Abstract
Seizure disorders are often associated with infectious etiologies. Infection, via the intracerebral (i.c.) route, of C57BL/6J mice with the Daniels (DA) strain of Theiler’s murine encephalomyelitis virus (TMEV) results in approximately 50% of the mice developing acute behavioral seizures. TMEV-DA is the wild-type strain of the virus that replicates within the parenchyma of the brain. A variant of TMEV-DA, TMEV-H101, does not replicate within the parenchyma of the brain. However, infection with TMEV-H101 via the i.c. route still results in approximately 40% of the mice developing acute behavioral seizures. Infiltrating macrophages producing interleukin-6 (IL-6) have been implicated in the induction of acute seizures following TMEV-DA infection. We examined macrophage infiltration and microglial activation within the brain and cytokine levels in the periphery in mice infected with TMEV-DA or TMEV-H101, and assessed the effects of the addition of recombinant IL-6 to the periphery in wild-type and IL-6 knockout mice infected with TMEV-DA. We found that pathologic levels of IL-6 in the periphery may play a role in the development of seizures when viral replication within the brain is limited. Examination of the role played by the peripheral immune system in the development of seizures/epilepsy in the TMEV-induced seizure model, the first viral infection driven model for epilepsy, could lead to the elucidation of novel therapeutics.
Keywords: Theiler’s murine encephalomyelitis virus, Viral encephalitis, Picornavirus, Innate immune response, Inflammation, Cytokines
Introduction
Seizure disorders can be associated with infectious etiologies. More specifically, certain viruses have been shown to incite an immune response driving seizures. Previous work from our laboratory has demonstrated that the cytokine interleukin (IL)-6 is involved in the induction of acute seizures when C57BL/6J mice are infected intracerebrally (i.c.) with the Daniels (DA) strain of Theiler’s murine encephalomyelitis virus (TMEV) (Cusick et al., 2013; Libbey et al., 2011a). Furthermore, macrophages migrating from the periphery to the central nervous system (CNS) were one cell-type that was correlated with both the production of IL-6 and the induction of seizures (Cusick et al., 2013). Limiting macrophage migration to the CNS during TMEV-DA infection suppressed the number of mice having seizures. Additionally, TMEV-DA-infected mice experiencing seizures had a significantly higher number of IL-6+ cells in the brain than control uninfected mice or infected mice that did not experience seizures. Finally, significantly more infiltrating macrophages were IL-6+ compared to resident microglia (Cusick et al., 2013). Therefore, macrophages producing IL-6 have been implicated in the development of seizures in a model where there is a productive viral infection of the CNS, as TMEV antigen can be detected in the CNS following TMEV-DA i.c. infection of C57BL/6J mice (Kirkman et al., 2010).
A mutant named TMEV-H101 was created inadvertently as a result of transcriptional errors by the T7 polymerase while using a modified full-length infectious cDNA clone of the TMEV-DA virus as template (Zurbriggen et al., 1991). The TMEV-H101 mutant virus encodes a point mutation (T101I) in viral protein 1 of the capsid. In addition, in sequencing the TMEV-H101 viral genome, there were also several nucleotide substitutions in the 5′ untranslated region as well as additional amino acid substitutions in the capsid protein coding region, suggesting that there are a number of perturbations in the viral genome important for the viral tropism (Tsunoda et al., 1997). TMEV antigen was not detected in the CNS (frontal lobe, septum, caudoputamen, hippocampus, thalamus, hypothalamus, midbrain or cortex) following TMEV-H101 i.c. infection of C57BL/6J mice (Cusick et al., 2014; Libbey et al., 2011b) despite the fact that virus is administered directly into the brain (i.c. infection); nonetheless, 40% of the TMEV-H101-infected mice experienced seizures, compared to 58% of the TMEV-DA-infected mice (Libbey et al., 2011b). Thus, with TMEV-H101 infection, seizures are induced in the absence of robust CNS virus replication in the parenchyma, suggesting that peripheral serum levels of IL-6 could be playing an important role in the induction of seizures in the absence of a productive infection within the CNS.
To determine if peripheral factors such as IL-6 are implicated in the induction of seizures, we examined the level of macrophage infiltration and microglial activation in the brains of TMEV-DA and TMEV-H101-infected mice. We found that macrophage infiltration/microglial activation was markedly reduced in TMEV-H101 infection. We assayed for the levels of cytokines in the periphery and found IL-6 to be significantly higher in TMEV-H101-infected mice, compared to TMEV-DA-infected mice. Based on this, we provided C57BL/6J mice with recombinant mouse IL-6 to determine its effect on seizure susceptibility and macrophage infiltration/microglial activation. We found that elevation of IL-6 levels in the periphery tended to induce a modest increase in both the number of mice experiencing seizures and the number of activated microglia and macrophages infiltrating the brain of TMEV-DA-infected C57BL/6J mice.
