Abstract
The validity of a comprehensive international neuropsychological (NP) test battery for detection of HIV associated neurocognitive disorders (HAND) in a Tamil speaking southern Indian cohort (69 HIV+ and 67 HIV−) was explored. The prevalence of HAND was significantly higher in the HIV+ versus HIV− group (33% vs.13%, p < 0.01). Impairment rates were highest in the motor and speed of information processing domains. An NP battery translated into Tamil appears to be a valid tool for assessing HAND because a prevalence it found of HAND in southern India is similar to that found elsewhere.
Keywords: Neurocognitive impairment (NCI), HIV-associated neurocognitive disorders (HAND), HIV infection, Cross-cultural assessment, India
Introduction
An estimated 2.1 million HIV− infected people are living in India in 2015 and 86,000 were newly infected occurred1. While the incidence is declining, consequences of HIV infection remain, including residual cognitive impairment that affects occupational and social functioning in some patients2.
HIV-associated cognitive deficits, known as HIV-associated neurocognitive disorders or HAND3, must be diagnosed by standardized neuropsychological (NP) testing4,5. Most data on NP testing for HAND come from studies conducted in the United States, Australia, and Europe. The prevalence and characteristics of cognitive complications of HAND in regions most affected by the disease, including India, have been studied less frequently, in part because of the need to translate the tests into local languages and to create normative data.
The goal of this study was to adapt the HIV Neurobehavioral Research Program (HNRP, University of California, San Diego) International NP test battery for use in Chennai, India and to explore its validity for the detection of HAND in patients on and off ART. This battery included 12 tests that yield 17 measures to evaluate multiple cognitive domains that are known to be vulnerable to HIV6–8.
Methods
Participants
Individuals (≥ 18 years old) presenting to Y. R. Gaitonde Center for AIDS Research and Education (YRG CARE) in Chennai, India for HIV testing or treatment were approached for participation in the study. Sixty-nine HIV+ and 67 HIV− men and women 19 to 62 years of age who consented and had no confounding condition that could impact their performance on NP testing were recruited from April 2010 to September 2013. Any human data included in this manuscript was obtained in compliance with regulations of the Indian Council of Medical Research and the Institutional Review Boards of the University of California, San Diego and YRG CARE. All participants provided written informed consent. HIV diagnosis was confirmed by Western blot testing.
Training and adaptation of the NP battery
We used the HNRP International NP battery, which has estimated prevalence of HAND in studies in Brazil9, Cameroon8, China10, India11,12, Nigeria13, South Africa, USA14, and Zambia15. Nurses were trained and certified to administer the battery. The cognitive and neuromedical procedures were adapted to local settings by translating all tests in the battery into Tamil. Many of the tests such as Trail Making Test-A, Pegboard, Color Trails Test, Brief Visuospatial Memory Test-Revised, Stroop, Hopkins Verbal Learning Test-Revised had been previously translated to Tamil at YRG CARE16. The remaining instruments in the HNRP battery were also reviewed by native Tamil speakers at YRG CARE, deemed to be acceptable with minimal changes, and translated into Tamil by professional translators, after obtaining permission from the test publishers. A second translation service independently performed back translation from Tamil into English.
Data analysis
We developed demographically corrected norms that adjust for age, education and gender for the NP tests using data from the HIV− participants. The statistical procedure to develop demographically corrected norms has been detailed earlier6,17. Briefly, raw scores for each test were converted to normally distributed scaled scores, with a mean of 10 and a standard deviation of 3 in the normative group. Age, education, and gender adjusted scores were then transformed to demographically corrected T-scores, which have a mean of 50 and a standard deviation of 10. Finally, these T-scores were used to compute mean T-scores for each NP test measure as well as Domain and Global Deficit Scores. The Global Deficit Score (GDS) was used for classification of overall impairment status on the battery. Deficit scores focus on impaired performance, as would be done by a clinician, and have been shown to be sensitive to HIV-related impairments6. Neurocognitive impairment (NCI) was defined as a GDS value ≥ 0.5. Comparisons between groups were made using chi-squared tests and t-tests as appropriate. Cohen’s d effect sizes were also calculated for group comparisons. Pearson correlation was used to test association between domain and global NP scores and numeric demographic covariates (age and education).
