Abstract
The aim of this study was to investigate the role of cytochrome P450-2E1 (CYP2E1) in aging-dependent kidney damage since it is poorly understood. Young (7 weeks) and aged female (16–17 months old) wild-type (WT) and Cyp2e1-null mice were used. Kidney histology showed that aged WT mice exhibited typical signs of kidney aging such as cell vacuolation, inflammatory cell infiltration, cellular apoptosis, glomerulonephropathy, and fibrosis, along with significantly elevated levels of renal TNF-α and serum creatinine than all other groups. Furthermore, the highest levels of renal hydrogen peroxide, protein carbonylation and nitration were observed in aged WT mice. These increases in the aged WT mice were accompanied by increased levels of iNOS and mitochondrial nitroxidative stress through altered amounts and activities of the mitochondrial complex proteins and significantly reduced levels of the antioxidant glutathione (GSH). In contrast, the aged Cyp2e1-null mice exhibited significantly higher antioxidant capacity with elevated heme oxygenase-1 and catalase activities compared to all other groups, while maintaining normal GSH levels with significantly less mitochondrial nitroxidative stress compared to the aged WT mice. Thus, CYP2E1 is important in causing aging-related kidney damage most likely through increasing nitroxidative stress and that CYP2E1 could be a potential target in preventing aging-related kidney diseases.
Keywords: Kidney, aging, CYP2E1, apoptosis, fibrosis, oxidative stress
1. Introduction
Aging is an inevitable gradual physiological process which affects all the organs of the body. It is characterized by a slow, but progressive, deterioration of the body ability to maintain normal homeostasis or to respond efficiently to various stimuli and stressors, including environmental agents, eventually leading to organ failure with increased morbidity and mortality (Lopez-Otin et al., 2013). A mounting number of theories have been proposed to explain the normal aging process (Lopez-Otin et al., 2013; Medvedev, 1990; Sergiev et al., 2015). The widely accepted theory for the aging process is “the free radical theory of aging” (Harman, 1956). This theory states that the highly reactive oxidative radicals may increase damage in aged tissues by the reaction of the free radicals with cellular macromolecules, leading to alteration and/or deterioration of normal cellular functions, resulting in organ damage. Constantly, reactive oxygen species (ROS) and reactive nitrogen species (RNS) were reported to increase progressively with aging leading to the accumulation of oxidized and/or nitrated proteins in aged organs (Chakravarti and Chakravarti, 2017; Reeg and Grune, 2015).
Kidneys are one of the most affected organs by aging, and aged kidneys have been reported in humans and many other species such as rats, mice, and hamsters. Aging causes significant changes both to the (sub-)organelle structures and physiological functions of the kidneys (Martin and Sheaff, 2007; Percy et al., 2008). Moreover, kidney aging and the deterioration of its functions may lead to the development of many other age-related diseases in various tissues (Martin and Sheaff, 2007). The aged kidneys undergo many significant changes during the aging process such as inflammation, glomerulonephropathy, glomerulosclerosis, cell apoptosis, and renal fibrosis which indirectly reflects kidney aging (Hodgin et al., 2015; Martin and Sheaff, 2007; Percy et al., 2008). Interestingly, and in agreement with the “free radical theory of aging” (Harman, 1956), oxidative stress along with oxidative protein modifications have been correlated with kidney toxicities, acute and chronic kidney diseases, and aged kidney with deteriorating functions (Hodgin et al., 2015; Hosohata, 2016; Martin and Sheaff, 2007; Pedraza-Chaverri et al., 2016; Percy et al., 2005; Percy et al., 2008; Poulianiti et al., 2016; Small et al., 2012).
The cytochrome P450-2E1 (CYP2E1) is an enzyme, constitutively expressed in hepatic and extrahepatic tissues, including the kidney (Guengerich et al., 1991). CYP2E1 is involved in the metabolism of many small molecule substrates such as alcohol (ethanol), drugs, solvents, procarcinogens, and fatty acids while its expression can be induced by many of its substrate compounds. In addition, various pathophysiological conditions such as obesity and diabetes as well as many hormones, gender, and nutrition factors may regulate CYP2E1 levels and activities. Interestingly, CYP2E1-related catalytic activity may produce the superoxide anion even in the absence of its substrates (Caro and Cederbaum, 2004;Novak and Woodcroft,2000; Song et al., 1996). The superoxide anion can become a potent oxidative radical that can cause severe tissue damage through interaction with other free radicals such as nitric oxide (NO) to produce a more potently toxic peroxynitrite and may also produce toxic hydroxyl radical in the presence of metals (Abdelmegeed et al., 2015; Abdelmegeed and Song, 2014; Song et al., 2014).
We have recently reported a role of CYP2E1 in liver aging-related liver fibrosis and we showed that oxidative stress played a significant role in this process (Abdelmegeed et al., 2016). However, the potential role of CYP2E1 in the process of aging-dependent kidney damage with the development cellular apoptosis, glomerulonephropathy, and fibrosis, was never been studied. Thus, this study was aimed to investigate the role of CYP2E1 in age-related renal changes by comparing the histological, biochemical and functional markers in young and aged wild-type (WT) and Cyp2e1-null mice.
2. Materials and methods
2.1. Materials
All chemicals used in this study were from Sigma Chemical (St. Louis, MO, USA), unless indicated otherwise. Specific antibodies against CYP2E1, inducible nitric oxide synthase (iNOS), 3-nitrotyrosine (3-NT), nicotinamide adenine dinucleotide phosphate oxidase (NADPH oxidase), myeloperoxidase, total mitochondrial electron transport chain proteins (OXPHOS), and β-actin were from Abcam Inc. (Cambridge, MA, USA). Antibodies against glutamate-cysteine ligase catalytic subunit (GCLC) and glutamate-cysteine ligase modifier subunit (GCLM) were from Aviva Systems Biology Corp. (San Diego, CA, USA). Rabbit polyclonal antibodies against CYP1A, 2B, or 4A were generous gifts from Dr. James Hardwick, Northeastern Ohio University College of Medicine, Rootstown, OH, USA. Respective antibodies against heme oxygenase-1 (HO-1), collagen 1A1, ATP synthase subunit beta (ATP5B), and secondary antibodies conjugated with alkaline phosphatase were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
2.2. Animals and tissue collection for histology analyses
Young (approximately 7 weeks) and old female (approximately 16–17 months) inbred WT and Cyp2e1-null mice on a 129/Svj background, used in this study, were obtained from Dr. Frank Gonzalez, National Cancer Institute, Bethesda, MD (Lee et al., 1996). Mice were divided into four groups (n=10/group): (1) young WT; (2) old WT; (3) young Cyp2e1-null; and (4) old Cyp2e1-null. All mice were fed with the NIH31 rodent diet (ContD, 7017 NIH-31 open formula mouse/rat sterilizable diet (Choi et al., 2017). Parts from the kidneys were fixed in 10% neutral formalin for histological analyses and the rest of the kidneys from each mouse was snap-frozen for further biochemical analyses and trunk blood was collected immediately after killing by decapitation of mice briefly sedated by CO2 exposure. The histological and fibrosis scoring evaluation were performed blindly by a board-certified pathologist, using hematoxylin and eosin (H&E)-, periodic acid-Schiff (PAS)-, and Sirius red-stained slides. Each section was evaluated for glomerulonephropathy, inflammatory cell foci, tubular cytoplasmic vacuolation, tubular degeneration and/or regeneration, and kidney fibrosis (Table. 1). The image was captured by Olympus BX-51 microscope equipped with Digital Camera DP-70 (Olympus, Japan). For the characterization of the magnitude of fibrosis in all four groups, fibrotic and total areas were determined and the percentage of fibrotic area was calculated using Image J software (National Institutes of Health, Bethesda, MD, USA) from various image fields per slide. Animal experiments were performed in accordance with the National Institutes of Health guidelines for small animals and approved by the NIAAA Institutional Animal Care and Use Committee.
Table 1.
Summary of histopathological evaluation of kidneys in young or aged WT and Cyp2e1-null mice.
| Wild type young | Wild type old | Cyp2e1-null young | Cyp2e1-null old | |
|---|---|---|---|---|
| Number of animals examined | 7 | 7 | 7 | 7 |
| Glomerulonephropathy | 0 | 7 | 0 | 4 |
| Slight | 5 | 4 | ||
| Moderate | 2 | |||
| Inflammatory cell foci | 1 | 7 | 1 | 4 |
| Slight | 1 | 2 | 1 | 1 |
| Moderate | 2 | 2 | ||
| Severe | 3 | 1 | ||
| Tubular cytoplasmic vacuolation | 0 | 4 | 0 | 2 |
| Slight | 4 | 2 | ||
| Tubular degeneration/regeneration | 0 | 6 | 0 | 2 |
| Slight | 4 | 1 | ||
| Moderate | 2 | 1 | ||
| Fibrosis | 0 | 5 | 0 | 2 |
| Slight | 2 | 1 | ||
| Moderate | 2 | 1 | ||
| Severe | 1 |
Grading System: slight, few glomeruli or tubules or surrounding cells affected; moderate, multiple glomeruli or tubules or surrounding cells affected; severe, diffuse tissue infiltration and/or damage.
