Structured Summary
Objective
To systematically review studies reporting risk of spontaneous abortion among pregnant women of typical reproductive potential with and without uterine leiomyomas.
Data Sources
We searched Pub Med, Embase, Web of Science, and ClinicalTrials.gov for publications from January 1970 to December 2016.
Method of Study Selection
We excluded studies that did not use imaging to uniformly document leiomyoma status of all participants, did not have a comparison group without leiomyomas, or primarily included women seeking care for recurrent miscarriage, infertility care or assisted reproductive technologies.
Tabulation, Integration, and Results
Two authors independently reviewed eligibility, extracted data, and assigned overall quality ratings based on predetermined criteria. Of 1,469 articles identified, nine were eligible. Five enrolled general obstetric populations and four included women undergoing amniocentesis. In five studies in general obstetric populations thatincluded21,829 pregnancies (1,394 women with leiomyomas and 20,435 without), only one adjusted for potential confounders. This meta-analysis revealed no increase in risk of spontaneous abortion among those with leiomyomas compared to those without (11.5% compared with 8.0%; Risk Ratio [RR]: 1.16, 95% Confidence Interval [CI]: 0.80 to 1.52). When bias from confounding was estimated for non-adjusted studies, the aggregate calculated risk ratio was 0.83 (95% CI 0.68–0.98).
Conclusion
Leiomyoma presence was not associated with increased risk of spontaneous abortion in an analysis of more than 20,000pregnant women. Failure of prior studies to adjust for confounders may have led to the common clinical belief that leiomyomas are a risk factor for spontaneous abortion.
Introduction
Uterine leiomyomas are benign smooth muscle tumors of the uterus estimated to be present in up to one in five women of reproductive age.1-3 Fibroids are commonly implicated by patients and clinicians as a cause of spontaneous abortion. However, a Cochrane systematic review and meta-analysis failed to demonstrate any difference in spontaneous abortion risk between women randomized to myomectomy versus no leiomyoma surgery prior to conception.4 Three systematic reviews since 2000 suggest leiomyoma status associate with spontaneous abortion risk.5-7 These reviews are restricted to or dominated by studies of women seeking reproductive assistance. Since it is understood that women seeking fertility treatment differ in spontaneous abortion risk and rates of successful pregnancy than the average woman, it may not be appropriate to base general understanding of risk associated with leiomyomas on studies of special populations. These considerations provide grounds for reexamine inguterine leiomyomas during pregnancy as risk factors for spontaneous abortionin populations more representative of all reproductive-age women
The purpose of this review is to quantify the association between leiomyoma presence during pregnancy and risk of spontaneous abortion with the hypothesis that uterine leiomyomas increase risk of spontaneous abortion for general obstetric populations. Specifically, we aimed to review studies on which current knowledge is based, calculate a summary effect estimate, and evaluate how leiomyoma location, number, and size modify associated risk.
Sources
The plan and protocol for literature search, study selection, data extraction, and analysis were developed by A.C.S. a priori and adhere to MOOSE guidelines for reporting meta-analyses and systematic reviews of observational studies.8
All studies published in academic journals were identified through searches of electronic databases (MEDLINE, Web of Science, Embase, and ClinicalTrials.gov) using the terms “fibroid, ” “leiomyoma, ” “leiomyomata, ” “myoma, ” “miscarriage, ” “abortion, ” “fertility, ” “fetal death, ” and “pregnancy loss” (see Appendix 1, available online at http://links.lww.com/xxx, for full search strategies). All studies published in English between January 1, 1970 and December 20, 2016 were included in the search. Reference lists of included studies were hand-searched to ensure no eligible reports were missing. A list of all identified studies is available upon request.
Study Selection
We included all studies that compared the risk of spontaneous abortion among pregnant women with leiomyomas to pregnant women without leiomyomas. Leiomyoma status had to be determined with imaging for all participants. Since we aim to assess the impact of leiomyomas on spontaneous abortion risk among women of typical reproductive potential, studies limited to women seeking care for recurrent miscarriage, infertility, orassisted reproductive technologies were excluded.
