Abstract
The potential of licorice dietary supplements to interact with drug metabolism was evaluated by testing extracts of three botanically identified licorice species (Glycyrrhiza glabra L., Glycyrrhiza uralensis Fish. ex DC. and Glycyrrhiza inflata Batalin) and 14 isolated licorice compounds for inhibition of 9 cytochrome P450 enzymes using a UHPLC-MS/MS cocktail assay. G. glabra showed moderate inhibitory effects against CYP2B6, CYP2C8, CYP2C9, and CYP2C19, and weak inhibition against CYP3A4 (testosterone). In contrast, G. uralensis strongly inhibited CYP2B6 and moderately inhibited CYP2C8, CYP2C9 and CYP2C19, and G. inflata strongly inhibited CYP2C enzymes and moderately inhibited CYP1A2, CYP2B6, CYP2D6, and CYP3A4 (midazolam). The licorice compounds isoliquiritigenin, licoricidin, licochalcone A, 18β-glycyrrhetinic acid, and glycycoumarin inhibited one or more members of the CYP2C family of enzymes. Glycycoumarin and licochalcone A inhibited CYP1A2, but only glycycoumarin inhibited CYP2B6. Isoliquiritigenin, glabridin and licoricidin competitively inhibited CYP3A4, while licochalcone A (specific to G. inflata roots) was a mechanism-based inhibitor. The three licorice species commonly used in botanical dietary supplements have varying potential for drug-botanical interactions as inhibitors of cytochrome P450 isoforms. Each species of licorice displays a unique profile of constituents with potential for drug interactions.
TOC Graphic
1. Introduction
Many consumers of botanical dietary supplements also take prescription drugs and over the counter medications (Gardiner et al., 2007a; Gardiner et al., 2007b). Concomitant use of botanical dietary supplements and drugs might cause drug-botanical interactions, resulting in increased or decreased drug exposure and consequently leading to amplified adverse effects or drug inefficacy, respectively (de Lima Toccafondo Vieira and Huang, 2012; Gurley, 2012). Therefore, it is important to understand the potential of botanical dietary supplements to alter the metabolism and disposition of drugs. Major mechanisms of drug-botanical interactions include inhibition and induction of drug metabolizing enzymes or transporters (Liu et al., 2011; Sprouse and van Breemen, 2016). Inhibition of metabolism can increase drug exposure and toxicity, whereas induction can lead to decreased drug exposure and loss of efficacy (Huang and Lesko, 2004; de Lima Toccafondo Vieira and Huang, 2012).
Hepatic metabolism is the primary clearance pathway for most prescription drugs. Over two-thirds of drug metabolism is mediated by cytochrome P450 (CYP) enzymes including CYP3A (46%), CYP2C9 (16%), CYP2C19 (12%), CYP2D6 (12%), CYP1A (9%), CYP2B6 (2%), and CYP2E1 (2%) (Pelkonen, et al., 2008; Preissner, et al., 2010; Rendic and Guengerich, 2015). Inhibition of CYP enzymes is the most common mechanism underlying drug-botanical interactions. Usually, CYP inhibition causes increased drug exposure and possible toxicity, except for drugs that require metabolic activation by CYP enzymes, when CYP inhibition may lead to loss of efficacy (Lin and Lu, 1998).
Licorice is the common name attributed to certain species of the genus Glycyrrhiza that are known to produce the saponin glycyrrhizin (Fig. 1), a natural sweetener responsible for the characteristic sweet taste of the roots. Traditionally, dried licorice roots have been used as a food flavoring agent and for various medicinal purposes since ancient times (Nassiri Asl and Hosseinzadeh, 2008; Hosseinzadeh and Nassiri-asl, 2015). As such, preparations of licorice roots have been reported to have significant anti-inflammatory, anti-viral, anti-carcinogenic, anti-allergic, hepatoprotective, and estrogenic effects (Fiore et al., 2008; Hajirahimkhan et al., 2013; Nassiri Asl and Hosseinzadeh, 2008). The genus Glycyrrhiza includes approximately 30 species, among which Glycyrrhiza glabra L., G. uralensis Fisch. ex DC. and G. inflata Batalin are the three most commonly used in various systems of traditional medicine. In the European and Chinese Pharmacopoeias, G. glabra, G. uralensis and G. inflata are used interchangeably (World Health Organization, 1999). Because of this botanical ambiguity, many licorice dietary supplements do not disclose which species are used in their formulation (Zhang and Ye, 2009; Simmler et al., 2015a).
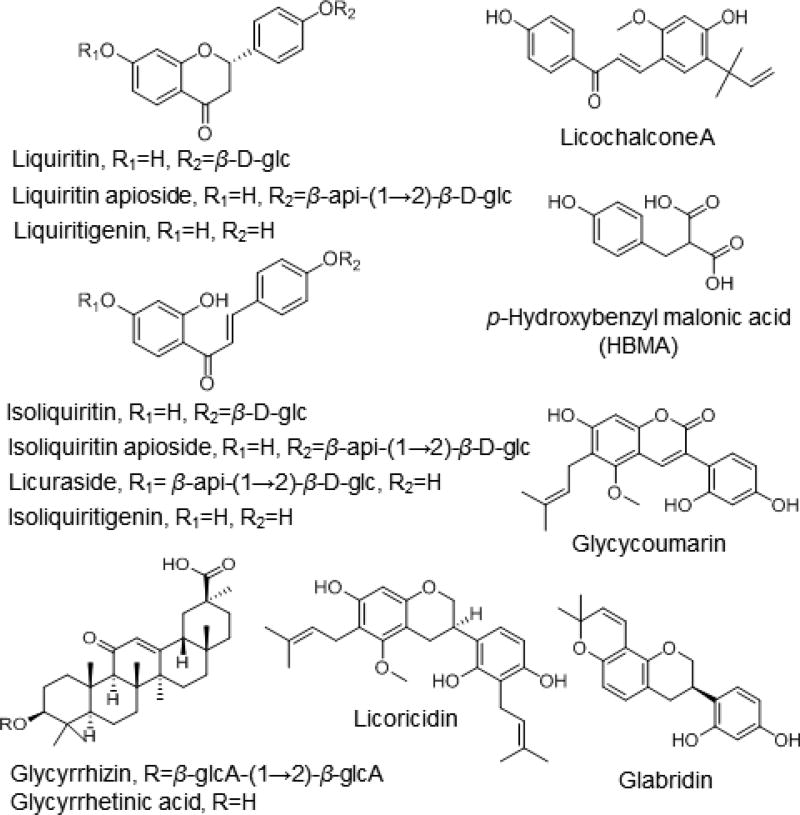
Fig. 1.
Chemical structures of the 14 licorice compounds tested individually for inhibition of cytochrome P450 enzymes.
