chemists on natural products with accelerating advances in neuroethology.
Keywords: Chemical Neuroethology, Natural Products, Cone Snail Venom, Toxin Cabals
Introduction
Animals evolve specific chemicals to interact with other animals that they may encounter such as potential prey, predators or competitors. Thus, a skunk uses volatile compounds to deter any potential predators and sessile marine animals (e.g., ascidians and sponges) produce chemical deterrents to avoid predation. The compounds that evolution has produced to mediate such chemical interactions are the natural products. Here, we refer to natural products using the most common definition from the field of organic chemistry, where natural products are virtually synonymous with ‘specialized metabolites’ or ‘secondary metabolites’. These are chemicals that are not part of central metabolism, but often have a role in interspecies interactions.
Because natural products have been explored mainly as drug leads, they have largely been studied by chemists and biochemists in the pharmaceutical industry (Davies 2006). In almost all cases, the biological role of a natural product is unknown, and the specific chemical interaction in a native biological context in which a particular compound is used has not been identified. Thus, a central principle that would seem obvious to neuroethologists is generally not addressed at all by the research community that presently studies natural products: Every natural product was evolved for a specific biological purpose.
Natural products may benefit the “producer” animal by changing the behavior of the “target” animal in ways advantageous to the producer. This is particularly obvious for a specific class of natural products, those directly injected into the targeted animal. Such natural products are produced in venoms, and envenomation typically causes a rapid and dramatic effect on the behavior of the animal injected with venom. Thus, venom components are a special class of natural products, delivered to the intended target by direct injection. This subclass of natural products does not have to traverse through barriers that non-injectable natural products must be able to pass — for this reason, there are convergent properties of venom components from various sources that differ from conventional natural products, even though the animals that produce them might be phylogenetically distant from each other. Most bioactive venom components that have been characterized are small polypeptides or peptides, generally highly structured. Structural stability is most often achieved through the formation of specific disulfide crosslinks.
In this review article, the biochemistry of venom components evolved by fish-hunting cone snails will be correlated both with the behavior of the predatory cone snail itself, and effects on the behavior of the envenomated animal. The latter will be described with a focus on the underlying mechanisms by which specific compounds in the venom elicit changes in behavior observed after envenomation. This article is deliberately highly selective, and is not intended to be comprehensive review of fish-hunting cone snail venoms. There is an expanding literature on these venoms, many of the publications are given in an appendix to this review; many of these studies, while adding useful data to the literature on these venoms, are only peripherally relevant to the major theme of this article. Our larger goal is to establish a new sub-discipline in the broader field of Neuroethology that we refer to as “Chemical Neuroethology”. We hope to demonstrate that a vast interdisciplinary scientific area is now ripe for development, one which can potentially link the very substantial work carried out by chemists on natural products with accelerating advances in neuroethology. At present, there is little overlap between the biochemistry of natural products and neuroethology but this largely neglected interface can (and should) be more systematically explored. Development of the interdisciplinary area should result in unique and powerful new pharmacological tools for investigating neuroethological mechanisms.
The emphasis in the last few decades of natural products research has been on developing an ever more sophisticated chemical and biochemical technology targeted to the discovery and chemical characterization of natural products (primarily for drug development), with very little progress in clarifying the biological foundation that led to the evolution of that specific compound. This is largely because funding for most of the research on natural products has been motivated by a desire to develop new therapeutic drugs, and is therefore mostly neglected by biologists. We assert in this review that a better balance between the chemistry and the biology of natural products, and in particular a strengthened link to neuroethology is long overdue, and should in fact facilitate the search for drug leads. However, a major goal of this review is to show how natural products can be used to uncover mechanisms at the molecular, cellular and circuit levels that underlie behavior, that might be difficult to discern using other methods
Biological Background
In this article, our general focus will be on the marine environment. An obvious feature of chemical interactions in the marine environment that differ from those on land is that if these interactions take place at a distance, the mediating chemicals (i.e., natural products) are not restricted by a requirement for the generation of volatile compounds (such as the interaction between a skunk and its potential predators). For marine animals, compounds that are water-soluble can mediate chemical interactions between two organisms. Thus, marine animals can secrete specific compounds into the water to affect the behavior of another animal in its ecological niche. We can expect that a rich cornucopia of marine natural products that affect behavior remains to be discovered. The work with venomous snails provides a very preliminary indication of just how much remains to be discovered.
The cone snail species discussed in this review are part of what is arguably the most significant radiation of marine snails that has ever occurred over the entire evolutionary history of molluscs, likely triggered by the extinction of ammonites at the end of the Cretaceous (Kohn 1985). Parallel to the expansion of mammals on land, a group of marine snails that are known collectively as the neogastropods expanded rapidly into diverse ecological niches; these are primarily predatory, carnivorous marine snails, and some of the largest shelled molluscs in the oceans today are the result of this adaptive expansion of neogastropod groups. These include lineages such as the Tonnoideans that feed primarily on Echinoderms, Mitrids that prey on sipunculid worms, Muricids that prey on bivalves and small specialized groups, such as the Colubrarids, the “vampires of the ocean”, that suck blood from fish (Oliverio and Modica 2010). The most biodiverse of the neogastropod lineages are the conoidean snails that are venomous, predatory snails, likely exceeding 10,000 species; most conoidean lineages appear to prey on polychaete worms. Among the most familiar of the conoidean snails (superfamily Conoidea) are the cone snails that belong to the family Conidae; it is estimated that there are 800 species, with a significant number still to be discovered. The other two groups of conoidean snails are the auger snails (family Terebridae) that comprise ca. 400 species, and the remainder, collectively called turrids, that probably exceed 10,000 species (Watkins et al. 2006). The latter are little known, since many are small forms that live in deep water marine environments.
Most living species in the family Conidae were traditionally assigned to a single genus, Conus. A recent molecular phylogeny of the family Conidae recognized three additional genera in the family, Conasprella, Californiconus and Profundiconus — altogether, these comprise ca. 100 species (Puillandre et al. 2014). The ~700 species that comprise the genus Conus are generally divided into three broad classes, the largest group are species that are worm hunting (vermivorous), but there are snail hunting (molluscivorous) and fish hunting (piscivorous) lineages as well. In this review, our focus will be on piscivorous cone snails. These are by far the best understood in terms of neuroethological mechanisms, but reference will be made to species in other lineages.
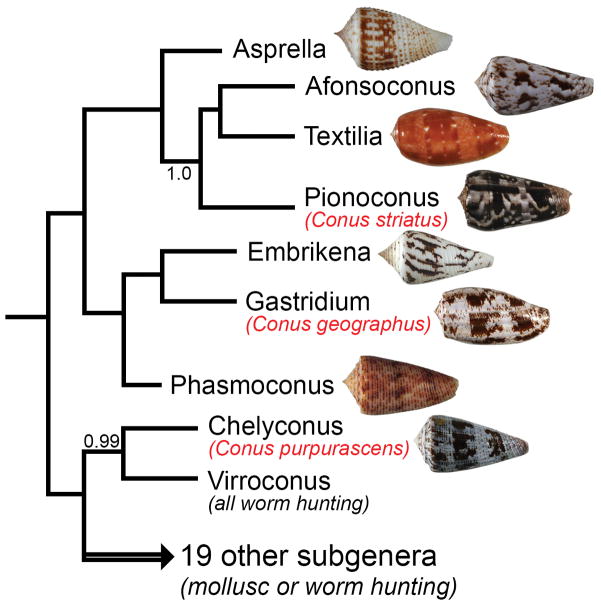
A recent revision of the taxonomy of the genus Conus, proposed ca. 60 sub-generic groups (Puillandre et al. 2014); 8 of these are likely to be primarily fish hunting (Olivera et al. 2015). The division of fish-hunting species into 8 clades is based primarily on molecular phylogenetic data using standard mitochondrial markers. The 8 fish-hunting clades vary considerably in their species richness and in biogeographic distribution; seven of the lineages are found in the Indo-Pacific marine province, and only one is in the New World and the Atlantic (Olivera et al. 2015). The 8 lineages are shown in Figure 1.
Figure 1.
Phylogenetic tree showing shells of fish-hunting cone snails, each representing one of the 8 subgenera. Among the shells illustrated are Conus rolani (subgenus Asprella); Conus kinoshitai, (subgenus Afonsoconus); Conus bullatus (subgenus Textilia); Conus striatus (subgenus Pionoconus); Conus pergrandis (subgenus Embrikena); Conus geographus (subgenus Gastridium); Conus moluccensis (subgenus Phasmoconus) and Conus purpurascens (subgenus Chelyconus). The focus of this review are 3 of the species shown, Conus striatus, Conus geographus and Conus purpurascens. All of the specimens illustrated are from the Philippines, except for Conus striatus (Madagascar) and Conus purpurascens (Mexico, Eastern Pacific).
The 3 fish-hunting cone snail species we focus on here each belong to a different subgenus. These species, Conus geographus (subgenus Gastridium), Conus striatus (subgenus Pionoconus) and Conus purpurascens (subgenus Chelyconus), have been among the most intensively studied cone snail species, and a comprehensive framework for correlating their venom biochemistry to their prey-capture behavior can be reconstructed. Conus geographus occurs in the Western Pacific and much of the Indian Ocean, with its biogeographic range overlapping considerably with Conus striatus. However, the latter is more widely distributed, and is found in the Hawaiian Archipelago (where Conus geographus does not occur). Conus purpurascens is found in the Panamic marine province, from the Sea of Cortez and the Clipperton Islands down to the Galapagos. All 3 species are relatively large, and can be quite common in some of the localities where they are found (Conus striatus and Conus geographus are consumed as food, primarily as components of bouillabaisse-like shellfish broths in the Central Philippines). We first describe the differences in the behavior of the 3 species when they forage for their fish prey.
Overview of Prey-Capture Behavior
Foraging and envenomation strategies
Although the relationship between the three different subgenera of fish-hunting cone snails covered here is not yet firmly established, the present molecular evidence is consistent with the two Indo-Pacific species, Conus geographus and Conus striatus being more closely related to each other than to the New World species, Conus purpurascens (Olivera et al. 2015; Puillandre et al. 2014). However, as we detail below, Conus purpurascens and Conus striatus are much more similar, both in their behavioral strategy for capturing fish, and in the effects of their venom on the fish prey; Conus geographus is divergent in both respects. As we discuss in a later section, the similarities in the fish-hunting behaviors and strategies of Conus striatus and Conus purpurascens appear to be a result of convergent evolution.
All fish-hunting cone snails are primarily nocturnal, and sense the presence of fish through exquisitely sensitive chemosensory mechanisms. Because cone snails forage at night, they are quiescent during daylight hours. We have maintained and observed all 3 Conus species described here in aquaria for extended periods of time. In aquaria, the snails are immobile in a lighted aquarium room, with Conus geographus lying on the surface, typically against any available rock or coral (with multiple individuals tending to gather next to each other). In contrast, both Conus striatus and Conus purpurascens bury themselves under the sand, and may not be visible at all. If these snails have not captured any fish for an extended period of time, introducing a fish into the aquarium will trigger an immediate reaction from the snails, even during the day.