Methods
Animals
Five week old, male C57BL/6J mice and C57BL/6J mice deficient in IL-6 (IL-6KO) were obtained from the Jackson Laboratory (Bar Harbor, ME). All animal experiments were reviewed and approved by the University of Utah Institutional Animal Care and Use Committee (Protocol #12-09006, #15-08004) and conducted in accordance with the guidelines prepared by the Committee on Care and Use of Laboratory Animals, Institute of Laboratory Animals Resources, National Research Council. All efforts were made to minimize suffering. Mice were euthanized through an overdose of isoflurane.
TMEV infection
Mice were anesthetized with isofluorane by inhalation and infected i.c. with 3 × 103, 3 × 104, or 3 × 105 plaque forming units (pfu) of TMEV-DA, TMEV-H101 (Tsunoda et al., 1997) or mock infected with phosphate-buffered saline (PBS) at a final volume of 20 μl per mouse. The site of injection was in the posterior parietal cortex of the right cerebral hemisphere to a depth of 2 mm [posterior (caudal) and medial of the right eye at approximately begma − 2 mm and interaural + 8 mm] (Kirkman et al., 2010). The needle had a William’s collar to limit penetration of the tip to 2 mm. The DA and H101 strains of TMEV were propagated as previously described (Tsunoda et al., 1997).
IL-6 treatment
C57BL/6J mice infected i.c. with 3 × 105 pfu of TMEV-DA were randomly divided into two treatment groups. One group (N=17) was treated, via intraperitoneal injection (i.p.), with 0.25 ng/g/mouse of recombinant mouse IL-6 (R&D Systems, Minneapolis, MN) and the second group (N=15) was treated i.p. with vehicle control (PBS, 25 μl). Treatment started on the day of infection and continued daily through day 14 post-infection (p.i.). TMEV-DA-infected IL-6KO mice were also treated with either recombinant mouse IL-6 (N=20) or vehicle (N=18).
Seizure scoring
Mice were monitored daily for seizures through day 21 p.i. The monitoring of seizure activity was performed as previously described (Libbey et al., 2011a). Briefly, mice were observed for 2 hours each day, at the same time of day, and mice were scored for 1 seizure per mouse per day. If more than 1 seizure was observed, then the highest scoring seizure was used to represent the data. Seizure activity was graded using the Racine scale: stage 1, mouth and facial movements; stage 2, head nodding; stage 3, forelimb clonus; stage 4, rearing; stage 5, rearing and falling (Benkovic et al., 2004; Racine, 1972). Seizure burden was analyzed by assessing both seizure incidence (numbers of mice having seizures, numbers of observed seizures per mouse) and seizure severity [cumulative seizure score per mouse, numbers of mice with the various maximum seizure scores (stages 1–5), numbers of seizures scored as stage 5].
Flow Cytometry
Flow cytometry was performed as previously described (Cusick et al., 2013). Briefly, on day 3 p.i., mice were euthanized and perfused with PBS. Subsequently, cells were mechanically isolated from the brains, incubated with trypsin for 30 minutes at 37°C and suspended in RPMI 1640 medium (Mediatech, Herndon, VA) supplemented with 10% Cosmic Calf Serum (CCS; Hyclone, Logan, UT) and with or without 1% L-glutamine (Mediatech), 1% antibiotics (Mediatech) and 50 μM 2-mercaptoethanol (Sigma, St. Louis, MO). Cells were further purified with Histopaque-1083 (Sigma). Cells were treated with Fc block (BD Bioscience, San Jose, CA), stained with the indicated anti-mouse antibodies for 30 minutes at 4°C [anti-CD45–v500 (BD Bioscience) and anti-CD11b–allophycocyanin (APC) (eBioscience, San Diego, CA)], and analyzed by flow cytometry on a BD FACSCanto II. Brain-derived cells were stained and analyzed individually for each mouse. Gating was determined based on fluorescence-minus-one (FMO) with isotype matched immunoglobulin controls. More specifically, FMO controls contained each antibody conjugate used in the experiment except one, with the addition of the appropriate isotype control for the excluded fluorochrome. This was performed for each fluorochrome. Flow cytometry data analysis was performed using FlowJo software (Tree Star, Inc., Ashland, OR).
Enzyme-linked immunosorbent assay (ELISA)
Samples of whole blood were collected, via cheek bleed, on days 3 and 7 p.i. from C57BL/6J mice infected i.c. with 3 × 105 pfu of TMEV-DA, TMEV-H101 or mock infected with PBS. The whole blood was centrifuged at 3,300 x g and 4°C for 30 minutes and the sera was removed and stored at −80°C until tested. The levels of IL-6, tumor necrosis factor (TNF) and IL-1β were measured in the sera using the OptEIA Mouse IL-6 ELISA Set, the OptEIA Mouse TNF ELISA Set and the OptEIA Mouse IL-1β ELISA Set, respectively, according to the manufacturer’s recommendation (BD Biosciences). Fluorescence was measured using a Wallac Victor 2 Multi-label Counter (PerkinElmer, Waltham, MA).
