Abstract
MV-NIS is an Edmonston-lineage oncolytic measles virus expressing the human sodium-iodide symporter--a means for monitoring by noninvasive imaging of radioiodine. Patients with relapsed, refractory myeloma who had explored all other treatment options were eligible for this Phase I trial. Cohort 1 was treated with intravenous MV-NIS, and Cohort 2 received cyclophosphamide two days prior to MV-NIS. Thirty-two patients were treated. Cohort 1 initially enrolled to 4 dose-levels without reaching MTD and subsequently to 2 higher dose-levels when improved virus manufacture technology made it possible. MTD was not reached in Cohort 1, and TCID50 1011 is the dose being used in a Phase II trial of single agent MV-NIS. Grade 3–4 AEs in both cohorts at all dose levels were: neutropenia (n=9); leukocyte count decreased (n=5); thrombocytopenia (n=2); and CD4 lymphocytes decreased, anemia and lymphopenia (each n=1). MV-N RNA sequences were amplified from gargle specimens, blood and urine. 123I scans were positive in 8 patients. One patient achieved a CR; transient drops in serum FLCs were seen in other patients. MV-NIS is capable of replicating before being cleared by the immune system. Oncolytic viruses offer a promising new modality for the targeted infection and destruction of disseminated myeloma.
Background
Multiple myeloma is not curable with current therapy and was expected to cause 11,240 deaths in the USA in 2015.(1) Median survival is approximately four years and new approaches to therapy are urgently required. The disease responds initially to alkylating agents, immune modulators, proteasome inhibitors, and corticosteroids, but eventually becomes refractory. New approaches are needed. Oncolytic immunotherapy is one such modality.(2, 3) Oncolytic viruses mediate their antitumor effects in two phases. Virus-infected tumor cells are killed during the oncolytic phase. Next uninfected tumor cells are killed by crossprimed tumor-specific cytotoxic T lymphocytes, in immunotherapeutic phase.
MV-NIS is a live attenuated measles virus, engineered to express the human thyroidal sodium-iodide symporter (NIS).(4, 5) The virus is selectively oncolytic, targeting and destroying tumor cells through CD46, a membrane regulator of complement activation that is known to be overexpressed on many human malignancies.(6–8) CD46 is the cellular receptor for MV-NIS, mediating both virus entry and subsequent cell killing through cell-cell fusion.(9) The CD46 tropism of attenuated Edmonston lineage measles viruses was acquired during tissue culture adaptation(10) and distinguishes them from wild-type measles viruses, which enter cells primarily through an alternative receptor, SLAM, expressed on activated T cells, B cells and monocytes(11) MV-NIS has dual tropism for both SLAM and CD46. The cytopathic effect of MV-NIS increases exponentially as the density of CD46 receptors on target cells increases and is therefore dramatic at high CD46 receptor densities (tumor) but minimal at low densities (normal tissues).(12) CD46 regulates complement activation by acting as a co-factor for factor I-mediated cleavage of C3b and thereby protects the cells on which it is expressed from complement-mediated lysis.(6, 7) MV-NIS demonstrated considerable oncolytic potency when administered intravenously to rodents bearing human myeloma xenografts.(4, 5) Intratumoral spread of the virus was monitored non-invasively by radioiodine imaging and the anti-neoplastic potency of the virus was significantly boosted by 131I.
Myeloma plasma cells over-express CD46 and are therefore highly susceptible to MV-NIS.(13) We hypothesized that systemic virus administration would be feasible in patients with advanced myeloma since these patients have greatly reduced circulating titers against common vaccine antigens, like measles.(14, 15) Cyclophosphamide was included as part of the treatment design to blunt too rapid an anamnestic response which would be a threat to viral propagation and tumor targeting.(16, 17)
It was our overall hypothesis is that MV-NIS administered intravenously to patients with advanced multiple myeloma would selectively propagate in myeloma deposits throughout the body in a safe fashion. If this primary hypothesis was satisfied, this should lead to tumor cell killing and reduction of tumor burden, which could be tested in a phase II setting. The primary hypothesis was tested in a phase I clinical trial (NCT00450814) by serial Q-RT-PCR and nuclear imaging of radioiodine uptake after MV-NIS therapy to monitor the changing number and location of virus-infected cells.
METHODS
Patients
Patients were eligible if they had relapsed or refractory myeloma, age 18 years or older, at least 2 or more prior non-overlapping chemotherapeutic combinations but did not have known standard therapy known to extend life expectancy, ECOG performance status (PS) 0, 1 or 2, life expectancy of greater than 12 weeks, adequate bone marrow reserve (neutrophil count ≥ 1×10(9)/L, platelets ≥ 50×10(9)/L, and hemoglobin ≥ 8.5 g/dL), adequate organ reserve (AST ≤2 times upper limit of normal, creatinine < 2 times upper limit of normal, total bilirubin ≤ 1.5×upper limit of normal, and an INR ≤ 1.4×ULN), a negative serum pregnancy test done ≤ 7 days prior to registration for women of childbearing potential, ability to provide written informed consent.
Patients were excluded if they had prior allogeneic stem cell transplantation, uncontrolled infection, chemotherapy within 3 weeks prior to registration, or immunotherapy or biologic therapy within 4 weeks of study registration, New York Heart Association classification III or IV, known symptomatic coronary artery disease, or known atrial fibrillation or SVT, active CNS disorders, HIV positive test result, allergy to oral iodine, previous exposure to heat inactivated measles virus vaccine, pregnant or nursing women, men or woman with reproductive capacity not willing to use contraception, and exposure to household contacts ≤15 months old or household contact with known immunodeficiency.
Study Oversight
This study was conducted in accordance with the principles of the Declaration of Helsinki. The protocol was approved by the local human investigations committee. Written informed consent was obtained from all the patients before screening.
Study Design
The primary goal of the phase I trial was to determine the maximum tolerated dose (MTD) of MV-NIS when administered with or without cyclophosphamide in patients with relapsed or refractory multiple myeloma. Secondary endpoints included: 1) determination of the regimen’s safety and toxicity profile; 2) hematologic response rate; 3) time course of viral gene expression and virus elimination; 4) biodistribution of virally infected cells at various times points after infection with MV-NIS using 123I nuclear imaging; 5) humoral responses to the injected virus.