When it senses fish, Conus geographus will begin moving, extending its siphon (a sensory organ that enables the snail to detect prey from a distance) back and forth -much like the trunk of an elephant, clearly sampling the water and moving systematically towards the fish. As the snail gets closer, it opens its large rostrum, which is funnel shaped (see Fig. 2). It will aim the open rostrum towards the fish and after a period of time, attempt to engulf the fish with the rostrum. Once a fish is completely engulfed by the rostrum, the snail closes the rostrum tightly so that it looks like a balloon, with a fish trapped inside. The snail then injects venom into the trapped fish although this can be difficult to directly observe once the fish is hidden inside the rostrum.
Figure 2.
Conus geographus foraging. In the top figure, the snail is moving towards fish that it has detected through its intake siphon. It has opened its rostrum, and is secreting a subset of venom components. Middle panel, The snail begins to engulf a fish; the fish has become quiescent and sufficiently disabled so that it is unable to swim away. Bottom panel, the snail has closed off its rostrum, fish are now trapped inside. The snail will then sting the fish and inject a small amount of venom that causes an irreversible block of neuromuscular transmission. The eye-stalk of the snail is visible on the middle and bottom panels; the snail can detect light, but the chemosensory system is primarily used to detect and locate fish prey.
In contrast, both Conus purpurascens and Conus striatus will typically remain buried under the sand, but their siphon will emerge when a fish is introduced. They maneuver so that their rostrum is partially sticking out of the sand. From within the rostrum, the long distensible proboscis is extended towards the fish. The proboscis of Conus striatus is yellow (see Fig. 3), while the proboscis of Conus purpurascens is bright red (see Fig. 4). The proboscis of both species can be extended many times the length of the shell, and the snail then strikes the fish, extruding a harpoon-like radular tooth from the end of the proboscis which serves as both harpoon and hypodermic needle. In a good strike, the fish is tethered, venom is injected through the hollow harpoon-like tooth, and the fish may struggle briefly, but within seconds it is immobilized in a tetanic paralysis, characterized by very stiff fins and a rigid body posture. The snail then retracts its proboscis, and the harpooned and immobilized fish is drawn towards the rostrum of the snail, and completely engulfed.
Figure 3.
Conus striatus foraging. Upper left, the snail has extended its yellow proboscis after detecting a fish. Right panel, the snail has just stung the fish, and the fish is immobilized with extremely stiff fins. The harpoon-like tooth extends out of the proboscis (see arrow). Lower left, the snail has retracted its proboscis, and is about to completely engulf the immobilized fish.
Figure 4.

Conus purpurascens capturing its prey. The top panel shows the buried snail extending it bright red proboscis towards the fish. In this specific case, the fish was stung in the mouth. The fish was tethered through the harpoon, the proboscis was retracted and in the middle panel, the snail is beginning to engulf the immobilized fish. Note the very stiff pectoral fin of the fish as it is being engulfed. Also note that a second Conus purpurascens has its siphon sticking out of the substrate, and is beginning to extend its proboscis towards the fish. In the lower panel, the immobilized fish is halfway engulfed by the rostrum.
In all three species, the fish that are within the rostrum are ultimately totally paralyzed and pre-digested, and the snail regurgitates most of the scales and bones of the fish, as well as the one harpoon used for injecting venom. In the case of Conus geographus, injection of venom takes place after the fish have been engulfed within the rostrum of the snail. In the case of Conus geographus, injection of venom takes place after the fish have been engulfed within the rostrum of the snail. This is difficult to directly observe but studies by Johnson and Stablum (1970) suggest that the injection occurs through the fish’s gill aperture and into the pharynx; Fish removed from the pharynx of Conus geographus showed hemorrhaging among the gill filaments and in one case a radula tooth was found between the gill filaments in the posterior portion of the pharynx.
It is clear that for Conus purpurascens and Conus striatus, venom is injected during the initial strike, since a tetanic paralysis is observed seconds afterward. Thus, at the behavioral level, Conus purpurascens and Conus striatus are similar in their strategy: both extend their proboscis, strike and tether the fish. By retracting their proboscis into the rostrum, the immobilized fish is then engulfed and predigested. Conus geographus however engulfs the fish first before injecting venom.
The radular tooth is used to inject venom in all three species. In Conus striatus and purpurascens; these are not only used as hypodermic needles, but also as harpoons to tether the fish after the initial strike. In these species, the tip of the radular tooth is shaped very much like a harpoon, and furthermore, there is a prominent barb (Figure 5), so that when the tooth pierces the skin of the fish, the fish is tethered and even if it tries to struggle loose, cannot escape as long as the snail is able to firmly grasp the tooth, which it achieves by powerful circular muscles at the end of the proboscis, and by grasping a ligament attached to the distal end of the tooth. In contrast, the radular tooth of Conus geographus resembles a hypodermic needle, not a barbed harpoon (Figure 5).
Figure 5.
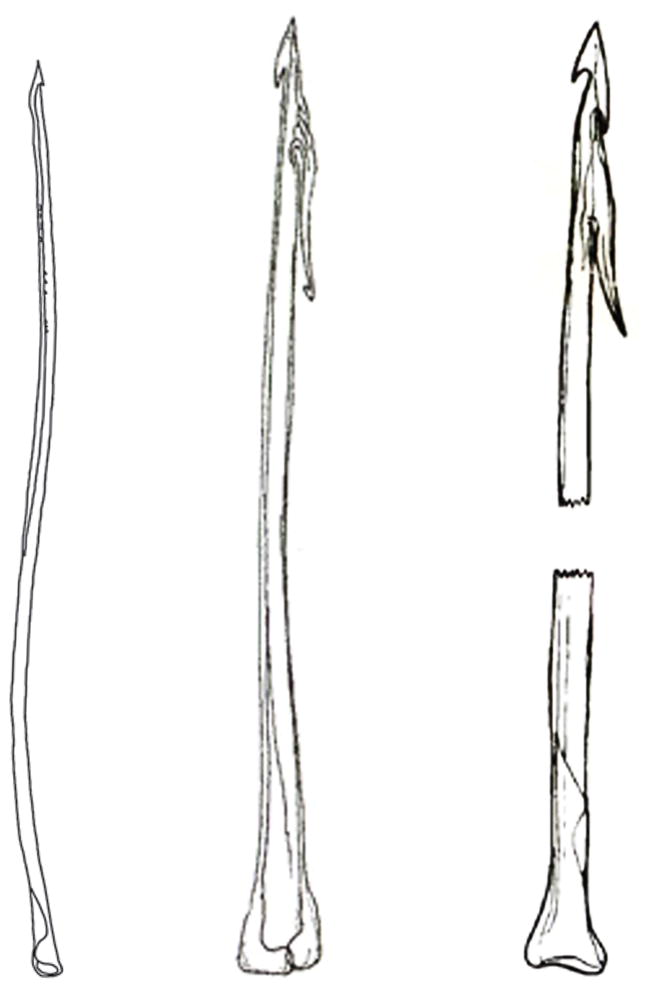
Comparison of radula teeth of fish-hunting cone snail that use a net-hunting (from left to right C. geographus) and taser-and-tether hunting strategy (C. striatus (middle) and C. purpurascens (right)). Reproduced from www.theconecollector.com
Rationale for behavioral differences
The striking behavioral similarities between Conus striatus and Conus purpurascens in their foraging behavior despite their considerable phylogenetic divergence contrasts with the behavior observed for Conus geographus (Olivera et al. 2015). These can be understood by different selection pressures. The behavior of Conus striatus and Conus purpurascens maximizes the probability that the predatory cone snail will capture a targeted fish. In contrast, the behavior of Conus geographus can be rationalized as a strategy primarily aimed at capturing as many fish as possible whenever the cone snail goes foraging (Olivera, personal observations). This selects for a different behavior, with additional consequences on all aspects of the biology of these species.
The behavior of the 3 species in captivity is revealing; Conus striatus and Conus purpurascens are routinely fed by tethering a fish with forceps; the snails extend their proboscis towards the tethered fish, harpoon the fish, thereby easily bagging their meal. After being fed such a tethered fish, even when only a small fish is presented to a snail that has not fed for an extended period, no attempt will be made to harpoon a second fish (Nybakken 1967). Indeed, if the snails miss, they will typically not extend their proboscis and harpoon another fish until the following day (if the tethered fish is pushed very close to the rostrum of the snail, they will open their rostrum and suck up the fish without injecting venom, personal observations). In contrast, although injection of venom is not directly observed (since this occurs within the rostrum of the snail), for Conus geographus, if a fish is released into the aquarium, the snail will attempt to capture it and if multiple fish are released, it will attempt to capture as many fish as possible. Thus, in the wild, Conus purpurascens and Conus striatus likely target a single individual fish during each foraging event. In contrast, we believe that Conus geographus primarily targets schools of fish, probably hiding in crevices in reefs, and that this species can routinely capture multiple fish each time it goes foraging.
Physiology and Pharmacology of Prey Capture: Conus geographus
Overview of the physiological strategy for prey capture by Conus geographus
The prey capture observations on Conus geographus described in the text below are based on hundreds of specimens, collected from various localities in the Philippines. When the snail goes foraging, the fish or school of fish that it targets need to be made more vulnerable and prone to capture. The snail uses a chemical warfare pre-capture strategy that makes the fish much more vulnerable. Thus, in the pre-capture stage, fish are believed to be systematically debilitated in at least two ways; their energy metabolism is altered and the fish become hypoglycemic (Safavi-Hemami et al. 2015b). Additionally, their sensory circuitry is attacked, so that they become unable to sense the approach of the predator and their impending engulfment by the snail (Kohn 2014). This pre-capture stage causes an obvious alteration in the behavior of the fish, and as a consequence, the fish not only do not swim away, but their swimming becomes highly erratic and aberrant. The pre-capture stage makes it possible for the snail to closely approach the fish or school of fish, and to engulf many fish at a time, or sequentially. Once engulfed, venom components are injected (Johnson and Stablum 1971) that completely block neuromuscular circuitry causing a total paralysis of each envenomated fish. The snail continues foraging and engulfing more debilitated fish, each then injected with the paralytic components of the venom. Since the fish that have been captured are paralyzed within the large rostrum of the snail, Conus geographus can continue foraging and scooping up as many debilitated fish as possible, even when multiple fish are already crammed into its large rostrum.