As in drug-drug interaction studies, in vitro models of drug-botanical interactions are usually evaluated to ascertain whether clinical investigations are indicated (van Breemen, 2015; Sprouse and van Breemen, 2016). Recombinant CYP enzymes, human hepatocytes or human liver microsomes are typically used with probe substrates to measure possible inhibition of enzyme activity caused by test compounds or botanical extracts (Li et al., 2015; Walsky and Obach, 2004). The current FDA guidance for drug interaction studies recommends inhibition studies of CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, and CYP3A (U.S. Food and Drug Administration, 2012). Although drug interaction studies are not specified in the FDA guidance for CYP2A6 and CYP2E1, these enzymes may also cause drug-botanical interactions. CYP2A6 is involved in the metabolism of some tobacco compounds including nicotine, cotinine and N1-nitrosonornicotine (Pelkonen et al., 2000), and CYP2E1 has been reported to metabolize ethanol, caffeine, acetaminophen, and chlorzoxazone (Hrycay and Bandiera, 2008; Dey 2013). In this study, we used a cocktail assay approach (Li et al., 2015) with ultrahigh-pressure liquid chromatography-tandem mass spectrometry (UHPLC-MS/MS) to measure possible inhibition of all seven CYP isoforms recommended by the FDA as well as CYP2A6 and CYP2E1 by extracts of G. glabra, G. uralensis and G. inflata and 14 compounds (Fig. 1) isolated from these species.
2. Materials and Methods
2.1 Materials and Chemicals
Phenacetin was purchased from Tokyo Chemical Industry (Tokyo, Japan). Coumarin, bupropion hydrochloride, tolbutamide, chlorzoxazone, troleandomycin, glutathione, superoxide dismutase from bovine liver, catalase from bovine liver, β-nicotinamide adenine dinucleotide phosphate (NADPH), formic acid, potassium phosphate monobasic, and potassium phosphate dibasic were purchased from Sigma-Aldrich (St. Louis, MO). Dextromethorphan hydrobromide was obtained from MP Biomedicals (Santa Ana, CA). [d6]-Hydroxybupropion was purchased from Santa Cruz Biotechnology (Dallas, TX), and midazolam and testosterone were obtained from Cerilliant Corporation (Round Rock, TX). [d5]-7-Hydroxycoumarin, [d7]-6β-hydroxytestosterone and [13C3]-1’-hydroxymidazolam were purchased from BD Gentest (Woburn, MA). Amodiaquine, [d5]-N-desethylamodiaquine, (S)-mephenytoin, [d4]-acetaminophen, [d2]-6-hydroxychlorxazone, [d9]-4-hydroxytolbutamide, [d3]-(±)-4’-hydroxymephenytoin, and [d3]-dextrorphan tartrate were purchased from Toronto Research Chemicals (Toronto, Canada). Glycycoumarin (92.3% w/w) was purchased from BioBioPha (Kunming Institute of Botany, China). The licorice compounds glycyrrhizin (95.0% w/w), 18β-glycyrrhetinic acid (97.0% w/w) glabridin (87.10% w/w), and licochalcone A (96.06% w/w) were purchased from Sigma-Aldrich (St. Louis, MO), whereas liquiritigenin (95.5% w/w), liquiritin (95.9% w/w), liquiritin apioside (88.0% w/w), isoliquiritigenin (95.5% w/w), isoliquiritin (89.7% w/w), isoliquiritin apioside/licuraside (74.8/23.8% w/w), and p-hydroxybenzylmalonic acid (90.0% w/w) were isolated as described previously (Simmler et al., 2013, 2014, 2015b). Licoricidin (95.2% w/w) was isolated using another approach as described previously (Bodet et al., 2008).
The purities of each purchased and isolated licorice compound were determined by 1D, 1H quantitative NMR with the 100% method, as described previously (Pauli et al., 2014).
Pooled mixed gender human liver microsomes from 200 donors were purchased from XenoTech (Lenexa, KS). Supersomes containing human recombinant CYP3A4, oxidoreductase and cytochrome b5 were obtained from Corning (Woburn, MA). LC-MS-grade acetonitrile and methanol were purchased from Thermo Fisher (Fair Lawn, NJ). Water was prepared using an Elga Purelab Ultra (Siemens Water Technologies, Woodridge, IL) water purification system.
2.2 Extraction and quantitative analysis of licorice compounds
Dried root materials of Glycyrrhiza. uralensis Fisch. ex. DC. and Glycyrrhiza glabra L. (Fabaceae) were purchased from a local supplier (Chicago, IL), and from Mountain Rose Herbs, respectively. Glycyrrhiza inflata Batalin was collected in Xinjiang province, China in 2012. Licorice powders were botanically identified through DNA-based identification, macroscopic and microscopic analyses, and comparison with voucher specimens at the Field Museum of Natural History (Chicago, IL) as well as DNA barcoding as described previously (Simmler et al., 2015a). The root powders from each species were extracted by maceration at room temperature with a solvent mixture consisting of ethanol (200 USP proof), isopropanol and water (90:5:5, v/v/v) and a plant powder/volume of solvent ratio of 1:15 (Hajirahimkhan et al., 2015). The quantitative analysis of 14 licorice constituents in the extracts was carried out using a UHPLC-MS/MS assay as described previously (Li, et al., 2016).
2.3 IC50 determination using cocktail assay
Using a cocktail assay, IC50 values were determined according to the method of Li et al. (2015). Briefly, licorice extracts (G. glabra, G. uralensis and G. inflata) at 11 concentrations from 0.005–250 µg/mL or licochalcone A from 0.001–100 µM were incubated with 1.3 mM NADPH, 0.2 mg/mL human liver microsomes, and a cocktail of 10 probe substrates (100 µM phenacetin for CYP1A2, 1.5 µM coumarin for CYP2A6, 12 µM bupropion for CYP2B6, 1 µM amodiaquine for CYP2C8, 100 µM tolbutamide for CYP2C9, 50 µM (S)-mephenytoin for CYP2C19, 2.5 µM dextromethorphan for CYP2D6, 15 µM chlorzoxazone for CYP2E1, and 2.5 µM midazolam and 50 µM testosterone for CYP3A4) in potassium phosphate buffer (100 µL, 0.1 M, pH 7.4) at 37°C for 10 min. The incubation time of 10 min was selected so that the metabolic transformation of the probe substrates remained linear. The reactions were terminated by adding 20 µL of a stop solution consisting of water/acetonitrile/formic acid, (92:5:3; v/v/v) containing stable isotope-labeled surrogate standards of each metabolite. The samples were then centrifuged at 13000 × g at 4°C for 15 min prior to analysis using UHPLC-MS/MS as described previously (Li et al., 2015). Probe substrates for CYP2B6 and CYP2E1 were used at initial concentrations of 0.1Km, while others were at Km concentrations. As described previously (Li et al., 2015), marker inhibitors of each of the cytochromes P450 were used as positive controls to confirm that the microsomes were susceptible to inhibition.
2.4 Single concentration inhibition potency screening assay
Fourteen licorice compounds (liquiritin, isoliquiritin, liquirtin apioside, liquirtigenin, isoliquiritigenin, glycyrrhizin, 18β-glycyrrhetinic acid, p-hydroxybenzylmalonic acid, licoricidin, glycycoumarin, licochalcone A, and glabridin and a mixture of isoliquiritin apioside/licuraside (74.76/23.75 % w/w)) (Fig. 1) at 10 µM each were incubated individually with the probe substrates cocktail as described above. Methanol (0.5 µL) was added as a solvent control, and percentages of control activity were calculated to determine inhibition potency.