2.3. Measurements of serum creatinine, total renal tumor necrosis factor-α (TNF-α), and glutathione (GSH) levels in young and aged mice
Intra-renal GSH in 50 mg kidney extracts were determined by using commercially available kits (Cayman Chemical, Ann Arbor, Michigan, USA). TNF-α levels were evaluated by a specific ELISA kit (Abcam Inc., Cambridge, MA, USA). The amounts of serum creatinine for each mouse of the four groups were determined using a detection kit from Cayman Inc., (Ann Arbor, MI). The manufacturer's protocols were followed for all commercial kits.
2.4. Immunoblot analysis for evaluating the levels of target proteins, oxidized proteins, and immunoprecipitated proteins
Total renal homogenates from four different mouse groups were prepared in ice-cold extraction buffer (50 mM Tris–Cl, pH 7.5, 1 mM EDTA, and 1% CHAPS), and mitochondrial fractions were prepared by differential centrifugation, as described previously (Abdelmegeed et al., 2016; Choi et al., 2016, 2017). All extraction buffers were pre-equilibrated with nitrogen gas to remove the dissolved oxygen. Protein concentrations for total protein lysates and mitochondrial lysates were determined using the bicinchoninic acid protein assay reagent (Pierce, Rockford, IL, USA). Equal amounts of lysates were resolved on 7 or 12% SDS-PAGE gels and electrophoretically transferred to nitrocellulose membranes (Hercules, CA, USA). Membranes were blocked with 5% (w/v) nonfat milk proteins, and then incubated with a specific primary antibody to each target protein in 3.5% (w/v) bovine serum albumin (BSA) overnight at 4°C. Following separate steps of washing to remove unbound antibodies, membranes were probed with a specific secondary antibody for 1 h. Image was detected by ECL SuperSignal West Pico Kit (Thermo Fisher scientific, Waltham, MA 02451). β-actin and ATP-5A were used as loading controls for whole kidney extracts and mitochondrial proteins, respectively. For protein carbonylation, an aliquot of the total renal lysates was derivatized with dinitrophenylhydrazine (DNPH) under acid denaturing conditions using Oxyblot protein oxidation detection kit (Millipore, Billerica, MA) by following the manufacturer’s instructions. A separate aliquot of the renal lysates, that had been acid-denatured as well but not treated with DNPH, were used as a negative control. For the immunoprecipitation experiment, two pooled samples were prepared from each of the four groups. Each sample with a total 500 µg of renal cell lysates was pooled equally from 5 mice/sample (n=10/group). Then, the specific antibody (5 µg) to collagen 1A1 was used for immunoprecipitation. Pierce™ protein A/G magnetic beads from Life Technologies (Grand Island, NY, USA) were used by following the manufacturer's protocol. The immunoprecipitated proteins were subsequently analyzed by immunoblot analysis using the specific antibody against collagen 1A1. Relative levels of target proteins were normalized to the levels of the loading controls, β-actin and ATP-5B, for total cell lysates and mitochondria, respectively, and collagen 1A1 for IP followed by immunoblot. Values for the young WT mice group were set at 100%.
2.5. Immunohistochemistry and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) analyses
Formalin-fixed kidney samples were processed and 5-µm thick paraffin sections were used for immunohistochemistry (IHC). In brief, deparaffinized kidney sections were subjected to 3% hydrogen peroxide followed by antigen retrieval. The sections were blocked with 2% (w/v) non-fat skim milk solution, and incubated with the primary antibody against myeloperoxidase, oxidized proteins, nitrated proteins detected by anti-3-NT antibody, or collagen type 1A1 at 4 °C overnight. After subsequent washing steps to remove the unbound antibody, the attached primary antibody was then linked to the dextran polymer per manufacturer's protocol (Envision kit, Dako, Carpinteria, CA, USA). The final reaction was performed by immersing the sections in a solution of 3,3′-diaminobenzidine (DAB). The sections were then counterstained with hematoxylin. For detection of DNA strand breaks to characterize apoptotic cell death in kidney tissues, the ApopTag Peroxidase in situ apoptosis detection kit (cat. # S7100) (Millipore, Billerica, MA) was used. TUNEL-positive kidney cells were counted in 10 high-power microscope fields for all four groups and tabulated.
2.6. Measurements of renal hydrogen peroxide (H2O2) levels, mitochondrial complexes activities, ATP levels, CYP2E1, and anti-oxidant enzyme activities
Hydrogen peroxide (H2O2) levels were measured using the hydrogen peroxide assay kit (Abcam Inc., Cambridge, MA, USA) following the colorimetric assay protocol and as previously described (Abdelmegeed et al., 2016). Mitochondrial complexes I, II, III, and IV activities were evaluated using mitochondrial proteins by commercial kits from Cayman Chemical Inc. (Ann Arbor, MI, USA) per manufacturer’s protocol. ATP levels in snap-frozen liver tissues were evaluated by using the ATP Assay Kit (Abcam Inc., Cambridge, MA, USA) following the manufacturer's protocol. CYP2E1 activity was measured by the rate of p-nitrophenol oxidation to p-nitrocatechol with 1 mg of renal cytosol/sample (Reinke and Moyer, 1985). The catalytic activities of total renal superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (Gpx) in the total renal lysates were determined by using the kits from Cayman (Ann Arbor, MI, USA), by following the manufacturer’s instruction.
2.7. Data evaluation
Data presented are mean ± SEM, n=8–10, unless indicated otherwise. All statistical analyses were performed using Prism7 software. Two-way ANOVA was performed to assess the differences in all the results. Only, when two-way ANOVA revealed the presence of age-genotype interaction, one-way ANOVA followed by post hoc Tukey's test at a significance level of 0.05 was performed to determine the differences between the four groups as previously described (Abdelmegeed et al., 2017; Abdelmegeed et al., 2016; Abdelmegeed et al., 2011).
3. Results
3.1. Increased renal histopathological changes in aged WT mice
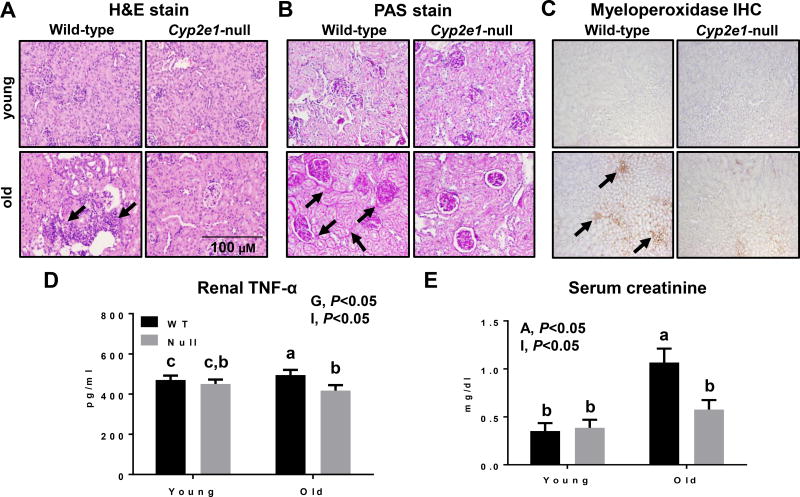
We first compared the respective kidney tissues of young (7-weeks) and aged (16-months) WT and Cyp2e1-null groups (n=10/group) to evaluate the age-related histological kidney changes. The kidneys of the aged WT mice appeared normal but slightly pale. We observed elevated inflammatory cell foci (often lymphocytes and neutrophils), tubular cytoplasmic vacuolation, tubular degeneration/regeneration with occasionally thickened basement membranes, glomerulonephropathy with hyaline material expanding the mesangium (PAS-positive), and enlarged glomerular tufts in aged WT mice. Additionally, the levels of renal fibrosis were markedly increased in aged WT compared to the corresponding Cyp2e1-null mice and young WT (Fig. 1A and B and Table 1). These histological results were supported by the significantly increased levels of neutrophil marker myeloperoxidase IHC (Fig. 1C), renal TNF-α contents (Fig. 1D), and serum creatinine levels (Fig. 1E) in aged WT mice compared to all other groups. The renal TNF-α levels were significantly affected by the genotype (i.e. gene effect), and there was a significant age-genotype interaction (Fig. 1D). One-way ANOVA revealed that TNF-α levels were highest in aged WT mice compared to all other groups (Fig. 1D). Serum creatinine, an indirect indicator of renal functions and which inversely related to the health of the kidneys, was significantly higher in aged mice (i.e., age effect), and a significant age-genotype interaction was monitored (Fig. 1E). The levels of serum creatinine in aged WT were significantly higher than all other groups, although aged Cyp2e1-null mice exhibited significantly higher levels than young mice (Fig. 1E). None of these structural or histopathological changes were detected in the young mice of both genotypes.
Figure 1.