There are a number of anatomical and ecological factors consistent with the reconstructed picture above. Conus geographus is unusually agile, much more active, and capable of much faster locomotion than most cone snail species. Furthermore, it has an extremely light shell, which helps in its unusual agility. Uniquely, this species is able to secrete a mucus thread that functions much like a spider’s silk line; the snail can essentially levitate from one surface (such as the edge of a reef) and lower itself gradually. Thus, in the coral reef habitats in which the snail is found, if there are fish schools hiding in crevices in the reef, the snail can approach the school by levitating downwards with its mucus thread, opening its rostrum, efficiently delivering at close range, the venom components important for debilitating the fish in the pre-capture stage.
Deconstruction of the physiological strategy: correlating behavior with venom composition
The physiological strategy can readily be understood by elucidating the mechanism of individual venom components used by the snail for prey capture. In the pre-capture phase, selected venom components are released into the water, eliciting a change in behavior and physiology of the fish. The most straightforward venom components to understand is a “weaponized” insulin that Conus geographus has evolved. When added to water, this venom insulin elicits hypoactivity in fish (Safavi-Hemami et al. 2015b). It was demonstrated that this venom component (Con-Ins G1) causes blood glucose levels to fall through binding the fish insulin receptor (Safavi-Hemami et al. 2015b), and even potently binds to the human insulin receptor (Menting et al. 2016). As will be discussed in more detail below, this insulin has striking similarity in its sequence and structure to fish insulin (and is very divergent from the native insulins used for insulin signaling in the snail itself). Notably, unlike endogenous fish insulins that are secreted by the β-cells of the pancreas as hexameric complexes, designed to act over a long period of time, the cone snail insulin is a monomer that acts very quickly (Menting et al. 2016), reflecting its streamlined role in prey capture. When tested in zebrafish, after the insulin is released into the water, it immediately impairs the swimming behavior of fish (Safavi-Hemami et al. 2015b), and is presumably taken up through the gills of the fish from which it rapidly enters the blood stream. However, this is only one of the venom components believed to be released by the snail.
Other venom components affect signaling events in the sensory circuitry, and appear to be primarily targeted to the hair-cell/lateral line system of fish, that allow fish to sensitively detect motion in the water. Prominent among this group of venom components are Conantokin G, an inhibitor of the NMDA receptor (Mena et al. 1990), and Contulakin G, an agonist of the neurotensin receptor (Craig et al. 1999). In addition, other peptides in the venom may potentially cause a decrease in the excitability of the targeted sensory circuitry (these may include inhibitors of the 5HT3 serotonin receptor (England and Gulyas 1998), and an agonist of the vasopressin receptor (Nielsen et al. 1994)), but the role of these putative molecular targets in the sensory circuitry of fish has not yet been comprehensively characterized.
Once the debilitated fish have been captured by the snail, a specific set of venom components is then injected to cause irreversible paralysis. These components are among the best understood cone snail venom peptides, and when injected individually into fish, cause a neuromuscular paralysis (Olivera 1997b). Each venom component targets a specific pharmacological site. The overall effect is a complete and potent inhibition of neuromuscular transmission.
One of these components, ω-conotoxin GVIA, acts at the pre-synaptic terminus of the neuromuscular junction (Olivera et al. 1984). This peptide binds to the voltage-gated calcium channels that effectively transduce the electrical signal from the motor axon action potential into the biochemical events that result in neurotransmitter release (Sato et al. 1993). At the fish neuromuscular junction, the predominant calcium channels are the Cav 2.2 (N-type) Ca channel subtype, and blocking these causes paralysis. A second component acts on the post-synaptic site of the neuromuscular junction; this peptide, α-conotoxin GI is a competitive antagonist of the nicotinc acetylcholine receptor (Cruz et al. 1978). Thus, this small, 13 amino acid peptide is analogous to the major paralytic toxins in the venoms of cobra-like snakes (the α-neurotoxins, such as α-bungarotoxin and α-cobatoxin). In effect, even if the pre-synaptic terminus could release the neurotransmitter acetylcholine, in the presence of α-conotoxin GI, the muscle action potential is not initiated because the nicotinic acetylcholine receptors are completely blocked and therefore do not open when the neurotransmitter is released. Together, ω-conotoxin GVIA acting at the pre-synaptic terminus and α-conotoxin GI acting at the post-synaptic terminus are a total barrier to synaptic transmission between the motor axon and the skeletal muscle. It almost seems like overkill, but in addition there is a third very potent venom component, μ-conotoxin GIIIA, which blocks the muscle action potential (Cruz et al. 1985; Wilson et al. 2011). This peptide alone is sufficient to paralyze a fish (and is probably also a major factor in human fatality caused by defensive stings of Conus geographus). The failure to transmit action potentials along the plasmalemma of skeletal muscles inhibits muscle contraction, resulting in paralysis even under conditions where synaptic transmission is normal. Thus, how a fish that has been engulfed within the rostrum of Conus geographus ends up being totally paralyzed is understood in considerable mechanistic detail since these 3 venom components, shown in Table 1, have been extensively studied by many laboratories and have become standard pharmacological tools in neuroscience research.
The elucidation of how Conus geographus paralyzes fish demonstrated a general principle: in order to achieve a specific physiological endpoint, what has evolved is analogous to a combination drug strategy — in effect, the cone snails always use not just one pharmacological agent, but a combination to achieve a specific physiological endpoint. We have suggested that these combinations be called “cabals” (since cabals are secret societies out to overthrow existing authority) (Olivera 1997b). Thus, the combination of the 3 peptides, ω-conotoxin GVIA, α-conotoxin GI and μ-conotoxin GIIIA comprise the motor cabal, a potent combination to completely block neuromuscular transmission.
The other physiological endpoint that we described above is attained by the venom components used in the pre-capture strategy, which together are referred to as the “nirvana cabal” (Olivera 1997a). The effects of these venom components on fish appeared to be mimic heroin addicts in an opium den. The fish appear quiescent, sedated and blissed out. In fact, they are severly debilitated by the combination of sensory deprivation and hypoglycemia.
Physiology and Pharmacology of Prey Capture: Conus striatus and Conus purpurascens
Overview of the Physiological Strategy
The overall physiological strategy for capturing a fish is broadly similar for Conus striatus and Conus purpurascens, and therefore these will be discussed together. These two species are in fact representative of many other fish-hunting cone snails in their overall envenomation strategy for the successful capture of a fish. Fish can swim away from predators that they can detect; hence, most fish-hunting cone snail species are active at night when they can ambush fish that are typically hidden so they are not attacked by sharks and other nocturnal predators. However, if they have not fed for some time, Conus striatus and Conus purpurascens can both also be active during the day if fish are present. These two species may in fact have strategies to attract the fish to come towards them during the day or attract nocturnal fish species. Conus purpurascens has a bright red proboscis, and it has been suggested that it can use this as a lure to have fish move within striking range (Olivera et al. 2015). The yellow proboscis of Conus striatus may have a similar function.
When these snails strike, a harpoon-like tooth is ejected out of the proboscis with sufficient force to pierce the skin and scales of the fish (Schulz et al. 2004). Venom components known as the “lightning-strike cabal” are injected and cause instantaneous tetanic paralysis by massive depolarization of all of the axons in the neighborhood of the injection site (Olivera 1997a). This has the same effect as applying a powerful taser to the injection site, and as a result, the fish experiences an electrical storm in its nervous system, but one that is induced pharmacologically (not electrically by a taser gun or an electric eel). Once immobilized, the tethered fish is drawn into the rostrum of the snail where it is engulfed and a second set of toxins that act somewhat more slowly (the “motor cabal”) causes a final flaccid and irreversible paralysis (Olivera 1997a). Thus, envenomation by Conus striatus and Conus purpurascens results in the same endpoint as envenomation by Conus geographus: a paralyzed fish within the rostrum of the snail, where pre-digestion occurs. In all 3 cases, the hard parts of the fish (scales, bones) and the harpoons used for injecting venom, are regurgitated by the snail, and the soft parts of the partly digested fish move further down into the gut. Thus, there are clear similarities and differences between the overall strategy of Conus striatus and Conus purpurascens vs. Conus geographus.
Deconstruction of physiological strategies, similarities and differences between Conus striatus and Conus purpurascens
For both of these fish-hunting Conus species, the envenomation and capture of prey can be divided into two distinct phases — the initial phase is massive hyperactivation of electrical activity of axons near the site of injection (and therefore ultimately, across the entire nervous system). This is what underlies the tetanic paralysis of the fish observed within a few seconds in a typical strike. The core strategy to achieve this is to use at least two distinct classes of pharmacological agents that target the voltage-gated ion channels responsible for normal action potential generation. One set of components are hydrophobic peptides that have a common mechanism of action, the δ-conotoxins. These peptides inhibit Na channel inactivation by binding to the voltage sensor and keeping the activated channel in an open state (Leipold et al. 2005). The net result of δ-conotoxin activity is a much greater influx of Na+ ions across the membrane, therefore increasing the depolarization of the membrane. Na channel inactivation is a major factor in the normal repolarization of an axonal membrane after an action potential. What makes the injection of the venom much more potent is that the δ-conotoxins are always paired with K-channel inhibitors (collectively known as κ-conotoxins) that block voltage-gated K channels (Terlau et al. 1996). After an action potential, in addition to Na channel inactivation, membrane repolarization occurs because of the efflux of K+ ions through voltage-gated K channels, which open after Na channels are activated. The combination of Na channel inactivation and K channel activation are key to the kinetics of membrane repolarization and to the duration of an action potential. If both Na-channel inactivation and K-channel conductance are blocked, the major mechanisms for repolarization of the membrane after an action potential do not function, and therefore any action potential reaching these axons is no longer transient, but would trigger and maintain a massive depolarization of the axonal membranes. This would generate trains of action potentials, in both anterograde and retrograde directions, resulting in an electrical storm within the nervous system of the envenomated fish. A massive transmitter release at neuromuscular junctions results in a prolonged contraction of skeletal muscles, which is the macroscopic phenotype observed (Terlau et al. 1996). There is considerable structural diversity in venom peptides targeted to K channels; a number of putative K-conotoxin type excitatory peptides have been characterized (Jakubowski et al. 2005; Teichert et al. 2006; Dutertre et al. 2010; Chun et al. 2012) with likely multiple K channel targeted peptides in a single venom. There are additional venom components that facilitate the effects of the δ and κ toxins; together, these comprise what we call the “lightning-strike cabal”. Among those whose mechanism is understood are the con-ikot-ikot peptides. The massive depolarization induced by δ- and κ-conotoxins would not normally be transmitted through synapses with undiminished intensity. If there is excessive neurotransmitter release through synapses, this does not translate into a correspondingly increased electrical signal on the post-synaptic side, because the post-synaptic receptors desensitize rapidly under normal conditions. However, the con-ikot-ikot peptides prevent AMPA receptors (which are found in the hair-cell circuitry of fish) to be resistant to desensitization (Walker et al. 2009). They remain in an open state in the presence of glutamate, and therefore the hyperexcitability in pre-synaptic axons is faithfully transmitted across the synapse, with a corresponding hyperexcitability of post-synaptic axons as well. There are several other excitatory peptides found in the venoms of Conus striatus and Conus purpurascens whose mechanisms have not been firmly established that likely contribute to, or facilitate the effects of the lightning-strike cabal (Kelley et al. 2006). A diverse set of excitatory peptides have been found in fish-hunting venoms, some of which may not be part of the lightning-strike cabal but have other physiological functions.