Inclusion screening and data extraction were performedusing standardized forms implemented in Research Electronic Data Capture (REDCap) (Appendix 2, available online at http://links.lww.com/xxx).9 The primary outcome was spontaneous abortion(definition varied across studies) among recognized pregnancies. Aspirational coding was completed for factors thought to be associated with both risk of spontaneous abortion among women without leiomyomas and with leiomyoma presence. Potential confounders included maternal age, race, alcohol, body mass index (BMI), parity, and prior terminations. Data was abstracted for leiomyoma characteristics (location, size, number) when available. Risk of bias was determined using the Newcastle-Ottawa Scale.10 Scores were converted to Agency for Healthcare Research and Quality (AHRQ) classifications of good, fair, or poor quality.11
Study eligibility, data extraction, and risk of bias were determined independently by two reviewers. Percent agreement between authors for these steps was 99.5%, 98.0%, and 95.8%, respectively. Discrepancies between the two reviewers were resolved by a third party blinded to the other reviewers’ decisions. Study authors were contacted for missing information.
A random-effects meta-analysis was performed to calculate pooled risk ratios (RR) and 95% confidence intervals (CI). We limited the meta-analysis to studies evaluating general obstetric populations (excluding studies limited to women undergoing amniocentesis) to arrive at an aggregate estimate most representative of the risk-relationship in women of typical reproductive potential. For the meta-analysis, adjusted point estimates were used when available. The Q and I2 statistics were used to test for heterogeneity between included studies. Begg's and Egger's tests were used to assess publication bias. We evaluated year of publication, study design, and study quality as potential sources of heterogeneity using meta-regression. Meta-analyses by leiomyoma location, size, and number were performed if at least three studies presented a measure of association for the characteristic.
Only one study adjusted for confounders. Therefore, we performed a secondary analysis where we used external estimates of confounding to account for bias attributable to unmeasured confounding in the studies that only reported crude estimates.12, 13 This method compares the adjusted and unadjusted estimates from a study that presents both models to quantify U, the multiplicative bias produced from confounding.12 This measure is used to estimate adjusted risk ratios in studies that do not measure and adjust for confounding factors using the equation: RRadjusted = RRunadjusted/U. We used estimates from the only study that presented adjusted models to estimate U (fully adjusted model included maternal age, race alcohol use, parity, and history of prior terminations). We then corrected the variance of the adjusted measures for the statistical error in the estimate of residual confounding.14 All analyses were performed in Stata 14.1 by A.C.S. (Stata Corp, College Station, TX).
Results
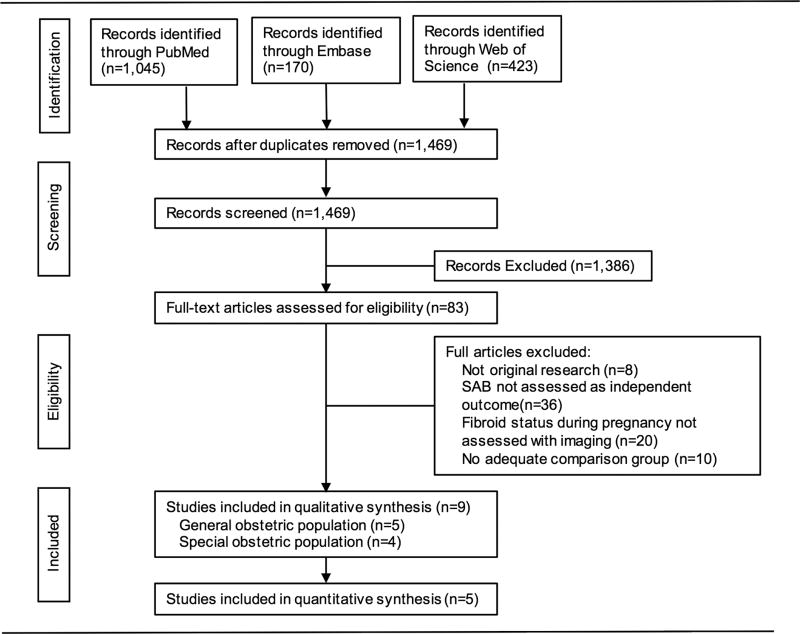
Of 1,468 studies screened, nine were included in the systematic review and five studies were included in the meta-analysis (Figure 1). Three of the studies were conducted in the United States, five in Europe, and one in the Middle East and study populations ranged from 200–21,219 participants. All publications included were observational cohorts and five enrolled a general obstetric population(Table 1)15-19 while four were restricted to women undergoing amniocentesis(Table 2).20-23 Three of the four studies among women undergoing amniocentesis reported leiomyoma presence increased risk of loss after the procedure by an estimated factor between 2.5 and 8.0 (Table 2). Since these studies were limited to special high-risk populations and required participant to have an ongoing pregnancy at the time of procedure, we describe these studies in the qualitative synthesis and exclude them in the quantitative analysis. Five studies were classified as good quality 15, 17, 19, 21, 23 and four as poor quality 16, 18, 20, 22 based on AHRQ standards for quality grading (Appendix 3, available online at http://links.lww.com/xxx).