Statistical analysis
The programs SigmaPlot (Systat Software, Inc., Chicago, IL) or StatView (SAS Institute Inc., Cary, NC) were used for all statistical analyses performed. The Student’s t test was performed for pairwise comparisons. Analysis of variance (ANOVA), followed when necessary by the Fisher’s Protected Least Significant Difference (PLSD) post hoc test, was performed to determine group differences for continuous data (observed seizures per mouse, cumulative seizure score per mouse, ELISA). The Chi-Square test was utilized for nominal data (seizures: yes or no; stage 5 seizure: yes or no; seizure score maximums: yes or no).
Results
Reducing H101 virus still induced seizures
I.c. infection of C57BL/6J mice with 3 × 105 pfu of the H101 mutant virus resulted in approximately 40% of the mice developing acute behavioral seizures (Libbey et al., 2011b). Here we reduced the amount of H101 mutant virus in the inoculum to see if fewer mice would experience seizures. We found that decreasing the amount of H101 virus reduced the numbers of mice experiencing seizures, from 43% (13/30) at 3 × 105 pfu, to 15% (3/20) at 3 × 104 pfu and 20% (4/20) at 3 × 103 pfu (Table 1). Therefore, although the group infected with 3 × 105 pfu of the H101 mutant virus had significantly more mice experiencing seizures than the group infected with 3 × 104 pfu of the H101 mutant virus (p<0.05, Chi-square), infection with lower amounts of H101 still resulted in a certain portion of mice developing seizures.
Table 1.
Seizure rate (Racine scale, stage 3–5) in mice infected with increasing TMEV-H101 viral doses.
p<0.05, compared to 3 × 104, chi-square test
PFU, plaque forming unit
calculated as: (number of mice with seizures/total number of mice) × 100
Ramified microglia versus infiltrating macrophages/activated microglia
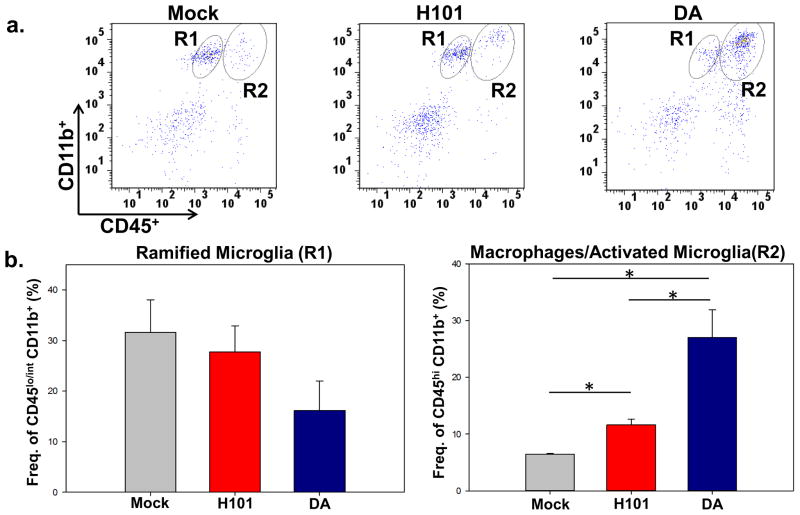
Peripheral macrophages were one of the dominant cell-types infiltrating the brain (Cusick et al., 2013; Howe et al., 2012a, b) and these cells were involved in the development of acute seizures following TMEV-DA infection (Cusick et al., 2013). As TMEV antigen was not detected in the CNS following TMEV-H101 infection (Cusick et al., 2014; Libbey et al., 2011b) despite the fact that virus is administered directly into the brain (i.c. infection), we tested whether macrophages were infiltrating the brain following TMEV-H101 infection. Phenotypic markers were used to differentiate ramified microglia from activated microglia/macrophages; ramified microglia have low to intermediate expression of CD45 and high CD11b expression on the cell surface, whereas activated microglia/macrophages express high levels of both CD45 and CD11b (Ford et al., 1995; Sedgwick et al., 1991). Far fewer activated microglia/macrophages (CD45hi CD11b+) were found to be infiltrating the brain of TMEV-H101-infected animals than TMEV-DA-infected animals (Figure 1A, R2). The number of activated microglia/macrophages (CD45hi CD11b+) was significantly higher (p<0.05, Student’s paired t test) in TMEV-H101-infected mice in comparison to mock infected mice; however the number of activated microglia/macrophages in TMEV-DA-infected mice was significantly higher (p<0.05, Student’s paired t test) than both the TMEV-H101-infected mice and the mock infected mice (Figure 1B, R2). Therefore, microglial activation and macrophage infiltration into the brain are reduced following TMEV-H101 infection compared to TMEV-DA infection. The number of ramified microglia (CD45lo/int CD11b+) was lower in the TMEV-H101-infected mice, and lower still in the TMEV-DA-infected mice, compared to mock infected mice, but this difference did not reach significance (Figure 1B, R1). Conversion of microglia from the ramified phenotype to the activated phenotype may account, either fully or in part, for this observed reduction in ramified microglia, although this has previously been discounted in TMEV-DA infection through the use of GFP chimeric mice (Cusick et al., 2013). The loss of ramified microglia through cell death is currently being explored.