Histological and biochemical changes in aged WT compared to other groups. Aging induced profound histopathological changes in kidneys, as evidenced by increased inflammatory cell foci, tubular cytoplasmic vacuolation, tubular degeneration, glomerulonephropathy, and kidney fibrosis in old WT mice, which were significantly decreased in aged Cyp2e1-null mice, as shown with: (A) H&E staining, (B) PAS staining, and (C) myeloperoxidase IHC (n=7/group). Black arrows represent (A) inflammatory foci, (B) glomerulonephropathy and tubular degeneration/regeneration, and (C) myeloperoxidase. The levels of (D) renal TNF-α, and (E) serum creatinine are shown (n=6–8/group). A, age; G, genotype; I, age-genotype interaction. Labeled means, which do not share a common letter, represent significant difference from each other. P < 0.05.
3.2. Increased apoptosis of kidney cells and kidney fibrosis in aged WT mice
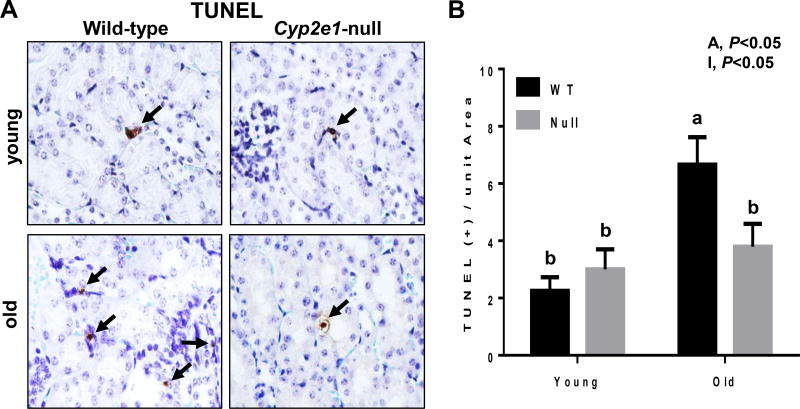
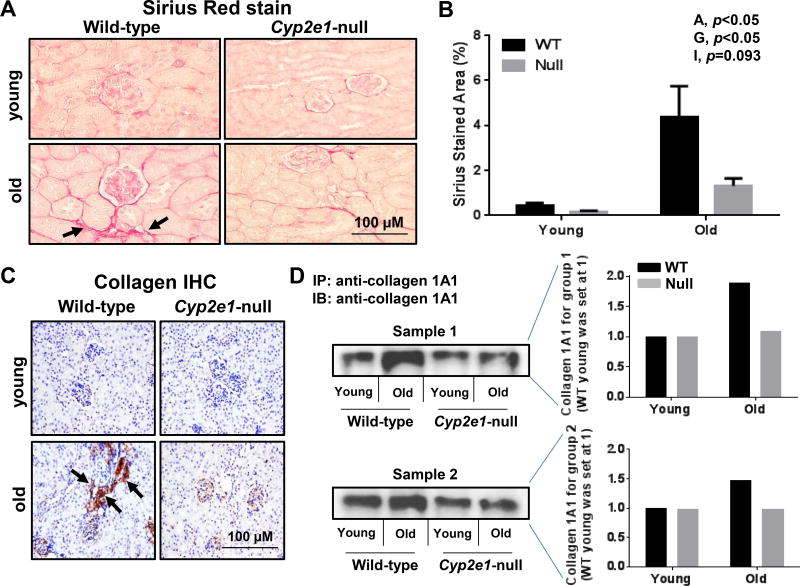
To evaluate any difference in the levels of cell apoptosis of the kidneys in all four groups, the TUNEL assay was performed. TUNEL-positive apoptotic kidney cells were remarkably elevated in the aged WT (Fig. 2A). Significant differences were observed in the levels of apoptotic kidney cells resulting from age effects (Fig. 2B). In addition, a significant age-genotype interaction was monitored, and further analysis revealed that aged WT mice showed the highest levels of apoptotic cells (Fig. 2B). In addition, kidney fibrosis, as evidenced by Sirius red staining, was significantly increased in aged WT, compared to all other groups (Fig. 3A and B, and Table 1). Two-way ANOVA revealed both significant age and genotype effects on kidney fibrosis (Fig. 3B). The accumulation of collagen fiber and development of renal fibrosis in aged WT mice were further confirmed by IHC for increased collagen formation and distribution determined with the specific anti-collagen 1A1 antibody (Fig. 3C), and by immunoprecipitation of two different samples (pooled from 5 mice in each group/sample) by using the specific collagen 1A1 followed by immunoblotting with the same antibody (Fig. 3D). Collectively, these results (Fig. 1–3) clearly demonstrate that the WT mice undergo more progressive aging-dependent renal histopathological and structural changes. In contrast, the corresponding aged Cyp2e1-null mice seem to exhibit far less histopathological changes and were resistant to aging-related kidney damage, suggesting an important role of CYP2E1 in regulating the kidney functions.
Figure 2.
Increased levels of apoptotic cells in kidneys of aged WT mice. (A) TUNEL-positive hepatocytes were marked with black arrows and (B) quantified in 10 high-power fields (n=7/group). A, age; I, age-genotype interaction. Labeled means, which do not share a common letter, indicate significant difference from each other. P < 0.05.
Figure 3.
Increased kidney fibrosis in aged WT mice. (A and B) Representative histological analysis of kidney sections (n=7/group), which show Sirius red stained collagen and percentage of fibrotic areas, respectively. The increased levels of collagen in aged WT mice were further proven by (C) collagen IHC analysis, and (D) immunoprecipitation followed by immunoblot analyses of two pooled samples (pooled from 5 mice) per each mouse group (n=10/group) and densitometric analysis, respectively. Specific anti-collagen 1A1 antibody was used for both C and D. A, age; G, genotype; I, age-genotype interaction. Labeled means, which do not share a common letter, reflect significant difference from each other. P < 0.05.
3.3. Elevation of oxidative stress and decreased anti-oxidant capacity in aged WT
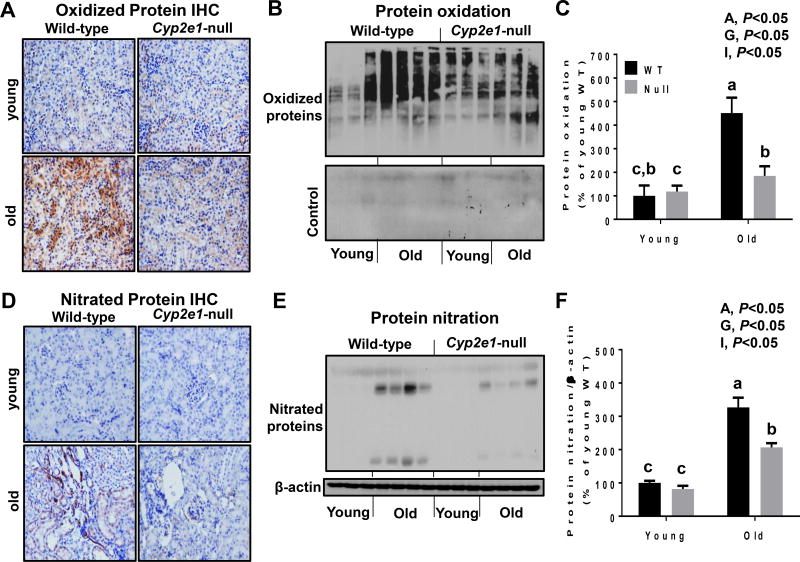
We further determined whether oxidative stress levels were increased in aged WT and correlated with the signs of aging-related renal damage. We first evaluated the levels of nitroxidative protein modification, a foot print of increased oxidative/nitrative stress. IHC evaluation showed remarkably higher levels of oxidized and nitrated proteins in the aged WT compared to those of all other groups (Fig. 4A and D, respectively). It is noteworthy to mention that the specificity of the oxidized proteins was confirmed by disappearance of the bands when they were not treated with DNPH (Fig. 4B). Oxyblot and immunoblot using anti-3-NT antibody followed by two-way ANOVA showed that the levels of renal oxidized (Fig. 4B and C) and nitrated proteins (Fig.4E and F) were significantly elevated in aged WT mice than young mice (i.e., age and genotype effects). In addition, a significant age-genotype interaction was observed with both oxidized and nitrated protein levels both of which were significantly higher in aged WT mice, compared to all other groups, although both of these covalently modified proteins were significantly higher in aged Cyp2e1-null mice compared to young groups (Fig. 4C and F).
Figure 4.
Increased levels of oxidized and nitrated proteins in aged WT mice. (A, D) Representative results of IHC (n=5–6/group) and (B–F) immunoblot and densitometric analyses (n=8–10/group) for (A, B) oxidized proteins and (D, E) nitrated proteins, as indicated. A, age; G, genotype; I, age-genotype interaction. Labeled means, which do not share a common letter, represent significant difference from each other. P < 0.05.