A comparison of the species described above Conus geographus, Conus striatus and Conus purpurascens can be carried out at several different levels. Conus striatus and Conus purpurascens are much more similar to each other behaviorally, and in overall physiological strategy for capturing prey; Conus geographus is clearly divergent in these respects from the two other species. Nevertheless, the genetic evidence reflected in the phylogenetic tree in Fig. 1 suggests that Conus striatus is in fact more closely related to Conus geographus than to Conus purpurascens. A comparison of the deconstructed mechanisms provides additional evidence for the more recent divergence of Conus striatus from Conus geographus. There are greater similarities at the mechanistic and molecular/biochemical level than is obvious from comparing behavior and macro-physiological mechanisms.
This is most strikingly illustrated by the “motor cabals” that have been elucidated for these 3 species. All piscivorous cone snails, as an endpoint of envenomation, achieve a complete paralysis of prey within the rostrum of the snail where pre-digestion will take place. Thus, all fish-hunting snails presumably have evolved a motor cabal, the components of which lead to an inhibition of neuromuscular transmission. The motor cabals of Conus geographus and Conus striatus are more similar to each other at the mechanistic level, and in their overall pharmacological targeting. Thus, both snails inhibit pre-synaptic Ca channels, and have evolved toxins that belong to the same gene family, the ω-conotoxins (Ramilo et al. 1992; Cruz et al. 1985). These have not been detected in C. purpurascens venom. Similarly, both snails inhibit the post-synaptic nicotinic acetylcholine receptor, using peptides that are structurally very similar, the α-conotoxins that belong to the α3/5 subfamily (Zafaralla et al. 1988; Cruz et al. 1978). Both of these components are absent in Conus purpurascens venom. Instead, Conus purpurascens uses another major inhibitor of the post-synaptic receptor, a structurally divergent peptide, αA-conotoxin PIVA (Hopkins et al. 1995). All of these are competitive antagonists of the nicotinic receptor.
Furthermore, it appears that while Conus striatus and Conus geographus both have multiple toxins for inhibiting neurotransmitter release by the pre-synaptic terminus through a block of voltage-gated Ca channels, the strategy that has evolved in Conus purpurascens is to have multiple toxins inhibit the post-synaptic nicotinic acetylcholine receptor. In addition to competitive antagonists, such as αA-conotoxin PIVA, non-competitive peptides, the ψ-conotoxin, are found in Conus purpurascens venom (Van Wagoner et al. 2003) (and are absent in Conus geographus and Conus striatus). The only known homologous components that are used by all 3 species to block the same molecular target at the neuromuscular circuitry are the μ-conotoxins that block the pore of the muscle Na channel subtype, Nav1.4 . A summary of these comparisons is shown in Table I. The similarities in the motor cabal of Conus geographus and striatus, and the divergence from Conus purpurascens suggest that when Conus striatus and Conus geographus diverged, their common ancestor was already piscivorous. Presumably, this ancestral species already expressed ω-conotoxins, α-conotoxins belonging to the α3/5 subfamily and μ-conotoxins in its venom to paralyze fish.
Table I.
Comparison of Motor Cabal Components
| Conus species | Specific Toxin | Sequences |
|---|---|---|
| Molecular Target: Post-synaptic nicotinic acetylcholine receptors | ||
| C. geographus | α GI |
|
| α GII |
|
|
| C. striatus | α SI |
|
| α SIA |
|
|
| C. purpurascens | αA PIVA |
|
| α PIA |
|
|
| φ PIIIE |
|
|
|
| ||
| Molecular Target: Pre-synaptic Ca Channels | ||
| C. geographus | ω GVIA |
|
| C. striatus | ω SVIB |
|
| C. purpurascens | (none found) | |
|
| ||
| Molecular Target: Na channels, Nav1.4 | ||
| C. geographus | μGIIIA |
|
| C. striatus | μSIIIA |
|
| C. purpurascens | μPIIIA |
|
Evolutionary Insights
Origins of fish hunting: evidence from the 3 fish-hunting Conus species
The analysis of mechanisms that underlie prey capture suggest the following evolutionary scenario: in the Indo-Pacific, an ancestral worm-hunting species led to a fish-hunting descendant which is potentially the ancestor of all fish-hunting cone snails (in 7 different subgenera) now found across the Indo-Pacific region (Olivera et al. 2015). However, this conversion from vermivory to piscivory did not occur only once; in the New World, a parallel event occurred, but this gave rise only to one living lineage, Chelyconus, with two extant species, Conus purpurascens in the Panamic marine province of the eastern Pacific, and Conus ermineus, which is spread throughout the tropical Atlantic (one of the very few Conus species that is found on both sides of the Atlantic). In addition to the analysis of the motor cabal mechanisms described above, a comparison of the lightning-strike cabal of Conus purpurascens vs. Conus striatus provides additional evidence for this scenario. If the two core components of the lightning-strike cabal, the peptides which prevent inactivation of voltage-gated Na channels and those that block K channels are compared, it is found that the δ-conotoxins are homologous and belong to the same genetic group in both species (in technical terms, the O1-gene superfamily). These δ-toxins that inhibit Na channel inactivation are in fact found broadly across the genus Conus, not only in fish-hunting species, but in mollusc-hunting and worm-hunting species as well (Fainzilber et al. 1991; Aman et al. 2015; Jin et al. 2015). These all share the same structural framework and hydrophobic character. Thus, early in the evolution of the genus Conus, δ-conotoxins probably became an integral part of the venom repertoire (Aman et al. 2015).
The contrast between δ-conotoxins that target voltage-gated Na channels and those peptides that block voltage-gated K channels (the pharmacological class of κ-conotoxins) could not be greater. The latter significantly diverge when different Conus subgenera are compared. Thus, there are several putative toxins that inhibit K channels in Conus striatus, and the best characterized are a class of peptides known as conkunitzins. The particular peptide found in Conus striatus venom, conkunitzin S1 blocks K channels that belong to the Shaker subfamily (Bayrhuber et al. 2005). In contrast, the peptide that blocks voltage-gated K channels in Conus purpurascens venom is entirely different in structure, and genetically (it belongs to the O1 gene superfamily) (Terlau et al. 1997). This peptide, κ-conotoxin PVIIA is unrelated to conkunitzin S1 found in Conus striatus venom, which belongs to the conkunitzin gene superfamily (Bayrhuber et al. 2005).
These results suggest a specific scenario for the origin of fish hunters. Certain lineages of worm-hunters were pre-adapted in that they had evolved δ-conotoxins that would deter fish from competing for their worm prey. If Na channels do not inactivate, this could cause firing of sensory circuitry leading to pain, and therefore this would be a good weapon to deter fish competitors (or potential predators). However, if in addition, a K-channel targeted peptide had been generated, then when fish were stung, it would not only feel pain, but go into a tetanic paralysis. This could therefore be the initiating event transforming a worm-hunting cone snail to a piscivorous lineage. This suggests that in the New World, an O1-superfamily peptide had sufficient affinity for voltage-gated K-channel in fish to cause the transition, while in the Indo-Pacific, a conkunitzin peptide may have been responsible. From this initial lineage of fish-hunting cone snails that primarily use the lightning-strike cabal to capture fish, a motor cabal was added, making certain that the fish prey became completely and irreversibly paralyzed within the rostrum of the snail.
We suggest that from the Indo-Pacific ancestor, the evolution of the Conus geographus lineage was a later stage. The motor cabal components remain homologous in structure and sequence in all fish-hunting cone snails in the Indo-Pacific, but the ability to capture multiple fish may have led to a replacement of lightning-strike cabal components by the nirvana cabal to debilitate multiple fish at one time. Thus, the ancestral state for fish-hunting Conus are lightning-strike cabal components, and a signature that is conserved from worm-hunting ancestors are δ-conotoxins that target voltage-gated Na channels in fish.
Other evolutionary insights from venom components
In the sections above, we have focused only on 3 species of Conus. We discuss general insights from the work that has been done on other Conus venom components to date. The genes encoding these venom components are among the fastest evolving genes known (Sunagar and Moran 2015; Chang and Duda 2012). Essentially, there is no overlap between the venom components of two different Conus species, even between closely related sister species (Barghi et al. 2015). Although there clearly is considerable conservation in the physiological strategy (and, as shown for Conus striatus and Conus purpurascens some convergent evolution) at the molecular level, conotoxin genes are subject to hypermutation and high rates of gene duplication coupled with positive selection (Chang and Duda 2012; Duda and Palumbi 1999), and each species has its own distinctive venom complement. The rationale that has been offered is that since venom peptides are meant to act on other animals in the same ecological niche as that specific Conus species, and every species presumably has its own distinctive ecological niche, then the precise spectrum of prey, predators and competitors differ for each species. Since the venom genes essentially reflect the biotic interactions of a particular species, these are the genes that need to change so that at the species level, the animal is as fit as possible for a particular ecological niche, and therefore these are the genes that diverge between species (Zugasti-Cruz et al. 2006). Thus, when combined with the combination strategy that the snails employ, these factors predict an enormous biochemical and pharmacological diversity of natural products (venom peptides) that have evolved in the 700 species of the genus Conus. Because of the accelerated evolution possible in this system, as a whole, the genus can adapt to ecological changes rapidly, which accounts for the generation of so much biodiversity.
Nevertheless, some intriguing evolutionary questions remain to be addressed, which are raised by the data collected so far. We have discussed Conus striatus and Conus purpurascens, and the fact that they occur in different biogeographic areas. What is remarkable however is that the subgenus to which each belongs is strikingly different in terms of species richness. Chelyconus, the only fish-hunting clade in the New World has only two known species, both broadly distributed biogeographically. In contrast, Pionoconus has 10 times as many species, and furthermore, it is not the only fish-hunting clade in the Indo-Pacific, but 6 other subgenera are present, one of which (Phasmoconus) is even more species rich (Olivera et al. 2015). Why has there been so much speciation of fish-hunting cone snails in the Indo-Pacific region, and essentially a single species in the entire Panamic and another closely-related one in the Atlantic Ocean (these two species, Conus purpurascens and Conus ermineus were clearly a single species until they became geographically isolated from each other). Is the lack of speciation in the New World because the initiating event that led to fish hunting from vermivory was much more recent? Is the reason ecological — are there more opportunities for different ecological niches in the Indo-Pacific? Each of these hypotheses makes very different predictions, which in principle can be tested in the near future.