Results
Description of the study population
A total of 136 (69 HIV+ and 67 HIV−) adults from both urban and rural settings were evaluated. As a group, HIV+ participants were older (p = 0.02), less educated (p = 0.001), and more likely to be male (p < 0.001) compared to HIV− participants (Table 1). However, the age and education ranges were comparable for HIV+ and HIV− groups such that lower education levels and higher ages were represented in the HIV− controls for the purpose of creating demographically correct T-scores. The demographic effects on the T-scores were non-significant in either group. Of the 69 HIV+ individuals, 40 (58%) were classified as having AIDS based on the WHO criteria. The mean estimated duration of infection was 5 years (SD 3.5). The HIV+ cohort was generally healthy, with a median current CD4+ cell count of 532 cells/μL (median, IQR 361, 749). Twenty-seven (39%) HIV+ participants were ART-naïve, 36 (52%) were on ART, and 6 (9%) were ART-experienced, but were not currently taking ART. The ART-naïve participants were less likely to have AIDS when compared to those on ART or who were ART-experienced. They were also younger and more likely to be female than those on ART (p values < 0.05).
Table 1.
Demographic and HIV disease characteristics of the cohort.
| Characteristics | HIV− (n = 67) | HIV+ (n = 69) | p- value |
|---|---|---|---|
| Age (mean, SD) | 34.1 (7.8) | 37.4 (8.1) | 0.02 |
| Education (mean, SD) | 12.1 (3.7) | 10.2 (2.9) | 0.001 |
| Gender (%Male) | 28.4 | 66.7 | <0.001 |
Note:
p < 0.05.
Error bars represent the 95% confidence interval.
NP test performance
As expected, substantial demographic effects were observed. In the HIV− group, older age was significantly associated with lower uncorrected Global mean scaled scores (r= −0.26, p = 0.03) and Speed of Information Processing domain scaled scores (r= −0.32; p = 0.001). Higher levels of education were also significantly associated with higher Global mean scaled score (r= 0.57; p < 0.001) and Domain scaled scores (Executive Functioning r = 0.36; p = 0.03; Verbal Fluency r = 0.45; p < 0.001; Working Memory r = 0.47; p < 0.001; Learning r = 0.48, p < 0.001; Memory r = 0.45, p < 0.001; Speed of Information Processing r = 0.65, p < 0.001). Women had better scores on memory and lower scores on motor functioning than men (p’s < 0.05).
After application of normative adjustments based upon the HIV− group, as expected demographic factors no longer influenced NP performance in either group. For the HIV− group, the mean T score for all measures was approximately 50 (SD 10), and all associations with demographics (age, education, gender) had p > 0.5.
NP test performance by HIV status
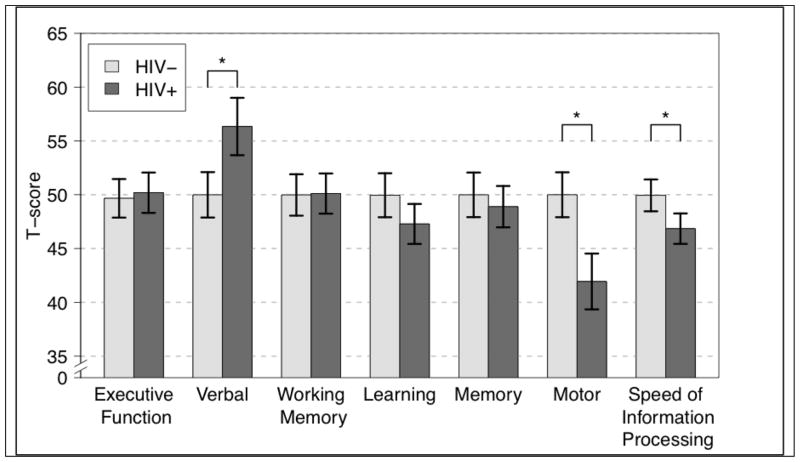