2.5 Single point time-dependent inhibition screening assay
Licorice extracts and compounds showing inhibition of CYP3A4 were tested further to determine their potential to function as CYP3A4 time-dependent inhibitors. Human liver microsomes (0.5 mg/mL) were incubated with extracts (200 µg/mL or 400 µg/mL) or compounds (100 µM or 50 µM) when using midazolam or testosterone as the substrate, respectively, in potassium phosphate buffer (100 µL, 0.1 M, pH 7.4) at 37°C in the presence and absence of 1.3 mM NADPH. After 30 min, a 5 µL aliquot of the incubation mixture was added to 95 µL potassium phosphate buffer containing 1.3 mM NADPH and a probe substrate at 5Km concentration. The reactions were terminated after incubating for 5 min and processed as described above. Addition of methanol solvent (0.5 µL) was used as a negative control and addition of 4 µM troleandomycin (a mechanism-based inhibitor of CYP3A4) (Polasek and Miners, 2006) was used as a positive control.
Two different calculations were used for evaluating the time-dependent inhibition data as indicated in equations (1) and (2). In equation (1), a ratio of the CYP3A4 activities in the experiment and negative control incubations was calculated (Atkinson et al., 2005); and in equation (2), a difference was calculated between the CYP3A4 activities in the negative control and the experiment (Obach et al., 2007). For both equations, the percentage of decrease in activity was determined with “A” defined as the activity of CYP3A4 (midazolam or testosterone).
| (1) |
| (2) |
2.6 KI and kinact determination
Incubations to determine Ki and kinact values contained 0.5 mg/mL human liver microsomes or 20 pmol/mL cDNA expressed CYP3A4, 1.3 mM NADPH, and licochalcone A at various concentrations (0, 50, 100, 200, and 300 µM for human liver microsomes or 0, 2, 5, 10, 20, and 50 µM for recombinant CYP3A4) in potassium phosphate buffer (100 µL, 0.1 M, pH 7.4). After 0, 4, 8, 12, 20, or 30 min incubation at 37°C, 5 µL aliquots were transferred to 95 µL potassium phosphate buffer containing 1.3 mM NADPH and midazolam at saturating concentration. After 5 min, the reactions were terminated and processed as described above.
2.7 Effect of washing to restore CYP3A4 activity
Human liver microsomes (0.2 mg/mL) or recombinant CYP3A4 (1 pmol/mL) were incubated with 50 µM or 10 µM licochalcone A, respectively, for 30 min at 37°C in the presence and absence of 1.3 mM NADPH. Preincubated samples were centrifuged at 10,000×g at 4°C for 30 min, and the protein pellets were re-suspended in potassium phosphate buffer (100 µL, 0.1 M, pH 7.4). Washing was repeated 2 more times. After washing, the remaining enzyme activity was measured using midazolam at the 5Km concentration.
2.8 Effects of glutathione, superoxide dismutase and catalase on CYP3A4 inactivation
Licochalcone A (100 µM or 20 µM) was preincubated with 1.3 mM NADPH and 0.5 mg/mL human liver microsomes or 20 pmol/mL recombinant CYP3A4 for 30 min at 37°C in the presence and absence of 2 mM glutathione, 1000 U/mL superoxide dismutase or 1000 U/mL catalase. The remaining enzyme activity was determined using midazolam at the 5Km concentration.
2.9 UHPLC-MS/MS of probe substrate metabolites
All metabolites and surrogate standards were analyzed by using UHPLC-MS/MS on a Shimadzu (Kyoto, Japan) Nexera UHPLC and LCMS-8050 triple quadrupole mass spectrometer as described previously (Li et al., 2015). Briefly, separations were carried out using a Waters (Milford, MA) ACQUITY UPLC BEH C18 column (2.1 × 100 mm, 1.7 µm) at a flow rate of 0.5 mL/min with a column oven temperature of 40°C. The primary metabolites of the 10 probe substrates and their corresponding isotope-labeled internal standards were separated using a 3-min linear gradient from 20% to 75% acetonitrile in water containing 0.1% formic acid. Tandem mass spectrometry quantitative analysis of the probe substrate metabolites was carried out using electrospray with polarity switching, collision-induced dissociation, and selected reaction monitoring. For additional details regarding the UHPLC-MS/MS method, see Li et al. (2015).
2.10 Data analysis
Quantitative UHPLC-MS/MS data were analyzed using Shimadzu LabSolutions software. The IC50, KI and kinact values were determined using the enzyme kinetics module of SigmaPlot (Systat Software, San Jose, CA). The percent of control activity, kobs,app and other calculations were carried out using Microsoft Excel (Seattle, WA).
3. Results
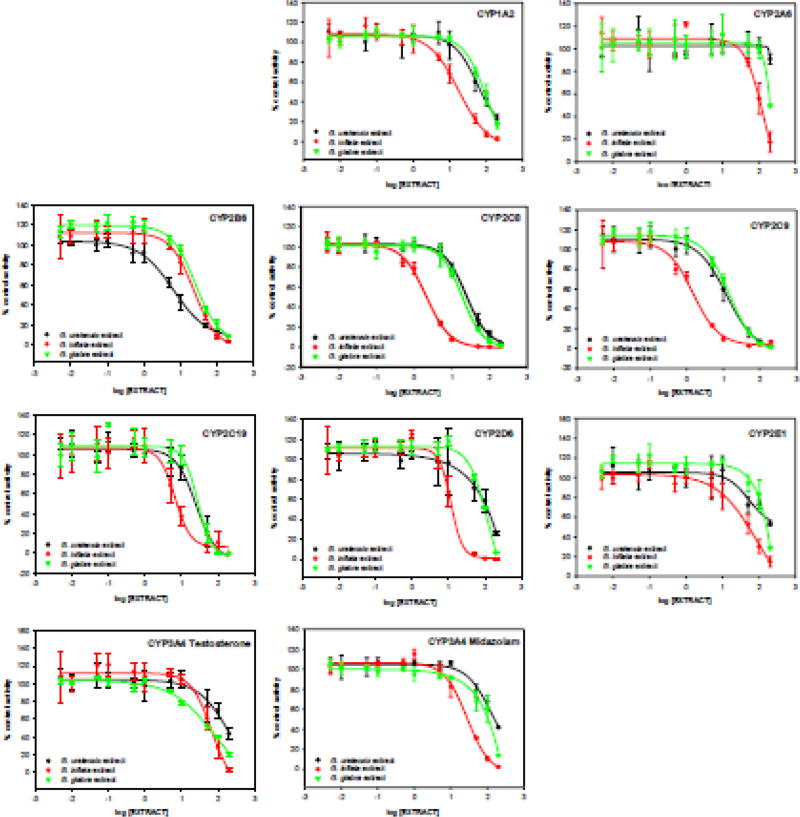
The inhibition curves (Fig. 2) and IC50 values (Table 1) for G. glabra, G. uralensis and G. inflata showed unique inhibitory effects indicating that not all species of licorice are equivalent with respect to drug interactions (pairwise comparison indicated that at least one pair of IC50 values was not the same with P-value<0.05). G. glabra extract displayed moderate inhibitory effects against CYP2B6, CYP2C8, CYP2C9, and CYP2C19, and weak inhibition against CYP3A4 (testosterone). In contrast, G. uralensis extract strongly inhibited CYP2B6 and moderately inhibited CYP2C8, CYP2C9 and CYP2C19, while G. inflata extract strongly inhibited enzymes in the CYP2C family and moderately inhibited CYP1A2, CYP2B6, CYP2D6, CYP3A4 (midazolam).