Fig. 1.
Differentiation between ramified microglia and macrophages/activated microglia in the brains of TMEV-infected mice. a. Representative flow cytometry plots of ramified microglia (CD45lo/int CD11b+, R1) and macrophages/activated microglia (CD45hi CD11b+, R2) isolated from the brain of either a mock infected mouse (left panel), a TMEV-H101-infected (3 × 105 pfu) mouse (center panel) or a TMEV-DA-infected (3 × 105 pfu) mouse (right panel). Gates were set according to FMO, as described in the Methods. b. Quantification of flow cytometry data from three separate experiments presented as the mean + standard error of the mean (SEM) with 4 mice per group. *p<0.05, Student’s paired t test
Peripheral cytokine levels
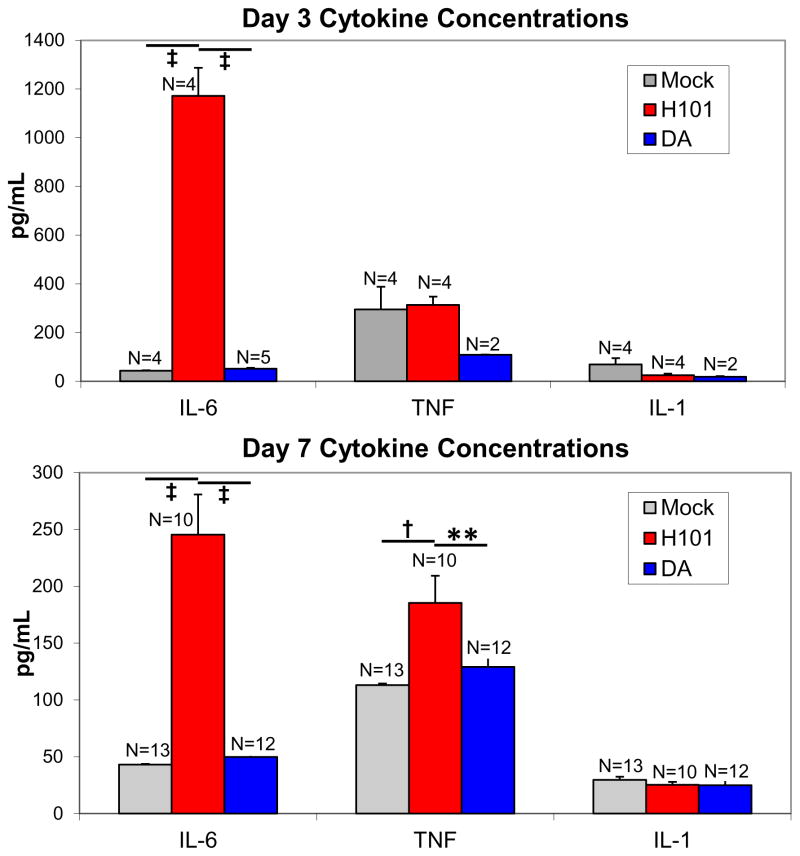
The levels of three common proinflammatory cytokines, TNF, IL-6 and IL-1, were assessed in the periphery of TMEV-infected mice by ELISA. Both IL-6 and TNF, but not IL-1, were previously shown, through the use of cytokine deficient mice and examination of cytokine mRNA expression levels in the brain, to correlate with seizures following infection with TMEV-DA (Kirkman et al., 2010). Also previously, in TMEV-DA infection, the numbers of monocytes/macrophages were shown to be significantly higher in the peripheral blood at 48 hrs p.i., compared to prior to infection (Cusick et al., 2013); however, the levels of cytokines in the periphery were not determined. The levels of IL-6 in the sera of TMEV-H101-infected mice at both days 3 (Figure 2, upper panel) and 7 (Figure 2, lower panel) p.i. were significantly higher than the levels in TMEV-DA-infected mice and mock infected mice (p<0.0001, ANOVA, Fisher’s PLSD). At day 3 p.i. (Figure 2, upper panel), there were no significant differences between the groups of mice for levels of either TNF or IL-1. At day 7 p.i. (Figure 2, lower panel), there was no significant differences between the groups of mice for level of IL-1; however the level of TNF in the sera of TMEV-H101-infected mice was significantly higher than the levels in TMEV-DA-infected mice (p<0.01, ANOVA, Fisher’s PLSD) and mock infected mice (p<0.001, ANOVA, Fisher’s PLSD). Therefore, the balance of cytokines, TNF and IL-6, in the sera is different between TMEV-DA infection and TMEV-H101 infection.
Fig. 2.