NCI, as measured by the global deficit score (GDS ≥ 0.50), was more prevalent in the HIV+ group than in the HIV− group (33.3% vs. 13.4%, p < 0.01, relative risk (RR) 2.5). Impairment rates by cognitive domain were highest in the Motor (40.9% vs. 11.9%, p < 0.0001, RR 3.4) and the Speed of Information Processing (29.4% vs. 11.9%, p = 0.01, RR 2.5) domains. As seen in Figure 1, at the domain level, the greatest group differences were Motor, Speed of Information Processing, and Verbal functioning. Comparisons of demographically corrected T-scores across all test measures (Table 2) showed that the NP performance of HIV+ participants was significantly worse than that of HIV− persons for the Trail Making Test A (Cohen’s d = −0.68; p < 0.0001), WAIS-III Digit Symbol Coding (Cohen’s d = −0.53; p < 0.01), WAIS-III Symbol Search (Cohen’s d = −0.49; p < 0.01), HVLT-R Learning (Cohen’s d = −0.79; p < 0.001), and grooved pegboard dominant (Cohen’s d = −0.67; p < 0.001) and non-dominant (Cohen’s d = −0.86; p < 0.0001) hand; the effects remained significant after correction for multiple comparisons using the false discovery rate (FDR) method. Small-to-medium HIV effects were also noted for Category errors (Cohen’s d = −0.34; p = 0.07), Color Trails I (Cohen’s d = −0.32; p = 0.06), and BVMT-R Delay (Cohen’s d = −.23; p = 0.18), although not statistically significant with the current sample size. On measures of verbal fluency, the opposite pattern was observed and HIV+ persons demonstrated higher T scores (Cohen’s d = 0.59; p < 0.001). Post-hoc analyses demonstrated that the HIV+ and HIV− participants had comparable, low rates of impairment in this domain (p > 0.5).
Figure 1.
Domain T-scores by HIV status.
Table 2.
Neuropsychological (NP) test battery and T-scores (mean ± SD) in the HIV− and HIV+ groups
| NP test (reference) | HIV− | HIV+ | p | d |
|---|---|---|---|---|
| Verbal Fluency | ||||
| Letter fluency | 50.0 (10) | 56.5 (11.8) | <0.001 | .59 |
| Executive function | ||||
| Category errors | 50.0 (10) | 46.8 (8.75) | 0.07 | −.34 |
| Stroop color-word interference | 50.0 (10) | 50.8 (10.1) | 0.65 | .08 |
| Color Trails II | 50.0 (10) | 50.9 (11.3) | 0.61 | .08 |
| Speed of information processing | ||||
| Trails A | 50.0 (10) | 42.6 (11.6) | <0.0001 | −.68 |
| WAIS-III Digit Symbol coding | 50.0 (10) | 45.2 (7.9) | <0.01 | −.53 |
| WAIS-III Symbol Search | 50.0 (10) | 45.9 (6.2) | <0.01 | −.49 |
| Stroop Color | 50.0 (10) | 50.5 (10.9) | 0.78 | .05 |
| Color Trails I | 50.0 (10) | 46.2 (13.2) | 0.06 | −.32 |
| Learning | ||||
| BVMT-R Learning | 50.0 (10) | 51.7 (11.1) | 0.33 | .16 |
| HVLT-R Learning | 50.0 (10) | 43.0 (7.4) | <0.001 | −.79 |
| Memory | ||||
| BVMT-R Delay Recall | 50 (10) | 47.6 (10.7) | 0.18 | −.23 |
| HVLT-R Delay Recall | 50.0 (10) | 49.9 (7.6) | 0.93 | −.01 |
| Working Memory | ||||
| PASAT 50 | 50.0 (10) | 50.9 (10.2) | 0.61 | .08 |
| WMS-III Spatial Span | 50.0 (10) | 49.2 (11.3) | 0.65 | −.07 |
| Motor Function | ||||
| Grooved Pegboard DH* | 50.0 (10) | 43.0 (10.7) | <0.001 | −.67 |
| Grooved Pegboard NDH** | 50.0 (10) | 40.8 (11.3) | <0.0001 | −.86 |
| Global Deficit Score | .22 (.24) | .36 (.30) | <0.01 | .51 |
The p values < 0.001 would withstand adjustment for multiple comparisons.
