Abstract
In 2005, two groups independently discovered that the G protein-coupled receptor GPR30 binds estradiol in cultured cells and, in response, initiates intracellular signaling cascades [69, 81]. GPR30 is now referred to as GPER, the G-protein coupled estrogen receptor [65]. While studies in animal models are illuminating GPER function, there is controversy as to whether GPER acts as an autonomous estrogen receptor in vivo, or whether GPER interacts with nuclear estrogen receptor signaling pathways in response to estrogens. Here, we review the evidence that GPER acts as an autonomous estrogen receptor in vivo and discuss experimental approaches to test this hypothesis directly. We propose that the degree to which GPER influences nuclear estrogen receptor signaling likely depends on cell type, developmental stage and pathology.
Estrogens exert diverse effects on organ systems throughout the body by binding to estrogen receptors. Estrogen receptors alpha and beta (ERα, ERβ), members of the nuclear receptor family [6, 16], were initially cloned and characterized as ligand-dependent transcription factors [22, 23]. Yet we now appreciate that the same nuclear estrogen receptor proteins and their alternatively spliced variants can also activate signaling pathways in the cytosol and at the plasma membrane, separate from their function as transcription factors [7, 13, 39, 51, 60]. Ligand bound ERs can associate with the plasma membrane [1, 47, 59, 61, 67] and influence kinase signaling cascades via direct interaction with Src [50, 51], B-Raf [73] and the p85α subunit of phosphatidylinositol-3-OH-kinase [72]. ERα can also interact directly with G proteins [35, 52, 87, 88] and with the integral membrane protein metabotropic glutamate receptor type 1a [13, 37]. Whereas ERα and ERβ function in the nucleus and at the plasma membrane, the G protein-coupled estrogen receptor (GPER/GPR30) represents a new class of estrogen receptor, an integral membrane protein restricted to cell membranes that cannot directly regulate gene expression [18, 65, 69, 81]. While GPER, unlike ERα and ERβ, is not a transcription factor and thus cannot directly regulate gene expression, GPER may regulate gene expression indirectly, by activating proteins that change transcription factor activity [3, 34, 56, 66].
MEMBRANE VS NUCLEAR ESTROGEN SIGNALING
ER splice variants in mammals
How do cells regulate ERα- and ERβ-dependent nuclear vs membrane associated signaling? This question remains an active area of inquiry. Alternative splicing of estrogen receptor genes has been demonstrated to influence membrane signaling. Alternative splicing of the human ESR1 gene results in multiple isoforms of ERα protein, where different isoforms differentially associate with cell membranes (Figure 1). The full-length protein encoded by the human ESR1 gene, hERα66, acts as a ligand-dependent transcription factor and, following post-translational modification, can anchor to the plasma membrane and activate signaling cascades [67, 68]. The alternatively spliced hERα46 protein lacks the N-terminal transactivation domain (AF-1 domain) [19] and is targeted to the plasma membrane of endothelial cells in a palmitoylation-dependent manner [39]. The alternatively spliced hERα36 protein lacks both N- and C-terminal transactivation domains (AF-1, AF-2 domains) [84] and is associated with the plasma membrane, where it activates MAPK pathway in a heterologous culture system (HEK293 cells) [85]. In heterologous cell culture systems, both hERα46 and hERα36 are less efficient transcription factors than hERα66 and are thought to predominantly function as membrane-associated estrogen receptors [39, 85]. When overexpressed in cultured HEK293 cells, hERα36 can act as a dominant negative and inhibit hERα66-and ERβ-dependent transcription in the nucleus [85]. ERα36 and ERα46 splice variants are expressed in mice and rats [29, 42], but their function is not well understood (Figure 1).
Figure 1. Estrogen receptor alpha (ERα) splice variants.
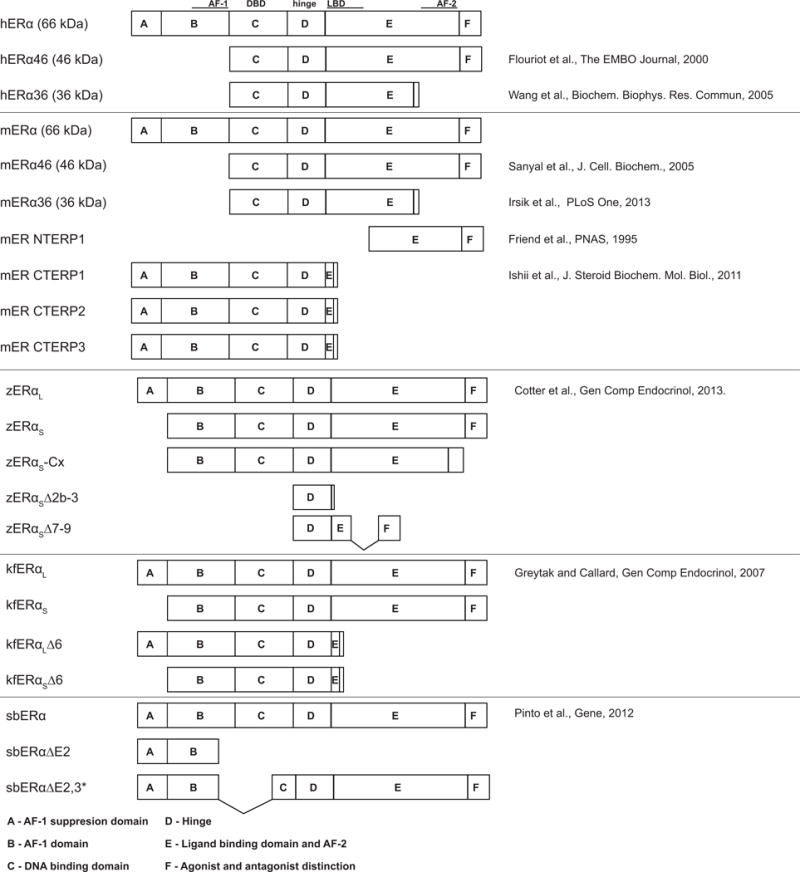
Depicted protein structures of full length and spice variants in human (h), mouse (m), zebrafish (z), killifish (k) and sea bream (sb). Functional regions (based on Krust et al, EMBO J 1986): AF-1, activating function 1 domain; DBD, DNA binding domain; LBD, ligand binding domain; AF-2, activating function 2 domain. Blank regions indicate amino acid sequence unique to the splice variant, except for kfERαL∆6 and kfERαS∆6 where the sequence following the E domain is identical. NTERP, N terminally truncated ER protein; CTERP, C terminally truncated ER protein.