The study consisted of two sequential dose escalation schemes to determine the maximum tolerated dose (MTD) of MV-NIS when given with or without cyclophosphamide. First, patients were to be accrued to the prescribed dose levels for one dose of single agent MV-NIS. Once the MTD was determined for MV-NIS alone, then a second stage of dose escalation was to be done to determine the MTD of MV-NIS when given in combination with of cyclophosphamide 10 mg/kg two days prior to the MV-NIS. The design was a standard 3+3 dose escalation, with a 6 week observation window. When the trial began in 2006, the manufacturing capabilities of MV-NIS were limited to a maximum dose of TCID50 of 109; therefore there were 4 dose levels in Stage I (no cyclophosphamide patients: TCID50 106, 107, 108, and 109) and 5 dose levels in Stage II (with cyclophosphamide: MTD/100, 3 × MTD/100, 9 × MTD/100, 27 × MTD/100, and 81 × MTD/100). TCID50 is defined as 50% tissue culture infective dose, which is the measure of infectious virus titer; more specifically, it quantifies the amount of virus required to produce a cytopathic effect in 50% of inoculated tissue culture cells. Due to new improved good manufacturing practices to produce higher titers of MV-NIS (See Supplemental Methods), in October 2011, higher doses of MV-NIS could be produced, so Stage II was suspended after 21 patients had been accrued to it, and Stage I was reopened to evaluate dose levels 5 and 6, which were to deliver MV-NIS at TCID50 1010 and 1011 (Supplemental Figure 1). Patients are notated in the results section by stage, dose level, and patient number (i.e. patient I.4.2 is stage I, dose level 4, patient 2).
All patients with intact thyroid glands (i.e. not previously surgically removed or ablated) received TSH suppressive doses of Cytomel® orally (25 mcg TID) for 4 days prior to 123I and through last dose required for imaging for the purpose reducing uptake of iodine by the thyroid gland by suppressing TSH dependent expression of the NIS protein in the thyroid follicle thereby recapitulating the anticipated biodistribution if therapeutic 131I were to be used in a successor trial.
The virus was given at the inpatient Clinical Research Unit by infusion in 250 ml of normal saline over 30 minutes for the first 26 patients. When higher doses were reached, the infusion rate was slowed to 60 minutes due to infusion related headache. Patients were closely monitored the inpatient Clinical Research Unit during infusion of the virus for acute febrile reaction. Per protocol, patients who developed febrile responses to the infusion were treated with acetaminophen 650 mg by mouth and diphenhydramine hydrochloride 50 mg intravenously or by mouth. For rigors, meperidine hydrochloride 50 mg was administered by vein.
Dose limiting toxicity was defined as follows as any grade 3 or higher AE attributed to therapy with the following exceptions. Lymphopenia was not considered a DLT. Neutropenia was considered a DLT if it was grade 3 lasting more than 7 days or grade 4. Thrombocytopenia was considered a DLT only if it was grade 4 lasting more than 7 days. Symptomatic measles infection lasting 6 or more days or a 10-fold increase in the measles N gene RNA copy number (copies per microgram RNA) between sequential samples at least 3 days apart after day +15 were also part of the definition of DLT.
Study Assessments
All patients underwent tumor assessments at baseline, including serum protein electrophoresis and quantitation immunoglobulin free light chains (FLC), MRI, PET-CT and 123I gamma camera imaging. All patients receiving a dose of drug were assessable for toxicity. All patients were assessed for hematologic response. Response was according the IMWG response criteria.(18) Adverse events (AE) were graded according to the Common Terminology Criteria for AE, version 3. The kinetics of virus spread and elimination and distribution of MV-NIS infected cells was monitored by serial measurements of viral RNA (Q-RT PCR of N gene) in mononuclear cells derived from blood, saliva and urine and by SPECT/CT whole body gamma camera imaging after oral 123I. AE and serum immunoglobulin free light chains were monitored for on days +3, +8, and +15 in all patients. Patients who had either detectable virus or positive scans at day 15 had additional testing on day 22 (and day 29) to document elimination of the virus and virus infected cells. Starting with patient II.1.2, the pharmacokinetic (blood Q-RT-PCR) studies were broadened to: end of infusion, 30 minutes post, 60 minutes post, 120 minutes post, and 240 minutes post. Patients had bone marrow biopsy on day +42 to determine response and viral persistence in bone marrow.
Patients were educated about the symptoms of a measles-like illness (coryza, malaise, fever, rash, lymphadenopathy and transient suppression of the immune system) and were told to report these symptoms immediately. The protocol prescribed that treatment for measles with ribavirin and immune globulin be implemented if the symptoms (including temperature ≥ 38.5° C) persist for as long as 6 days or earlier at the treating physician’s discretion. The other contingency for instituting anti-viral therapy was a 10-fold increase in the copy number (copies per microgram RNA) between sequential samples at least 3 days apart after day +15. This increase beyond day 15 would be considered a DLT.
Laboratory Correlates
Assessment of viremia and viral shedding
Mononuclear cells were isolated from blood, biopsy specimens, throat washings and urine according to protocol, and viral replication quantitative RT-PCR to determine virus RNA copy number was done.(19) Peripheral blood was collected in PAXgene tubes. Oral secretion samples were collected using Scope mouthwash. RNA was extracted using the Qiagen RNeasy Total RNA Kit and Qiagen QIAshredder. Q-RT-PCR was done using primers, the TaqMan One-step RT-PCR Master Mix Reagents Kit. (Applied Biosystems,Foster City, CA), on a Stratagene MX4000 Multiplex Quantitative PCR System – a spectrophotometric thermocycler (Stratagene, LaJolla, CA).
The description of the remaining laboratory correlates are contained in the supplementary materials.