Figure 1.
Flow diagram of studies identified in the systematic review.
Table 1. Characteristics of studies from general obstetric populations.
| Study | Study Years | Study Design | No. of Participants* | Population | Definition of Exposure | Confounders Adjusted | Definition of Outcome† | Risk‡ | Quality§ |
|---|---|---|---|---|---|---|---|---|---|
| Exacoustòs et al,17 1993 Italy | 1984–1990 | Retrospective cohort | 12,708 (492/12,216) | Exposed and unexposed reportedly matched by age and parity | Leiomyoma(s) 3 cm in diameter on ultrasound prior to 20 weeks gestation | None | Loss prior to 20 weeks | 7.7% v. 6.8% | Good |
| Mollica et al,16 1996 Italy | 1983–1994 | Retrospective cohort | 2,551 (88/2,463) | Exposed randomized to clinical protocol and matched with unexposed receiving routine obstetric care | Leiomyoma(s) on ultrasound | None | — | 13.6% v. 9.3% | Poor |
| Benson et al,15 2001 USA | 1991–1993 | Retrospective cohort | 858 (143/715) | Exposed and unexposed matched 1:5 by age and parity | Leiomyoma(s) on first trimester ultrasound | None | Loss prior to 25 weeks | 14.0% v. 7.6% | Good |
| Majeed et al,18 2011 Pakistan | 2008–2010 | Retrospective cohort | 200 (100/100) | Exposed matched with random sample of unexposed | Leiomyoma(s) on ultrasound | None | Loss prior to 24 weeks | 11% v. 5% | Poor |
| Hartmann et al,19 2017 USA | 2000–2012 | Prospective cohort | 5,512 (571/4,941) | Women enrolled prior to conception or before 12 weeks gestation | Leiomyoma(s) 0.5 cm on research ultrasound | Maternal age, race/ethnicity, alcohol use, prior terminations, parity | Loss prior to 20 weeks | 14.0% v. 10.9% | Good |
—, not reported
Reported as total (with leiomyomas/without leiomyomas)
In definition of outcome, weeks refer to weeks of gestation
Proportion of pregnancies ending in spontaneous abortion exposed versus unexposed
Grading based on the Newcastle-Ottawa Scale
Table 2. Characteristics of studies restricted to women undergoing amniocentesis*.
| Study | Study Years | No. of Participants† | Population | Definition of Exposure | Confounders Adjusted | Definition of Outcome‡ | Estimate of Effect (95% CI) |
|---|---|---|---|---|---|---|---|
| Salvador et al,21 2002 USA | 1994–2000 | 256 (128/128) | Women undergoing amniocentesis; exposed and unexposed matched by age and parity | Leiomyoma(s)1 cm in diameter on ultrasound | None | Loss between 15 and 24 weeks | Unadjusted RR: 8.0 (1.02–63.04) |
