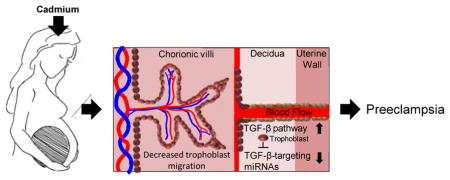
Graphical Abstract
1. Introduction
Previous studies have identified significant associations between exposures to the toxic metal cadmium (Cd) and the development of preeclampsia (PE) 1, 2. PE is a pregnancy disorder that is characterized by hypertension and proteinuria that can cause adverse health effects for mother and child, and sometimes death 3. The placenta has been identified as the potential etiologic factor in PE development, as well as a target organ for Cd accumulation2. Cd exposure can occur through various routes, such as cigarette smoke and ingesting contaminated food from Cd-containing soils 4. In addition, Cd exposure through the consumption of contaminated drinking water remains a serious concern as millions of women in the U.S. and globally use unregulated drinking water that may contain elevated Cd levels5. Recently, supporting prior research on the links between Cd exposure and PE1, 6–8, we demonstrated that elevated placental Cd levels significantly increased the odds of developing PE1. However, the underlying mechanism of Cd-associated PE is still unknown.
Disruption in growth factor signaling has been postulated as a mechanism for PE development. The vascular endothelial growth factor (VEGF) and its receptor Fms-related tyrosin kinase 1 (Flt1) are important for placental vasculogenesis and have been suggested as potential biomarkers for early detection of PE 9. Additionally, disrupted signaling of the transforming growth factor beta (TGF-β) pathway has been highlighted as a feature of PE. For example, elevated expression of the TGF-β pathway ligand TGFB1 was identified in the serum of preeclamptic women compared to women that were normotensive10. In prior studies, we observed that expression of genes belonging to the TGF-β pathway was enhanced in preeclamptic placentas 11. Moreover, microRNAs (miRNAs) that target the TGF-β pathway were dysregulated in preeclamptic placentas in association with Cd 7. In the same study, placental trophoblast cells treated with Cd displayed increased expression of the TGF-β pathway and dysregulation of TGF-β-targeting miRNAs following Cd treatment. These data support that the TGF-β pathway may be controlled epigenetically and showcase epigenetic dysfunction as a hallmark of PE 12. Cd may also play a role in influencing cell migration, where treatment with Cd previously inhibited in vitro migration in immortalized human trophoblast cells (HTR-8/SVneo) through actin skeleton reorganization 13, a possible effect of Cd-induced expression of the TGF-β pathway.
We hypothesized that Cd exposure results in increased expression of the TGF-β pathway through miRNA-regulated mechanisms in placental trophoblast cells. The hypothesized impact of this dysregulation is inhibition of trophoblast cell migration and thus proper implantation of the placenta. To test this hypothesis, we utilized JEG-3 cells, a human placental trophoblast cell line that posesses similar biological characteristics of first trimester trophoblast cells and has been used in previous studies to evaluate the effects of environmental contaminants 7, 14 in relation to Cd exposure, pathway modulation and miRNA expression.
2. Methods
Cell culture methodology
JEG-3 cells, a human placental trophoblast cell line, were used for all cell culture analyses as previously published7. Cultured media contained Dulbecco’s Eagle Minimum Essential Media with 10% FBS, sodium pyruvate, and penicillin/streptomycin. Cells were incubated at 37°C with 5% CO2. Cd chloride (CdCl2) was dissolved into the media by vortex for final concentrations of 0.028, 1, 10, and 25 uM and 5mL of Cd-containing media that was added to cells for 48 hours. TGFB1 (R&D Systems, Minneapolis, MN) and TGF-β receptor I/II Inhibitor LY2109761 (Selleckchem, Houston, TX) was added to cultured media for a final concentration of 10ng/mL and 5uM for 24 hours prior to Cd treatment, respectively.
Cell viability assay
JEG-3 cells were seeded in 96-well black clear bottom plates at 10,000/mL with a total of 200uL of cultured media. Dilutions of (CdCl2) were carried out starting at 2,000 uM until a final concentration of 0.001 uM was reached. Experiments were carried out in biological triplicate. Following a 48-hour incubation, media was removed and cell viability measured by the CellTiter-Glo Luminescent Cell Viability Assay (Promega, Madison, WI). Luminescence was measured using a Promega GloMax Microplate Luminometer.