Evaluation of MV-NIS Biodistribution Using SPECT/CT
Radioiodine uptake was visualized on SPECT/CT scans obtained 1–2 hours after oral administration of 5 mCi 123I. The scans were obtained at baseline and on days 8, and 15 post-virus administration using a Phillips Brightview SPECT/CT scanner. To suppress thyroidal NIS expression, liothyronine sodium (25 mcg, three times daily) was administered orally for 4 days prior to the first scan and was continued until completion of the final scan. Those patients with a positive scan at day +15 had additional scans at day +22 and +29 until scans were negative. On day +8 dosimetry was done (day +8, 1–2 hours post 123I, day +8, 6 hours post 123I, and day +9, which was 24 hours post 123I. Initial patients had gamma camera scans, but the protocol was amended to use SPECT-CT. The pre-scan and the “6 hour scan” day 8 covered 80 cm of body length (chest and abdomen); subsequent scans covered 40–80 cm (chest plus or minus abdomen) depending on regions of interest.
Data analysis
AE were summarized including those events attributed to experimental agents. Exploratory analyses to identify predictors for response and correlations between viral persistence and baseline measures were performed using Fisher’s exact test, Wilcoxon rank sum, Spearman rho tests as appropriate. JMP 12.1 software was used (SAS, NC).
RESULTS
Patients
Thirty-two patients were treated on the phase I protocol. Three patients were replaced for lack of protocol completion. Baseline demographics for the 29 evaluable patients are shown in Table 1. The median age of participants was 62 (range: 43–82). The median number of prior regimens was 5 (range: 2–15). Stage I initially enrolled 13 patients receiving one dose 106, 107 108 or 109 TCID50 of MV-NIS. Patient I.4.2 was replaced because he developed influenza pneumonia 4 days after treatment with MV-NIS. For the influenza pneumonia he was hospitalized and unable to complete protocol assessments.
Table 1.
Baseline characteristics
| Cohort 1 (N=21) |
Cohort 2 (N=8) |
Total (N=29) |
|
|---|---|---|---|
| Age | |||
| Median (range) | 63.0 (43.0–82.0) | 61.0 (49.0–81.0) | 62.0 (43.0–82.0) |
| Gender | |||
| Female | 12 (57.1%) | 2 (25.0%) | 14 (48.3%) |
| Male | 9 (42.9%) | 6 (75.0%) | 15 (51.7%) |
| Race | |||
| White | 21 (100.0%) | 8 (100.0%) | 29 (100.0%) |
| Ethnicity | |||
| Not Hispanic or Latino | 21 (100.0%) | 8 (100.0%) | 29 (100.0%) |
| Performance Score | |||
| 0 | 9 (42.9%) | 5 (62.5%) | 14 (48.3%) |
| 1 | 8 (38.1%) | 2 (25.0%) | 10 (34.5%) |
| 2 | 4 (19.0%) | 1 (12.5%) | 5 (17.2%) |
| Durie-Salmon Stage at On Study | |||
| Missing | 1 | 0 | 1 |
| IA | 4 (20.0%) | 0 (0.0%) | 4 (14.3%) |
| IIA | 5 (25.0%) | 4 (50.0%) | 9 (32.1%) |
| IIIA | 11 (55.0%) | 4 (50.0%) | 15 (53.6%) |
| Prior Transplant | |||
| Yes | 16 (76.2%) | 7 (87.5%) | 23 (79.3%) |
| No | 5 (23.8%) | 1 (12.5%) | 6 (20.7%) |
| Number of Previous Regimens | |||
| Median (range) | 6.0 (2.0–15.0) | 5.0 (4.0–9.0) | 5.0 (2.0–15.0) |
| 2 – 4 | 6 (28.6%) | 2 (25%) | 8 (27.5%) |
| 5 – 7 | 9 (42.8%) | 4 (50.0%) | 13 (44.8%) |
| 8+ | 6 (28.5%) | 2 (25.0%) | 8 (27.5%) |
| Months from Diagnosis to On Study | |||
| Median (range) | 82.1 (8.6–163.8) | 83.7 (7.0–119.7) | 82.1 (7.0–163.8) |
Cohort I: Single agent MV-NIS; Cohort II: Cyclophosphamide and MV-NIS
Because no DLT was observed at doses 106, 107 108 or 109 TCID50, as per protocol, Stage 2, which included 10 mg/kg of cyclophosphamide 2 days prior to MV-NIS, began to accrue patients (Supplemental Figure 1). Eight patients were enrolled receiving doses of TCID50 107, 3×107, and 9×107. At trial conception, manufacturing allowed doses only up to 109 TCID50, but technology improvements subsequently allowed for two higher dose levels (1010 and 1011 TCID50). Therefore, Stage II accrual was suspended, and Stage I accrual was resumed to test these 2 higher levels. Four patients received 1010, and 7 patients received 1011. Patient I.5.3 was replaced because of inability to follow through on trial follow-up due to rapidly progressive myeloma. Patient I.6.1 was replaced because one tenth of the expected dose was administered due to infusion reaction (headache). One patient experienced a DLT on dose level 6 (patient I.6.7, neutrophils). In Stage I, MTD was not reached, and TCID50 1011 was deemed the treatment dose for phase II study. Accrual to Stage II (with cyclophosphamide) remains suspended such that single agent MV-NIS could be more formally assessed as a Phase 2 trial.
Adverse Events
Table 2 and Supplementary Table 1 summarize the frequency of AE deemed to be at least possibly related to MV-NIS therapy in the 29 evaluable patients. Grade 1–2 AEs seen in at least 5 patients were: nausea (n=10); chills (n=9); leukopenia (n=8); fever (n=7); and diarrhea and neutropenia, (each n=5). Grade 3–4 AEs deemed at least possibly related to protocol therapy in both cohorts at all dose levels were: neutropenia (n=9); leukocyte count decreased (n=5); thrombocytopenia (n=2); and CD4 lymphocytes decreased, anemia and lymphopenia (each n=1). One patient treated in cohort 2, dose level 3 (e.g. CTX and TCID50 9x107) had a grade 3 left ventricular failure possibly related to therapy.
Table 2.