Fig. 2.
Inhibition curves for the inhibition of cytochrome P450 enzymes by extracts G. uralensis, G. inflata and G. glabra obtained using a cocktail assay. Activity is expressed as a percentage of remaining activity compared with the control containing no inhibitor. Experiments were carried out three times.
Table 1.
IC50 values for inhibition of cytochrome P450 enzymes in human liver microsomes by licochalcone A and extracts of G. uralensis, G. inflata and G. glabra (mean ± standard deviation).
| Enzyme | Substrate |
G. glabra IC50 (µg/mL) |
G. uralensis IC50 (µg/mL) |
G. inflata IC50 (µg/mL) |
Licochalcone A IC50 (µg/mL) |
|---|---|---|---|---|---|
| CYP1A2 | Phenacetin | 83.04 ± 16.96 | 68.25 ± 26.75 | 18.07 ± 4.14 | 12.40 ± 3.47 |
| CYP2A6 | Coumarin | >100 | >100 | >100 | >25 |
| CYP2B6 | Bupropion | 19.58 ± 1.80 | 6.51 ± 0.97 | 23.89 ± 4.55 | 17.96 ± 4.58 |
| CYP2C8 | Amodiaquine | 17.06 ± 1.54 | 24.03 ± 3.00 | 2.08 ± 0.15 | 1.05 ± 0.08 |
| CYP2C9 | Tolbutamide | 12.36 ± 1.14 | 12.65 ± 2.57 | 1.50 ± 0.19 | 0.78 ± 0.09 |
| CYP2C19 | (S)-Mephenytoin | 19.68 ± 2.70 | 25.46 ± 5.12 | 6.75 ± 0.96 | 3.60 ± 0.79 |
| CYP2D6 | Dextromethorphan | >100 | >100 | 11.73 ± 1.20 | 17.23 ± 1.68 |
| CYP3A4 | Midazolam | >100 | >100 | 15.47 ± 2.19 | 16.05 ± 1.91 |
| Testosterone | 55.64 ± 18.49 | >100 | 70.22 ± 21.80 | 6.78 ± 1.27 | |
| CYP2E1 | Chloroxazone | >100 | >100 | >100 | >25 |
To help explain the differences in the inhibition profiles of the three studied licorice species, UHPLC-MS/MS was used to measure the levels of 14 characteristic marker compounds (Fig. 1 and Table 2). The profiles of these compounds were unique for each of the licorice species. For example, G. glabra contained relatively more flavonoid and chalcone apiosides and was the only species to contain glabridin. Licoricidin and glycycoumarin were most abundant in G. uralensis extract, and only G. inflata contained licochalcone A.
Table 2.
Concentrations (% w/w) of the 14 licorice constituents in each tested licorice extract.
| Licorice compound | G. glabra | G. uralensis | G. inflata |
|---|---|---|---|
|
| |||
| % Weight compound / weight extract | |||
|
| |||
| Liquiritin | 0.31 | 3.83 | 1.33 |
| Isoliquiritin | 0.07 | 0.33 | 0.18 |
| Liquiritin apioside | 9.10 | 1.09 | 7.70 |
| Isoliquiritin apioside | 2.52 | 0.13 | 1.99 |
| Licuraside | 1.25 | 0.05 | 0.17 |
| Liquiritigenin | 0.07 | 0.23 | 0.26 |
| Isoliquiritigenin | 0.05 | 0.07 | 0.15 |
| Glycyrrhizin | 0.55 | 4.20 | 1.42 |
| Glabridin | 0.46 | 0 | 0 |
| Glycyrrhetinic acid | 0.03 | 0.01 | 0.20 |
| Licoricidin | 0 | 0.08 | 0 |
| HBMAa | 3.45 | 1.88 | 5.06 |
| Glycycoumarin | 0 | 0.13 | 0.005 |
| Licochalcone A | 0 | 0 | 5.24 |
HBMA = p-hydroxy-benzylmalonic acid
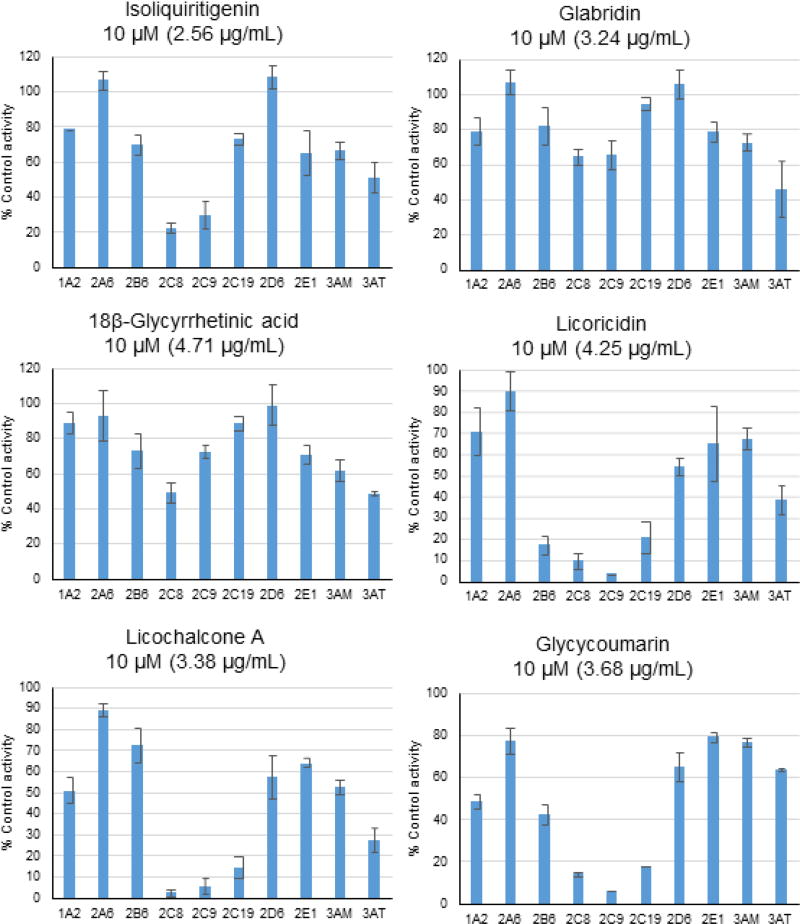
All 14 licorice constituents were assayed for inhibition of 9 cytochrome P450 isoforms using the probe substrate cocktail. Six of these compounds, isoliquiritigenin, glabridin, 18β-glycyrrhetinic acid, licoricidin, glycycoumarin, and licochalcone A, showed more than 50% inhibition of one or more cytochrome P450 enzymes at a test concentration of 10 µM (Fig. 3). Occurring in G. glabra, G. uralensis and G. inflata extracts, isoliquiritigenin inhibited CYP2C8 and CYP2C9 and 18β-glycyrrhetinic acid was a weak inhibitor of CYP2C8 and CYP3A4 (testosterone). Found only in G. glabra, glabridin inhibited CYP3A4 (testosterone). Licoricidin, unique to the tested G. uralensis extract, inhibited CYP2B6, CYP2C8, CYP2C9, CYP2C19, and CYP3A4 (testosterone). Glycycoumarin (found in G. uralensis and G. inflata but not in G. glabra) inhibited CYP1A2, CYP2B6 and CYP2C. Licochalcone A, abundant only in the G. inflata extract (5.24% w/w, Table 2), strongly inhibited CYP2C8 and CYP2C9, was a moderate inhibitor of CYP2C19 and CYP3A4 (testosterone), and weakly inhibited CYP1A2. IC50 values were determined for licochalcone A (Table 1) and ranged from high nM levels for inhibition of CYP2C9 to less than 20 µM for CYP1A2.