Transwells
Cells were seeded into a Corning 8.0 uM pore transwell chamber (Sigma-Aldrich) at 5 ×104 with 200uL of Dulbecco’s Eagle Minimum Essential Media. A total of 500uL of media was placed inside the bottom well. Cells were incubated at 37°C with 5% CO2 for 48 hours and then fixed with formaldehyde. Once non-migrated cells were removed from inside the chamber, fixed cells were stained with crystal violet and membranes imaged using an Olympus IX81 Inverted Light Microscope. All images were taken at 10X magnification. 12 non-overlapping images from each membrane were captured and used with ImageJ64 (version 10.2) to measure the percentage area of migrated cells. Experiments were carried out in biological triplicate, and any outliers removed prior to statistical analyses.
Quantitative PCR analysis
RNA was collected using the AllPrep DNA/RNA/miRNA Universal Kit (Qiagen, Valencia CA) according to manufacturer’s instructions. The quantity of isolated RNA was measured with the Nanodrop 1000 spectrophotometer, and its integrity verified by the Agilent 2100 Bioanalyzer. cDNA was generated using either Qiagen’s RT2 First Strand kit or miScript II RT Kit for gene and miRNA analysis, respectively. miRNA expression was assessed using Qiagen’s miScript Primer Assays and SYBR Green PCR kits (Valencia, CA). The miRNA U6 was used as a housekeeping gene. To evaluate the expression of genes within the TGF-β pathway, the TGF-β/BMP Signaling Pathway RT2 Profiler PCR Array (Valencia, CA) was used, which tests 84 genes related to TGF-β signal transduction. Ct values from the RT2 Profiler PCR Arrays were analyzed for subsequent statistical testing. Qiagen’s one-step QuantiTect SYBR Green RT-PCR kit and QuantiTect Primer Assays (Valencia, CA) were used to evaluate expression of key members of the TGF-β pathway. Experiments were carried out in biological triplicate, and any outliers removed prior to statistical analyses.
Transfections
JEG-3 cells were seeded in 6-well cell culture plates with 2mL of cultured media. Once cells were ~75% confluent, cells were transfected with 5 nM of the miR26a inhibitor or mimic (Exiqon, Woburn, MA) using Invitrogen’s Lipofectamine 2000 Reagent and protocol. Control cells were treated with Lipofectamine 2000 Reagent without RNA. Cells treated with Cd were transfected 24 hours after the 48-hour incubation.
Statistical Analysis
To assess gene expression and miRNA differences in cells, raw CT values from quantitative PCR were used to calculate delta CT values. Partek Genomics Suite 6.4 (St Louis, Missouri) was used to complete analysis of covariance (ANCOVA). Statistically differences in mRNA and miRNA expression was defined as a false discovery rate-corrected p-value (q-value) < 0.05.
Migration percentages from 12 non-overlapping images from each transwell were averaged and t-tests were performed to assess statistical significance. Cells without Cd treatment were used as controls to assess migration differences of cells treated with Cd. To examine TGF-β-associated differences, cells treated with Cd and TGFB1 were compared to cells treated with TGFB1 only (control) and cells treated with Cd and LY2109761 were compared to cell treated with LY2109761 alone (control). Treatment with Lipofectamine only was used as a control to analyze alterations in migration between cells transfected with the miR26a Inhibitor and Mimic. P-values less than 0.05 were defined as statistically significant.