Peripheral cytokine levels in mice infected with TMEV-H101, TMEV-DA or mock infected. Mice were infected with 3 × 105 pfu of virus, or injected with PBS, and serum was collected at days 3 and 7 p.i. and assayed for IL-6, TNF and IL-1. **, p<0.01; †, p<0.001; ‡, p<0.0001 (ANOVA, Fisher’s PLSD). Data represents the mean + SEM. The total numbers of mice (N) are shown over the individual bars of the graph
IL-6 Treatment
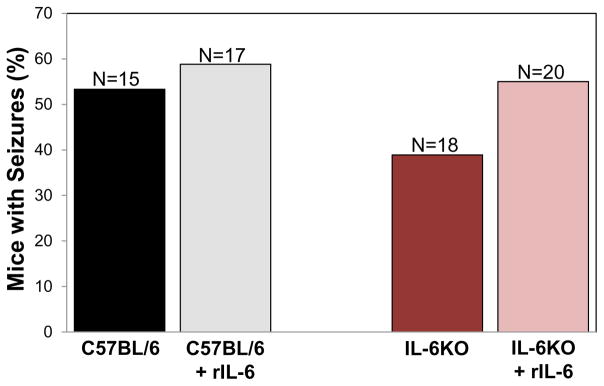
It has previously been shown that overexpression of IL-6 within the CNS results in spontaneous seizures (Campbell et al., 1993). To test whether peripherally increasing the amount of IL-6 present in the mouse would result in an increase in the number of mice experiencing seizures, C57BL/6J mice infected with 3 × 105 pfu of TMEV-DA were treated i.p. with recombinant mouse IL-6. This amount of IL-6 approximately achieved levels found in sera of mice infected with 3 × 105 pfu of TMEV-H101 (data not shown). A modest increase in the number of mice having seizures was observed in the C57BL/6J mice treated with recombinant mouse IL-6, compare to the vehicle-treated mice; this difference did not reach significance (Figure 3). Previously we demonstrated that IL-6KO mice experienced seizures at a significantly reduced rate compared to wild-type C57BL/6J mice when infected with TMEV-DA: 15% compared to 52.4%, respectively (Kirkman et al., 2010). Here IL-6KO mice were infected with 3 × 105 pfu of TMEV-DA and treated peripherally with recombinant mouse IL-6 to determine whether the addition of IL-6 to these deficient mice would restore the number of mice experiencing seizures to the wild-type level. Treatment with recombinant mouse IL-6 modestly increased the number of IL-6KO mice experiencing seizures, compared to the vehicle treated mice; again this modest increase did not reach significance (Figure 3). The number of IL-6KO mice experiencing seizures following treatment with recombinant mouse IL-6 (55%) emulated the level observed for wild-type mice (53.3%). Therefore, the addition of recombinant IL-6 to both wild-type C57BL/6J mice and IL-6KO mice tended to increase the number of mice experiencing seizures. The time course of seizure occurrence varied slightly between wild-type C57BL/6J mice and IL-6KO mice in that wild-type C57BL/6J mice experienced seizures between days 3 and 7 p.i. and IL-6KO mice experienced seizures between days 3 and 10 p.i. The addition of recombinant IL-6 to wild-type C57BL/6J mice altered the time course of seizure occurrence such that these mice experienced seizures between days 3 and 12 p.i., compared to between days 3 and 7 p.i. without the addition of recombinant IL-6. Addition of recombinant IL-6 to IL-6KO mice did not alter the time course of seizure occurrence and mice experienced seizures between days 3 and 10 p.i. There were no significant differences between any of the four groups of mice in numbers of observed seizures per mouse (wild-type C57BL/6J, 15 mice had 17 seizures; wild-type C57BL/6J plus recombinant IL-6, 17 mice had 16 seizures; IL-6KO, 18 mice had 16 seizures; IL-6KO plus recombinant IL-6, 20 mice had 23 seizures), cumulative seizure score per mouse (wild-type C57BL/6J, 5.2 ± 1.5; wild-type C57BL/6J plus recombinant IL-6, 4.1 ± 1.0; IL-6KO, 4.3 ± 1.5; IL-6KO plus recombinant IL-6, 5.6 ± 1.4; mean ± standard error of the mean [SEM]) or in the numbers of mice with the various maximum seizures scores (Table 2). Finally, of all of the seizures that were observed, the numbers of seizures that were scored as a stage 5 on the Racine scale (the most severe seizure) were significantly higher (p<0.05, Chi-square) in the IL-6KO plus recombinant IL-6 group (20/23, 87%) compared to the wild-type C57BL/6J plus recombinant IL-6 group (8/16, 50%). The other two groups fell in-between (wild-type C57BL/6J, 11/17, 64.7%; IL-6KO, 13/16, 81.3%) and were not significantly different.
Fig. 3.
Seizure rate (Racine scale, stage 3 to 5) in mice treated with recombinant mouse IL-6. C57BL/6J and IL-6KO mice were infected with 3 × 105 pfu of TMEV-DA, treated with recombinant mouse IL-6 (rIL-6), or vehicle, and observed for the development of seizures through day 21 p.i. The total numbers of mice infected (N) are shown over the individual bars of the graph. Percent mice with seizures is calculated as follows: (number of mice with seizures/total number of mice) × 100
Table 2.