Fig. 3.
Inhibition of CYP enzymes by isoliquiritigenin, glabridin, glycyrrhetinic acid, licoricidin, glycycoumarin, and licochalcone A at 10 µM. Activity is expressed as a percentage of remaining activity compared with the control containing no inhibitor. Experiments were carried out in duplicate. 3AM: CYP3A4 (midazolam); 3AT: CYP3A4 (testosterone).
Inhibitors of CYP3A4, G. glabra extract, G. inflata extract and licochalcone A were tested for possible time-dependent inhibition. The G. inflata extract showed time-dependent inhibition of CYP3A4 (midazolam) (Table 3), and licochalcone A, a major constituent of the G. inflata extract, also demonstrated time-dependent inhibition. However, neither lichochalcone A nor the G. inflata extract produced time-dependent inhibition of CYP3A4 metabolism of testosterone (Table 3).
Table 3.
Percentage of CYP3A4 activity decrease after incubation with licorice extract or single compound using a single point time-dependent inhibition screening assay. Experiments were carried out in duplicate.
| Activity decrease % | ||||
|---|---|---|---|---|
|
|
||||
| 3A4 (midazolam) | 3A4 (testosterone) | |||
|
|
||||
|
Equation 1 (Obach et al. 2007) |
Equation 2 (Atkinson et al. 2005) |
Equation 1 (Obach et al. 2007) |
Equation 2 (Atkinson et al. 2005) |
|
| G. glabra | Not determined | Not determined | −3.1 | −6.6 |
| G. inflata | 10.8 | 34.7 | −1.6 | −2.9 |
| Licochalcone A | 23.2 | 58.0 | 0.9 | 1.4 |
| Troleandomycin | 75.6 | 72.8 | 26.8 | 28.3 |
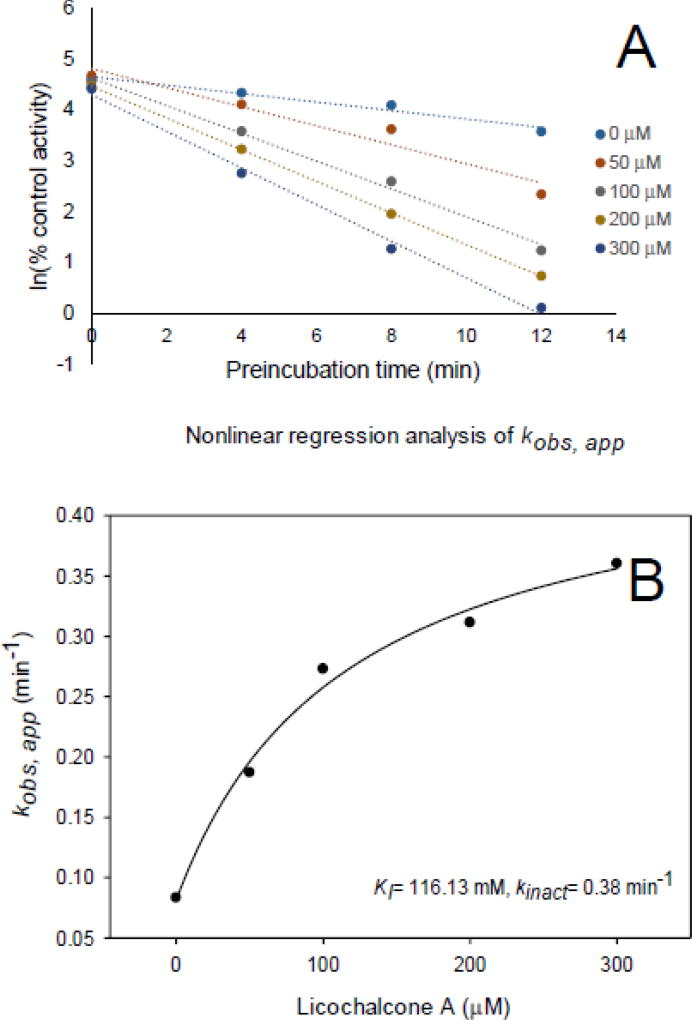
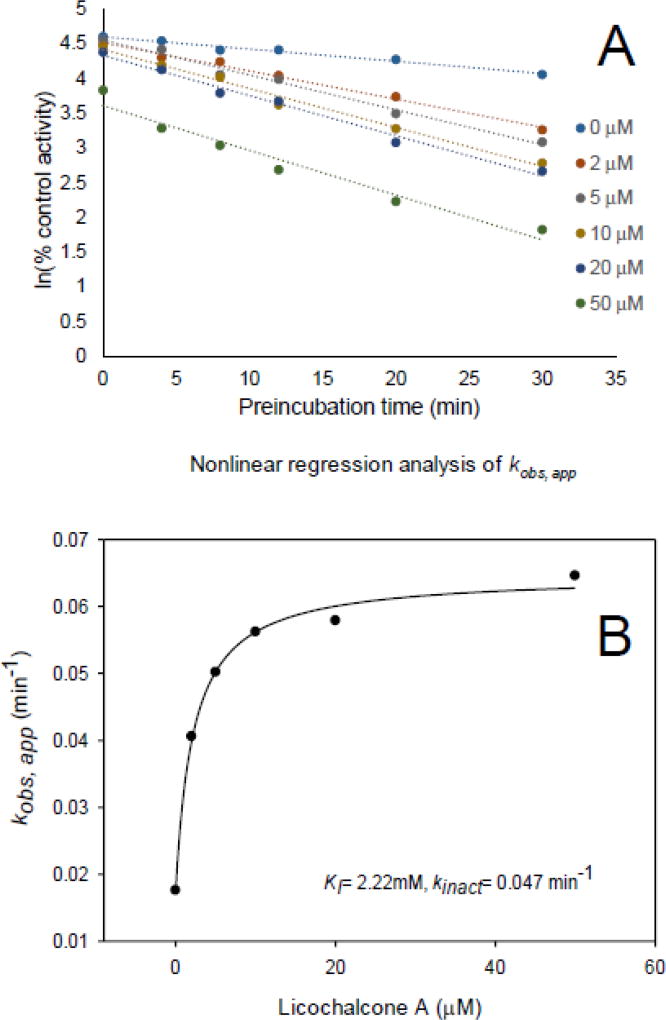
The kinetic constants for the inactivation of CYP3A4 (midazolam) by licochalcone A were determined using human liver microsomes and recombinant CYP3A4. After estimating the initial rate constants (kobs,app), KI and kinact were determined using nonlinear regression analysis (Fig. 4 and Fig. 5). The KI and kinact of licochalcone A for human liver microsomes were 116.13 µM and 0.38 min−1, respectively, and using recombinant human CYP3A4, they were 2.22 µM and 0.047 min−1. Efficiencies of inactivation were 0.0033 µM−1min−1 and 0.021 µM−1min−1 for human liver microsomes and recombinant CYP3A4, respectively. After washing out the inhibitor licochalcone A, the remaining enzyme activities were 57% for the human liver microsomes and 16.7% for recombinant CYP3A4, which indicated that washing did not restore CYP3A4 activity. Addition of glutathione, superoxide dismutase or catalase to the incubation did not prevent CYP3A4 inactivation by licochalcone A (Table 4). Therefore, licochalcone A was determined to be a mechanism based inhibitor of CYP3A4.