3. Results
3.1 Cd reduces placental trophoblast migration in JEG3 cells
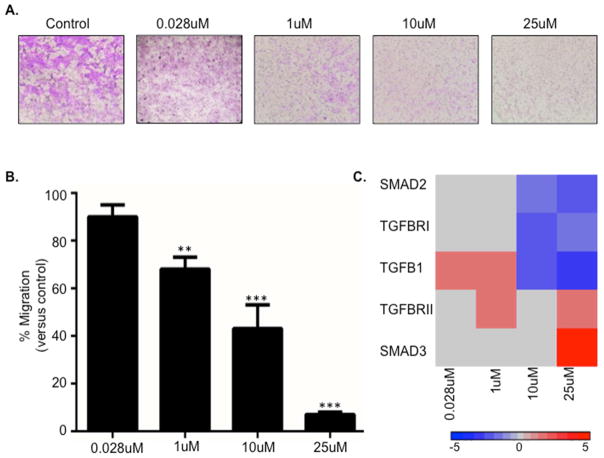
JEG3 cells were treated with 0.028, 1, 10, and 25 uM CdCl2. These concentrations span a range of environmentally relevant low (i.e. <5% cytotoxity) to high (i.e. >35% cytotoxicity) Cd toxicity concentrations respectively (Supplemental Figure 1). Following a 48-hour incubation, cells treated with Cd displayed diminished ability to migrate compared to untreated cells, which was enhanced with higher concentrations of Cd (Figure 1A and B). Specifically, the 1 uM concentration resulted in a significant reduction in migration at 30% migration versus control. The 10 uM and 25uM concentrations resulted in a 50% and 90% reduction in migration respectively (Figure 1B).
Figure 1. Cadmium (Cd) inhibits placental cell migration.
(A) Representative images of crystal violet-stained transwell membranes from JEG-3 cells treated for 48 hours with 0.028, 1, 10, and 25uM Cd. (B) Quantitative results of the transwell analysis. Cd-treated cells were compared to untreated cells to determine significance. *=p<0.05; **=p<0.01; ***=p<.001 (C) Heatmap displaying quantitative PCR results of TGF-β pathway genes using RNA isolated from JEG-3 cells treated with 0.028, 1, 10, and 25uM Cd.
3.2 Increased signaling through the TGF-β pathway results in decreased trophoblast migration
To assess whether Cd impacts the mRNA expression of key regulators of the TGF-β pathway in the placental trophoblast cells, qRT-PCR was used. Cd was found to induce the expression of transforming growth factor beta (TGFB1) at the lower concentrations (0.028 and 1uM) (Figure 1C and Supplemental Table 1). Higher concentrations (10uM and 25uM) increased SMAD family member 3 (SMAD3) and decreased TGFB1, transforming growth factor beta receptor 1 (TGFBRI), and SMAD family member 2 (SMAD2) expression (Figure 1C). Interestingly, transforming growth factor beta receptor 2 (TGFBRII) displayed increased mRNA expression with a range of low and high Cd concentrations.
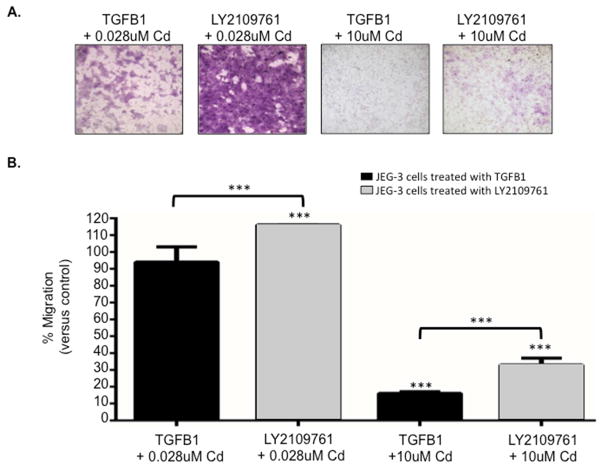
Next, we investigated the role of the TGF-β pathway in Cd-associated placental cell migration. JEG-3 cells were first treated with either the TGFB1 ligand or the TGF-β receptorI/II inhibitor LY2109761 for 24 hours followed by a 48-hour treatment with 0.028 or 10uM Cd. Cells treated with the ligand TGFB1 and exposed to Cd displayed significantly decreased migration compared to control (Figure 2). In contrast, treatment with the inhibitor LY2109761 improved Cd-associated migration with 0.028 and 10uM Cd. Interestingly, cell migration was improved 10-fold with inhibition of the TGF-β pathway with low and high Cd concentrations, indicating that this pathway indeed influences Cd-associated placental cell migration (Figure 2B).
Figure 2. The TGF-β pathway facilitates Cd-induced migration inhibition in placental cells.