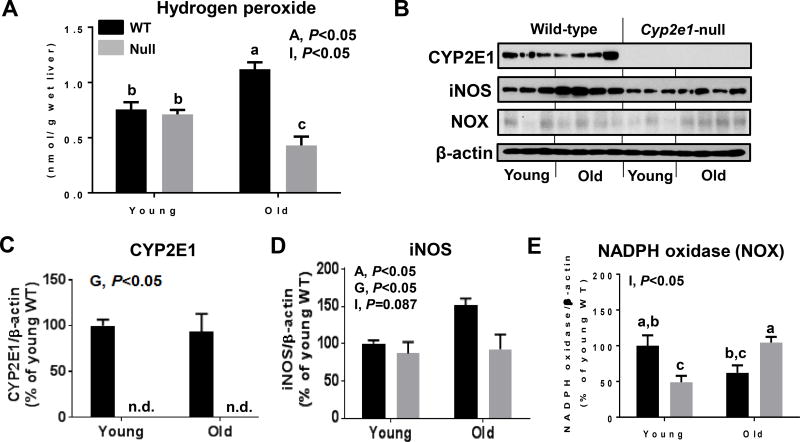
Indeed, the levels of H2O2 were higher in aged mice than young mice (i.e., age effect) and there was a significant age-genotype interaction (Fig. 5A). One-way ANOVA revealed that aged WT mice exhibited highest H2O2 levels, while aged Cyp2e1-null mice showed lowest levels compared to all other groups. Since oxidative stress may result from the imbalance between oxidative radical production and their removal by the antioxidant defense system, we evaluated potential alterations of some nitroxidative marker proteins and the mitochondrial electron transport chain proteins as well as antioxidant molecule GSH and other antioxidant enzymes. We first investigated some potential sources of the nitroxidative stress. As expected, there was a significant difference in the CYP2E1 levels in WT mice compared to Cyp2e1-null mice (i.e., genotype effect) while the amounts were similar in young and aged WT mice (Fig. 5B and C), and cytosolic CYP2E1 activity exhibited similar pattern (data not shown). The iNOS levels were significantly higher in aged WT mice compared to all other groups (Fig. 5B and D). Interestingly, NADPH oxidase protein exhibited a significant age-genotype interaction, and further statistical analysis showed that the levels of NADPH oxidase in aged Cyp2e1-null mice were significantly higher than their corresponding young controls and aged WT mice, although the levels were not higher than young WT mice (Fig. 5B and E). Xanthine oxidase, however, did not exhibit significant changes in aged versus young groups (data not shown).
Figure 5.
Increased levels of H2O2 and some nitroxidative marker proteins in aged WT mice. Equal amounts of whole renal lysate proteins were (n=8–10/group) analyzed to determine the levels of: (A) H2O2, and (B–E) other proteins, as indicated by immunoblot and densitometric analyses of (B, C) CYP2E1, (B, D) iNOS, and (B, E) NADPH oxidase. A, age; G, genotype; I, age-genotype interaction. Labeled means, which do not share a common letter, indicate significant difference from each other. P < 0.05.
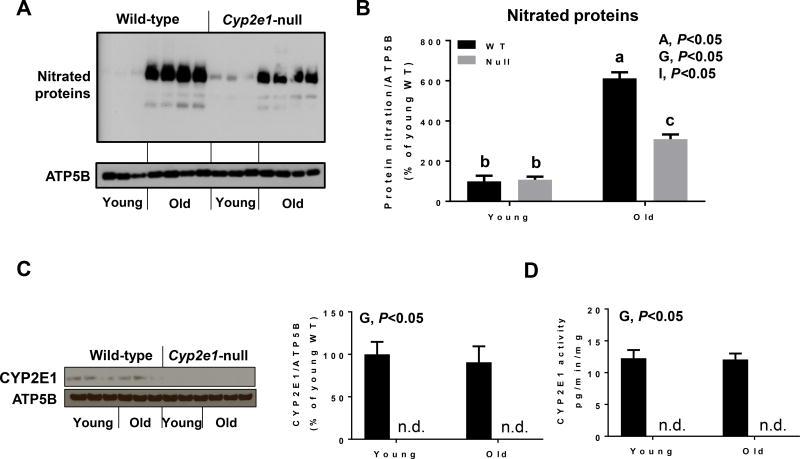
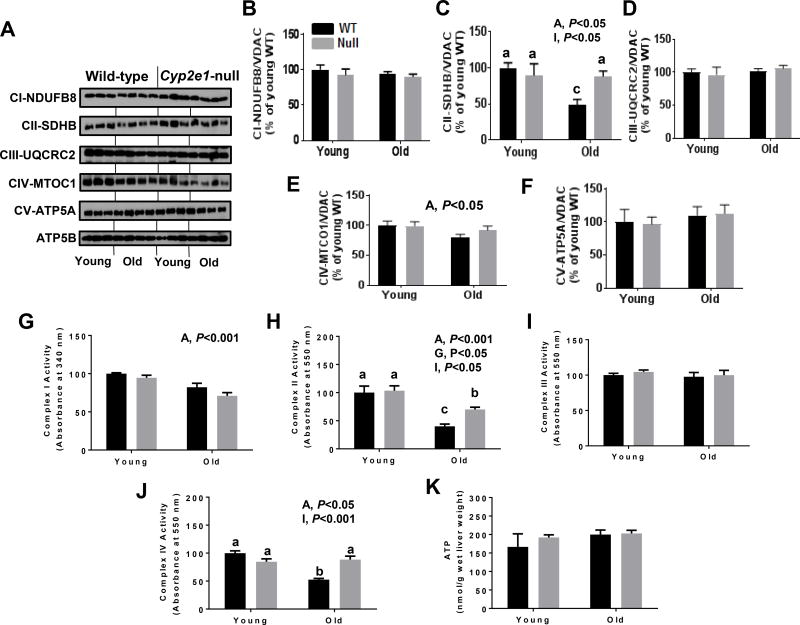
Since alteration of mitochondria functions might be another major source of elevated oxidative stress and the iNOS levels in the old WT mice were highest, we first evaluated whether mitochondrial proteins were differentially nitrated in aged WT mice compared to all other groups (Fig. 6A and B). Immunoblot analysis revealed that there was a significant increase in nitrated proteins in aged WT mice (i.e., age and gene effects), and there was a significant age-genotype interaction. Further one-way ANOVA showed that the levels of nitrated proteins were significantly higher in aged WT mice than all other groups, even though the levels observed in aged Cyp2e1-null mice were significantly higher than the young groups (Fig. 6A and B). Since CYP2E1 is also present in mitochondria, we evaluated its protein levels and activities in the kidneys of all groups. However, we only monitored a significant gene effect without any age effect for both levels and activities (Fig. 6C and D). We then analyzed the levels of mitochondrial complex proteins using the total OXPHOS rodent WB antibody cocktail, which contains 5 mouse monoclonal antibodies against CI subunit NDUFB8, CII-SDHB, CIII-Core protein 2 (UQCR2), CIV subunit 1 (MTCO1) and CV alpha subunit (ATP-5A) as well as complexes activities. Immunoblot and statistical analyses showed no significant changes in the levels of the subunits of CI, CIII, and CV (Fig. 7A, B, D, and F). In contrast, significant age effect and age-genotype interaction was observed for CII (SDHB) subunit protein levels (Fig. 7A and C). One-way ANOVA revealed that the amount of CII (SDHB) subunit was significantly lower in aged WT than all other groups and that such alteration was absent in Cyp2e1-null mice regardless of their age (Fig. 7A and C). Further, the levels of CIV (MTCO1) subunit in aged mice were lower than young counterparts (i.e., age effect) (Fig. 7A and E). Since immunoblot measurement of the functional proteins mainly detects one subunit per complex which might not necessarily represent the complex state, we therefore evaluated the activities of mitochondrial complexes. Complex I activity measurement revealed lower activity levels in aged mice than young mice (i.e., age effect) regardless of the gene effect (Fig. 7G). Significant age and gene effects and age-genotype interaction for CII activities were monitored (Fig. 7H). One-way ANOVA revealed that the CII complex activity levels were lowest in the aged WT mice among all other groups despite the fact that CII complex activity levels were significantly lower in aged Cyp2e1-null mice compared to their young counterparts and young WT mice (Fig. 7H). Complex III activity exhibited no significant changes related to age or gene (Fig. 7I). Significant age and age-genotype interactions for CIV activities were recorded (Fig. 7J). One-way ANOVA revealed that the CIV complex activity levels were the lowest in the aged WT mice among all other groups (Fig. 7J). We did not, however, observe any significant alteration in ATP levels in the aged mice groups compared to the young groups (Fig. 7K). Taken together (Fig. 1, Fig. 5, Fig. 6, and Fig. 7), these data suggest that elevated inflammation, iNOS, and mitochondrial dysfunction via alteration of the mitochondrial electron transport chain proteins and activities are likely involved in the increased nitroxidative stress during the kidney aging process and that this combination is more prominent in the aged WT than in the aged Cyp2e1-null mice.
Figure 6.
Increased levels of nitrated mitochondrial proteins and altered levels of mitochondrial CYP2E1 levels and activities in the kidneys of aged WT mice. Equal amounts of renal mitochondrial proteins were analyzed to determine the levels of (A, B) nitrated mitochondrial proteins, (C,D) CYP2E1 protein levels, and (E) CYP2E1 activity (n=8–10/group). A, age; G, genotype; I, age-genotype interaction. Labeled means, which do not share a common letter, reflect significant difference from each other. P < 0.05.
Figure 7.