Evolution viewed through a single locus: the venom insulin genes
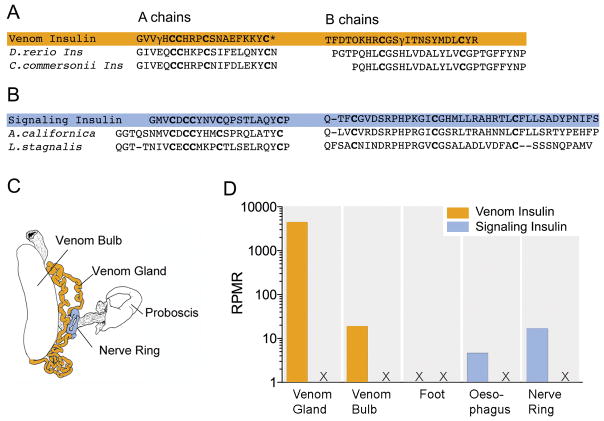
Another way to gain an overview of the evolution of the whole genus is by focusing on a single gene, and a particularly revealing genetic locus is that which codes for the venom insulin. As we outlined above, this was first discovered in Conus geographus as a component of the nirvana cabal; in essence, Conus geographus uses this to debilitate its prey by making it hypoglycemic (Safavi-Hemami et al. 2015a). Using transcriptome analysis, we can now query which venoms contain an insulin and which do not. For example, no insulin is expressed at a detectable level in either Conus striatus or Conus ermineus, a close relative of Conus purpurascens from the Chelyconus subgenus (transcriptome data for Conus purpurascens is not yet available). The advantage of using insulin as an evolutionary marker is because the insulin peptide superfamily (comprising insulin, insulin-like peptides, insulin-like growth factors and relaxins) is very well characterized in many vertebrates and invertebrates species (Blumenthal 2010; Ebberink et al. 1989; Floyd et al. 1999; Shabanpoor et al. 2009; Smit et al. 1998). The physiological function of insulin has remained mostly conserved throughout evolution, including regulation of glucose homeostasis, neuronal signaling, memory and reproduction (Ebberink et al. 1989; Blumenthal 2010). However, structurally insulins expressed in molluscs can be easily distinguished from their vertebrate homologues; in all vertebrates, including fish, insulin consists of A and B chains, cross-linked by two disulfide bonds and a third disulfide bridge within the A chain (Adams et al. 1969). Molluscan insulins are distinct in having longer A and B chains and an additional disulphide bridge between the A and B chain (Ebberink et al. 1989). As shown in Figure 6, transcriptome analysis of different tissues of C. geographus and several other species revealed that cone snails express at least two classes of insulins: a highly conserved insulin is expressed in their neuroendocrine tissue (presumably this conventional signaling insulin is finely tuned to the Conus insulin receptor, which also evolves very slowly) and a rapidly diverging insulin that is specifically expressed in the snail’s venom gland (Safavi-Hemami et al. 2016). These two distinct classes are referred to as signaling and venom insulins. Dramatically elevated evolutionary rates for the venom insulin class strongly suggests that these venom components frequently experience directional selection to target heterospecific insulin receptors in a changing mix of prey, predators and competitors. This is best illustrated for Con-Ins G1, the venom insulin discovered and functionally characterized from Conus geographus (Safavi-Hemami et al. 2015b). Unlike molluscan signaling insulins, Con-Ins G1 shares the disulfide framework and sequence length with signaling insulins found in fish. Moreover, (see Figure 6) ~ 90 % of amino acids of the A chain are similar to fish insulins. Similarities between Con-ins G1 and fish insulins are lower in the B chain (~70%). However, these differences render Con-Ins G1 faster acting than fish insulin and highlight its streamlined role for predation (Menting et al. 2016). Similarly, mollusc-hunting snails, such as Conus textile and Conus marmoreus, express a venom insulin that has more similarities to endogenous molluscan insulins than to any other group, including fish and worm insulins (Safavi-Hemami et al. 2015b). Thus, had one not already known that Conus geographus preys on fish and Conus textile and Conus marmoreus are snail hunters one could have easily deduced this information from investigating their venom insulin sequences.
Figure 6.
Similarities in the cysteine arrangement, chain lengths, and amino acid residues between the A and B chains of (A) the venom insulin Con-Ins G1 from Conus geographus and fish insulins from Danio rerio (zebrafish) and Catostomus commersonii (white sucker) and (B) between the cone snail signaling insulin and endogenous insulins from the 2 molluscs Aplysia californica (Californian sea hare) and Lymnea stagnalis (giant pond snail), highlighting that Con-ins G1 specifically evolved to mimic fish insulin in order to efficiently target the metabolism of its fish prey. (C) Anatomical drawing showing the two tissue types with highest expression levels of venom- and signaling insulins in Conus geographus. (D) Venom insulins were most highly expressed in the venom gland whereas signaling insulins were only detected in tissues associated with neuroendocrine function (oesophagus and nerve ring). Numbers represent insulin sequencing reads per million total reads (rprm, log scale). Adapted from (Safavi-Hemami et al. 2016).
Distribution of venom insulins in Conus
The pattern of venom insulin expression within Conus initially seems random; some species of Conus do not appear to express any venom insulin, such as Conus striatus and Conus purpurascens, the two species described above. However, venom insulins are broadly expressed across the genus (Safavi-Hemami et al. 2016). Not only are they found in fish-hunting cone snails such as Conus geographus (and its close relative Conus tulipa), but in some other lineages of fish-hunting cones (but not in others), as well as in some mollusc-hunting and worm-hunting species. So far, the best explanation for the evolution of venom insulin in some species is that it may be part of a “pre-capture strategy”, similar to that used by Conus geographus.
In two cases where venom insulin is expressed in other Conus species, the snails do not exhibit the rostrum-engulfment behavior of Conus geographus and Conus tulipa. Instead in one fish-hunting species (Conus c.f. sulcatus) and a mollusc-hunting species (Conus textile) that express venom insulin, it appears that the snail injects its prey multiple times (unpublished observations). Thus, venom insulins may be used as part of a “pre-capture strategy”, debilitating the prey with an initial bolus of insulin and other venom components that is injected at a distance, without tethering the prey. Once prey becomes unable to defend itself or to escape from the initial bolus of venom components injected, this then allows the cone snail to inject another set of venom components that ultimately result in the complete paralysis of the prey. However, this is just an initial correlation between behavior and the presence or absence of venom insulins — notably, Conus striatus and Conus purpurascens only sting their prey once, and therefore in a sense, there is no separate, defined pre-capture stage. Of course, with the lightning-strike cabal components of their venoms, it would seem that the effects of insulin on the prey would be superfluous and unnecessary, given the very rapid immobilization elicited by other venom components in these species.
Bioactive cone snail venom components do not have to be peptidic or polypeptidic
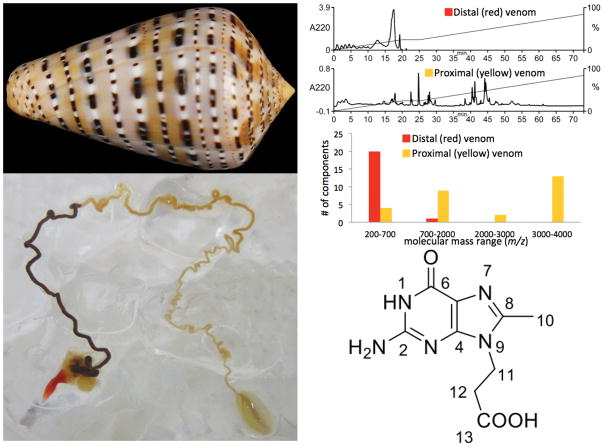
It is possible to infer that some Conus species use a pre-capture strategy, even though their behavior has never actually been observed. The evidence for this is that some Conus have venom glands that are clearly compartmentalized into distinct sectors. One of these is the Eastern Atlantic species Conus genuanus, which has a dramatic difference in color between the venom duct closest to the pharynx and proboscis (which would therefore be expected to be extruded from the gland and injected into prey first), while the segment proximal to the venom bulb is whitish in color (see Figure 7) (Neves et al. 2015). These two segments of the duct have recently been analyzed, and a surprising result was obtained: the components in the red segment (presumably used in a pre-capture strategy) are all small molecules with very minor peptidic or polypeptidic components detected. The bioactivity of the venom in this segment of the venom gland was analyzed, and neuroactivity was assessed both by direct injection into the CNS of a mouse, as well as effects on dissociated sensory neurons using a calcium imaging method, Constellation Pharmacology.
Figure 7.
Analysis of Conus genuanus venom glands. Top left, the shell of Conus genuanus, a worm-hunting species. Lower left, the venom duct of a specimen collected in the Cape Verde Islands; note the red segment close to the pharynx, and the yellowish segment proximal to the venom bulb. Upper right, analysis of the venom by high-performance liquid chromatography and by mass spectrometry. Note that the venom components in the red segment elute earlier on the HPLC column, and have a lower molecular weight, consistent with most of these bing small molecule natural products, and not peptides, which are the major components of the yellowish segment of the venom gland. Lower right, the structure of genuanosine, a bioactive component isolated from the red segment of the venom gland. This unique derivative of guanine has never previously been characterized from any source, and therefore is a unique bioactive natural product, the first small molecule venom component from a cone snail that may be part of its “pre-capture strategy”.
A bioactive component of the red segment of the duct was purified and characterized, and surprisingly, proved to be a unique nucleotide (see Figure 7) (Neves et al. 2015). The work on Conus genuanus venom is therefore notable in that it unequivocally demonstrates that bioactive components of Conus venoms are not necessarily peptides or polypeptides, but may be small molecule natural products. Thus, it is likely that small molecule natural products are widely distributed in Conus venoms, but because these are generally more difficult to characterize than the disulfide-rich peptides, this is the first chemical characterization of any bioactive non-peptidic venom component (other than serotonin) (McIntosh et al. 1993) reported in the literature.
Implications for Future Research on the Chemical Biology of Natural Products
The case studies described above integrating behavior of cone snails and their fish prey with the natural products that mediate these interactions provide the basis for a potential new framework for investigating natural products. At the level of whole organisms, a key issue that needs to be addressed is what behavioral changes do natural products elicit in a targeted animal that are advantageous to the producer? The cone snail case studies suggest that in order to effectively achieve changes in behavior, there are specific physiological endpoints that must be achieved with multiple natural products involved, each acting through its own specific mechanism. The idea that the producer evolved a “cabal” of natural products to change the behavior of potential predators, prey or competitors is strongly suggested by the three case studies analyzed. In parallel, each individual natural product needs to be comprehensively characterized to be able to address its native role; it is insufficient to just carry out a chemical and biochemical characterization. Every natural product presumably has a specific molecular mechanism, and must bind to a physiologically-relevant molecular target in the organism whose behavior the producer wants to alter. How binding of the natural product to the molecular target changes the function of that molecular target, and the circuit in which the molecular target functions will also need to be elucidated. In this context, the results obtained from the cone snail case studies so far are highly relevant for charting out what we might expect in the future. Two components of nirvana cabal from Conus geographus have been investigated in a pre-clinical setting for the treatment of epilepsy (conantokin-G) and chronic pain (contulakin-G) and the venom insulin from the same species holds potential for diabetes. Thus, addressing the central biological issues regarding chemical interactions between one cone snail species, Conus geographus, has led to the identification of three drug targets.