Adverse Events At Least Possibly Related, all grades, by dose level
| Body System | Stage I | Stage II | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DL1 | DL 2 | DL 3 | DL 4 | DL 5 | DL 6* | DL 1 | DL 2 | DL 3 | ||||||||||||||||||
| Grade | 1 | 2 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 1 | 2 | 3 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 |
| Hematology | 0 | 2 | 0 | 1 | 1 | 0 | 0 | 2 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 2 | 3 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Pulmonary | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Coagulation | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Constitutional | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 2 | 3 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Dermatology/Skin | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Gastrointestinal | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 1 | 3 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 1 | 0 |
| Hepatic | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Endocrine | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Infection/FN | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ocular/Vision | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pain | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Syndromes | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cardiovascular | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Recommended phase 2 dose
Measles immunity pre-and post -treatment
Pre-existing and post-therapy measles virus immunity was tested using two methods. By ELISA and neutralizing assay titer, 10 of 31 and 13 of the 31 patients had pre-existing measles immunity (Figure 1 and Supplementary Table 2). Post treatment 25 of 27, and 27 of 27 were measles positive by ELISA and neutralizing antibody, respectively.
Figure 1.
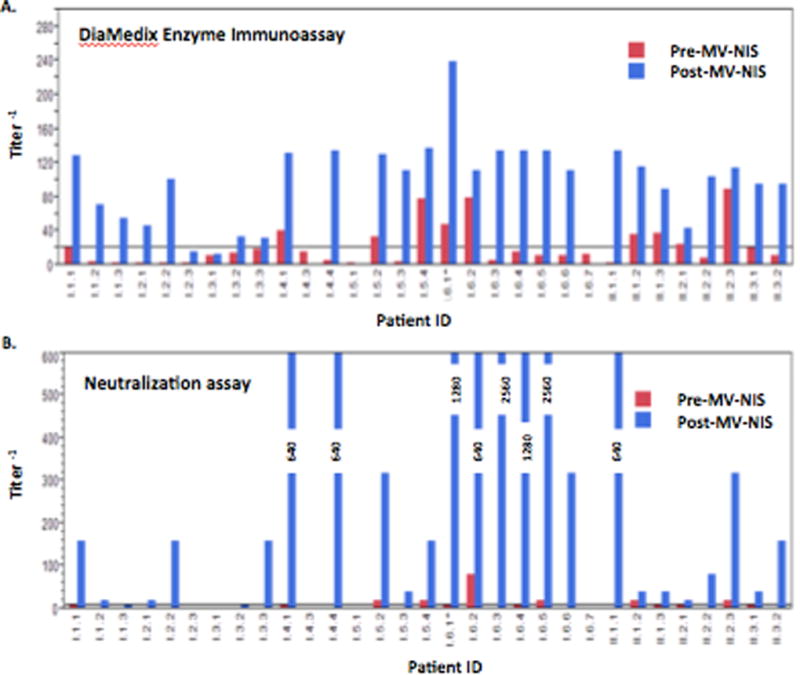
Humoral response to measles
A. DiaMedix Enzyme Immunoassay. Non-reactive is less than 1:20 (horizontal line)
B. Neutralization assay. Negative is less than 1:10 (horizontal line)
Assessment of baseline bone marrow’s infectivity ex vivo
Twenty patients had enough bone marrow to perform ex vivo infectivity experiments (Supplementary Table 2). As shown in Supplemental Figure 2A, the CD138-positive cells were more easily infected than the CD138-negative cells with respective median (range) of 52% (43%, 63%) versus 5% (1%, 18%). The CD138 positive cells expressed significantly higher numbers of surface CD46 molecules than the CD138 negative cells (Supplemental Figure 2B).
Pharmacokinetics/Pharmacodynamics
Serial measurements of blood N-gene were performed in the first 4 hours of therapy in patients II.1.2 onward (Figure 2). Virus cleared from blood by 4 hours for patients receiving less than 108 even when administered with CTX. For 2 of the 3 patients receiving TCID50 109, N-gene was measurable up through day 3 but not beyond. Once patients were treated with doses of TCID501011, N-gene was detected in 2 of 4 patients at day 8 and 1 of 4 at day 15 and the highest peaks were achieved in those patients given this highest dose.
Figure 2.
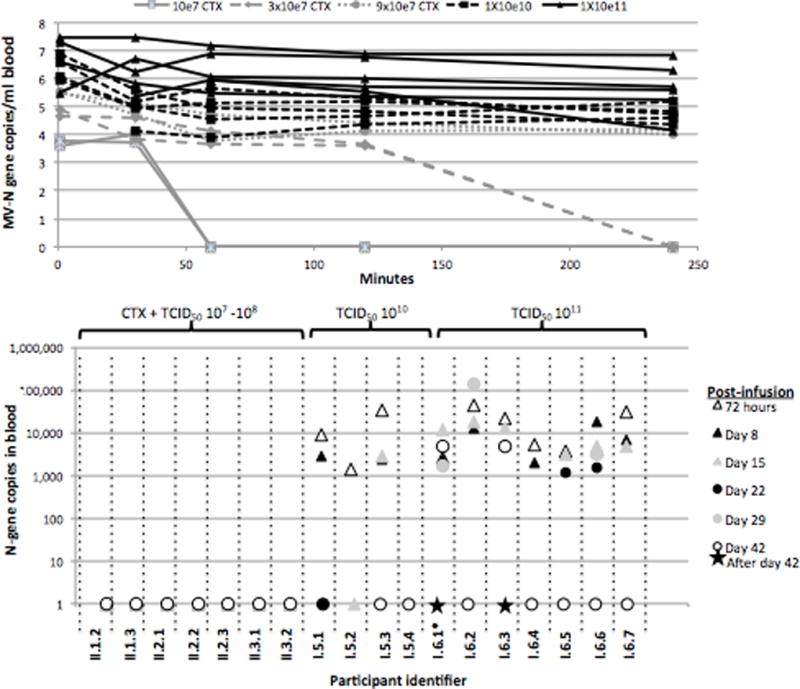
Summary of pharmacokinetics and correlative studies by dose level. The infusion rate of virus was 100 mL over 30 minutes for all patients through patient I.5.4. For patients I.6.1-I.6.3, the intent was the same, but at this dose of virus, actual infusion rates were 85 minutes, 156 minutes, and 99 minutes respectively due to infusion reactions (see text) requiring halting and resumption of drug. Patient I.6.1 received only 11 mL of drug due to infusion reaction, and was therefore replaced. Thereafter, the protocol was modified to a 250 mL over 2.5 hours in for patients I.6.4-I.6.6. The infusion rate for patient I.6.7 was adjusted to 250 mL over 1 hour.