More prominent alteration in the protein levels and activities of mitochondrial electron transport chain complex proteins in the kidneys of aged WT mice. Equal amounts of renal mitochondrial proteins were analyzed to determine the protein levels (A–F) and mitochondrial electron transport chain complex activities (G–K) as indicated.. A, age; G, genotype; I, age-genotype interaction. Labeled means, which do not share a common letter, reflect significant difference from each other. P < 0.05.
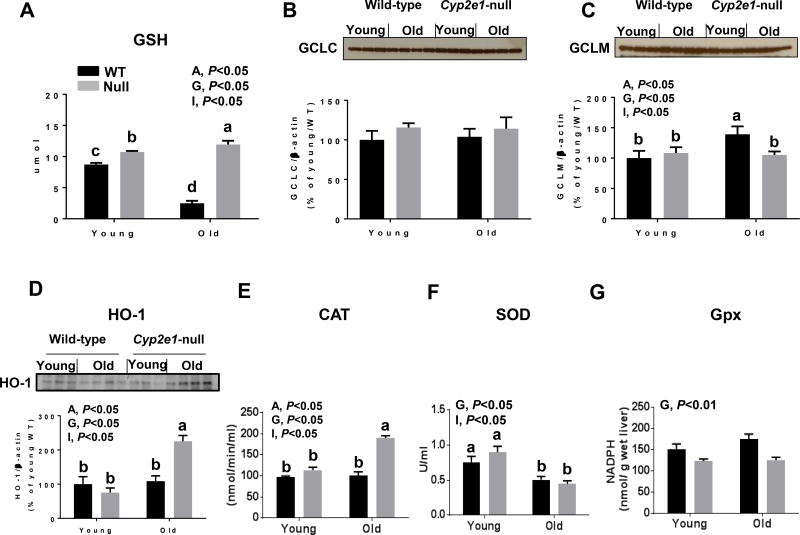
Following the evaluation of various sources of oxygen radical production, we next evaluated some cellular defense systems against oxygen radicals. We observed significant age effects and age-genotype interaction for both GSH levels and HO-1 protein levels (Fig. 8A and D). One-way ANOVA showed that GSH levels were lowest in aged WT mice (Fig. 8A). The evaluation of GCLC revealed no differences among the groups, although there was a tendency for higher levels in the KO mouse groups (Fig. 8B). GCLM levels revealed that there was a significant age, genotype, and age-genotype interaction (Fig. 8C). Further analysis of GCLM showed that aged WT exhibited the highest levels of GCLM (Fig. 8C). This data suggests that the decreased levels of GSH in aged WT mice was probably due to increased levels of oxidation and conjugation and that the increase of GCLM levels might be adaptive response to decreased GSH levels. In addition HO-1 levels were highest in aged Cyp2e1-null (Fig. 8D), compared to all other groups. Catalase activity was significantly affected by age and genotype and there was a significant age-genotype interaction (Fig. 8E). Catalase activity was highest in aged Cyp2e1-null mice, compared to all other groups (Fig. 8E). Both SOD and Gpx activities were significantly affected by genotype, however, a significant age-genotype interaction was only monitored for SOD activity (Fig. 8F and G). Further analysis of SOD activity showed that its levels were similarly significantly lower in aged mice than young mice (Fig. 8F).
Figure 8.
High levels of renal antioxidant capacity in aged Cyp2e1-null mice. (A–G) Equal amounts of whole kidney lysates were used to measure the relative levels and densitometric analyses of (A) GSH, (B) GCLC, (C) GCLM, (D) HO-1, and the renal activities of (E) catalase, (F) superoxide dismutase, and (G) Gpx in different mouse groups, as indicated. A, age; G, genotype; I, age-genotype interaction. Labeled means, which do not share a common letter, represent significant difference from each other. P < 0.05.
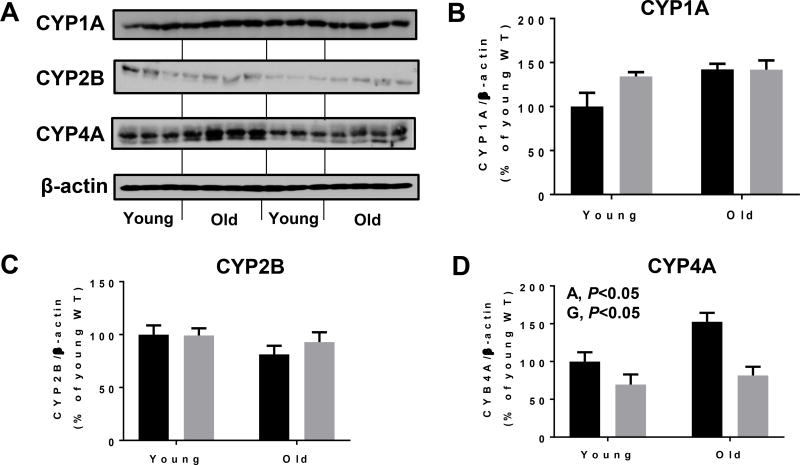
We finally determined the levels of few other renal cytochrome P450 proteins to evaluate whether these proteins could be altered during the aging or contribute to aging-related damage, since CYP4A is also known to produce ROS, as previously reviewed (Hardwick et al., 2009). We did not monitor any significant changes in the protein levels of CYP1A and 2B among the groups (Fig. 9A, B and C). CYP4A, however, exhibited a significant difference depending on both age and genotype. Aged WT mice exhibited highest CYP4A levels among all the groups (Fig. 9A and D), suggesting a potential differential regulation of some cytochrome P450 proteins upon CYP2E1 deletion and during aging process.
Figure 9.
Changes in the levels of some cytochrome P450 proteins in young and aged WT and Cyp2e1-null mice. Equal amounts of whole kidney lysate proteins were used for immunoblot analyses. Representative results of immunoblot analysis and densitometric analysis results (n=8–10/group) are presented for the levels of (A, B) CYP1A, (A, C), CYP2B and (A, D) CYP4A. A, age; G, genotype.
Collectively, these results (Fig. 1–9) suggest that oxidative stress is increased in parallel with the kidney aging process in WT mice. Furthermore, since all these changes were not as prominent in aged Cyp2e1-null mice, it is reasonable to assume that CYP2E1 plays at least a partial role in the aging-related kidney disease such as inflammation, apoptosis, glomerulonephropathy, and fibrosis.
4. Discussion
Mice exhibited many similarities to humans suffering from age-related kidney changes such as inflammation, apoptosis, glomerulonephropathy, and fibrosis (Hodgin et al., 2015; Martin and Sheaff, 2007; Percy et al., 2005; Percy et al., 2008; Sataranatarajan et al., 2012). In addition, mice have a short life span and thus they are suitable models to investigate the aging process and potential underlying mechanism(s). Interestingly, women seem to be less prone to the decreased kidney functions until reaching menopause when aging accelerates, probably due to the reduced effects of estrogen and lower estrogen receptor expression (Gross et al., 2005). This notion was supported by the exacerbation of declining kidney functions and increased glomerulosclerosis in aged female rats (Fortepiani et al., 2003). The constitutive expression of CYP2E1, an established source ROS production, has been suggested to be significantly higher in female than male mice (Konstandi et al., 2013), although it has been suggested otherwise previously (Chanas et al., 2003). In addition, physiological implications of our rodent results would need to be evaluated in senior (female) people who may use CYP2E1 substrate or inducer drugs such as acetaminophen (APAP), chlorzoxazone, and isoniazid since a multivariate logistic regression report showed that some females, who used high doses of APAP as a pain killer, exhibited increased risks of decreased renal function (Curhan et al., 2004). Thus, we decided to evaluate the effect of CYP2E1 using female mice to determine the aging-related kidney damage. However, it is not very well-established whether males or females are more sensitive to kidney damage in response to different drugs and chemicals particularly during aging state. Thus, it is still important to evaluate the role of CYP2E1 in promoting aging-related kidney damage in male mice to compare the effects observed with females to determine potential sex-dependent difference(s) in this process.
CYP2E1 is a well-established producer of high levels of ROS (Caro and Cederbaum, 2004; Novak and Woodcroft, 2000; Song et al., 1996) and is involved in promoting various post-translational modifications such as protein oxidation, nitration, phosphorylation, adduct formations, among others. (Abdelmegeed et al., 2015; Abdelmegeed and Song, 2014; Song et al., 2013, 2014). CYP2E1 has been suggested in mediating kidney injury and toxicity in response to acetaminophen (Ko et al., 2017), myoglobinuric acute kidney injury, which resembles rhabdomyolysis (Wang et al., 2014), acute rejection in kidney transplantation recipients (Kim et al., 2014), chronic ethanol ingestion (Latchoumycandane et al., 2014), chloroform (Sasso et al., 2013), and cisplatin (Liu and Baliga, 2003), to name a few, mainly via nitroxidative stress-mediated events. All these reports support the hypothesis that CYP2E1 in mice is likely to play an important role in promoting aging-related kidney disorders, possibly via increased levels of nitroxidative stress. In this study, we have evaluated age-related changes in histochemical features and biochemical parameters and these outcomes were compared between aged WT mice and other groups, including aged Cyp2e1-null mice, to further characterize the involvement of CYP2E1 in aging-related kidney damage.