Indeed, even for the best-studied cone snail, Conus geographus, there is much that remains to be done. Thus, the presence of a second venom insulin that is similar to the endogenous insulins of molluscs suggests that this venom component is not targeted to the fish prey of Conus geographus, but rather to molluscan predators or competitors. At the macroscopic level, we do not know the spectrum of potential predators and competitors on which this particular venom component is meant to act. Thus, of the ca. 100 different venom components expressed in the venom gland of Conus geographus, to date we only understand the probable physiological role of a minor fraction of these and even though this species is the most comprehensively understood at the present time. This underscores the extent of our ignorance, and highlights the opportunities available in integrating natural products research with the study of the behavior of organisms and their biotic interactions.
In rich biodiverse marine communities such as coral reefs, chemical interactions between organisms may be particularly intense. A specific biological scenario where there is clear evidence for complex chemical interactions is in how planktonic larvae of such animals as ascidians, barnacles or bivalves such as oysters, make a decision where to settle down for their sessile adult form (Fusetani 2011). Clearly, the organisms that have already settled at a site have a strong interest in who their neighbors are going to be, and evidence that the residents can send both attractant and deterrent natural product signals to larvae that are swimming around has been obtained, but the neuroethological mechanisms and the molecular targets for each individual natural products are not known.
General issues raised regarding future natural product research
The work on Conus venom peptides suggests that to be effective biological agents at the macroscopic level, multiple natural products, each acting through a different molecular mechanism, are probably required to effectively change the behavior of the targeted animal. Thus, we can expect suites of natural products to act together; it clearly will be a challenge to integrate these bioactivities. A second insight from the work on cone snail venoms is that because each species has its own spectrum of potential prey, predators and competitors, the natural products used for chemical interactions will be highly species-specific. The implications for the chemical diversity that this would generate are staggering.
If organisms have evolved so many different natural products, why has it been a challenge to identify and characterize these? One possible reason is the lack of biological insights in searching for natural products. The typical scenario used to identify bioactive natural products from the marine environment is to harvest 1 kilo of every organism found, preserve the tissue and later grind it up and each sample so collected is analyzed, for bioactivity using some set of assays. If this approach had been used on cone snails, it is doubtful that any of the venom peptides would have been detected. Not surprisingly, the natural product community has focused on sessile marine organisms, such as sponges and ascidians, largely because these have been productive in yielding novel natural products. However, it is likely that only the major defensive compounds, spread throughout the body can be detected by standard methods. Most animals would be expected to produce natural products in specialized glands and tissues (such as the cone snail venom glands). Indeed, even in sessile invertebrates when distributions of natural products in animals is studied, precise patterns are observed that reflect the ecology of compounds (Sharp et al. 2007; Thompson 1985). It seems reasonable to hypothesize that in order to understand chemical interactions, knowledge about the anatomy of the producer organisms is key to knowing where to look. If this hypothesis is true, then a vast, previously hidden chemical diversity awaits discovery. The technological improvement in analytical techniques as well as the possibility of using genomic approaches makes the exploration of this putative, uncharted chemical diversity eminently feasible at the present time.
A final issue is whether insights gained from cone snail venom components will apply generally to animals that produce non-peptidic natural products. One point of view is that the delivery system which all venomous animals have evolved has lowered the barrier to evolving a complex suite of natural products. In the Conoidean snails, it is likely that each of the over 10,000 species has a distinct repertoire of between 70–200 genes encoding bioactive venom components. Should we expect a similar number of natural products from a predatory, non-venomous neogastropod snail? If indeed the evolution of a delivery system has allowed venomous animals to more easily generate a rich diversity of bioactive compounds acting on their targeted prey, predators and competitors, then the prediction would be that non-venomous animals are likely to have evolved a smaller number of bioactive natural products to interact with other animals in their environment.
However, there are a number of indications that this is not necessarily true. Many marine natural products are in fact generated by bacterial symbionts (Haygood et al. 1999); this allows the very rapid evolution of new compounds, since novelty is not limited by the generation time of the marine animal itself, but rather can proceed much more rapidly through the symbionts. Single microbial genomes can hold upwards of 10 or more natural product biosynthetic pathways, leading potentially to hundreds of products. For example, the human microbiota has the capacity to produce thousands of natural products (Cimermancic et al. 2014; Donia et al. 2014). Furthermore, symbiotic bacteria have recently been shown to use biosynthetic pathways for their natural products that are “diversity generating”, meaning that the pathways are amenable to creating many different compounds (Tianero et al. 2016).
Is it possible then that while Conoidean snails generate the large majority of the natural products that they use for chemical interactions through genes encoded in their genome, that other neogastropods generate non-peptidic natural products? These could be made by the animals, or more efficiently by cultivating the appropriate bacterial symbionts. Would we also expect between 70–200 genes operating, both in the genome of the producer marine animal itself as well as in the metagenome comprising its spectrum of bacterial symbionts, and that together these generate an equal complexity of non-peptidic natural products? These are clearly issues for which we do not yet have definitive answers, but which can be addressed; the discovery that even cone snails produce non-peptidic natural products suggests that in any case, there will be a substantial chemical diversity that can be accessed, if the biology and chemistry are integrated together — this is what will break open the scientific field that lies between Neuroethology and research on natural products as it is carried out today, a field that we suggest be called “Chemical Neuroethology”.
Acknowledgments
The research of the authors was supported by the National Institute of General Medical Science GM048677 (to BMO), Marie Curie Fellowship (to HS-H), Esther Fujimoto Memorial Fellowship (to SR), and by NIH U01TW008163(to EWS).
Appendix
The model of Conus geographus behavior coordinated with envenomation described above comes from decades of study in multiple labs; a recent publication (Dutertre et al. 2014b) would modify this model. We believe that the interesting hypothesis proposed in this publication requires more experimental data; an explanation that rationalizes all of the present experimental observations is provided below.
The prey capture observations on Conus geographus described in the text above were based on hundreds of specimens, collected from various localities in the Philippines. These observations have been widely corroborated, including in a publicly accessible video (see C. geographus, Silent Assassin from the BBC that shows a freely moving Conus geographus engulfing an enormous fish without extending its proboscis. In this video, after being totally engulfed, the fish struggles and jerks, and then becomes paralyzed, presumably in response to the envenomating sting. See https://www.youtube.com/watch?v=FYh2zeAsRXY). However, a diversity of foraging behavior has been reported for Conus geographus from some Australian localities; as documented by Bingham and co-workers for Australian specimens (Bingham et al. 2012). Even in this survey of Australian specimens, most C. geographus engulf their prey before envenomation, but a minority (5/27) were observed to extend their proboscis in the presence of prey. Behavioral divergence between different populations of conspecific cone snails is clearly a topic that needs to be systematically explored.
Based on their study of the minor fraction of Australian Conus geographus that extend their proboscis when presented with fish prey, Dutertre, Lewis and coworkers proposed that Conus geographus could secrete two types of venom, one for predation and another exclusively for defense. In essence, the predation venom components were equivalent to the lightning-strike cabal described in this review, while the defensive venom contained motor cabal components. An alternative explanation for the observations on the behavioral cadre of Conus geographus that inject venom before completely engulfing their prey is that motor cabal components are used both for prey capture, as well as for defense. This is supported by findings of Dutertre et al. (2014) on the presence of motor cabal toxins (μ-GIIIA and ω-GVIA) in the defense-evoked venom and a study by Bingham et al. (2012) that showed that these compounds were also present in the predatory venom of C. geographus.. The ecological conditions of the Australian Conus geographus cadre presumably make direct injection of lightning-strike cabal components more effective than release into the water (but would therefore result in the snail only being able to capture one fish prey per foraging event). Conus geographus specimens from the Philippines can be fed fish sequentially in aquaria (which is not true for other fish-hunting Conus species), and after being fed one or two fish, if the snail is presented with another fish prey, it opens its rostrum and totally paralyzed fish are visible within the open rostrum. These observations are consistent with the snail being able to inject multiple prey sequentially, and suggest that paralytic components of venom are injected into each engulfed fish. In contrast, when the snail is threatened, it can clearly inject a larger amount of venom (we have observed that if the shell of a Conus geographus specimen is being broken, it will extend its proboscis and spray out several hundred microliters of venom). Thus, several experimental questions should be addressed for the Australian Conus geographus with the unusual behavior. Do these snails sting fish prey a second time after they are engulfed? How much does the volume of venom injected vary in the two types of stings as the stimulus is varied? These are experimental issues that need to be resolved to elucidate the role of motor cabal components. A fundamental conceptual issue is that there is no evidence that fish are the main predators—why would venom components purely evolved for defense be so specific for the vertebrate neuromuscular junction; this suggests that selection pressure was based on the prey type.
Professor J.P. Bingham, University of Hawaii, is probably the most knowledgeable authority on the ecology of Conus geographus in Australia, and has kindly provided a potential rationale for the divergence in behavior observed in some Australian specimens. C. geographus that extend their proboscis towards fish prey are enriched in the Boult Reefs at the Great Barrier Reef in which staghorn coral is prevalent (Bingham et al. 2012). This may be an environment in which releasing venom components and engulfing prey is a less effective strategy, selecting for behavior in which the snails directly inject lightning-strike cabal venom components into potential prey. This possibility is made more likely by our unpublished observations that certain lineages of fish-hunting cone snails that do appear to have lightning-strike cabal-like components inject their prey twice, the first injection resulting in quiescent (but not paralyzed) fish.
The diversity of prey capture behavior documented above is presumably reflected in variability in venom composition. The striking interspecific divergence in the venom components of different Conus species is well documented, but the extent of intraspecific divergence is less well studied. However, a number of recent studies have been carried out, both on Conus geographus and other fish-hunting cone snails which allow a comparison between venoms of different individuals of the same species; these reports suggest considerable intraspecific variation in venom components (Rodriguez et al. 2015; Dutertre et al. 2014a; Dutertre et al. 2010; Himaya et al. 2015) when these analyses are carried out on milked venom of the same individual (Biass et al. 2009); the results also suggest that even within a single individual, there can be variation in venom components as a function of time.