DH: Dominant hand;
NDH: Non-dominant hand
NP test performance by ART status
Within the HIV+ cohort, individuals who were not taking ART (ART-naïve or ART-experienced but not currently taking ART) performed comparably to those who were on ART across all cognitive domains, including the global T-score (p values > 0.1). The current CD4+ T-cell counts in the 3 groups differed considerably, but not significantly (median [IQR]: ART-naïve 499 [387-701], current ART 588 [339-777], and ART-experienced 367 [180-651]). The CD4 cell counts in these groups probably reflects: a) policy-driven selection of only patients with low CD4 counts (<350) for ART, b) their increased CD4 counts in response to treatment, and c) a trend (non-significant) toward the return of previously ART treated patients to their former CD4 depleted state while off treatment.
Comparison of prevalence of HIV effect on cognition in other international settings
This neuropsychological battery has been translated and adapted for use in three other non-US studies in India11, China10 and Zambia18. The prevalence of NCI in HIV-infected patients in this study (33%) is similar to the findings of four other studies (33–45%) shown in Table 3.
Table 3.
HAND prevalence in other international settings using the HNRC international neuropsychological test battery.
| Region | N | Age (Mean, SD) | Education (Mean, SD) | % Male | NCI Prevalence |
|---|---|---|---|---|---|
| China (Anhui)10 | 108 | 40.8 (6.4) | 5.5 (2.2) | 67% | 34% |
| India (Pune)11 | 92 | 35.6 (6.9) | 9.1 (2.5) | 59% | 45% |
| India (Chennai) (Present study) | 69 | 37.4 (8.1) | 10.2 (2.9) | 67% | 33% |
| USA14 | 843 | 42.7 (8.8) | 13 (2.5) | 79% | 36% |
| Zambia18 | 266 | 40.7 (0.73) | 9.9(2.2) | 40% | 35% |
Discussion
This study confirmed that the HNRP International NP battery, when translated into Tamil, was understood by participants from southern India and identified a similar prevalence of neurocognitive deficits from HIV infection as found in multiple other diverse geographic and linguistic settings4,10,16,19.
Studies of HAND have mainly been conducted in cohorts infected with HIV-1 genetic subtype (i.e., clade) B. However, HIV-1 subtype C is the most prevalent clade in the world and is predominant in India, as well as in parts of Africa20. The prevalence of NCI associated with subtype C has ranged from higher 21,22 to similar 9 when compared with subtype B.
Clade C differs from B in ways that may affect HAND. For example, the HIV subtype C tat protein has reduced chemoattractant properties compared with subtype B23 and, thus, might be expected to result in lower rates of HAND. In contrast, subtype C from southern African countries is associated with higher HAND prevalence than subtype B in Western countries22. This finding may be because the Southeast Asian subtype C variants have greater tat induced neurovirulence21.
Several studies indicate that Clade C is associated with cognitive impairment12,16. In one of the first efforts to delineate the cognitive sequelae of HIV-1 infection in southern India, Yepthomi and colleagues compared cognitive functioning in HIV+ and HIV− controls16. Relative to education-matched healthy controls, their sample of treatment-naive HIV+ participants performed significantly worse on measures of motor functioning, speed of information processing, inhibition-switching, as well as learning and memory. Participants were assessed in either Tamil or Telegu, two southern Indian languages. The proportion of individuals speaking Telegu in the HIV+ group was significantly lower compared to the HIV− group, warranting cautious interpretation of the group differences in cognitive functioning. Other studies in different geographically/linguistically distinct regions of India have reported mild to moderate cognitive deficits in individuals with HIV-1 Clade C infection11,12,24. Our findings are consistent with prior reports of HIV-associated cognitive impairments in Clade C infected cohorts. Of note, these studies in Karnataka12, Chandigarh24, and Pune11, as well as our study in Chennai, vary in their study design (e.g., presence or absence of control group), HIV disease characteristics, and assessment approach (e.g., batteries and individual tests used, language of administration). Thus, direct comparisons across studies are not possible; however, there is evidence for a general trend of HIV-associated neurocognitive disorders in diverse Indian cohorts.