A. Blood N-gene levels by Q-RT-PCR over first 72 hours by participant
B. Blood N-gene levels by Q-RT-PCR from day 3 post infusion to day 42 by participant
No patients treated with doses below TCID50 109 had persistent viremia detected at 72 hours (Figures 2 and 3). All but 2 patients (I.4.1 and I.5.4) treated with TCID50109 or higher had persistent viremia detected at 72 hours; these 2 patients had positive MV titers at baseline. Their respective ELISA titers were 40.4 and 77.8, and their respective neutralizing antibody titers were 1:10 and 1:20. Four patients (I.6.1, I.6.2, I.6.3, and I.6.6) had persistent viremia after day 29. Patients I.6.2 and I.6.6 cleared by day 42, but documented clearance of viremia was not demonstrated until days 70 and day 150 for patients I.6.1 and I.6.3, respectively.
Figure 3.
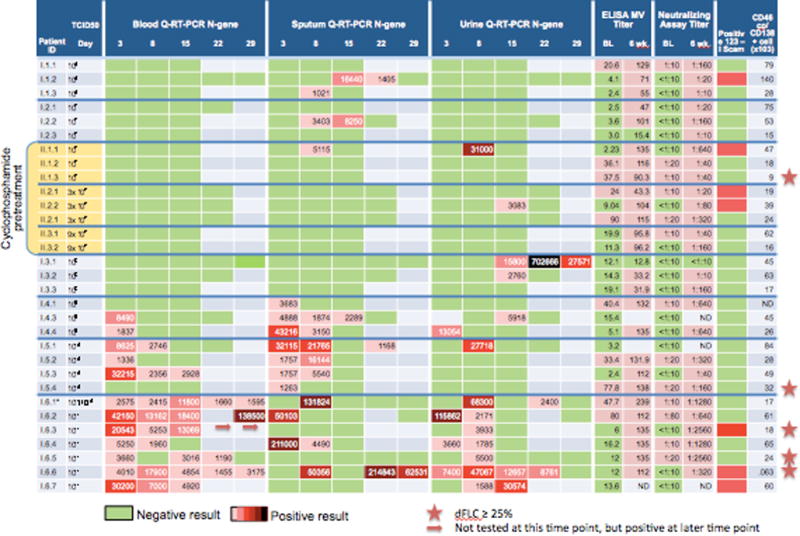
Pharmacokinetics in the context of measles virus titer, 123I SPECT / CT scan, and CD46 density on bone marrow plasma cells.
In contrast to detectable N-gene in the blood being present only at the highest dosing levels, detection of viral genomes in the sputum at the TCID50 106 and 107 levels at days 8, 15 and 22 was sporadic (Figure 3). At TCID50 109 and higher dosing viral genome was detected in the sputum in the majority of patients. Quite similarly, in the urine, genome was sporadically present in the urine with doses of less than TCID50 1011, but in all cases at doses of TCID50 1011.
Additional baseline and 6 week post-therapy correlative results are shown in Supplementary Table 1. Predictors for persistent viremia were sought. There was an inverse correlation between baseline T-cells (both CD4 and CD8) and viremia at day 22 (rho -0.7080, p=0.01 and rho =-0.7251, p=0.0076, respectively). Baseline anti-measles titers either by ELISA or by neutralizing antibody assay were not associated with duration of viremia or level of viremia in either the full dataset or the smaller subset of patients treated at the higher levels, though this may be limited by the fact that the majority of patients had very low titers.
Response
There was one major hematologic response as has been reported previously.(20) Patient I.6.3, who was treated at TCID50 1011, achieved a complete response that persisted for 9 months; she had isolated relapse in her skull without recurrent marrow involvement. This lesion was irradiated, and she remained disease free for an additional 19 months thereafter at which time she had a second isolated relapse in her sternum. Four other patients had transient reductions in their serum immunoglobulin free light chain of at least 25% during the first 4 weeks of therapy (Figure 4), one of whom was treated with cyclophosphamide and MV-NIS at TCID50 of 9 × 107 and the remainder who were treated with single agent MV-NIS. One of these patients, (I.6.6) had subjective softening and shrinking of her extramedullary plasmacytomas of her back and thighs.
Figure 4.
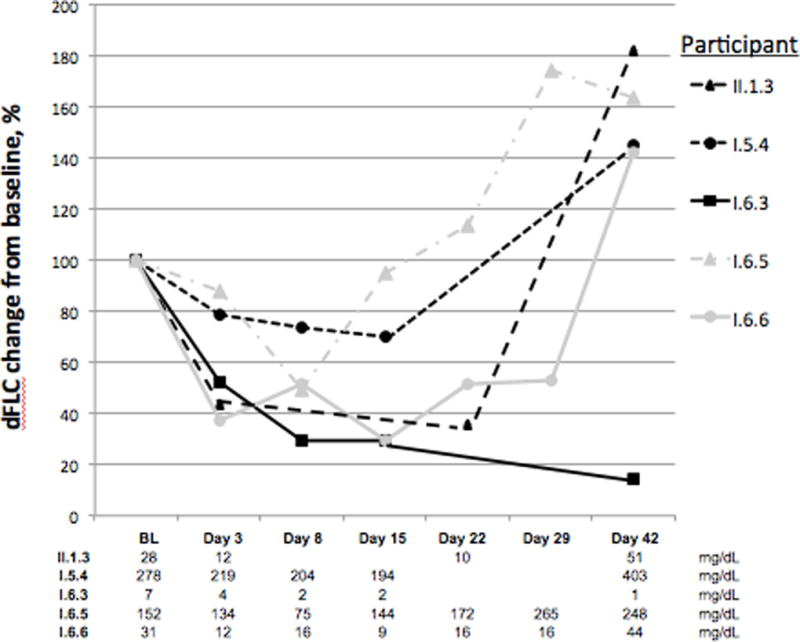
Reductions in difference of involved and uninvolved serum immunoglobulin free light chain (dFLC) among informative participants
Whether one focuses on the 11 patients treated at TCID501010 or higher or all 32 participants, those patients with a 25% dFLC reduction tended to have bone marrow plasma cells with lower levels of CD46 and higher levels of SLAM expression (Table 3). Surprisingly there was no difference in the baseline measles titers either by ELISA or by neutralizing antibodies. The ex vivo testing of measles infectivity at baseline was not informative in the FLC reduction group since only 1 patient had enough bone marrow plasma cells to perform the experiment (Patient I.5.4). His infectivity was 53%, which was comparable to that of the other 19 patients who had the assay performed but no reductions in FLC.