References
- Adams MJ, Blundell TL, Dodson EJ, Dodson GG, Vijayan M, Baker EN, Harding MM, Hodkin DC, Rimmer B, Sheat S. Structure of Rhombohedral 2 Zinc Insulin Crystals. Nature. 1969;224:491–495. doi: 10.1038/224491a0. [DOI] [Google Scholar]
- Aman JW, Imperial JS, Ueberheide B, Zhang M-M, Aguilar M, Taylor D, Watkins M, Yoshikami D, Showers-Corneli P, Safavi-Hemami H, Biggs J, Teichert RW, Olivera BM. Insights into the origins of fish hunting in venomous cone snails from studies of Conus tessulatus. Proc Natl Acad Sci USA. 2015;112 doi: 10.1073/pnas.1424435112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barghi N, Concepcion GP, Olivera BM, Lluisma AO. Comparison of the Venom Peptides and Their Expression in Closely Related Conus Species: Insights into Adaptive Post-speciation Evolution of Conus Exogenomes. Genome biology and evolution. 2015;7(6):1797–1814. doi: 10.1093/gbe/evv109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayrhuber M, Vijayan V, Ferber M, Graf R, Korukottu J. Conkunitzin-S1 is the first member of a new Kunitz-type neurotoxin family. Structural and functional characterization. J Biol Chem. 2005:280. doi: 10.1074/jbc.C500064200. [DOI] [PubMed] [Google Scholar]
- Biass D, Dutertre S, Gerbault A, Gerbault A, Menou J-L, Offord R, Offord R, Favreau P, Stocklin R, Stocklin R. Comparative proteomic study of the venom of the piscivorous cone snail Conus consors. J Proteomics. 2009;72(2):210–218. doi: 10.1016/j.jprot.2009.01.019. [DOI] [PubMed] [Google Scholar]
- Bingham J-P, Baker MR, Chun JB. Analysis of a cone snail’s killer cocktail – The milked venom of Conus geographus() Toxicon : official journal of the International Society on Toxinology. 2012;60(6):1166–1170. doi: 10.1016/j.toxicon.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal S. From insulin and insulin-like activity to the insulin superfamily of growth-promoting peptides: a 20th-century odyssey. Perspectives in biology and medicine. 2010;53(4):491–508. doi: 10.1353/pbm.2010.0001. [DOI] [PubMed] [Google Scholar]
- Chang D, Duda TFJ. Extensive and continuous duplication facilitates rapid evolution and diversification of gene families. Molecular Biology and Evolution. 2012;29(8):2019–2029. doi: 10.1093/molbev/mss068. [DOI] [PubMed] [Google Scholar]
- Chun JB, Baker MR, Kim DH, Leroy M, Toribo P, Bingham JP. Cone snail milked venom dynamics--a quantitative study of Conus purpurascens. Toxicon. 2012;60(1):83–94. doi: 10.1016/j.toxicon.2012.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimermancic P, Medema MH, Claesen J, Kurita K, Wieland Brown LC, Mavrommatis K, Pati A, Godfrey PA, Koehrsen M, Clardy J, Birren BW, Takano E, Sali A, Linington RG, Fischbach MA. Insights into secondary metabolism from a global analysis of prokaryotic biosynthetic gene clusters. Cell. 2014;158(2):412–421. doi: 10.1016/j.cell.2014.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AG, Norberg T, Griffin D, Hoeger C, Akhtar M, Schmidt K, Low W, Dykert J, Richelson E, Navarro V, Mazella J, Watkins M, Hillyard D, Imperial J, Cruz LJ, Olivera BM. Contulakin-G, an O-glycosylated invertebrate neurotensin. J Biol Chem. 1999;274 doi: 10.1074/jbc.274.20.13752. [DOI] [PubMed] [Google Scholar]
- Cruz LJ, Gray WR, Olivera BM. Purification and properties of a myotoxin from Conus geographus venom. Archives of Biochemistry and Biophysics. 1978;190:539–548. doi: 10.1016/0003-9861(78)90308-9. [DOI] [PubMed] [Google Scholar]
- Cruz LJ, Gray WR, Olivera BM, Zeikus RD, Kerr L, Yoshikami D, Moczydlowski E. Conus geographus toxins that discriminate between neuronal and muscle sodium channels. J Biol Chem. 1985;260 [PubMed] [Google Scholar]
- Davies J. Are antibiotics naturally antibiotics? J Ind Microbiol Biotechnol. 2006;33(7):496–499. doi: 10.1007/s10295-006-0112-5. [DOI] [PubMed] [Google Scholar]
- Donia MS, Cimermancic P, Schulze CJ, Wieland Brown LC, Martin J, Mitreva M, Clardy J, Linington RG, Fischbach MA. A systematic analysis of biosynthetic gene clusters in the human microbiome reveals a common family of antibiotics. Cell. 2014;158(6):1402–1414. doi: 10.1016/j.cell.2014.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duda TF, Palumbi SR. Molecular genetics of ecological diversification: Duplication and rapid evolution of toxin genes of the venomous gastropod Conus. Proceedings of the National Academy of Sciences. 1999;96:6820–6823. doi: 10.1073/pnas.96.12.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutertre S, Biass D, Stöcklin R, Favreau P. Dramatic intraspecimen variations within the injected venom of Conus consors: An unsuspected contribution to venom diversity. Toxicon. 2010;55(8):1453–1462. doi: 10.1016/j.toxicon.2010.02.025. doi: http://dx.doi.org/10.1016/j.toxicon.2010.02.025. [DOI] [PubMed] [Google Scholar]
- Dutertre S, Jin A-H, Alewood PF, Lewis RJ. Intraspecific variations in Conus geographus defence-evoked venom and estimation of the human lethal dose. Toxicon. 2014a;91:135–144. doi: 10.1016/j.toxicon.2014.09.011. doi: http://dx.doi.org/10.1016/j.toxicon.2014.09.011. [DOI] [PubMed] [Google Scholar]
- Dutertre S, Jin A-H, Vetter I, Hamilton B, Sunagar K, Lavergne V, Dutertre V, Fry BG, Antunes A, Venter DJ, Alewood PF, Lewis RJ. Evolution of separate predation- and defence-evoked venoms in carnivorous cone snails. Nature Communications. 2014b;5:3521. doi: 10.1038/ncomms4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebberink RHM, Smit AB, Van Minnen J. The Insulin Family: Evolution of Structure and Function in Vertebrates and Invertebrates. Biological Bulletin. 1989;177:176–182. [Google Scholar]
- England LJ, Gulyas J. Inactivation of a serotonin-gated ion channel by a polypeptide toxin from marine snails (vol 281, pg 575, 1998) Science. 1998;282(5388):417–417. doi: 10.1126/science.281.5376.575. [DOI] [PubMed] [Google Scholar]
- Fainzilber M, Gordon D, Hasson A, Spira ME, Zlotkin E. Mollusc-specific toxins from the venom of Conus textile neovicarius. Eur J Biochem. 1991;202 doi: 10.1111/j.1432-1033.1991.tb16412.x. [DOI] [PubMed] [Google Scholar]
- Floyd PD, Li L, Rubakhin SS, Sweedler JV, Horn CC, Kupfermann I, Alexeeva VY, Ellis TA, Dembrow NC, Weiss KR, Vilim FS. Insulin prohormone processing, distribution, and relation to metabolism in Aplysia californica. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19(18):7732–7741. doi: 10.1523/JNEUROSCI.19-18-07732.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusetani N. Antifouling marine natural products. Nat Prod Rep. 2011;28(2):400–410. doi: 10.1039/c0np00034e. [DOI] [PubMed] [Google Scholar]
- Haygood MG, Schmidt EW, Davidson SK, Faulkner DJ. Microbial symbionts of marine invertebrates: opportunities for microbial biotechnology. J Mol Microbiol Biotechnol. 1999;1(1):33–43. [PubMed] [Google Scholar]
- Himaya SWA, Jin A-H, Dutertre S, Giacomotto J, Mohialdeen H, Vetter I, Alewood PF, Lewis RJ. Comparative Venomics Reveals the Complex Prey Capture Strategy of the Piscivorous Cone Snail Conus catus. Journal of Proteome Research. 2015;14(10):4372–4381. doi: 10.1021/acs.jproteome.5b00630. [DOI] [PubMed] [Google Scholar]
- Hopkins C, Grilley M, Miller C, Shon KJ, Cruz LJ, Gray WR, Dykert J, Rivier J, Yoshikami D, Olivera BM. A new family of Conus peptides targeted to the nicotinic acetylcholine receptor. J Biol Chem. 1995;270 doi: 10.1074/jbc.270.38.22361. [DOI] [PubMed] [Google Scholar]
- Jakubowski JA, Kelley WP, Sweedler JV, Gilly WF, Schulz JR. Intraspecific variation of venom injected by fish-hunting <em>Conus</em> snails. Journal of Experimental Biology. 2005;208(15):2873. doi: 10.1242/jeb.01713. [DOI] [PubMed] [Google Scholar]
- Jin A-H, Israel MR, Inserra MC, Smith JJ, Lewis RJ, Alewood PF, Vetter I, Dutertre S. δ-Conotoxin SuVIA suggests an evolutionary link between ancestral predator defence and the origin of fish-hunting behaviour in carnivorous cone snails. Proceedings of the Royal Society B: Biological Sciences. 2015;282(1811) doi: 10.1098/rspb.2015.0817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C, Stablum W. Observations on feeding behavior of Conus geographus (Gastropoda:Toxoglossa) Pacific Science. 1971;25(1):109–111. doi: http://hdl.handle.net/10125/6112. [Google Scholar]
- Kelley WP, Schulz JR, Jakubowski JA, Gilly WF, Sweedler JV. Two Toxins from Conus striatus that Individually Induce Tetanic Paralysis. Biochemistry. 2006;45(47):14212–14222. doi: 10.1021/bi061485s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn AJ. Evolutionary ecology of Conus on Indo-Pacific coral reefs. Proceedings of the Fifth International Coral Reef Congress; 27 May 1985; 1985. pp. 139–144. vol Symposia and Seminars. [Google Scholar]
- Kohn AJ. Conus of the Southeastern United States and Caribbean. Princeton University Press; Princeton, UNITED STATES: 2014. [Google Scholar]
- Leipold E, Hansel A, Olivera BM, Terlau H, Heinemann SH. Molecular interaction of delta-conotoxins with voltage-gated sodium channels. FEBS Lett. 2005;579 doi: 10.1016/j.febslet.2005.05.077. [DOI] [PubMed] [Google Scholar]