Studies of cohorts in resource-rich settings (RRS) suggest that antiretroviral therapy (ART), especially with better CNS penetration, may be associated with prevention or reversal of NCI in some cohorts25. A recent report from Pune, India suggests that ART initiation in individuals with advanced immunosuppression (CD4 < 200) is associated with modest neurocognitive improvement11. However, long term effects of ART on cognitive sequelae of HIV-1 subtype C remain unclear.
Similar to other studies in China, Cameroon, and United States, we found significant HIV effects in the domains of motor function, speed of information processing and learning26. Although HIV-associated neural injury is not limited to any brain region, cognitive impairments in HIV appear to be preferentially associated with damage to frontostriatal circuits27. Involvement of these regions is thought to underlie the deficits in learning, motor function, and speed of information processing observed in HIV+ persons28. In cohorts in RRS, impairments in learning and memory are typically seen in individuals with advanced HIV-induced immunosuppression. 28. In our study, 58% of participants met criteria for AIDS, which may help to explain the high prevalence of deficits that we observed for the learning domain.
Raw test scores for the domain of verbal fluency were similar in HIV+ and HIV− subjects, consistent with other studies29. Contrary to expectations, we found that HIV+ patients performed better on demographically-corrected measures of verbal fluency compared to HIV− controls. This unexpected result raises the possibility that the normative corrections, developed from a relatively small, HIV−, control group, inadequately controlled for the effects of factors such as bilingualism and quality of education, which might impact performance on language measures30. This study was not designed to capture these variables.
Future studies may address this issue by assessing larger, well-matched samples using this NP battery. In US cohorts, the HIV effect in the domain of language and verbal fluency is small28. Data comparing pre- and post-combination ART (cART) rates of impairment indicate that verbal impairment was more common in the pre-cART era and has since decreased4. On the other hand, the evidence for an HIV effect on tests of verbal fluency in southern India cohorts is variable. For example, Das Gupta et al. 2007 reported significant differences in phonemic fluency but not animal fluency12. This issue warrants closer investigation to fully characterize the effects of HIV infection on language functioning and other factors that may influence this relationship in a South Indian context.
Among HIV+ individuals, NCI did not differ by ART status. Although, the prevalence of impairment in this cohort was similar to that seen in other international settings, it was at the lower end of the range (Table 3). Due to missing data to accurately determine HIV disease status (viral load, CD4+ nadir, CD4+ count for subjects not on ART), we cannot state whether individuals not on ART had relatively intact immune systems and, therefore, lower likelihood of HAND. Similarly, due to the cross-sectional study design, we cannot determine whether those on ART had restored their immune systems and consequently resolved HAND. Data regarding duration of ART use were not collected; thus we cannot comment on the relationship between length of treatment and HAND rates. The relationship between cognitive status and ART history warrants closer examination in longitudinal studies of this population.
This study has several limitations. First, the normative group used in the study was small. Larger samples are needed to more accurately characterize how demographic variables influence NP performance in HIV− South Indians. A better balance in terms of education, gender, and age in the control group would permit the development of more robust normative corrections. To the extent that occupational attainment may improve the estimate of premorbid level of cognition, future studies may also consider examining the relationship between this variable and cognitive performance. Second, factors that may impact test performance, such as quality of education and bilingualism, were not assessed and may have helped to understand the HIV effect observed on tests of verbal fluency. Differences between the HIV+ and HIV− groups in demographic characteristics may have impacted the accuracy with which the norming algorithm can predict the expected performance for less educated and older HIV+ individuals. Third, HIV from these patients was not genotyped. Instead, most participants were assumed to have HIV-1 subtype C infection based on reports of >90% infections in Chennai attributed to this subtype. Finally, measures of mood and everyday functioning, which would have better characterized neurobehavioral sequelae of HIV infection in southern India, were not included in this study.
Conclusions
Our study confirms the utility of the HNRP NP assessment tools for detecting HIV-induced NCI in a new linguistic and cultural setting. Our results suggest that they may be valuable in assessing NP performance in the HIV− Indian population, with and without other CNS diseases.