Fig. 4.
Determination of KI and kinact for inactivation of CYP3A4 (midazolam) by licochalcone A. (A) Time- and concentration-dependent inactivation of CYP3A4 (midazolam) by licochalcone A. (B) Non-linear regression analysis of kobs,app. Activity is expressed as a percentage of remaining activity compared with the control containing no inhibitor. Experiments were carried out three times.
Fig. 5.
Determination of KI and kinact for inactivation of recombinant CYP3A4 (midazolam) by licochalcone A. (A) Time- and concentration-dependent inactivation of recombinant CYP3A4 (midazolam) by licochalcone A. (B) Non-linear regression analysis of kobs,app. Activity is expressed as a percentage of remaining activity compared with the control containing no inhibitor. Experiments were carried out three times.
Table 4.
Effects of trapping agents on the inactivation of CYP3A4 by licochalcone A.
| Assay components | % Control activitya |
|
|---|---|---|
|
| ||
| Human liver microsomes | Licochalcone A | 100 |
| Licochalcone A, NADPH | 49.5 | |
| Licochalcone A, NADPH, glutathione | 38.5 | |
| Licochalcone A, NADPH, superoxide dismutase | 46.9 | |
| Licochalcone A, NADPH, catalase | 42.4 | |
|
| ||
| Recombinant human CYP3A4 | Licochalcone A | 100 |
| Licochalcone A, NADPH | 42.0 | |
| Licochalcone A, NADPH, glutathione | 32.4 | |
| Licochalcone A, NADPH, superoxide dismutase | 28.1 | |
| Licochalcone A, NADPH, catalase | 38.8 | |
mean of two experiments
4. Discussion
Kent et al. (2002) found moderate inhibition of CYP3A4 by an acetone extract of G. glabra, and we found weak inhibition of this drug metabolizing enzyme as well as moderate inhibition of CYP2B6, CYP2C8, CYP2C9, and CYP2C19 by an ethanolic extract (Table 1). In previous studies of G. uralensis, Tsukamoto et al. (2005) reported moderate inhibition of CYP3A4 by a methanol extract, and Qiao et al., (2014) reported strong inhibition of CYP2C9 but no effects on CYP3A4 by an ethanol extract or an ethyl acetate extract. In our studies using an ethanolic extract of G. uralensis, the results of Qiao, et al. (2014) were confirmed with respect to inhibition of CYP2C9 and lack of inhibition of CYP3A4 (Table 1).
Variations between laboratories regarding the potential for inhibition of cytochrome P450 enzymes by botanical dietary supplements are probably due to several factors including differing plant materials, extraction procedures and assay procedures. These differences emphasize the need for chemically standardizing the licorice extracts prior to biological evaluation (van Breemen, 2015). Nevertheless, there are striking similarities between the results from various laboratories regarding the potential for the licorice species G. glabra and G. uralensis to cause drug-botanical interactions.
Not investigated previously by other laboratories, the G. inflata extract showed the most potent inhibition of cytochrome P450 enzymes (with IC50 values in the low µg/mL range) among the three licorice species commonly used in botanical dietary supplements. In addition, the G. inflata extract inhibited more cytochrome P450 enzymes including CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, and CYP3A4 (Table 1). In assays of isolated licorice compounds, licochalcone A, which is a unique and abundant secondary metabolite of G. inflata (Table 2), was a major contributor to the inhibitory activity (Table 1).
In all, 14 licorice constituents were screened at 10 µM for possible inhibition of cytochrome P450 enzymes, and isoliquiritigenin, glabridin, 18β-glycyrrhetinic acid, licoricidin, glycycoumarin, and licochalcone A displayed the most significant inhibitory activities (Fig. 3). The mixture of isoliquiritin apioside/licuraside showed no inhibition, which is consistent with a previous report (Qiao et al., 2014). In a study using recombinant enzymes instead of human liver microsomes, Kent et al., (2002) found that glabridin irreversibly inhibited CYP3A4 and CYP2B6 and reversibly inhibited CYP2C9. Our data confirmed inhibition of CYP3A4 (testosterone) by glabridin but not inhibition of CYP2B6 or CYP2C9, which might be the result of differences between assays. In a human pharmacokinetics study, Aoki et al. (2007) reported that a single oral dose of a licorice dietary supplement containing 1% glabridin produced maximum plasma concentrations of up to 8 nM glabridin with a mean half-life of 10.7 h.
Our data indicate that isoliquiritigenin is a weak inhibitor of CYP3A4 (testosterone) and a moderate inhibitor of CYP2C9 (Table 1), and these results are consistent with those of Qiao et al. (2014). Like Qiao et al., we also found that glycycoumarin inhibited CYP1A2, CYP2C9, CYP2C19, and that liquiritin, liquiritin apioside, isoliquiritin, and isoliquiritin apioside did not inhibit any cytochrome P450 enzymes. However, we did not observe significant inhibition of CYP2D6 by glycycoumarin or inhibition of CY1A2 by liquiritigenin. Note that Qiao et al. (2014) did not assay for inhibition of CYP2B6 or CYP2C8 and only tested compounds isolated from G. uralensis, which included 7 of the 14 licorice compounds reported here.
Abundant only in G. inflata (Table 2), licochalcone A contributed significantly to the inhibition activities of G. inflata. Licochalcone A strongly inhibited CYP2C and, like the G. inflata extract, was a mechanism-based inhibitor of CYP3A4, which is the most abundant isoform in the human liver and is responsible for the metabolism of many prescribed drugs. In vitro detection of time-dependent inhibition of CYP3A4 has been suggested to correlate well with clinically relevant drug-botanical interactions (Clarke and Jones, 2008; Grimm et al., 2009).
Two equations were used to evaluate the experimental data for time-dependent inhibition of CYP3A4; note that equation 1 responds only to irreversible inhibition whereas equation 2 responds to both reversible and irreversible inhibition. For example, the positive control troleandomycin, known to only irreversibly inhibit CYP3A4, gave the same results using both equations (Table 4). However, time-dependent inhibition by G. inflata and licochalcone A showed different results using the two equations because they inhibited CYP3A4 (midazolam) both reversibly and time-dependently (Table 4). Further investigation, performed herein, confirmed that licochalcone A is a mechanism-based inhibitor of CYP3A4 (midazolam).