Seizures, Racine Scale – Maximum severity of disease.a
| Strain | Treatment | Number of mice | Number (%) of mice with maximum clinical stages | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| 1 | 2 | 3 | 4 | 5 | |||
| C57BL/6J | PBS | 8 | 0 | 0 | 0 | 0 | 8 (100) |
| rIL-6 | 10 | 0 | 0 | 0 | 3 (30) | 7 (70) | |
| IL-6KO | PBS | 7 | 0 | 0 | 0 | 0 | 7 (100) |
| rIL-6 | 11 | 0 | 0 | 0 | 1 (9.1) | 10 (90.9) | |
IL-6KO, Interleukin-6 knockout; PBS, phosphate-buffered saline; rIL-6, recombinant IL-6
C57BL/6J mice infected with 3 × 105 pfu of TMEV-DA
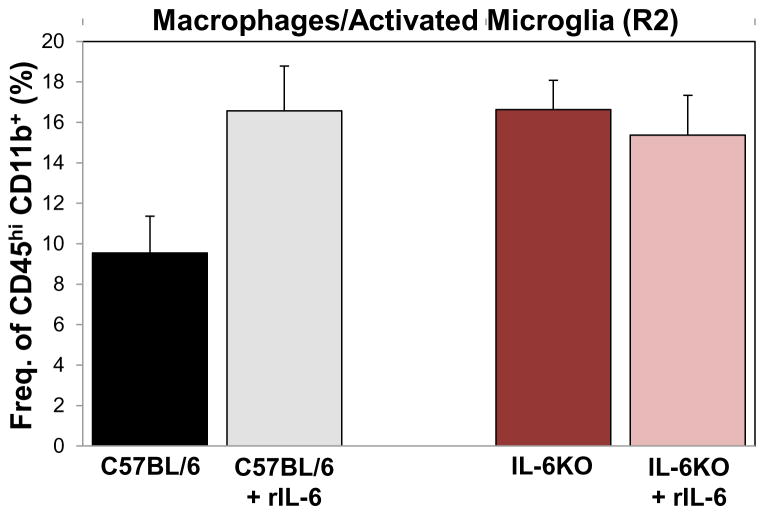
To test the effects of peripheral IL-6 on the amount of activated microglia and macrophages infiltrating the brain, CD45hi CD11b+ (R2) cells were differentiated from ramified microglia (CD45lo/int CD11b+) using phenotypic markers and flow cytometry, as before, on day 3 p.i. The amounts of activated microglia and macrophages infiltrating the brain were examined in wild-type C57BL/6J mice, IL-6KO mice, C57BL/6J mice treated with recombinant mouse IL-6 and IL-6KO mice treated with recombinant mouse IL-6, all infected with 3 × 105 pfu of TMEV-DA. The addition of recombinant mouse IL-6 to C57BL/6J mice tended to increase the amount of activated microglia/macrophages in the brain, compared to wild-type C57BL/6J mice, although this difference did not reach significance (p=0.07) (Figure 4). Surprisingly, the IL-6KO mice had more activated microglia/macrophages in the brain, compared to wild-type C57BL/6J mice (Figure 4). The addition of recombinant mouse IL-6 to IL-6KO mice made no difference to the amount of activated microglia/macrophages in the brain (Figure 4).
Fig. 4.
Macrophages/activated microglia in the brain of TMEV-infected mice with and without the elevation of peripheral IL-6. Quantification of macrophages/activated microglia (CD45hi CD11b+, R2) isolated, on day 3 post TMEV-DA infection (3 × 105 pfu), from the brains of either C57BL/6J mice, C57BL/6J mice treated with recombinant IL-6 (rIL-6), IL-6KO mice or IL-6KO mice treated with rIL-6. Data represents the mean + SEM with 3 mice per group
Discussion
TMEV is a naturally occurring enteric pathogen of the mouse (Theiler, 1937; Theiler and Gard, 1940). It is a non-enveloped, positive-sense, ssRNA virus of the family Picornaviridae. C57BL/6J mice infected with TMEV-DA develop acute encephalitis. The DA strain of TMEV is known to infect neurons (Dal Canto and Lipton, 1982; Lipton, 1975) and has a tropism for the hippocampus. The C57BL/6J mice are able to clear the virus by approximately day 28 p.i. and are immune to subsequent TMEV-DA viral infections (Libbey and Fujinami, 2011). C57BL/6J mice infected with TMEV-H101 experienced profound immunosuppression, H101 viral antigen is not detected in the brain parenchyma regardless of the fact that virus is administered directly into the brain (i.c. infection), but infection leads to greater than 90% mortality by day 7 p.i. (Cusick et al., 2014). The alterations in the TMEV-H101 viral genome, especially in the capsid protein coding region, are likely the cause of these differences in tropism and pathogenesis. Despite the differences in tropism and pathogenesis between the two viruses, infection with both TMEV-DA and TMEV-H101 results in the development of acute behavioral seizures in a significant portion of the infected mice, 58% and 40%, respectively (Libbey et al., 2011b). The role of infiltrating macrophages/activated microglia and IL-6 in the development of seizures has been demonstrated in TMEV-DA infection (Cusick et al., 2013; Libbey et al., 2011a). Here we explored the role of peripherally produced IL-6 in the development of seizures in TMEV-H101 infection.