(A) Representative images of crystal violet-stained transwell membranes from JEG-3 cells treated for 24 hours with either TGFB1 or the TGF-β receptorI/II inhibitor LY2109761 followed by a 48-hour treatment with 0.028 or 10uM Cd. (B) Quantitative results of the transwell analysis. Cells treated with TGFB1 and Cd was compared to cells treated with TGFB1 only (control) to determine significance, while cell treated with LY2109761 and Cd were compared to cells treated with LY2109761 only (control). *=p<0.05; **=p<0.01; ***=p<.001
3.3 miRNA-mediated inhibition of the TGF-β pathway improves trophoblast migration
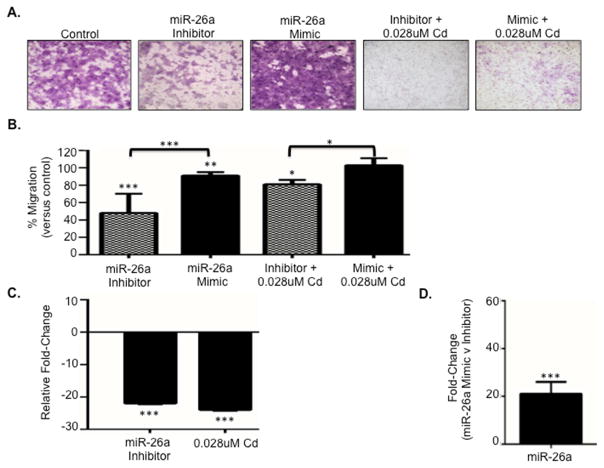
In prior work we identified a subset of 15 TGF-β pathway-targeting miRNAs that were associated with Cd in normotensive placenta, preeclamptic placenta, and placental trophoblast cells 7. From the subset, miR-26a was selected as a miRNA that targets the TGF-β pathway 15 to assess epigenetic regulation of the TGF-β pathway and establish its influence on placental cell migration. JEG-3 cells treated without or with 0.028uM Cd for 48 hours were subsequently transfected with either a miR-26a inhibitor (to reduce expression) or mimic (to increase expression) using Lipofectamine. Following transfection, cells treated with the inhibitor displayed significantly decreased migration without Cd (p=6.48e-8) and with Cd (p=0.03) compared to control (Figure 3A and B). In contrast, transfection with the mimic enriched placental cell migration in cells without Cd (p=0.001) and with Cd (not significant).
Figure 3. miR-26a expression regulates placental cell migration.
(A) Representative images of crystal violet-stained transwell membranes from JEG-3 cells treated with or without Cd and transfected with a miR-26a inhibitor or mimic. (B) Bar graph depicting quantitative data from transwell analysis of JEG-3 cells with and without Cd treatment in combination with transfections with a miR-26a inhibitor or mimic. *=p<0.05; **=p<0.01; ***=p<.001 (C) Quantitative PCR (qPCR) of decreased miR-26a expression in cells transfected with the miR-26a Inhibitor and cells treated with 48-hour 0.028uM Cd compared to control. (D) qPCR of miR-26a expression in cells transfected with the miR-26a mimic compared to cells transfected with the inhibitor.
Quantitative PCR (qPCR) of RNA isolated from JEG-3 cells transfected with the inhibitor confirmed that miR-26a expression was decreased in cells with reduced migration (Figure 3C and Supplemental Table 2) and increased in cells transfected with the mimic (Figure 3D) that displayed enhanced cellular migration. Moreover, 84 TGF-β pathway-associated genes were assessed in placental cells by qPCR following transfection with the miR-26a inhibitor. Out of the 84 assessed genes, 65 (77%) genes were upregulated (Supplemental Table 3), with 17 out of the 19 significantly altered genes displaying enhanced expression. Among these were SMAD3, SMAD5, transforming growth factor beta receptor associated protein 1 (TGFBRAP1), and SMAD specific E3 ubiquitin protein ligase 1 (SMURF1). Expression of SMAD1 was increased, but failed to reach statistical significance (Supplemental Table 3).