Licochalcone A, one of the major chalcones in G. inflata (5.24% w/w), contributed significantly to the cytochrome P450 enzyme inhibition observed for G. inflata. Previously, He et al. (2015) reported that licochalcone A strongly inhibited CYP1A2, CYP2C and CYP3A4 (testosterone). In our assay, licochalcone A strongly inhibited CYP2C and CYP3A4 (testosterone), and moderately inhibited CYP1A2, CYP2B6, CYP2D6 and CYP3A4 (midazolam). Importantly, we determined that licochalcone A inactivated CYP3A4 (midazolam) in a mechanism-based manner, although the underlying chemical reaction remains unclear. In a rat study, Choi et al. (2014) found that CYP3A4 metabolism of orally administered nifedipine was significantly inhibited by co-administration of licochalcone A at either 2.0 or 10 mg/kg, thereby increasing the serum half-life and the area under the plasma concentration-time curve for nifedipine.
Conclusions
Extracts from each of the three licorice species used most often in botanical dietary supplements, G. glabra, G. uralensis and G. inflata, were found to inhibit several drug-metabolizing cytochrome P450 enzymes. Several licorice characteristic compounds responsible for inhibiting these enzymes were identified, and among them were the species-specific compounds glabridin, glycycoumarin and more importantly licochalcone A. Because each of the licorice species showed a unique pattern of enzyme inhibition, different drug-botanical interactions may be expected for each. Therefore, it is important that the licorice raw materials used for the production of dietary supplements be botanically identified to the species level with a full disclosure of both the species identity and mode of preparation/extraction on the product labels.
Acknowledgments
The authors thank Shimadzu Instruments for providing the UHPLC-MS/MS system used during this investigation and Dr. Stefan Gafner for providing the licoricidin standard. This work was supported by U.S. National Institute of Health grants P50 AT000155 from the Office of Dietary Supplements and the National Center for Complementary and Integrative Health and R01 AT007659 from the National Center for Complementary and Integrative Health and the Office of the Director.
List of non-standard abbreviations
- UHPLC-MS/MS
ultrahigh-pressure liquid chromatography/tandem mass spectrometry
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aoki F, Nakagawa K, Kitano M, Ikematsu H, Nakamura K, Yokota S, Tominaga Y, Arai N, Mae TJ. Clinical safety of licorice flavonoid oil (LFO) and pharmacokinetics of glabridin in healthy humans. Am. Coll. Nutr. 2007;26:209–218. doi: 10.1080/07315724.2007.10719603. [DOI] [PubMed] [Google Scholar]
- Atkinson A, Kenny JR, Grime K. Automated assessment of time-dependent inhibition of human cytochrome P450 enzymes using liquid chromatography-tandem mass spectrometry analysis. Drug Metab. Dispos. 2005;33:1637–1647. doi: 10.1124/dmd.105.005579. [DOI] [PubMed] [Google Scholar]
- Bodet C, La VD, Gafner S, Bergeron C, Grenier D. A licorice extract reduces lipopolysaccharide-induced proinflammatory cytokine secretion by macrophages and whole blood. J. Periodontol. 2008;79:1752–1761. doi: 10.1902/jop.2008.080052. [DOI] [PubMed] [Google Scholar]
- Choi J-S, Choi J-S, Choi D-H. Effects of licochalcone A on the bioavailability and pharmacokineticcs of niffendipine in rats: possible role of intestinal CYP3A4 and P-gp inhibition by licochalcone A. Biopharm. Drug Dispos. 2014;35:382–390. doi: 10.1002/bdd.1905. [DOI] [PubMed] [Google Scholar]
- Clarke SE, Jones BC. Human cytochromes P450 and their role in metabolism-based drug-drug interactions. In: Rodrigues AD, editor. Drug-drug Interactions. Inform Healthcare; New York: 2008. pp. 53–85. [Google Scholar]
- Dey A, editor. Cytochrome P450 2E1: Its Role in Disease and Drug Metabolism. first. Springer Netherlands; Dordrecht, The Netherlands: 2013. [Google Scholar]
- Fiore C, Eisenhut M, Krausse R, Ragazzi E, Pellati D, Armanini D, Bielenberg J. Antiviral effects of Glycyrrhiza species. Phytother. Res. 2008;22:141–148. doi: 10.1002/ptr.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner P, Graham R, Legedza ATR, Ahn AC, Eisenberg DM, Phillips RS. Factors associated with herbal therapy use by adults in the United States. Altern. Ther. 2007a;13:22–29. [PubMed] [Google Scholar]
- Gardiner P, Kemper KJ, Legedza A, Phillips RS. Factors associated with herb and dietary supplement use by young adults in the United States. BMC Complement. Altern. Med. 2007b;7:39. doi: 10.1186/1472-6882-7-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm S, Einolf H, Hall S, He K. The conduct of in vitro studies to address time-dependent inhibition of drug-metabolizing enzymes: a perspective of the pharmaceutical research and manufacturers of America. Drug Metab. Dispos. 2009;37:1355–1370. doi: 10.1124/dmd.109.026716. [DOI] [PubMed] [Google Scholar]
- Gurley BJ. Pharmacokinetic herb-drug interactions (Part 1): origins, mechanisms, and the impact of botanical dietary supplements. Planta Med. 2012;78:1478–1489. doi: 10.1055/s-0031-1298273. [DOI] [PubMed] [Google Scholar]
- Hajirahimkhan A, Simmler C, Yuan Y, Anderson JR, Chen S-N, Nikolić D, Dietz BM, Pauli GF, van Breemen RB, Bolton JL. Evaluation of estrogenic activity of licorice species in comparison with hops used in botanicals for menopausal symptoms. PLoS One. 2013;8(7):e67947. doi: 10.1371/journal.pone.0067947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajirahimkhan A, Simmler C, Dong H, Lantvit DD, Li G, Chen S-N, Nikolić D, Pauli GF, van Breemen RB, Dietz BM, Bolton JL. Induction of NAD(P)H:quinone oxidoreductase 1 (NQO1) by Glycyrrhiza species used for women’s health: differential effects of the Michael acceptors isoliquiritigenin and licochalcone A. Chem. Res. Toxicol. 2015;28:2130–2141. doi: 10.1021/acs.chemrestox.5b00310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Wu J, Ning J, Hou J, Xin H, He Y, Ge G, Xu W. Inhibition of human cytochrome P450 enzymes by licochalcone A, a naturally occurring constituent of licorice. Toxicol. Vitr. 2015;29:1569–1576. doi: 10.1016/j.tiv.2015.06.014. [DOI] [PubMed] [Google Scholar]
- Hosseinzadeh H, Nassiri-Asl M. Pharmacological effects of Glycyrrhiza spp. and its bioactive constituents: update and review. Phyther. Res. 2015;29:1868–1886. doi: 10.1002/ptr.5487. [DOI] [PubMed] [Google Scholar]
- Hrycay EG, Bandiera SM. Cytochrome P450 enzymes. In: Gad SC, editor. Preclinical Development Handbook: ADME and Biopharmaceutical Properties. first. John Wiley & Sons; Hoboken, NJ: 2008. pp. 627–696. [Google Scholar]