| Cignini et al,20 2011 Italy | 1999–2005 | 21,219 (2,497/18,722) | Women undergoing second-trimester amniocentesis | Leiomyoma(s)>20 mm in diameter on pre-procedural ultrasound | None | Loss within four weeks of procedure | Unadjusted RR: 3.0 (0.58–15.45) |
| Corrado et al,22 2012 Italy | 2001–2009 | 2,990 (166/2,824) | Women undergoing amniocentesis, consecutive subjects | Leiomyoma(s)>2 cm in diameter on pre-procedural ultrasound at 15-19 weeks gestation | None | Loss prior to 24 weeks | Unadjusted OR: 3.4 (1.2–9.0) |
| Theodora et al,23 2016 Greece | 2004–2010 | 6,752 (165/6,587) | Women undergoing second trimester amniocentesis | Leiomyoma(s)>20 mm in diameter on ultrasound | Age, bleeding in pregnancy, prior terminations, prior first trimester miscarriages, amniotic fluid staining | Loss prior to 24 weeks | Unadjusted OR: 2.71 (1.08–6.80) Adjusted OR: 2.52 (1.01–6.40) |
CI, confidence interval; RR, risk ratio; OR, odds ratio
All studies were retrospective cohorts
Reported as total (with leiomyomas/without leiomyomas)
In definition of outcome, weeks refer to weeks of gestation
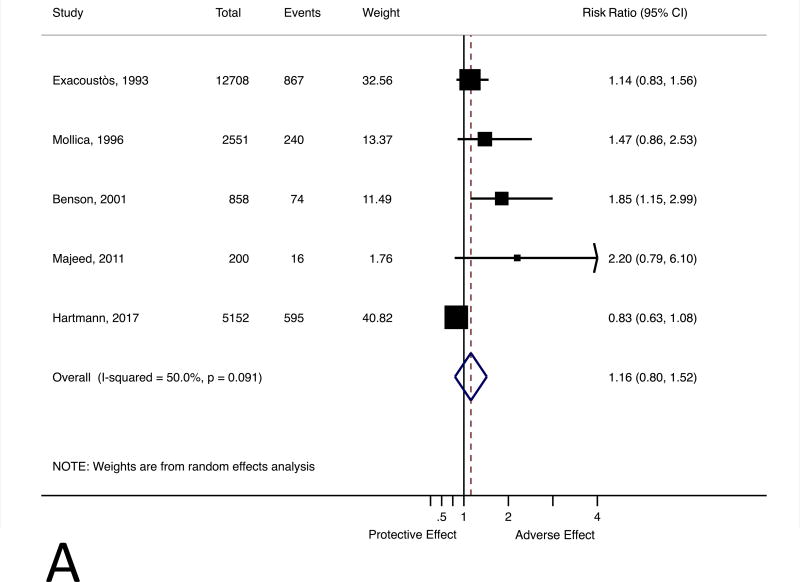
Our meta-analysis included five studies with 21,829 participants from general obstetric populations(1,394 women with leiomyomas and 20,435 without),.15-19 The meta-estimate doesnot suggest uterine leiomyomas are associated with an increased risk of spontaneous abortion (RR 1.16, 95% CI 0.80 to1.52) (Figure 2, Panel A). Heterogeneity of included studies was low (Q-statistic 8.01, p-value 0.09, τ2 0.07) with some true between-study variation (I2 50.0%). Year of publication, study design (retrospective cohort v. prospective cohort), and study quality did not explain any additional between-study heterogeneity (analysis not shown). There were too few studies for the Begg's and Egger's tests to detect evidence of publication bias (P=0.21and P=0.08). A trim-and-fill analysis predicted two missing studies pulling the corrected point estimate towards the null (RR 1.04; 95% CI 0.74 to 1.48).
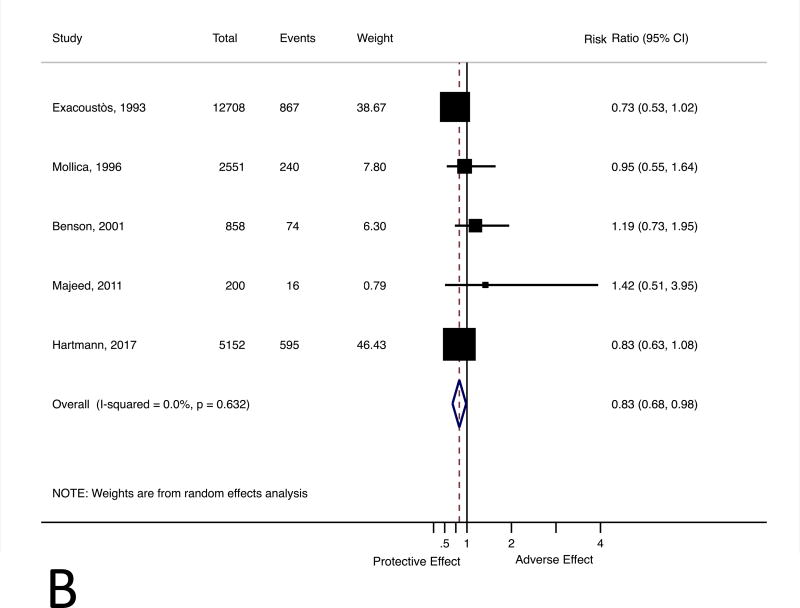
Figure 2.
Forest plot for the association between uterine leiomyomas and risk of spontaneous abortion (Panel A). Forest plot for the association between uterine leiomyomas and risk of spontaneous abortion with crude point estimates adjusted using external estimate of confounding (Panel B). CI, confidence interval.