Table 3.
Comparisons of baseline values of patients with FLC reduction versus those without, median (range)
| Including only highest dose levels a | Including all dose levels | ||||
|---|---|---|---|---|---|
| 25% FLC reduction | No FLC reduction |
p | No FLC reduction | p b | |
| N | 4 | 7 | 27 | ||
| Baseline measles titer, Diamedex assay | 12 (6, 77.8) | 16.2 (3.2, 80) | NS | 13.95 (2.23, 90) | NS |
| Baseline measles titer, Neutralization assay | 10 (0, 20) | 10 (0, 80) | NS | 0 (0, 80) | NS |
| BMPC | 4.5 (1, 54) | 30 (4, 80) | NS | 40 (4, 100) | 0.04 |
| CD46 MFI of PC’s | 119 (44, 180) | 380 (192, 626) | 0.01 | 254(115, 626) | 0.01 |
| CD46/BMPC cell, (x1000) | 21.0 (0.06, 32.8) | 59.8 (17.4, 84.9) | 0.06 | 45.5 (15.1, 140.8) | 0.02 |
| CD46/ CD138- cells, (x1000) | 7.4 (1.0, 12.3) | 5.9 (5.5, 14.3) | NS | 101.1 (4.5, 14.3) | NS |
| SLAM, % | 49 (16, 100) | 9 (1, 57) | 0.03 | 7.35 (0.4, 97) | 0.05 |
| SLAM MFI BMPC | 38 (11, 135) | 37 (4, 76) | NS | 19.5 (3.1, 144) | NS |
| SLAM MFI CD138- cells | 6 (6, 16) | 8 (3, 10) | NS | 5.5 (2.4, 11) | NS |
| Day 15 N-gene in blood by Q-RT-PCR | 3,935 (0, 13039) | 2,928 (0, 18,400) | NS | 0 (0, 18,400) | 0.04 |
BMPC, bone marrow plasma cells; MFI, mean fluorescent intensity; BMPC, bone marrow plasma cells; NS, not significant
Stage I patients at TCID50 1010 and 1011
Comparator of “25% FLC reduction” group includes 5 patients, i.e. the 4 patients treated at 1011 TCID50 or higher and the one Stage II patient treated with CTX and only 107 TCID50
Correlative studies
There was no correlation between baseline CD46 density or SLAM density on the bone marrow plasma cells or non-plasma cells and ex vivo infectivity by MV nor was there any correlation between baseline CD46 density or SLAM density and persistent viremia at day 8 or 15. However, CD46 density was, in all cases, higher on the neoplastic plasma cells compared to non-plasma cells and this was correlated with their higher susceptibility to MV infection. There was a relationship between the quantity of CD8 (suppressor T cells) in the peripheral blood and the ex vivo infectivity of the CD138+ bone marrow cells (rho=0.6233, p=0.0044). None of the baseline characteristics (numbers of peripheral blood T-cells, B-cells, or NK cells) predicted for high viral titers at day 15 in the overall dataset except for PK at 60 minutes (rho 0.70, p=0.002).
Imaging
Of the 31 patients, 8 had some degree of 123I uptake on their SPECT/CT scans. Three were in the stage 2 group, i.e. they received CTX pre-MV-NIS. Four of the other five positive patients were treated at TCID50 1010 or higher. In most patients, not all lesions had uptake, and uptake was modest. An example of a 123I scan was seen in participant I.6.6 (Figure 5). We could identify no baseline features predicted for positive 123I-SPECT/CT scans.
Figure 5.