Acknowledgments
All the authors declare no financial or other relationships that could be interpreted as a conflict of interest affecting this manuscript. We would like to thank the participants and the YRG CARE study team (Kavitha Balaji, Beulah Faith, Chenchulakshmi, Christina, and Jabin Sharma) for their invaluable contribution. This study was supported by a grant from Tibotec’s REACH (Research and Education in HIV/AIDS for Resource-Poor Countries) Initiative (SLL; NK), the National Institutes of Health career development awards (ARB: K23 MH085512, SLL: K24 MH097673), UCSD’s HIV Neurobehavioral Research Center (P30 MH62512: PI: RH) and the University of California, San Diego, Center for AIDS Research (CFAR), an NIH-funded program (P30 AI036214), which is supported by the following NIH Institutes and Centers: NIAID, NCI, NIMH, NIDA, NICHD, NHLBI, NIA, NIGMS, and NIDDK.
References
- 1.Global AIDS Update - 2016. UN Joint Programme on HIV/AIDS (UNAIDS); Jun, 2016. [Google Scholar]
- 2.Casaletto KB, Weber E, Iudicello JE, Woods SP. Real-World Impact of HIV-Associated Neurocognitive Impairment. In: Chiaravalloti ND, Goverover Y, editors. Changes in the Brain: Impact on Daily Life. New York, NY: Springer; 2017. pp. 211–45. [Google Scholar]
- 3.Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69(18):1789–99. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heaton RK, Franklin DR, Ellis RJ, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17(1):3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thames AD, Streiff V, Patel SM, Panos SE, Castellon SA, Hinkin CH. The role of HIV infection, cognition, and depression in risky decision-making. J Neuropsychiatry Clin Neurosci. 2012;24(3):340–8. doi: 10.1176/appi.neuropsych.11110340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carey CL, Woods SP, Rippeth JD, et al. Initial validation of a screening battery for the detection of HIV-associated cognitive impairment. Clin Neuropsychol. 2004;18(2):234–48. doi: 10.1080/13854040490501448. [DOI] [PubMed] [Google Scholar]
- 7.Cysique LA, Jin H, Franklin DR, Jr, et al. Neurobehavioral effects of HIV-1 infection in China and the United States: a pilot study. J Int Neuropsychol Soc. 2007;13(5):781–90. doi: 10.1017/S1355617707071007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanmogne GD, Kuate CT, Cysique LA, et al. HIV-associated neurocognitive disorders in sub-Saharan Africa: a pilot study in Cameroon. BMC Neurol. 2010;10:60. doi: 10.1186/1471-2377-10-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Almeida SM, Ribeiro CE, de Pereira AP, et al. Neurocognitive impairment in HIV-1 clade C- versus B-infected individuals in Southern Brazil. J Neurovirol. 2013;19(6):550–6. doi: 10.1007/s13365-013-0215-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heaton RK, Cysique LA, Jin H, et al. Neurobehavioral effects of human immunodeficiency virus infection among former plasma donors in rural China. J Neurovirol. 2008;14(6):536–49. doi: 10.1080/13550280802378880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghate M, Mehendale S, Meyer R, et al. The effects of antiretroviral treatment initiation on cognition in HIV-infected individuals with advanced disease in Pune, India. J Neurovirol. 2015;21(4):391–8. doi: 10.1007/s13365-015-0329-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta JD, Satishchandra P, Gopukumar K, et al. Neuropsychological deficits in human immunodeficiency virus type 1 clade C-seropositive adults from South India. J Neurovirol. 2007;13(3):195–202. doi: 10.1080/13550280701258407. [DOI] [PubMed] [Google Scholar]
- 13.Akolo C, Royal W, 3rd, Cherner M, et al. Neurocognitive impairment associated with predominantly early stage HIV infection in Abuja, Nigeria. J Neurovirol. 2014;20(4):380–7. doi: 10.1007/s13365-014-0254-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heaton RK, Clifford DB, Franklin DR, Jr, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75(23):2087–96. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kabuba N, Menon JA, Franklin DR, Jr, Heaton RK, Hestad KA. HIV- and AIDS-associated neurocognitive functioning in Zambia - a perspective based on differences between the genders. Neuropsychiatr Dis Treat. 2016;12:2021–8. doi: 10.2147/NDT.S105481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yepthomi T, Paul R, Vallabhaneni S, et al. Neurocognitive consequences of HIV in southern India: a preliminary study of clade C virus. J Int Neuropsychol Soc. 2006;12(3):424–30. doi: 10.1017/s1355617706060516. [DOI] [PubMed] [Google Scholar]