Our results showed that the deletion of Cyp2e1 gene significantly prevented and/or decreased the prominent features associated with kidney aging (Martin and Sheaff, 2007; Percy et al., 2005) such as inflammation, apoptosis, and fibrosis, as observed in aged WT compared to all other groups. It is well-recognized that aging process, including kidney aging, is correlated and/or facilitated with increased levels of oxidative radicals, particularly in chronic basis (Percy et al., 2005). The increased oxidative stress levels might be originated from normal metabolism of endogenous and exogenous compounds, leading to oxidative damage (Gregg et al., 2012) to cellular macromolecules such as proteins and lipids. Indeed, nitroxidative post-translational protein modifications are considered a well-established marker for oxidative stress during the aging process, which may negatively affect the functions of the modified target proteins prior to the observation of a full-blown organ damage (Abdelmegeed et al., 2013; Chakravarti and Chakravarti, 2017; Moon et al., 2008; Ortuno-Sahagun et al., 2014; Percy et al., 2005). Consistent with these reports, we found that the levels of renal H2O2, protein oxidation, and nitration, were significantly higher in aged WT compared to the aged Cyp2e1-null mice and young mouse groups.
The development of oxidative stress-mediated cell/organ damage, including the kidneys, is related to the imbalance between the production of oxidants and the defense capacity by the anti-oxidant system (Small et al., 2012; Song et al., 2014). There are many endogenous sources of ROS and RNS such as CYP2E1, iNOS, NADPH-oxidase, xanthine oxidase, and the mitochondrial electron transport chain, inflammatory cells, among others (Abdelmegeed and Song, 2014; Small et al., 2012; Song et al., 2014). Indeed, we monitored significantly higher levels of inflammation and oxidative stress, and lower antioxidant capacity in aged WT mice compared to other groups. The differential regulations of many proteins in NADPH oxidase, HO-1, catalase, and GSH were observed between aged WT and aged Cyp2e1-null mice. The deletions of one of the genes, particularly an important gene like CYP2E1, would possibly alter the expression of many other genes and proteins, either to compensate for the effect of the deleted gene or as an expected result if that gene’s regulation is affected by the deleted gene. Some of the genes might induce ROS to compensate for CYP2E1 deletion as certain basal levels of ROS is required for normal signaling and organs’ functions. Thus, it is possible that the upregulation of NADPH oxidase in the aged Cyp2e1-null mice, might be partly due to the deletion of CYP2E1 as an alternative source of oxidative stress as monitored in the aged Cyp2e1-null mice. In contrast, the decrease of NADPH oxidase in aged WT mice might be a defense mechanism by the cells to minimize the production of ROS. Similarly, the upregulation of catalase, HO-1, and the persistence of GSH levels in the aged KO mice might be due to the relatively more intact adaptive mechanisms in the aged KO mice than the aged WT mice. It is also reasonable to assume that there might be differential temporal efficiency in the adaptation mechanism between WT and Cyp2e1-null mice, and to assume that the WT mice have been exposed to the higher levels of ROS much earlier than the aged Cyp2e1-null mice, leading to earlier consumption of adaptive mechanisms’ capacity in the aged WT mice. However, eventually similar effects will take place in the aged Cyp2e1-null mice, but at later time point. The temporal regulation of different renal oxidative stress-inducing proteins antioxidant adaptive mechanisms will require further investigation.
We also observed changes in the levels of the mitochondrial electron transport chain protein complexes levels and activities. Despite the most significant changes in CII-SDHB protein levels in the aged WT mice, activity measurements revealed that complexes I, II, and IV were also affected. The discrepancy between immunoblot data and activity measurements might be attributed to (1) immunoblot data only evaluate one of the subunits of the each complex, and (2) enzyme activity might be inhibited by oxidative stress even when protein levels are similar (Moon et al., 2006; Moon et al., 2008),. This data suggests a potential role of mitochondrial impairment with increased production of ROS such as superoxide anion and H2O2, further leading to increased levels of mitochondrial protein nitration in aged WT to a higher degree compared with aged Cyp2e1-null mice. This is not surprising since it was previously reported that mitochondria isolated from old animals released higher levels of oxidative radicals than young animals (Sohal and Sohal, 1991). In addition, there was an inverse relation between the increased ROS production by mitochondria and the life span (Lambert et al., 2007). Furthermore, our previous reports showed causal roles of mitochondrial dysfunction in promoting full-blown tissue injury usually observed at later time points following exposure to CYP2E1 substrates such as APAP (Abdelmegeed et al., 2013; Abdelmegeed et al., 2010) and carbon tetrachloride (Jang et al., 2015) or under pathological conditions (Moon et al., 2006; Moon et al., 2008). The potential role of mitochondria in mediating the aging-related renal damage in WT mice warrant further investigation with treatment agents targeting mitochondria, such as antioxidant mito-Q (Akbar et al., 2016; Song et al., 2014), to establish their importance in the aging process and disease manifestation. Interestingly, despite the fact that mitochondrial respiration is essential for the production of ATP and that oxidatively-damaged mitochondria may produce less amounts of ATP, in our study, the levels of ATP in aged mice were not significantly changed, suggesting an adaptive mechanism (Anantharaju et al., 2002; Abdelmegeed et al., 2016). The intracellular GSH constitutes a major antioxidant in many organs, including the kidney (Small et al., 2012). In addition, there is a systematic elimination of many oxygen radicals by the coordinated action of many anti-oxidant enzymes such as SOD, CAT, and GPx (Small et al., 2012). Any disruption or suppression of the activities of one or more of these enzymes presumably would contribute to the increased oxidative stress. Furthermore, decreased expression of other anti-oxidant defense systems such as HO-1, might also lead to increased oxidative stress. Indeed, it has been shown that the induction of HO-1 was protective against in some models of nephrotoxicity (Liu and Baliga, 2003; Shiraishi et al., 2000). Our data showed that the overall antioxidant adaptive response to increased oxidative stress levels seems to be significantly stronger in the aged Cyp2e1-null mice than the aged WT group. The increase of antioxidant defense in aged Cyp2e1-null mice might explain the lower levels of accumulated oxidatively modified proteins along with the decreased levels of oxidative radicals compared to aged WT mice.
The amounts of several other hepatic cytochrome P450 proteins such as CYP1A and CYP2B exhibited no significant changes among the groups, although CYP4A were significantly higher in the aged WT mice compared to all other groups. These results suggest that there is a differential regulation in the levels of some other P450 proteins between the aged WT and the corresponding Cyp2e1-null mice. Since P450-mediated metabolisms produce ROS and the importance of P450 isoforms in the development of kidney toxicities has been reported (Carroll et al., 1997; Fan et al., 2015; Hirt and Jacobson, 1991), it becomes important to evaluate the implications of these differential regulations on the aging-related kidney diseases in future studies.
Taken together, our data clearly showed that a significant difference exists in the cellular redox balances between the aged WT and the corresponding Cyp2e1-null mice. The differences in oxidative stress levels could probably result from increased ROS/RNS production from inflammation, iNOS, and through the altered mitochondrial electron transport chain function, along with the decreased antioxidant capacity consisting of GSH, HO-1 and catalase activity in aged WT mice compared to aged Cyp2e1-null mice. Even though there was no additional increase in the CYP2E1 levels in aged WT mice, the increased levels of oxidative stress in WT mice could have been produced through CYP2E1-mediated metabolism of endogenous substrates throughout the life span. This is not surprising since CYP2E1 is known to increase the levels of ROS and RNS even in the absence of its substrates (Caro and Cederbaum, 2004; Song et al., 1996). It is also possible that the presence of CYP2E1, regardless of its levels, might exert its permissive action for other proteins, for example iNOS and CYP4A. It is possible that CYP2E1 was upregulated at certain time points during the ~17 month duration and then was returned to control levels at the time of tissue collection. Thus, the increased levels of CYP2E1-related inflammation and nitroxidative stress might play a significant role in the advancement of aging-related kidney damage such as apoptosis, glomerulonephropathy and fibrosis.
5. Conclusion
Our results revealed that CYP2E1 appears to be involved in the development of aging-related kidney disorders. This can be explained by the fact that elevated iNOS, altered mitochondrial electron transport chain, and permissive CYP2E1 increase the production of ROS/RNS and elevated nitrated proteins presumably via peroxynitrite in aged WT mice due to the redox imbalance resulting from increased oxidative stress along with decreased adaptive antioxidant responses. These changes likely increase inflammation and nitroxidative protein modifications, which can negatively affect the vital functions of the kidneys, leading to the development of the pathological features observed in the aged kidneys. Our results demonstrate for the first time the novel role of CYP2E1 in the kidney aging process in rodents, rendering CYP2E1 a potential target for translational research in preventing aging-related kidney damage as well as other kidney diseases.
Supplementary Material
Highlights.
-
*
The role of CYP2E1 in aging-related kidney damage is largely unknown.
-
*
Young and aged female wild-type (WT) and Cyp2e1-null mice were evaluated.