Only one of the five studies in the meta-analysis adjusted for confounders of the relationship between leiomyomas and spontaneous abortion risk. We approximated adjusted point estimates for the four studies that reported crude resultsusing the data from Hartmann and colleaguesas an external estimate of confounding.19 The meta-analysis with the adjusted estimatesd emonstrated leiomyoma status is not associated with increased spontaneous abortion risk (RR 0.83, 95% CI 0.68 to 0.98) (Figure 2, Panel B). Two studies reported risk of spontaneous abortion by leiomyoma number or size: one presented crude results that suggest risk of loss increases with leiomyoma size and number15 and the other presented adjusted results that do not indicate a dose-dependent trend by either leiomyoma characteristic(Table 3).19
Table 3. Leiomyoma size and number and association with spontaneous abortion by study.
| Study | Categories | n | Outcome Measure |
|---|---|---|---|
| Benson et al. | Prevalence of SAB† | ||
| Leiomyoma size | No leiomyomas | 715 | 7.6% |
| <2 cm | 39 | 20.5% | |
| 2-4 cm | 58 | 8.6% | |
| >4 cm | 46 | 15.2% | |
| Leiomyoma number | No leiomyomas | 715 | 7.6% |
| Single leiomyoma | 88 | 8.0% | |
| 2 | 25 | 24.0% | |
| 3 | 8 | 12.5% | |
| 4+ | 22 | 27.3% | |
| Hartmann et al. | Adjusted HR (95% CI) | ||
| Leiomyoma size* | No leiomyomas | 4,741 | 1.00 [referent] |
| Lowest quartile | 143 | 1.12 (0.74–1.68) | |
| 2nd quartile | 140 | 1.02 (0.65–1.59) | |
| 3rd quartile | 141 | 0.52 (029–0.91) | |
| Top quartile | 140 | 0.62 (0.34–1.14) | |
| Leiomyoma number | No leiomyomas | 4,741 | 1.00 [referent] |
| 1 | 398 | 0.84 (0.62–1.15) | |
| 2+ | 166 | 0.79 (0.51–1.23) |
SAB, spontaneous abortion; HR, hazard ratio; CI, confidence interval
Leiomyoma largest dimension: Lowest quartile [0.51–1.36 cm), 2nd quartile [1.36–2.35 cm), 3rd quartile [2.35–3.62 cm), Top quartile [3.62–13.20 cm]
Discussion
This meta-analysis, including21,829 pregnancies from five cohort studies, indicates uterine leiomyoma presence is not associated with increased risk of spontaneous abortion among general obstetric populations. Strikingly few studies rigorously examine the association between uterineleiomyomas and spontaneous abortion risk. Many neglect common confounders such as maternal age and race, which are known to be related to spontaneous abortion risk and leiomyoma presence.24, 25 This is the first review on the association between leiomyoma presence and spontaneous abortion that specifically evaluates studies of general obstetric populations and the only review that quantitatively accounts for bias due to potential confounders. Our findings challenge common clinical belief and should cause us to reconsider understanding of uterine leiomyomas as a risk factor for spontaneous abortion in women of typical reproductive potential. The misconception that leiomyomas increase risk of spontaneous abortion in the general population may lead to undue anxiety for patients with leiomyomas, inappropriate risk counseling, or the recommendation of unnecessary surgeries.
Three past reviews on this association estimate uterine leiomyomas increase risk of spontaneous abortion by between 24 and 75%.5-7 These reviews are either intentionally7 or incidentally5, 6 dominated by studies of special populations, such as women seeking fertility treatment or with a history of recurrent miscarriage. None of these reviews quantitatively address possible bias due to maternal age or race in their main summary estimates.5-7
Women in this review were already pregnant or had to achieve pregnancy to be enrolled in the included cohorts and are a distinct population from women who are unable to conceive naturally. Submucosal or large intramural leiomyomas may decrease fertility by impeding implantation,5 and leiomyomas contributing to this phenotype may affect risk of loss differently from those characterized in this review. Therefore, the women in this review are different from those included in past reviews and the risk association described here more likely characterizes the relationship between leiomyomas and spontaneous abortion risk in a population of women of average reproductive potential.