- 17.Norman MA, Moore DJ, Taylor M, et al. Demographically corrected norms for African Americans and Caucasians on the Hopkins Verbal Learning Test-Revised, Brief Visuospatial Memory Test-Revised, Stroop Color and Word Test, and Wisconsin Card Sorting Test 64-Card Version. J Clin Exp Neuropsychol. 2011;33(7):793–804. doi: 10.1080/13803395.2011.559157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kabuba N, Anitha Menon J, Franklin DR, Jr, Heaton RK, Hestad KA. Use of Western Neuropsychological Test Battery in Detecting HIV-Associated Neurocognitive Disorders (HAND) in Zambia. AIDS Behav. 2016 doi: 10.1007/s10461-016-1443-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robertson K, Kumwenda J, Supparatpinyo K, et al. A multinational study of neurological performance in antiretroviral therapy-naive HIV-1-infected persons in diverse resource-constrained settings. J Neurovirol. 2011;17(5):438–47. doi: 10.1007/s13365-011-0044-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buonaguro L, Tornesello ML, Buonaguro FM. Human immunodeficiency virus type 1 subtype distribution in the worldwide epidemic: pathogenetic and therapeutic implications. J Virol. 2007;81(19):10209–19. doi: 10.1128/JVI.00872-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rao VR, Neogi U, Talboom JS, et al. Clade C HIV-1 isolates circulating in Southern Africa exhibit a greater frequency of dicysteine motif-containing Tat variants than those in Southeast Asia and cause increased neurovirulence. Retrovirology. 2013;10:61. doi: 10.1186/1742-4690-10-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joska JA, Westgarth-Taylor J, Myer L, et al. Characterization of HIV-Associated Neurocognitive Disorders among individuals starting antiretroviral therapy in South Africa. AIDS Behav. 2011;15(6):1197–203. doi: 10.1007/s10461-010-9744-6. [DOI] [PubMed] [Google Scholar]
- 23.Ranga U, Shankarappa R, Siddappa NB, et al. Tat protein of human immunodeficiency virus type 1 subtype C strains is a defective chemokine. J Virol. 2004;78(5):2586–90. doi: 10.1128/JVI.78.5.2586-2590.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cook R, Waldrop-Valverde D, Sharma A, et al. Cognitive functioning, depression, and HIV medication adherence in India: a randomized pilot trial. Health Psychol Behav Med. 2014;2(1):640–52. doi: 10.1080/21642850.2014.913487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smurzynski M, Wu K, Letendre S, et al. Effects of central nervous system antiretroviral penetration on cognitive functioning in the ALLRT cohort. AIDS. 2011;25(3):357–65. doi: 10.1097/QAD.0b013e32834171f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Casaletto KB, Umlauf A, Beaumont J, et al. Demographically Corrected Normative Standards for the English Version of the NIH Toolbox Cognition Battery. J Int Neuropsychol Soc. 2015;21(5):378–91. doi: 10.1017/S1355617715000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jernigan TL, Archibald SL, Fennema-Notestine C, et al. Clinical factors related to brain structure in HIV: the CHARTER study. J Neurovirol. 2011;17(3):248–57. doi: 10.1007/s13365-011-0032-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reger M, Welsh R, Razani J, Martin DJ, Boone KB. A meta-analysis of the neuropsychological sequelae of HIV infection. J Int Neuropsychol Soc. 2002;8(3):410–24. doi: 10.1017/s1355617702813212. [DOI] [PubMed] [Google Scholar]
- 29.Iudicello JE, Woods SP, Parsons TD, Moran LM, Carey CL, Grant I. Verbal fluency in HIV infection: a meta-analytic review. J Int Neuropsychol Soc. 2007;13(1):183–9. doi: 10.1017/S1355617707070221. [DOI] [PubMed] [Google Scholar]
- 30.Kamat R, Ghate M, Gollan TH, et al. Effects of Marathi-Hindi bilingualism on neuropsychological performance. J Int Neuropsychol Soc. 2012;18(2):305–13. doi: 10.1017/S1355617711001731. [DOI] [PMC free article] [PubMed] [Google Scholar]