An initial finding from the current study is that, in addition to the differences in tropism and pathogenesis between TMEV-DA and TMEV-H101, there is a difference in the correlation between initial viral dose and the development of seizures. For TMEV-DA there was a direct, step-wise correlation between the viral dose and the percentage of mice that developed seizures; as the dose increased, the number of mice having seizures increased (Libbey et al., 2011b). For TMEV-H101 there is no direct, step-wise correlation between the viral dose and the number of mice having seizures (Table 1); however, mice receiving any dose lower than 3 × 105 pfu had fewer seizures.
Although TMEV-H101 does not replicate or cause inflammation within the brain parenchyma despite being administered directly into the brain (i.c. infection), robust pachymeningeal and leptomeningeal inflammatory responses, consisting of both polymorphonuclear and mononuclear cells, are apparent during acute viral infection (Tsunoda et al., 1997). Therefore, we determined whether there was a difference in the numbers of infiltrating macrophages/activated microglia in the brains of TMEV-H101-infected mice compared to TMEV-DA-infected mice. We found that there was a significant decrease in the number of infiltrating macrophages/activated microglia in the brains of TMEV-H101-infected mice compared to TMEV-DA-infected mice (Figure 1).
There are three barrier systems around the CNS: the mid arachnoid layer of the meninges, the blood-cerebrospinal barrier and the blood-brain barrier (Nieuwenhuys et al., 2008). The involvement of the meninges, which interacts with both the CNS and the periphery, in addition to the presence of inflammatory responses in both thigh and back muscles (myositis) during acute TMEV-H101 infection (Tsunoda et al., 1997), suggests that the periphery may play a larger role in TMEV-H101 infection than in TMEV-DA infection.
Since IL-6 has been implicated in the induction of acute seizures following TMEV-DA infection, the peripheral cytokine milieu, to include IL-6, was examined. In exploring the contribution of the peripheral cytokine milieu to the development of seizures in TMEV-infected mice, several observations were made. First we found increased levels of IL-6 (day 3 and day 7 p.i.) and increased levels of TNF (day 7 p.i.) in the peripheral blood of TMEV-H101-infected mice compared to TMEV-DA-infected mice (Figure 2). Next we found that elevating the levels of IL-6 in the periphery tended to modestly increase both the numbers of mice having seizures (more so in the IL-6KO mice) and the numbers of activated microglia and peripheral macrophages infiltrating into the brains (more so in the C57BL/6J mice) following TMEV-DA infection (Figures 3 and 4). This suggests that peripheral IL-6 may play a role in the development of seizures when IL-6 is lacking (IL-6KO mice) or reduced (TMEV-H101 infection) from the CNS cytokine milieu. Peripheral IL-6 may also influence the activation of microglia and the recruitment of macrophages into the CNS when IL-6 is present in the CNS cytokine milieu (TMEV-DA infection of C57BL/6J mice). In the IL-6KO mice there is an imbalance in the innate immune response to infection. As a possible means of compensating for this imbalance, the TMEV-DA-infected IL-6KO mice recruit more macrophages/activate more microglia, compared to the TMEV-DA-infected C57BL/6J mice, in the CNS through the release of inflammatory cytokines other than IL-6; however these infiltrating macrophages/activated microglia are not themselves producing IL-6. Therefore there would be no apparent effect on macrophage infiltration/microglial activation in TMEV-DA-infected IL-6KO mice.
Overall, these results suggest that the peripheral cytokine milieu, in addition to the CNS cytokine milieu, may play a role in the development of seizures and that pathological levels of IL-6 may induce seizures even when there is a lack of viral replication within the CNS. Examination of the role played by the peripheral immune system in the development of seizures/epilepsy in the TMEV-induced seizure model could lead to the elucidation of novel therapeutics.
Acknowledgments
We would like to thank Jordan T. Sim, BA, and Mitchell A. Wilson for excellent technical assistance, Karen S. Wilcox, PhD and F. Lynn Sonderegger, PhD, for helpful discussions, and Daniel J. Harper for the outstanding preparation of the manuscript.