4. Discussion
Increased levels of cadmium assessed in serum and placental tissue is established to increase the risk of preeclampsia1, 2. Additionally, increased expression of members of the TGF- β pathway has been established to be elevated in PE2, 7, 10, 11, 16. In prior work, we identified a subset of TGF-β pathway-targeting miRNAs that were associated with Cd in the preeclamptic placenta and placental trophoblast cells 7. In the present study, we investigated the impact of Cd on signaling within the TGF-β pathway, the role of the TGF-β pathway as a mediator of trophoblast migration, and the effect of TGF-β pathway-associated miRNAs on placental cell migration capacity. The results demonstrate that even at environmentally relevant concentrations, Cd reduces placental trophoblast migration and that the TGF-β pathway enhances this effect via miRNA control serving as a molecular mediator. These results are critical in the study of environmental contaminants that impact placental function and disease and are highly relevant to understanding a mechanistic basis for the risk of developing PE.
The results from this study highlight the role and impact of the TGF-β pathway in relation to placental trophoblast migration, a mechanism that is likely tied to cytoskeleton redisposition13. The TGF-β pathway mediates the regulation of focal complexes and extracellular matrix reorganization17, two factors that influence cellular migration. Increased signaling of the TGF-β pathway can induce mitogen-activated protein kinases (MAP kinases), extracellular signal-regulated kinase (ERK) 1 and 2, and c-jun N-terminal kinase (JNK) to reorganize cytoskeleton structure to regulate cell migration 18. Thus, aberrant signaling in placental trophoblast can affect placental trophoblast migration to increase risk of PE. The inability of the placenta to properly implant to the uterus may also contribute to PE3, 9. Efficient placental implantation is dependent on the ability of trophoblast cells to invade the mother’s vasculature to create proper blood flow to the placenta19. Differentiation of progenitor cells to extravillous trophoblasts (EVTs) that are capable of migrating and invading has been established as a key upstream regulator of trophoblastic motility, with signaling of SMAD 1/520 and the Wnt pathway21 highlighted as promoters. In contrast to the TGF-β pathway, activation of the Wnt pathway increased trophoblast migration, invasion, and the expression of pro-migratory genes in trophoblasts22, 23, possibly through a T-cell factor-4-mediated mechanism21, suggesting the Wnt pathway is an antagonist of the TGF-β pathway and could be used to counter the effects observed in Cd-associated PE.
We hypothesized that increased signaling of the TGF-β pathway would reduce trophoblast migration while decreased expression would increase migration. In support of this, placental trophoblast treated with Cd and the TGFB1 ligand displayed diminished cellular migration. TGFB1 treatment in Cd-treated trophoblast likely enhanced TGF-β pathway signaling, resulting in the significant reduction in migration compared to trophoblast treated with Cd and a TGF-β receptor inhibitor. These results showcase a role for the TGF-β pathway in Cd-mediated cellular migration in placental trophoblast cells.
To assess for the impact of epigenetic regulation of the TGF-β pathway on placental cell migration, miR-26a was selected for analysis. This miRNA has been shown to target and decrease the expression of the TGF-β pathway transcription factor SMAD family member 1 (SMAD1)15. The a priori hypothesis was that the decreased expression of miR-26a would result in increased expression of the TGF-β pathway members resulting in reduced migration of placental trophoblasts. The results highlighted that decreased expression of miR-26a achieved through transfection of the JEG3 cells with the miRNA inhibitor resulted in a modest increase in SMAD1 expression, as well as significantly enhanced expression of SMAD3 and SMAD5. This suggests possible crosstalk between TGF-β pathway-targeting miRNAs and other members of the pathway. Moreover, in line with the underlying hypothesis, reduction in the expression of miR-26a via the inhibitor resulted in significantly decreased placental trophoblast migration, corroborating a previous study that observed decreased cellular migration and expression of TGF-β pathway-targeting miRNAs in colorectal carcinoma cells following TGF-β treatment24. In contrast, migration was comparable to control cells in placental cells overexpressing miR-26a with or without Cd treatment. miRNA dysfunction is a hallmark of PE12 and is postulated as a cause due to miRNAs being critical regulators of cell function by mediating gene expression. In addition, numerous members of the TGF-β pathway are targeted by miRNAs25 further underlying the importance of understanding the mechanism of miRNA-mediated PE. These data highlight that similar to Cd treatment, reduced expression of TGF-β pathway-targeting miRNAs results in diminished trophoblast migration. Therefore, bolstering our hypothesis that Cd regulates the TGF-β pathway epigenetically through miRNA, impeding placental trophoblast migration.