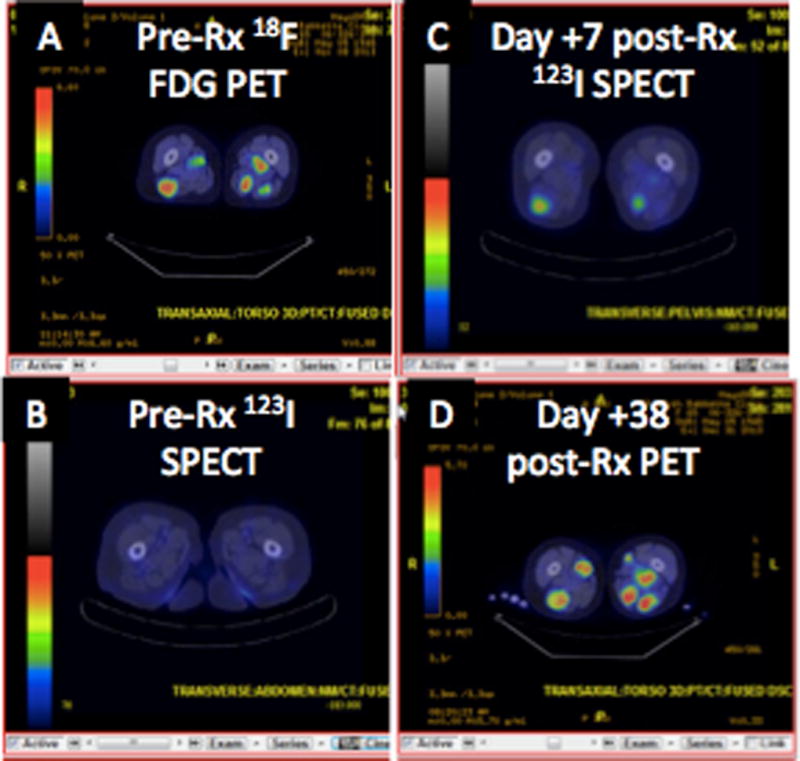
Nuclear imaging in representative patient
Representative scans of patient I.6.6
A. Baseline 18F-FDG PET scan showing subcutaneous plasmacytomas in legs
B. Baseline pre-treatment 123I SPECT/CT shows no iodine uptake
C. Day +7 post MV-NIS therapy shows 123I uptake in representative subcutanous plasmacytomas
D. Disease progression by day 38 as shown by 18F-FDG PET.
DISCUSSION
Herein, we describe a completed Phase 1 trial using a modified measles virus through an intravenous route for disseminated malignancy. We have demonstrated systemic administration of MV-NIS in patients with myeloma may be a safe and novel approach to salvage patients with multiply relapsed and refractory disease. Even at the highest doses, MV-NIS was reasonably well tolerated with the exception of significant infusion reactions (fever, chills, and gastrointestinal upset) proximal to the therapy. We found that infusion of TCID50 1011 was not practical over 30 minutes, predominantly due to headache, but is better tolerated with a less rapid infusion. The most significant AE’s at the phase 2 recommended dose level (TCID50 1011) were short-lived constitutional and gastrointestinal symptoms along with transient cytopenias.
We collected a large amount of data on our subjects, which is informative for the Phase 2 trial. First, there was a clear dose response in terms of higher blood stream levels achieved and more sustained viremia with higher doses infused. Viral shedding in the sputum and urine was typically limited to the first 8 to 15 days after therapy. In fact, among the 11 patients treated at TCID50 1010 or greater, there were 3 transient FLC responses and one durable hematological response. Second, undetectable measles titers are found in 58–68% of patients with multiply relapsed myeloma. Although the patient who had the dramatic hematologic response had undetectable measles virus titers and prolonged viremia, there was no clear pattern of negative baseline measles titers or persistent viremia among the other 4 patients with transient dFLC responses.
Neither ex vivo infectivity experiments nor CD46 expression on the bone marrow plasma cells predicted for transient response, levels of viremia, nor positive 123I scans. Any conclusions, however, about the predictive value of these correlates is limited by the small sample size of patients treated at the recommended phase II dose. These parameters will be explored further in the phase II.
The relative safety of this drug, the one dramatic response and the 4 transient responses make future study possible and hopefully fruitful. If single agent MV-NIS does not prove to be as effective as hoped, there are several potential strategies available. Preliminary data demonstrate that infecting mesenchymal cells with MV-NIS and using them as carriers allow the virus to evade the immune system more effectively.(21) Use of combination therapy with immune modulating drugs like high-dose cyclophosphamide or with immune check-point inhibitors is also another potential strategy to enhance efficacy of this novel agent.(17) Assuming one can achieve a more predictable and robust NIS mediated radioisotope enhancement, using therapeutic 131I along with the MN-NIS may also be an option for enhancing tumor kill.(5)
Supplementary Material
The design was a standard 3+3 dose escalation, first with single agent MV-NIS (Stage I) followed by a second cohort of patients who were to received pretreatment with immunosuppressive doses of cyclophosphamide 2 days before treatment with MV-NIS (Stage II). Due to novel and improved manufacturing processes, Stage II was suspended such that higher doses of single agent MV-NIS could be tested.
Ex vivo infectivity of CD138+ and CD138- by measles virus encoding enhanced green fluorescent protein (MV-eGFP) at MOI =1.0 for 2 hours at 37 °C. A. To demonstrate the MV-induced cytopathic effects, at the end of incubation period the virus was removed and the cells were maintained in 10% FBS-RPMI containing 1 ng/mL IL-6. At 24, 48, 72, and 96 hours after infection, the cells were photographed under blue light.
B. To quantitate the relative levels of viral infection per cell, at the end of incubation period, 12-well plates were seeded at 500,000 cells/well and maintained in the presence of fusion-inhibitory peptide. At 96 hours post infection flow cytometry was performed to ascertain the GFP expression per cell. CD46 expression per cell was quantitated using BD QuantiBRITE beads.
Acknowledgments
This work was supported by funds from the NIH/NCI (R01CA125614, R01CA168719, UL1 TR000135), Al and Mary Agnes McQuinn, the Siebens Foundation, the Richard M Schulze Family Foundation. The NCI (RAID Program) supported cGMP virus manufacture and toxicology/pharmacology studies.