- Huang S-M, Lesko LJ. Drug-drug, drug-dietary supplement, and drug-citrus fruit and other food interactions: what have we learned? J. Clin. Pharmacol. 2004;44:559–569. doi: 10.1177/0091270004265367. [DOI] [PubMed] [Google Scholar]
- Kent UM, Aviram M, Rosenblat M, Hollenberg PF. The licorice root derived isoflavan glabridin inhibits the activities of human cytochrome P450S 3A4, 2B6, and 2C9. Drug Metab. Dispos. 2002;30:709–715. doi: 10.1124/dmd.30.6.709. [DOI] [PubMed] [Google Scholar]
- Li G, Huang K, Nikolic D, van Breemen RB. High-throughput cytochrome P450 cocktail inhibition assay for assessing drug-drug and drug-botanical interactions. Drug Metab. Dispos. 2015;43:1670–1678. doi: 10.1124/dmd.115.065987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Nikolic D, van Breemen RB. Identification and chemical standardization of licorice raw materials and dietary supplements using UHPLC-MS/MS. J. Agric. Food Chem. 2016;64:8062–8070. doi: 10.1021/acs.jafc.6b02954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lima Toccafondo Vieira M, Huang S. Botanical-drug interactions: a scientific perspective. Planta Med. 2012;78:1400–1415. doi: 10.1055/s-0032-1315145. [DOI] [PubMed] [Google Scholar]
- Lin JH, Lu AY. Inhibition and induction of cytochrome P450 and the clinical implications. Clin. Pharmacokinet. 1998;35:361–390. doi: 10.2165/00003088-199835050-00003. [DOI] [PubMed] [Google Scholar]
- Liu C-X, Yi X-L, Si D-Y, Xiao X-F, He X, Li Y-Z. Herb-drug interactions involving drug metabolizing enzymes and transporters. Curr. Drug Metab. 2011;12:835–849. doi: 10.2174/138920011797470083. [DOI] [PubMed] [Google Scholar]
- Nassiri Asl M, Hosseinzadeh H. Review of pharmacological effects of Glycyrrhiza sp. and its bioactive compounds. Phytother. Res. 2008;22:709–724. doi: 10.1002/ptr.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obach RS, Walsky RL, Venkatakrishnan K. Mechanism-based inactivation of human cytrochrome P450 enzymes and the prediction of drug-drug interactions. Drug Metab. Dispos. 2007;35:246–255. doi: 10.1124/dmd.106.012633. [DOI] [PubMed] [Google Scholar]
- Pauli GF, Chen S-N, Simmler C, Lankin DC, Gödecke T, Jaki BU, Friesen JB, McAlpine JB, Napolitano JG. Importance of purity evaluation and the 360 potential of quantitative 1H NMR as a purity assay. J. Med. Chem. 2014;57:9220–9231. doi: 10.1021/jm500734a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelkonen O, Rautio A, Raunio H, Pasanen M. CYP2A6: A human coumarin 7-hydroxylase. Toxicology. 2000;144:139–147. doi: 10.1016/s0300-483x(99)00200-0. [DOI] [PubMed] [Google Scholar]
- Pelkonen O, Turpeinen M, Hakkola J, Honkakoski P, Hukkanen J, Raunio H. Inhibition and induction of human cytochrome P450 enzymes: current status. Arch. Toxicol. 2008;82:667–715. doi: 10.1007/s00204-008-0332-8. [DOI] [PubMed] [Google Scholar]
- Polasek TM, Miners JO. Quantitative prediction of macrolide drug-drug interaction potential from in vitro studies using testosterone as the human cytochrome P4503A substrate. Eur. J. Clin. Pharmacol. 2006;62:203–208. doi: 10.1007/s00228-005-0091-x. [DOI] [PubMed] [Google Scholar]
- Preissner S, Kroll K, Dunkel M, Senger C, Goldsobel G, Kuzman D, Guenther S, Winnenburg R, Schroeder M, Preissner R. SuperCYP: A comprehensive database on cytochrome P450 enzymes including a tool for analysis of CYP-drug interactions. Nucleic Acids Res. 2010;38:D237–D243. doi: 10.1093/nar/gkp970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao X, Ji S, Yu S-W, Lin X-H, Jin H-W, Duan Y-K, Zhang L-R, Guo D-A, Ye M. Identification of key licorice constituents which interact with cytochrome P450: evaluation by LC/MS/MS cocktail assay and metabolic profiling. AAPS J. 2014;16:101–113. doi: 10.1208/s12248-013-9544-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendic S, Guengerich FP. Survey of human oxidoreductases and cytochrome P450 enzymes involved in the metabolism of xenobiotic and natural chemicals. Chem. Res. Toxicol. 2015;28:38–42. doi: 10.1021/tx500444e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmler C, Hajirahimkhan A, Lankin DC, Bolton JL, Jones T, Soejarto DD, Chen S-N, Pauli GF. Dynamic residual complexity of the isoliquiritigenin– liquiritigenin interconversion during bioassay. J. Agric. Food Chem. 2013;61:2146–2157. doi: 10.1021/jf304445p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmler C, Jones T, Anderson JR, Nikolić DC, van Breemen RB, Soejarto DD, Chen S-N, Pauli GF. Species-specific standardisation of licorice by metabolomic profiling of flavanones and chalcones. Phytochem. Anal. 2014;25:378–388. doi: 10.1002/pca.2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmler C, Anderson JR, Gauthier L, Lankin DC, McAlpine JB, Chen S-N, Pauli GF. Metabolite profiling and classification of DNA-authenticated licorice botanicals. J. Nat. Prod. 2015a;78:2007–2022. doi: 10.1021/acs.jnatprod.5b00342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmler C, Nikolic D, Lankin DC, Yu Y, Friesen JB, van Breemen RB, Lecomte A, Le Quémener C, Audo G, Pauli GF. Orthogonal analysis underscores the relevance of primary and secondary metabolites in licorice. J. Nat. Prod. 2015b;77:1806–1816. doi: 10.1021/np5001945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprouse AA, van Breemen RB. Pharmacokinetic interactions between drugs and botanical dietary supplements. Drug Metab. Dispos. 2016;44:162–171. doi: 10.1124/dmd.115.066902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto S, Aburatani M, Yoshida T, Yamashita Y. CYP3A4 inhibitors isolated from licorice. Biol. Pharm. Bull. 2005;28:2002–2004. doi: 10.1248/bpb.28.2000. [DOI] [PubMed] [Google Scholar]
- U.S. Food and Drug Administration. Guidance for Industry - Drug interaction studies study design, data analysis, implications for dosing, and labeling recommendations 2012 [Google Scholar]
- van Breemen RB. Development of safe and effective botanical dietary supplements. J. Med. Chem. 2015;58:8360–8372. doi: 10.1021/acs.jmedchem.5b00417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsky RL, Obach RS. Validated assays for human cytochrome P450 activities. Drug Metab. Dispos. 2004;32:647–660. doi: 10.1124/dmd.32.6.647. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Radix Glycyrrhizae. Vol. 1. Geneva, Switzerland: 1999. WHO monographs on selected medicinal plants; pp. 183–194. [Google Scholar]
- Zhang Q, Ye M. Chemical analysis of the Chinese herbal medicine Gan-Cao (licorice) J. Chromatogr. A. 2009;1216:1954–1969. doi: 10.1016/j.chroma.2008.07.072. [DOI] [PubMed] [Google Scholar]