In conclusion, there are three major findings from the present study. First, in support of prior work that showed an impact of Cd on migration13, we confirm that exposure in placental trophoblast JEG3 cells decreases placental trophoblast migration. Second, the results demonstrate that an increase in signaling of the TGF-β pathway results in decreased trophoblast migration. Finally, the data support that miRNAs are critical for the TGF-β pathway-associated effect on cellular migration in placental trophoblast cells. In future, these miRNAs can be examined as targets for epigenetic reprogramming to repair cellular mobility. Investigating the underlying mechanisms for PE and potential environmental influences can provide further insight into molecular targets for reducing disease risk.
Supplementary Material
Cadmium treatment decreases placental trophoblast migration.
Increased TGF-β pathway signaling results in diminished trophoblast migration.
TGF-β pathway-targeting miRNAs are critical in regulating trophoblast migration.
miRNAs have the potential as therapeutic targets for cadmium-associated PE.
Acknowledgments
This research was supported by grants from the National Institutes of Health: R01 ES019315, P42 ES005948, and T32 ES007126.
Footnotes
The authors claim no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Laine JE, Ray P, Bodnar W, Cable PH, Boggess K, Offenbacher S, Fry RC. Placental Cadmium Levels Are Associated with Increased Preeclampsia Risk. PLoS One. 2015;10:e0139341. doi: 10.1371/journal.pone.0139341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pollack AZ, Ranasinghe S, Sjaarda LA, Mumford SL. Cadmium and Reproductive Health in Women: A Systematic Review of the Epidemiologic Evidence. Curr Environ Health Rep. 2014;1:172–184. doi: 10.1007/s40572-014-0013-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mol BJ, Roberts CT, Thangaratinam S, Magee LA, de Groot CJM, Hofmeyr GJ. Pre-eclampsia. Lancet. 2016;387:999–1011. doi: 10.1016/S0140-6736(15)00070-7. [DOI] [PubMed] [Google Scholar]
- 4.Satarug S, Garrett SH, Sens MA, Sens DA. Cadmium, environmental exposure, and health outcomes. Environ Health Perspect. 2010;118:182–190. doi: 10.1289/ehp.0901234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanders AP, Desrosiers TA, Warren JL, Herring AH, Enright D, Olshan AF, Meyer RE, Fry RC. Association between arsenic, cadmium, manganese, and lead levels in private wells and birth defects prevalence in North Carolina: a semi-ecologic study. BMC Public Health. 2014;14:955. doi: 10.1186/1471-2458-14-955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Saleh I, Shinwari N, Mashhour A, Mohamed Gel D, Rabah A. Heavy metals (lead, cadmium and mercury) in maternal, cord blood and placenta of healthy women. Int J Hyg Environ Health. 2011;214:79–101. doi: 10.1016/j.ijheh.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Brooks SA, Martin E, Smeester L, Grace MR, Boggess K, Fry RC. miRNAs as common regulators of the transforming growth factor (TGF)-beta pathway in the preeclamptic placenta and cadmium-treated trophoblasts: Links between the environment, the epigenome and preeclampsia. Food Chem Toxicol. 2016;98:50–57. doi: 10.1016/j.fct.2016.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Esteban-Vasallo MD, Aragones N, Pollan M, Lopez-Abente G, Perez-Gomez B. Mercury, cadmium, and lead levels in human placenta: a systematic review. Environ Health Perspect. 2012;120:1369–1377. doi: 10.1289/ehp.1204952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nikuei P, Malekzadeh K, Rajaei M, Nejatizadeh A, Ghasemi N. The imbalance in expression of angiogenic and anti-angiogenic factors as candidate predictive biomarker in preeclampsia. Int J Reprod Biomed. 2015;13:251–262. [PMC free article] [PubMed] [Google Scholar]