Footnotes
DISCLOSURES
SJR Co-founder, CEO and Board Member of Vyriad. KWP Co-founder and CTO of Vyriad (Chief Technical Officer). MF Co-founder of Vyriad. All three also hold equity in Vyriad.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Vacchelli E, Eggermont A, Sautes-Fridman C, Galon J, Zitvogel L, Kroemer G, et al. Trial watch: Oncolytic viruses for cancer therapy. Oncoimmunology. 2013;2(6):e24612. doi: 10.4161/onci.24612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galanis E, Atherton PJ, Maurer MJ, Knutson KL, Dowdy SC, Cliby WA, et al. Oncolytic measles virus expressing the sodium iodide symporter to treat drug-resistant ovarian cancer. Cancer Res. 2015;75(1):22–30. doi: 10.1158/0008-5472.CAN-14-2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Myers RM, Greiner SM, Harvey ME, Griesmann G, Kuffel MJ, Buhrow SA, et al. Preclinical pharmacology and toxicology of intravenous MV-NIS, an oncolytic measles virus administered with or without cyclophosphamide. Clin Pharmacol Ther. 2007;82(6):700–10. doi: 10.1038/sj.clpt.6100409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dingli D, Peng KW, Harvey ME, Greipp PR, O'Connor MK, Cattaneo R, et al. Image-guided radiovirotherapy for multiple myeloma using a recombinant measles virus expressing the thyroidal sodium iodide symporter. Blood. 2004;103(5):1641–6. doi: 10.1182/blood-2003-07-2233. [DOI] [PubMed] [Google Scholar]
- 6.Jurianz K, Ziegler S, Garcia-Schuler H, Kraus S, Bohana-Kashtan O, Fishelson Z, et al. Complement resistance of tumor cells: basal and induced mechanisms. Mol Immunol. 1999;36(13–14):929–39. doi: 10.1016/s0161-5890(99)00115-7. [DOI] [PubMed] [Google Scholar]
- 7.Liszewski MK, Post TW, Atkinson JP. Membrane cofactor protein (MCP or CD46): newest member of the regulators of complement activation gene cluster. Annu Rev Immunol. 1991;9:431–55. doi: 10.1146/annurev.iy.09.040191.002243. [DOI] [PubMed] [Google Scholar]
- 8.Seya T, Matsumoto M, Hara T, Hatanaka M, Masaoka T, Akedo H. Distribution of C3-step regulatory proteins of the complement system, CD35 (CR1), CD46 (MCP), and CD55 (DAF), in hematological malignancies. Leuk Lymphoma. 1994;12(5–6):395–400. doi: 10.3109/10428199409073780. [DOI] [PubMed] [Google Scholar]
- 9.Dorig RE, Marcil A, Chopra A, Richardson CD. The human CD46 molecule is a receptor for measles virus (Edmonston strain) Cell. 1993;75(2):295–305. doi: 10.1016/0092-8674(93)80071-l. [DOI] [PubMed] [Google Scholar]
- 10.Nielsen L, Blixenkrone-Moller M, Thylstrup M, Hansen NJ, Bolt G. Adaptation of wild-type measles virus to CD46 receptor usage. Arch Virol. 2001;146(2):197–208. doi: 10.1007/s007050170169. [DOI] [PubMed] [Google Scholar]
- 11.Tatsuo H, Ono N, Tanaka K, Yanagi Y. SLAM (CDw150) is a cellular receptor for measles virus. Nature. 2000;406(6798):893–7. doi: 10.1038/35022579. [DOI] [PubMed] [Google Scholar]
- 12.Anderson BD, Nakamura T, Russell SJ, Peng KW. High CD46 receptor density determines preferential killing of tumor cells by oncolytic measles virus. Cancer Res. 2004;64(14):4919–26. doi: 10.1158/0008-5472.CAN-04-0884. [DOI] [PubMed] [Google Scholar]
- 13.Ong HT, Timm MM, Greipp PR, Witzig TE, Dispenzieri A, Russell SJ, et al. Oncolytic measles virus targets high CD46 expression on multiple myeloma cells. Exp Hematol. 2006;34(6):713–20. doi: 10.1016/j.exphem.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Bjerrum OW, Mansa B. Antibodies to common viruses in sera from patients with multiple myeloma. Acta Pathol Microbiol Immunol Scand [B] 1982;90(6):397–401. doi: 10.1111/j.1699-0463.1982.tb00137.x. [DOI] [PubMed] [Google Scholar]
- 15.Heath RB, Fairley GH, Malpas JS. Production of antibodies against viruses in leukemia and related diseases. British Journal of Haematology. 1964;10:365–70. doi: 10.1111/j.1365-2141.1964.tb00713.x. [DOI] [PubMed] [Google Scholar]
- 16.Moschella F, Proietti E, Capone I, Belardelli F. Combination strategies for enhancing the efficacy of immunotherapy in cancer patients. Ann N Y Acad Sci. 2010;1194:169–78. doi: 10.1111/j.1749-6632.2010.05464.x. [DOI] [PubMed] [Google Scholar]
- 17.Peng KW, Myers R, Greenslade A, Mader E, Greiner S, Federspiel MJ, et al. Using clinically approved cyclophosphamide regimens to control the humoral immune response to oncolytic viruses. Gene Ther. 2013;20(3):255–61. doi: 10.1038/gt.2012.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Durie BG, Harousseau JL, Miguel JS, Blade J, Barlogie B, Anderson K, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20(9):1467–73. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 19.Wong ML, Medrano JF. Real-time PCR for mRNA quantitation. Biotechniques. 2005;39(1):75–85. doi: 10.2144/05391RV01. [DOI] [PubMed] [Google Scholar]
- 20.Russell SJ, Federspiel MJ, Peng KW, Tong C, Dingli D, Morice WG, et al. Remission of disseminated cancer after systemic oncolytic virotherapy. Mayo Clin Proc. 2014;89(7):926–33. doi: 10.1016/j.mayocp.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ong HT, Federspiel MJ, Guo CM, Ooi LL, Russell SJ, Peng KW, et al. Systemically delivered measles virus-infected mesenchymal stem cells can evade host immunity to inhibit liver cancer growth. J Hepatol. 2013;59(5):999–1006. doi: 10.1016/j.jhep.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The design was a standard 3+3 dose escalation, first with single agent MV-NIS (Stage I) followed by a second cohort of patients who were to received pretreatment with immunosuppressive doses of cyclophosphamide 2 days before treatment with MV-NIS (Stage II). Due to novel and improved manufacturing processes, Stage II was suspended such that higher doses of single agent MV-NIS could be tested.
Ex vivo infectivity of CD138+ and CD138- by measles virus encoding enhanced green fluorescent protein (MV-eGFP) at MOI =1.0 for 2 hours at 37 °C. A. To demonstrate the MV-induced cytopathic effects, at the end of incubation period the virus was removed and the cells were maintained in 10% FBS-RPMI containing 1 ng/mL IL-6. At 24, 48, 72, and 96 hours after infection, the cells were photographed under blue light.
B. To quantitate the relative levels of viral infection per cell, at the end of incubation period, 12-well plates were seeded at 500,000 cells/well and maintained in the presence of fusion-inhibitory peptide. At 96 hours post infection flow cytometry was performed to ascertain the GFP expression per cell. CD46 expression per cell was quantitated using BD QuantiBRITE beads.