This work was supported by NIH T32AI055434 (M.F.C.), 1R01NS065714 (R.S.F.) and the Emma Mary Deland Foundation (R.S.F.). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Benkovic SA, O’Callaghan JP, Miller DB. Sensitive indicators of injury reveal hippocampal damage in C57BL/6J mice treated with kainic acid in the absence of tonic-clonic seizures. Brain Res. 2004;1024:59–76. doi: 10.1016/j.brainres.2004.07.021. [DOI] [PubMed] [Google Scholar]
- Campbell IL, Abraham CR, Masliah E, Kemper P, Inglis JD, Oldstone MB, Mucke L. Neurologic disease induced in transgenic mice by cerebral overexpression of interleukin 6. Proc Natl Acad Sci USA. 1993;90:10061–10065. doi: 10.1073/pnas.90.21.10061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusick MF, Libbey JE, Doty DJ, Fujinami RS. DA virus mutant H101 has altered CNS pathogenesis and causes immunosuppression. Journal Neuroimmunol. 2014;277:118–126. doi: 10.1016/j.jneuroim.2014.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusick MF, Libbey JE, Patel DC, Doty DJ, Fujinami RS. Infiltrating macrophages are key to the development of seizures following virus infection. J Virol. 2013;87:1849–1860. doi: 10.1128/JVI.02747-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Canto MC, Lipton HL. Ultrastructural immunohistochemical localization of virus in acute and chronic demyelinating Theiler’s virus infection. Am J Pathol. 1982;106:20–29. [PMC free article] [PubMed] [Google Scholar]
- Ford AL, Goodsall AL, Hickey WF, Sedgwick JD. Normal adult ramified microglia separated from other central nervous system macrophages by flow cytometric sorting. Phenotypic differences defined and direct ex vivo antigen presentation to myelin basic protein-reactive CD4+ T cells compared. J Immunol. 1995;154:4309–4321. [PubMed] [Google Scholar]
- Howe CL, LaFrance-Corey RG, Sundsbak RS, LaFrance SJ. Inflammtory monocytes damage the hippocampus during acute picornavirus infection of the brain. J Neuroinflammation. 2012a;9:50. doi: 10.1186/1742-2094-9-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe CL, LaFrance-Corey RG, Sundsbak RS, Sauer BM, LaFrance SJ, Buenz EJ, Schmalstieg WF. Hippocampal protection in mice with an attenuated inflammatory monocyte response to acute CNS picornavirus infection. Sci Rep. 2012b;2:545. doi: 10.1038/srep00545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkman NJ, Libbey JE, Wilcox KS, White HS, Fujinami RS. Innate but not adaptive immune responses contribute to behavioral seizures following viral infection. Epilepsia. 2010;51:454–464. doi: 10.1111/j.1528-1167.2009.02390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libbey JE, Fujinami RS. Neurotropic viral infections leading to epilepsy: focus on Theiler’s murine encephalomyelitis virus. Future Virol. 2011;6:1339–1350. doi: 10.2217/fvl.11.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libbey JE, Kennett NJ, Wilcox KS, White HS, Fujinami RS. Interleukin-6, produced by resident cells of the central nervous system and infiltrating cells, contributes to the development of seizures following viral infection. J Virol. 2011a;85:6913–6922. doi: 10.1128/JVI.00458-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libbey JE, Kennett NJ, Wilcox KS, White HS, Fujinami RS. Lack of correlation of central nervous system inflammation and neuropathology with the development of seizures following acute virus infection. J Virol. 2011b;85:8149–8157. doi: 10.1128/JVI.00730-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton HL. Theiler’s virus infection in mice: an unusual biphasic disease process leading to demyelination. Infect Immun. 1975;11:1147–1155. doi: 10.1128/iai.11.5.1147-1155.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuys R, Voogd J, van Huijzen C. The Human Central Nervous System. 4. Springer-Verlag; New York: 2008. Blood supply, meninges and cerebrospinal fluid circulation; pp. 95–135. [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Sedgwick JD, Schwender S, Imrich H, Dorries R, Butcher GW, ter Meulen V. Isolation and direct characterization of resident microglial cells from the normal and inflamed central nervous system. Proc Natl Acad Sci USA. 1991;88:7438–7442. doi: 10.1073/pnas.88.16.7438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theiler M. Spontaneous Encephalomyelitis of Mice, a New Virus Disease. J Exp Med. 1937;65:705–719. doi: 10.1084/jem.65.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theiler M, Gard S. Encephalomyelitis of Mice: I. Characteristics and Pathogenesis of the Virus. J Exp Med. 1940;72:49–67. doi: 10.1084/jem.72.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunoda I, McCright IJ, Kuang LQ, Zurbriggen A, Fujinami RS. Hydrocephalus in mice infected with a Theiler’s murine encephalomyelitis virus variant. J Neuropathol Exp Neurol. 1997;56:1302–1313. doi: 10.1097/00005072-199712000-00005. [DOI] [PubMed] [Google Scholar]
- Zurbriggen A, Thomas C, Yamada M, Roos RP, Fujinami RS. Direct evidence of a role for amino acid 101 of VP-1 in central nervous system disease in Theiler’s murine encephalomyelitis virus infection. J Virol. 1991;65:1929–1937. doi: 10.1128/jvi.65.4.1929-1937.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]