- 10.Peracoli MT, Menegon FT, Borges VT, de Araujo Costa RA, Thomazini-Santos IA, Peracoli JC. Platelet aggregation and TGF-beta(1) plasma levels in pregnant women with preeclampsia. J Reprod Immunol. 2008;79:79–84. doi: 10.1016/j.jri.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 11.Martin E, Ray PD, Smeester L, Grace MR, Boggess K, Fry RC. Epigenetics and Preeclampsia: Defining Functional Epimutations in the Preeclamptic Placenta Related to the TGF-beta Pathway. PLoS One. 2015;10:e0141294. doi: 10.1371/journal.pone.0141294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Munaut C, Tebache L, Blacher S, Noel A, Nisolle M, Chantraine F. Dysregulated circulating miRNAs in preeclampsia. Biomed Rep. 2016;5:686–692. doi: 10.3892/br.2016.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alvarez MM, Chakraborty C. Cadmium inhibits motility factor-dependent migration of human trophoblast cells. Toxicol In Vitro. 2011;25:1926–1933. doi: 10.1016/j.tiv.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 14.Guan HY, Sun K, Yang KP. The ERK1/2 Signaling Pathway Regulates 11beta-Hydroxysteroid Dehydrogenase Type 2 Expression in Human Trophoblast Cells Through a Transcriptional Mechanism. Biology of Reproduction. 2013:89. doi: 10.1095/biolreprod.113.110924. [DOI] [PubMed] [Google Scholar]
- 15.Leeper NJ, Raiesdana A, Kojima Y, Chun HJ, Azuma J, Maegdefessel L, Kundu RK, Quertermous T, Tsao PS, Spin JM. MicroRNA-26a is a novel regulator of vascular smooth muscle cell function. J Cell Physiol. 2011;226:1035–1043. doi: 10.1002/jcp.22422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shaarawy M, El Meleigy M, Rasheed K. Maternal serum transforming growth factor beta-2 in preeclampsia and eclampsia, a potential biomarker for the assessment of disease severity and fetal outcome. J Soc Gynecol Investig. 2001;8:27–31. [PubMed] [Google Scholar]
- 17.Cichon MA, Radisky DC. Extracellular matrix as a contextual determinant of transforming growth factor-beta signaling in epithelial-mesenchymal transition and in cancer. Cell Adh Migr. 2014;8:588–594. doi: 10.4161/19336918.2014.972788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu J, Lamouille S, Derynck R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009;19:156–172. doi: 10.1038/cr.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lafontaine L, Chaudhry P, Lafleur MJ, Van Themsche C, Soares MJ, Asselin E. Transforming growth factor Beta regulates proliferation and invasion of rat placental cell lines. Biol Reprod. 2011;84:553–559. doi: 10.1095/biolreprod.110.086348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cabrera-Sharp V, Read JE, Richardson S, Kowalski AA, Antczak DF, Cartwright JE, Mukherjee A, de Mestre AM. SMAD1/5 signaling in the early equine placenta regulates trophoblast differentiation and chorionic gonadotropin secretion. Endocrinology. 2014;155:3054–3064. doi: 10.1210/en.2013-2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meinhardt G, Haider S, Haslinger P, Proestling K, Fiala C, Pollheimer J, Knofler M. Wnt-dependent T-cell factor-4 controls human etravillous trophoblast motility. Endocrinology. 2014;155:1908–1920. doi: 10.1210/en.2013-2042. [DOI] [PubMed] [Google Scholar]
- 22.Pollheimer J, Loregger T, Sonderegger S, Saleh L, Bauer S, Bilban M, Czerwenka K, Husslein P, Knofler M. Activation of the canonical wingless/T-cell factor signaling pathway promotes invasive differentiation of human trophoblast. Am J Pathol. 2006;168:1134–1147. doi: 10.2353/ajpath.2006.050686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sonderegger S, Haslinger P, Sabri A, Leisser C, Otten JV, Fiala C, Knofler M. Wingless (Wnt)-3A induces trophoblast migration and matrix metalloproteinase-2 secretion through canonical Wnt signaling and protein kinase B/AKT activation. Endocrinology. 2010;151:211–220. doi: 10.1210/en.2009-0557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang H, Nie L, Wu L, Liu Q, Guo X. NR2F2 inhibits Smad7 expression and promotes TGF-beta-dependent epithelial-mesenchymal transition of CRC via transactivation of miR-21. Biochem Biophys Res Commun. 2017;485:181–188. doi: 10.1016/j.bbrc.2017.02.049. [DOI] [PubMed] [Google Scholar]
- 25.Butz H, Racz K, Hunyady L, Patocs A. Crosstalk between TGF-beta signaling and the microRNA machinery. Trends Pharmacol Sci. 2012;33:382–393. doi: 10.1016/j.tips.2012.04.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.