The methods for the execution of this meta-analysis were rigorous and quantitatively strong. We are the first to present all relevant studies conducted in general obstetric populationson this association. While several of the original studies do not present adjusted analyses, we use a method considered to be an effective tool for minimizingbias from unmeasured confounders.12 Application of this method indicates crude estimates are biased, and the fact that all studies describing a significant risk association between leiomyomas and spontaneous abortion risk are unadjusted should provide an impetus forreevaluating prior beliefs.
This meta-analysis should be interpreted in light of the following considerations. To optimally determine case-status and capture spontaneous abortion events, participants should be enrolled prior to conception or in early pregnancy and undergo standardized imaging for leiomyoma assessment. This design is resource intensive and difficult to implement on a large scale, and accordingly, four of the five studies included in the meta-analysis were retrospective analyses.15-18 These studies are subject to selection bias since they depend on care utilization, rely on availability of imaging data, and recruit participants solely from academic medical centers. Methods for defining exposure and outcome status varied between studies. One study only counted women as exposed if they had a leiomyoma with a dimension greater than three centimeters17 and three studies did not provide a minimum measurement threshold in their exposure definition.15, 16, 18 Variation in exposure definitions may introduce heterogeneity secondary to differential exposure classification. The gestational age cut off for spontaneous abortion definitions ranged from 20 to 25 weeks’ gestation.26 Since losses are concentrated in early pregnancy with very few occurring beyond 20 weeks, we do not anticipate these differences to materially impact the summary estimate. Since four of the five studies fail to adjust for pertinent confounders, we attempted to account for bias using external estimates of unmeasured confounding.15-18 Our ability to estimate a bias-free summary measure using this method is dependent on how well the bias present in Hartmann et al. reflects confounding present in other studies.12, 13
In conclusion, this systematic review and meta-analysis does not indicate that leiomyoma presence, location, number, or sizeis related to spontaneous abortion riskin general obstetric populations.
Supplementary Material
Acknowledgments
Supported by CTSA award number UL1TR000445 from the National Center for Advancing Translational Sciences and by the Public Health Service award (T32 GM07347) from the National Institute of General Medical Sciences for the Vanderbilt Medical-Scientist Training Program.
The authors thank Nila Sathe (Vanderbilt's Evidence-Based Practice Center) for assistance in designing the protocol and Rachel Lane Walden (Vanderbilt Biomedical Library Services) for refining the search criteria.
Footnotes
Financial Disclosure: The authors did not report any potential conflicts of interest.
Each author has indicated that he or she has met the journal's requirements for authorship.
References
- 1.Laughlin SK, Baird DD, Savitz DA, Herring AH, Hartmann KE. Prevalence of uterine leiomyomas in the first trimester of pregnancy: an ultrasound-screening study. Obstet Gynecol. 2009;113:630–5. doi: 10.1097/AOG.0b013e318197bbaf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100–7. doi: 10.1067/mob.2003.99. [DOI] [PubMed] [Google Scholar]
- 3.Wise LA, Laughlin-Tommaso SK. Epidemiology of Uterine Fibroids: From Menarche to Menopause. Clin Obstet Gynecol. 2016;59:2–24. doi: 10.1097/GRF.0000000000000164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klatsky PC, Tran ND, Caughey AB, Fujimoto VY. Fibroids and reproductive outcomes: a systematic literature review from conception to delivery. Am J Obstet Gynecol. 2008;198:357–66. doi: 10.1016/j.ajog.2007.12.039. [DOI] [PubMed] [Google Scholar]
- 5.Pritts EA, Parker WH, Olive DL. Fibroids and infertility: an updated systematic review of the evidence. Fertil Steril. 2009;91:1215–23. doi: 10.1016/j.fertnstert.2008.01.051. [DOI] [PubMed] [Google Scholar]
- 6.Sunkara SK, Khairy M, El-Toukhy T, Khalaf Y, Coomarasamy A. The effect of intramural fibroids without uterine cavity involvement on the outcome of IVF treatment: a systematic review and meta-analysis. Hum Reprod. 2010;25:418–29. doi: 10.1093/humrep/dep396. [DOI] [PubMed] [Google Scholar]
- 7.Metwally M, Cheong Y, Horne A. Surgical treatment of fibroids for subfertility. Cochrane Database of Systematic Reviews. 2012;(11) doi: 10.1002/14651858.CD003857.pub3. [DOI] [PubMed] [Google Scholar]
- 8.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: A proposal for reporting. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 9.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale for assessing the quality of nonrandomised studies in meta-analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Retrieved May 15, 2016.