-
*
Aged WT mice exhibited highest levels of inflammation, nitroxidative stress, apoptosis, and fibrosis.
-
*
Cyp2e1-null mice were resistant to aging-related kidney damage partly due to significantly high levels of antioxidant capacity.
-
*
CYP2E1 is important in mediating aging-related kidney damage.
Acknowledgments
This research was supported by the Intramural Research Program of National Institute on Alcohol Abuse and Alcoholism. We are thankful to Dr. Frank J. Gonzalez (National Cancer Institute, NIH, Bethesda, MD, USA) and Dr. James P. Hardwick (Northeastern Ohio University College of Medicine, Rootstown, OH, USA) for providing the WT and Cyp2e1-null mice and the polyclonal antibodies to different P450 isoforms, respectively. We also thank Dr. Klaus Gawrisch for supporting this study.
Abbreviations
- APAP
acetaminophen
- ATP5B
ATP synthase subunit Beta
- CAT
catalase
- Cytochrome P450
CYP
- DNPH
dinitrophenylhydrazine
- GCLC
glutamate-cysteine ligase catalytic subunit
- GCLM
glutamate-cysteine ligase modifier subunit
- GSH
glutathione
- Gpx
glutathione peroxidase
- HO-1
heme oxygenase-1
- H2O2
hydrogen peroxide
- IHC
immunohistochemistry
- iNOS
inducible nitric oxide synthase
- NADPH oxidase
nicotinamide adenine dinucleotide phosphate oxidase
- NO
nitric oxide
- 3-NT
3-nitrotyrosine
- PAS
periodic acid-Schiff
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- TNFα
tumor necrosis factor-α
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick end labeling
- WT
wild-type.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statements
The authors declared no conflicts of interest relevant to this article.
References
- Abdelmegeed MA, Choi Y, Godlewski G, Ha SK, Banerjee A, Jang S, Song BJ. Cytochrome P450-2E1 promotes fast food-mediated hepatic fibrosis. Sci Rep. 2017;7:39764. doi: 10.1038/srep39764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelmegeed MA, Choi Y, Ha SK, Song BJ. Cytochrome P450-2E1 promotes aging-related hepatic steatosis, apoptosis and fibrosis through increased nitroxidative stress. Free Radic Biol Med. 2016;91:188–202. doi: 10.1016/j.freeradbiomed.2015.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelmegeed MA, Ha SK, Choi Y, Akbar M, Song BJ. Role of CYP2E1 in mitochondrial dysfunction and hepatic tissue injury in alcoholic and non-alcoholic diseases. Curr Mol Pharmacol. 2015 doi: 10.2174/1874467208666150817111114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelmegeed MA, Jang S, Banerjee A, Hardwick JP, Song BJ. Robust protein nitration contributes to acetaminophen-induced mitochondrial dysfunction and acute liver injury. Free Radic Biol Med. 2013;60:211–222. doi: 10.1016/j.freeradbiomed.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelmegeed MA, Moon KH, Chen C, Gonzalez FJ, Song BJ. Role of cytochrome P450 2E1 in protein nitration and ubiquitin-mediated degradation during acetaminophen toxicity. Biochem Pharmacol. 2010;79:57–66. doi: 10.1016/j.bcp.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelmegeed MA, Song BJ. Functional roles of protein nitration in acute and chronic liver diseases. Oxid Med Cell Longev. 2014;2014:149627. doi: 10.1155/2014/149627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelmegeed MA, Yoo SH, Henderson LE, Gonzalez FJ, Woodcroft KJ, Song BJ. PPARalpha expression protects male mice from high fat-induced nonalcoholic fatty liver. J Nutr. 2011;141:603–610. doi: 10.3945/jn.110.135210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbar M, Essa MM, Daradkeh G, Abdelmegeed MA, Choi Y, Mahmood L, Song BJ. Mitochondrial dysfunction and cell death in neurodegenerative diseases through nitroxidative stress. Brain Res. 2016;1637:34–55. doi: 10.1016/j.brainres.2016.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anantharaju A, Feller A, Chedid A. Aging Liver. A review. Gerontology. 2002;48:343–353. doi: 10.1159/000065506. [DOI] [PubMed] [Google Scholar]
- Caro AA, Cederbaum AI. Oxidative stress, toxicology, and pharmacology of CYP2E1. Annu Rev Pharmacol Toxicol. 2004;44:27–42. doi: 10.1146/annurev.pharmtox.44.101802.121704. [DOI] [PubMed] [Google Scholar]
- Carroll MA, Balazy M, Huang DD, Rybalova S, Falck JR, McGiff JC. Cytochrome P450-derived renal HETEs: storage and release. Kidney Int. 1997;51:1696–1702. doi: 10.1038/ki.1997.234. [DOI] [PubMed] [Google Scholar]
- Chakravarti B, Chakravarti DN. Protein Tyrosine Nitration: Role in Aging. Curr Aging Sci. 2017 doi: 10.2174/1874609810666170315112634. [DOI] [PubMed] [Google Scholar]
- Chanas B, Wang H, Ghanayem BI. Differential metabolism of acrylonitrile to cyanide is responsible for the greater sensitivity of male vs female mice: role of CYP2E1 and epoxide hydrolases. Toxicol Appl Pharmacol. 2003;193:293–302. doi: 10.1016/j.taap.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Choi Y, Abdelmegeed MA, Song BJ. Preventive effects of dietary walnuts on high-fat-induced hepatic fat accumulation, oxidative stress and apoptosis in mice. J Nutr Biochem. 2016;38:70–80. doi: 10.1016/j.jnutbio.2016.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y, Abdelmegeed MA, Song BJ. Diet high in fructose promotes liver steatosis and hepatocyte apoptosis in C57BL/6J female mice: Role of disturbed lipid homeostasis and increased oxidative stress. Food Chem Toxicol. 2017;103:111–121. doi: 10.1016/j.fct.2017.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curhan GC, Knight EL, Rosner B, Hankinson SE, Stampfer MJ. Lifetime nonnarcotic analgesic use and decline in renal function in women. Arch Intern Med. 2004;164:1519–1524. doi: 10.1001/archinte.164.14.1519. [DOI] [PubMed] [Google Scholar]
- Fan F, Muroya Y, Roman RJ. Cytochrome P450 eicosanoids in hypertension and renal disease. Curr Opin Nephrol Hypertens. 2015;24:37–46. doi: 10.1097/MNH.0000000000000088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortepiani LA, Zhang H, Racusen L, Roberts LJ, 2nd, Reckelhoff JF. Characterization of an animal model of postmenopausal hypertension in spontaneously hypertensive rats. Hypertension. 2003;41:640–645. doi: 10.1161/01.HYP.0000046924.94886.EF. [DOI] [PubMed] [Google Scholar]
- Gregg SQ, Gutierrez V, Robinson AR, Woodell T, Nakao A, Ross MA, Michalopoulos GK, Rigatti L, Rothermel CE, Kamileri I, Garinis GA, Stolz DB, Niedernhofer LJ. A mouse model of accelerated liver aging caused by a defect in DNA repair. Hepatology. 2012;55:609–621. doi: 10.1002/hep.24713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross ML, Ritz E, Korsch M, Adamczak M, Weckbach M, Mall G, Berger I, Hansen A, Amann K. Effects of estrogens on cardiovascular structure in uninephrectomized SHRsp rats. Kidney Int. 2005;67:849–857. doi: 10.1111/j.1523-1755.2005.00149.x. [DOI] [PubMed] [Google Scholar]
- Guengerich FP, Kim DH, Iwasaki M. Role of human cytochrome P-450 IIE1 in the oxidation of many low molecular weight cancer suspects. Chem Res Toxicol. 1991;4:168–179. doi: 10.1021/tx00020a008. [DOI] [PubMed] [Google Scholar]
- Hardwick JP, Osei-Hyiaman D, Wiland H, Abdelmegeed MA, Song BJ. PPAR/RXR Regulation of Fatty Acid Metabolism and Fatty Acid omega-Hydroxylase (CYP4) Isozymes: Implications for Prevention of Lipotoxicity in Fatty Liver Disease. PPAR Res. 2009;2009:952734. doi: 10.1155/2009/952734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Hirt DL, Jacobson HR. Functional effects of cytochrome P450 arachidonate metabolites in the kidney. Semin Nephrol. 1991;11:148–155. [PubMed] [Google Scholar]