- McIntosh JM, Foderaro TA, Li W, Ireland CM, Olivera BM. Presence of serotonin in the venom of Conus imperialis. Toxicon. 1993;31(12):1561–1566. doi: 10.1016/0041-0101(93)90340-o. [DOI] [PubMed] [Google Scholar]
- Mena EE, Gullak MF, Pagnozzi MJ, Richter KE, Rivier J, Cruz LJ, Olivera BM. Conantokin-G: a novel peptide antagonist to the N-methyl-D-aspartic acid (NMDA) receptor. Neuroscience Letters. 1990;118(2):241–244. doi: 10.1016/0304-3940(90)90637-o. [DOI] [PubMed] [Google Scholar]
- Menting JG, Gajewiak J, MacRaild CA, Chou DH, Disotuar MM, Smith NA, Miller C, Erchegyi J, Rivier JE, Olivera BM, Forbes BE, Smith BJ, Norton RS, Safavi-Hemami H, Lawrence MC. A minimized human insulin-receptor-binding motif revealed in a Conus geographus venom insulin. Nature structural & molecular biology. 2016 doi: 10.1038/nsmb.3292. [DOI] [PubMed] [Google Scholar]
- Neves JL, Lin Z, Imperial JS, Antunes A, Vasconcelos V, Olivera BM, Schmidt EW. Small Molecules in the Cone Snail Arsenal. Organic letters. 2015;17(20):4933–4935. doi: 10.1021/acs.orglett.5b02389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen DB, Dykert J, Rivier JE, McIntosh JM. Isolation of Lys-conopressin-G from the venom of the worm-hunting snail, Conus imperialis. Toxicon. 1994;32(7):845–848. doi: 10.1016/0041-0101(94)90009-4. [DOI] [PubMed] [Google Scholar]
- Nybakken J. Preliminary observations on the feeding behavior of Conus purpurascens Broderip, 1833. Veliger. 1967;10:55–57. [Google Scholar]
- Olivera BM. Conus Venom Peptides, Receptor and Ion Channel Targets, and Drug Design: 50 Million Years of Neuropharmacology. Molecular biology of the cell. 1997a;8:2101–2109. doi: 10.1091/mbc.8.11.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivera BM. E.E. Just Lecture, 1996. Conus venom peptides, receptor and ion channel targets, and drug design: 50 million years of neuropharmacology. Molecular biology of the cell. 1997b;8(11):2101–2109. doi: 10.1091/mbc.8.11.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivera BM, McIntosh JM, Cruz LJ, Luque FA, Gray WR. Purification and sequence of a presynaptic peptide toxin from Conus geographus venom. Biochemistry. 1984;23 doi: 10.1021/bi00317a001. [DOI] [PubMed] [Google Scholar]
- Olivera BM, Seger J, Horvath MP, Fedosov AE. Prey-Capture Strategies of Fish-Hunting Cone Snails: Behavior, Neurobiology and Evolution. Brain, behavior and evolution. 2015;86(1):58–74. doi: 10.1159/000438449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliverio M, Modica MV. Relationships of the haematophagous marine snailColubraria(Rachiglossa: Colubrariidae), within the neogastropod phylogenetic framework. Zool J Linnean Soc. 2010:158. [Google Scholar]
- Puillandre N, Bouchet P, Duda TF, Jr, Kauferstein S, Kohn AJ, Olivera BM, Watkins M, Meyer C. Molecular phylogeny and evolution of the cone snails (Gastropoda, Conoidea) Molecular phylogenetics and evolution. 2014;78:290–303. doi: 10.1016/j.ympev.2014.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramilo CA, Zafaralla GC, Nadasdi L, Hammerland LG, Yoshikami D, Gray WR, Kristipati R, Ramachandran J, Miljanich G, Olivera BM. Novel alpha- and omega-conotoxins from Conus striatus venom. Biochemistry. 1992;31 doi: 10.1021/bi00156a009. [DOI] [PubMed] [Google Scholar]
- Rodriguez AM, Dutertre S, Lewis RJ, Marí F. Intraspecific variations in Conus purpurascens injected venom using LC/MALDI-TOF-MS and LC-ESI-TripleTOF-MS. Analytical and Bioanalytical Chemistry. 2015;407(20):6105–6116. doi: 10.1007/s00216-015-8787-y. [DOI] [PubMed] [Google Scholar]
- Safavi-Hemami H, Gajewiak J, Karanth S, Robinson SD, Ueberheide B, Douglass AD, Schlegel A, Imperial JS, Watkins M, Bandyopadhyay PK, Yandell M, Li Q, Purcell AW, Norton RS, Ellgaard L, Olivera BM. Specialized insulin is used for chemical warfare by fish-hunting cone snails. Proc Natl Acad Sci U S A. 2015a;112(6):1743–1748. doi: 10.1073/pnas.1423857112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safavi-Hemami H, Gajewiak J, Karanth S, Robinson SD, Ueberheide B, Douglass AD, Schlegel A, Imperial JS, Watkins M, Bandyopadhyay PK, Yandell M, Li Q, Purcell AW, Norton RS, Ellgaard L, Olivera BM. Specialized insulin is used for chemical warfare by fish-hunting cone snails. Proc Natl Acad Sci USA. 2015b;112 doi: 10.1073/pnas.1423857112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safavi-Hemami H, Lu A, Li Q, Fedosov AE, Biggs J, Showers Corneli P, Seger J, Yandell M, Olivera BM. Venom Insulins of Cone Snails Diversify Rapidly and Track Prey Taxa. Mol Biol Evol. 2016 doi: 10.1093/molbev/msw174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Park NG, Kohno T, Maeda T, Kim JI, Kato R, Takahashi M. Role of basic residues for the binding of omega-conotoxin GVIA to N-type calcium channels. Biochemical and biophysical research communications. 1993;194(3):1292–1296. doi: 10.1006/bbrc.1993.1964. [DOI] [PubMed] [Google Scholar]
- Schulz JR, Norton AG, Gilly WF. The projectile tooth of a fish-hunting cone snail: Conus catus injects venom into fish prey using a high-speed ballistic mechanism. Biological Bulletin. 2004;207:77–79. doi: 10.2307/1543581. [DOI] [PubMed] [Google Scholar]
- Shabanpoor F, Separovic F, Wade JD. The human insulin superfamily of polypeptide hormones. Vitamins and hormones. 2009;80:1–31. doi: 10.1016/s0083-6729(08)00601-8. [DOI] [PubMed] [Google Scholar]
- Sharp KH, Davidson SK, Haygood MG. Localization of ‘Candidatus Endobugula sertula’ and the bryostatins throughout the life cycle of the bryozoan Bugula neritina. ISME J. 2007;1(8):693–702. doi: 10.1038/ismej.2007.78. [DOI] [PubMed] [Google Scholar]
- Smit AB, van Kesteren RE, Li KW, Van Minnen J, Spijker S, Van Heerikhuizen H, Geraerts WP. Towards understanding the role of insulin in the brain: lessons from insulin-related signaling systems in the invertebrate brain. Progress in neurobiology. 1998;54(1):35–54. doi: 10.1016/s0301-0082(97)00063-4. [DOI] [PubMed] [Google Scholar]
- Sunagar K, Moran Y. The Rise and Fall of an Evolutionary Innovation: Contrasting Strategies of Venom Evolution in Ancient and Young Animals. PLoS genetics. 2015;11(10):e1005596. doi: 10.1371/journal.pgen.1005596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teichert RW, López-Vera E, Gulyas J, Watkins M, Rivier J, Olivera BM. Definition and Characterization of the Short αA-Conotoxins: A Single Residue Determines Dissociation Kinetics from the Fetal Muscle Nicotinic Acetylcholine Receptor. Biochemistry. 2006;45(4):1304–1312. doi: 10.1021/bi052016d. [DOI] [PubMed] [Google Scholar]
- Terlau H, Jacobson R, Shon KJ, Stocker M, Stuhmer W, Olivera BM. K-conotoxin PVIIa, a Conus peptide targeted to potassium channels H. Pflugers Archiv-European Journal of Physiology. 1997;433(6):O103–O103. [Google Scholar]
- Terlau H, Shon KJ, Grilley M, Stocker M, Stuhmer W, Olivera BM. Strategy for rapid immobilization of prey by a fish-hunting marine snail. Nature. 1996;381(6578):148–151. doi: 10.1038/381148a0. [DOI] [PubMed] [Google Scholar]
- Thompson JE. Exudation of biologically-active metabolites in the sponge Aplysina fistularis - I. Biological evidence. Marine Biology. 1985;88(1):23–26. doi: 10.1007/BF00393039. [DOI] [Google Scholar]
- Tianero MD, Pierce E, Raghuraman S, Sardar D, McIntosh JA, Heemstra JR, Schonrock Z, Covington BC, Maschek JA, Cox JE, Bachmann BO, Olivera BM, Ruffner DE, Schmidt EW. Metabolic model for diversity-generating biosynthesis. Proc Natl Acad Sci U S A. 2016;113(7):1772–1777. doi: 10.1073/pnas.1525438113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wagoner RM, Jacobsen RB, Olivera BM, Ireland CM. Characterization and three-dimensional structure determination of psi-conotoxin Piiif, a novel noncompetitive antagonist of nicotinic acetylcholine receptors. Biochemistry. 2003;42 doi: 10.1021/bi0272757. [DOI] [PubMed] [Google Scholar]
- Walker CS, Jensen S, Ellison M, Matta JA, Lee WY, Imperial JS, Duclos N, Brockie PJ, Madsen DM, Isaac JTR, Olivera B, Maricq AV. A Novel Conus Snail Polypeptide Causes Excitotoxicity by Blocking Desensitization of AMPA Receptors. Current Biology. 2009;19(11):900–908. doi: 10.1016/j.cub.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins M, Hillyard DR, Olivera BM. Genes expressed in a turrid venom duct: divergence and similarity to conotoxins. J Mol Evol. 2006:62. doi: 10.1007/s00239-005-0010-x. [DOI] [PubMed] [Google Scholar]
- Wilson MJ, Yoshikami D, Azam L, Gajewiak J, Olivera BM, Bulaj G, Zhang M-M. mu-Conotoxins that differentially block sodium channels NaV1.1 through 1.8 identify those responsible for action potentials in sciatic nerve. Proc Natl Acad Sci USA. 2011;108 doi: 10.1073/pnas.1107027108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafaralla GC, Ramilo C, Gray WR, Karlstrom R, Olivera BM, Cruz LJ. Phylogenetic specificity of cholinergic ligands: alpha-conotoxin SI. Biochemistry. 1988;27 doi: 10.1021/bi00418a065. [DOI] [PubMed] [Google Scholar]
- Zugasti-Cruz A, Maillo M, Lopez-Vera E, Falcon A, Heimer de la Cotera EP, Olivera BM, Aguilar MB. Amino acid sequence and biological activity of a gamma-conotoxin-like peptide from the worm-hunting snail Conus austini. Peptides. 2006;27 doi: 10.1016/j.peptides.2005.07.021. [DOI] [PubMed] [Google Scholar]