- 11.Viswanathan M, Ansari MT, Berkman ND, Chang S, Hartling L, McPheeters LM, et al. Agency for Healthcare Research and Quality Methods Guide for Comparative Effectiveness Reviews. 2012. Assessing the Risk of Bias of Individual Studies in Systematic Reviews of Health Care Interventions. AHRQ Publication No 12-EHC047-EF. [PubMed] [Google Scholar]
- 12.Rothman KJ, Greenland S, Lash TL. Chapter 33: Meta-analysis Modern Epidemiology. 3rd. Philadelphia (PA): Lippincott Williams & Wilkins; 2008. pp. 660–2. [Google Scholar]
- 13.Bross ID. Pertinency of an extraneous variable. J Chron Dis. 1967;20:487–95. doi: 10.1016/0021-9681(67)90080-x. [DOI] [PubMed] [Google Scholar]
- 14.Greenland S, Mickey R. Closed-form and dually consistent methods for 2x2xK and IxJxK tables. App Stat. 1988;37:335–43. [Google Scholar]
- 15.Benson CB, Chow JS, Chang-Lee W, Hill JA, Doubilet PM. Outcome of pregnancies in women with uterine leiomyomas identified by sonography in the first trimester J Clin Ultrasound. 2001;29:261–4. doi: 10.1002/jcu.1031. [DOI] [PubMed] [Google Scholar]
- 16.Mollica G, Pittini L, Minganti E, Perri G, Pansini F. Elective uterine myomectomy in pregnant women. Clin Exp Obstet Gyn. 1996;23:168–72. [PubMed] [Google Scholar]
- 17.Exacoustos C, Rosati P. Ultrasound diagnosis of uterine myomas and complications in pregnancy. Obstet Gynecol. 1993;82:97–101. [PubMed] [Google Scholar]
- 18.Majeed T, Waheed F, Sattar Y, Mobusher I, Saba K. Impact of uterine fibroids on the obstetric performance of the women; Complications and pregnancy outcome. Pak J Med Sci. 2011;5:274–7. [Google Scholar]
- 19.Hartmann KE, Velez Edwards DR, Savitz DA, Jonsson Funk ML, Wu P, Baird DD. Prospective cohort study of uterine fibroids and miscarriage risk. Am J Epidemiol. 2017 doi: 10.1093/aje/kwx062. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cignini P, Mobili L, D'Emidio L, Mangiafico L, Coco C, Giorlandino C. Uterine fibroids and risk for complications following second-trimester amniocentesis. J Reprod Med. 2011;56:393–7. [PubMed] [Google Scholar]
- 21.Salvador E, Bienstock J, Blakemore KJ, Pressman E. Leiomyomata uteri, genetic amniocentesis, and the risk of second-trimester spontaneous abortion. Am J Obstet Gynecol. 2002;186:913–5. doi: 10.1067/mob.2002.123988. [DOI] [PubMed] [Google Scholar]
- 22.Corrado F, Cannata ML, La Galia T, Magliarditi M, Imbruglia L, D'Anna R, et al. Pregnancy outcome following mid-trimester amniocentesis. J Obstet Gynaecol. 2012;32:117–9. doi: 10.3109/01443615.2011.633717. [DOI] [PubMed] [Google Scholar]
- 23.Theodora M, Antsaklis A, Antsaklis P, Blanas K, Daskalakis G, Sindos M, et al. Fetal loss following second trimester amniocentesis. Who is at greater risk? How to counsel pregnant women? J Matern-Fetal Neo M. 2016;29:590–5. doi: 10.3109/14767058.2015.1012061. [DOI] [PubMed] [Google Scholar]
- 24.Laughlin SK, Baird DD, Savitz DA, Herring AH, Hartmann KE. Prevalence of Uterine Leiomyomas in the First Trimester of Pregnancy An Ultrasound-Screening Study. Obstet Gynecol. 2009;113:630–5. doi: 10.1097/AOG.0b013e318197bbaf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mukherjee S, Edwards DRV, Baird DD, Savitz DA, Hartmann KE. Risk of Miscarriage Among Black Women and White Women in a US Prospective Cohort Study. Am J Epidemiol. 2013;177:1271–8. doi: 10.1093/aje/kws393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Avalos LA, Galindo C, Li DK. A Systematic Review to Calculate Background Miscarriage Rates using Life Table Analysis. Birth Defects Res A. 2012;94:417–23. doi: 10.1002/bdra.23014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.