- Hodgin JB, Bitzer M, Wickman L, Afshinnia F, Wang SQ, O'Connor C, Yang Y, Meadowbrooke C, Chowdhury M, Kikuchi M, Wiggins JE, Wiggins RC. Glomerular Aging and Focal Global Glomerulosclerosis: A Podometric Perspective. J Am Soc Nephrol. 2015;26:3162–3178. doi: 10.1681/ASN.2014080752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosohata K. Role of Oxidative Stress in Drug-Induced Kidney Injury. Int J Mol Sci. 2016;17 doi: 10.3390/ijms17111826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S, Yu LR, Abdelmegeed MA, Gao Y, Banerjee A, Song BJ. Critical role of c-jun N-terminal protein kinase in promoting mitochondrial dysfunction and acute liver injury. Redox Biol. 2015;6:552–564. doi: 10.1016/j.redox.2015.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SK, Park HJ, Seok H, Jeon HS, Lee TW, Lee SH, Moon JY, Ihm CG, Kim TH, Kim YH, Kang SW, Park SJ, Jeong KH, Chung JH. Association studies of cytochrome P450, family 2, subfamily E, polypeptide 1 (CYP2E1) gene polymorphisms with acute rejection in kidney transplantation recipients. Clin Transplant. 2014;28:707–712. doi: 10.1111/ctr.12369. [DOI] [PubMed] [Google Scholar]
- Ko JW, Shin JY, Kim JW, Park SH, Shin NR, Lee IC, Shin IS, Moon C, Kim SH, Kim SH, Kim JC. Protective effects of diallyl disulfide against acetaminophen-induced nephrotoxicity: A possible role of CYP2E1 and NF-kappaB. Food Chem Toxicol. 2017;102:156–165. doi: 10.1016/j.fct.2017.02.021. [DOI] [PubMed] [Google Scholar]
- Konstandi M, Cheng J, Gonzalez FJ. Sex steroid hormones regulate constitutive expression of Cyp2e1 in female mouse liver. Am J Physiol Endocrinol Metab. 2013;304:E1118–1128. doi: 10.1152/ajpendo.00585.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert AJ, Boysen HM, Buckingham JA, Yang T, Podlutsky A, Austad SN, Kunz TH, Buffenstein R, Brand MD. Low rates of hydrogen peroxide production by isolated heart mitochondria associate with long maximum lifespan in vertebrate homeotherms. Aging Cell. 2007;6:607–618. doi: 10.1111/j.1474-9726.2007.00312.x. [DOI] [PubMed] [Google Scholar]
- Latchoumycandane C, Nagy LE, McIntyre TM. Chronic ethanol ingestion induces oxidative kidney injury through taurine-inhibitable inflammation. Free Radic Biol Med. 2014;69:403–416. doi: 10.1016/j.freeradbiomed.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SS, Buters JT, Pineau T, Fernandez-Salguero P, Gonzalez FJ. Role of CYP2E1 in the hepatotoxicity of acetaminophen. J Biol Chem. 1996;271:12063–12067. doi: 10.1074/jbc.271.20.12063. [DOI] [PubMed] [Google Scholar]
- Liu H, Baliga R. Cytochrome P450 2E1 null mice provide novel protection against cisplatin-induced nephrotoxicity and apoptosis. Kidney Int. 2003;63:1687–1696. doi: 10.1046/j.1523-1755.2003.00908.x. [DOI] [PubMed] [Google Scholar]
- Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JE, Sheaff MT. Renal ageing. J Pathol. 2007;211:198–205. doi: 10.1002/path.2111. [DOI] [PubMed] [Google Scholar]
- Medvedev ZA. An attempt at a rational classification of theories of ageing. Biol Rev Camb Philos Soc. 1990;65:375–398. doi: 10.1111/j.1469-185x.1990.tb01428.x. [DOI] [PubMed] [Google Scholar]
- Moon KH, Hood BL, Kim BJ, Hardwick JP, Conrads TP, Veenstra TD, Song BJ. Inactivation of oxidized and S-nitrosylated mitochondrial proteins in alcoholic fatty liver of rats. Hepatology. 2006;44:1218–1230. doi: 10.1002/hep.21372. [DOI] [PubMed] [Google Scholar]
- Moon KH, Hood BL, Mukhopadhyay P, Rajesh M, Abdelmegeed MA, Kwon YI, Conrads TP, Veenstra TD, Song BJ, Pacher P. Oxidative inactivation of key mitochondrial proteins leads to dysfunction and injury in hepatic ischemia reperfusion. Gastroenterology. 2008;135:1344–1357. doi: 10.1053/j.gastro.2008.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak RF, Woodcroft KJ. The alcohol-inducible form of cytochrome P450 (CYP 2E1): role in toxicology and regulation of expression. Arch Pharm Res. 2000;23:267–282. doi: 10.1007/BF02975435. [DOI] [PubMed] [Google Scholar]
- Ortuno-Sahagun D, Pallas M, Rojas-Mayorquin AE. Oxidative stress in aging: advances in proteomic approaches. Oxid Med Cell Longev. 2014;2014:573208. doi: 10.1155/2014/573208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedraza-Chaverri J, Sanchez-Lozada LG, Osorio-Alonso H, Tapia E, Scholze A. New Pathogenic Concepts and Therapeutic Approaches to Oxidative Stress in Chronic Kidney Disease. Oxid Med Cell Longev. 2016;2016:6043601. doi: 10.1155/2016/6043601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percy C, Pat B, Poronnik P, Gobe G. Role of oxidative stress in age-associated chronic kidney pathologies. Adv Chronic Kidney Dis. 2005;12:78–83. doi: 10.1053/j.ackd.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Percy CJ, Power D, Gobe GC. Renal ageing: changes in the cellular mechanism of energy metabolism and oxidant handling. Nephrology (Carlton) 2008;13:147–152. doi: 10.1111/j.1440-1797.2008.00924.x. [DOI] [PubMed] [Google Scholar]
- Poulianiti KP, Kaltsatou A, Mitrou GI, Jamurtas AZ, Koutedakis Y, Maridaki M, Stefanidis I, Sakkas GK, Karatzaferi C. Systemic Redox Imbalance in Chronic Kidney Disease: A Systematic Review. Oxid Med Cell Longev. 2016;2016:8598253. doi: 10.1155/2016/8598253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeg S, Grune T. Protein Oxidation in Aging: Does It Play a Role in Aging Progression? Antioxid Redox Signal. 2015;23:239–255. doi: 10.1089/ars.2014.6062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinke LA, Moyer MJ. p-Nitrophenol hydroxylation. A microsomal oxidation which is highly inducible by ethanol. Drug Metab Dispos. 1985;13:548–552. [PubMed] [Google Scholar]
- Sasso AF, Schlosser PM, Kedderis GL, Genter MB, Snawder JE, Li Z, Rieth S, Lipscomb JC. Application of an updated physiologically based pharmacokinetic model for chloroform to evaluate CYP2E1-mediated renal toxicity in rats and mice. Toxicol Sci. 2013;131:360–374. doi: 10.1093/toxsci/kfs320. [DOI] [PubMed] [Google Scholar]
- Sataranatarajan K, Feliers D, Mariappan MM, Lee HJ, Lee MJ, Day RT, Yalamanchili HB, Choudhury GG, Barnes JL, Van Remmen H, Richardson A, Kasinath BS. Molecular events in matrix protein metabolism in the aging kidney. Aging Cell. 2012;11:1065–1073. doi: 10.1111/acel.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergiev PV, Dontsova OA, Berezkin GV. Theories of aging: an ever-evolving field. Acta Naturae. 2015;7:9–18. [PMC free article] [PubMed] [Google Scholar]
- Shiraishi F, Curtis LM, Truong L, Poss K, Visner GA, Madsen K, Nick HS, Agarwal A. Heme oxygenase-1 gene ablation or expression modulates cisplatin-induced renal tubular apoptosis. Am J Physiol Renal Physiol. 2000;278:F726–736. doi: 10.1152/ajprenal.2000.278.5.F726. [DOI] [PubMed] [Google Scholar]
- Small DM, Coombes JS, Bennett N, Johnson DW, Gobe GC. Oxidative stress, anti-oxidant therapies and chronic kidney disease. Nephrology (Carlton) 2012;17:311–321. doi: 10.1111/j.1440-1797.2012.01572.x. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Sohal BH. Hydrogen peroxide release by mitochondria increases during aging. Mech Ageing Dev. 1991;57:187–202. doi: 10.1016/0047-6374(91)90034-w. [DOI] [PubMed] [Google Scholar]
- Song BJ, Koop DR, Cederbaum AI, Ingelman-Sundberg M, Nanji A. Ethanol-inducible cytochrome P450 (CYP2E1): biochemistry, molecular biology and clinical relevance: 1996 update. Alcohol Clin Exp Res. 1996;20:138A–146A. doi: 10.1111/j.1530-0277.1996.tb01764.x. [DOI] [PubMed] [Google Scholar]
- Song BJ, Abdelmegeed MA, Henderson LE, Yoo SH, Wan J, Purohit V, Hardwick JP, Moon KH. Increased nitroxidative stress promotes mitochondrial dysfunction in alcoholic and nonalcoholic fatty liver disease. Oxid Med Cell Longev. 2013;2013:781050. doi: 10.1155/2013/781050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song BJ, Akbar M, Abdelmegeed MA, Byun K, Lee B, Yoon SK, Hardwick JP. Mitochondrial dysfunction and tissue injury by alcohol, high fat, nonalcoholic substances and pathological conditions through post-translational protein modifications. Redox Biol. 2014;3:109–123. doi: 10.1016/j.redox.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Shah SV, Liu H, Baliga R. Inhibition of cytochrome P450 2E1 and activation of transcription factor Nrf2 are renoprotective in myoglobinuric acute kidney injury. Kidney Int. 2014;86:338–349. doi: 10.1038/ki.2014.65. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.