In contrast to nuclear estrogen receptors that are alternatively spliced and signal in the nucleus and at cell membranes, GPER is a seven transmembrane GPCR with no known alternatively spliced isoforms. GPER has been detected at the plasma membrane and at intracellular membranes, such as the endoplasmic reticulum [2, 8, 9, 17, 20, 46, 62, 69, 86].
ER splice variants in teleosts
As in humans, many teleost fish species express alternatively spliced variants of ERα, including sea bream [64], killifish [24] and zebrafish [10] (Figure 1). Though six splice variants have been identified in zebrafish, none appear orthologous to the two physiologically relevant human ERα splice variants hERα36 and hERα46. Additionally, no functional role has been identified yet for any ERα splice variant in zebrafish. Thus, it is not known whether zebrafish ERα splice variants preferentially signal at the cytosol or cell membranes.
GPER signaling in teleosts
GPER mediates oocyte maturation in teleosts [44, 57, 58, 63]. Oocytes mature in the presence of progestins, while estrogens block maturation. In oocytes from Atlantic croaker (Micropogonias undulates), common carp (Cyprinus carpio) and zebrafish (Danio rerio), the GPER agonist G1 blocked progestin-induced maturation [44, 57]. In the absence of exogenous progestins, G1 reduced spontaneous maturation of croaker and zebrafish oocytes [57], while the GPER antagonist G15 increased spontaneous maturation of carp and zebrafish oocytes [44, 63], suggesting that estrogens act via GPER to block oocyte maturation. In zebrafish oocytes, G15 blocked the inhibitory effects of estradiol. Additionally, reducing GPER function using either anti-sense morpholino oligonucleotides or pre-treatment with GPER antibodies blocked the inhibitory effects of estradiol [57, 63]. Knockdown of ERα did not alter the response to estradiol, suggesting that estradiol acts via GPER and not via ERα to block oocyte maturation [58]. However, the involvement of ERβ, or of alternatively spliced variants of ERα, has not been explored.
GPER signaling in mammals
Several groups have independently generated GPER mutant mice. Disruption of the first two transmembrane domains of the GPER gene by insertion of a lacZ reporter gene resulted in mice with no gross changes in fat mass, growth or reproductive ability [30]. Disrupting the GPER gene with a neomycin resistance cassette resulted in mice that were viable and fertile with no gross abnormalities in physical, immunological, or neurological development [83]. However, Haas et al. later reported that these mice exhibited increased body weight and abdominal fat compared to wildtype [25]. Two groups independently deleted the complete GPER open reading frame using Cre-mediated recombination: one by deleting the entire open reading frame [45], the other by deleting exon 3 entirely (open reading frame plus additional untranslated regions) [55]. The former mutant mice exhibited hyperglycemia, impaired glucose tolerance, reduced body growth, and increased mean arterial blood pressure associated with decreased insulin production in female mice [45], while the latter mutant mice exhibited no abnormalities in blood pressure, growth, fertility or expression of estrogen responsive genes [55]. Subsequent studies have identified potential roles for GPER, but few have utilized the available ERα and ERβ mutant mice, together with GPER, to directly test whether GPER and ERs interact in a number of physiological processes. Additionally, double and triple combination mutants would help further our understanding of these complex interactions between estrogen receptors.
EVIDENCE FOR GPER INTERACTING WITH ER SIGNALING
GPER activity leads to increased expression of ERα36
Evidence that GPER upregulates hERα36 expression comes from studies in cultured cells. In HEK293 and COS7 cells lacking endogenous GPER and hERα36 expression, overexpression of GPER increased expression of hERα36 but not hERα66 [34]. Conversely, in the SK-BR-3 breast cancer cell line, knockdown of GPER reduced hERα36 expression, while knockdown of hERα36 had no effect on GPER expression [34]. These results suggest that under certain contexts, GPER can upregulate ERα expression.
Note that these results do not exclude GPER acting as an autonomous estrogen receptor. Temporal differences in GPER activity could explain how GPER can act autonomously and influence ER expression in the same cells. Immediately following GPER activation, GPER may activate G proteins and other signaling proteins independently of ERα. Such downstream signaling pathways may ultimately upregulate hERα36, but this may occur hours following initial GPER activity.
GPER and ERα proteins interact
Two independent studies provide evidence for a physical association between GPER and ERα proteins in human primary monocytes (hPM) and in a human endometrial adenocarcinoma cell line (Ishikawa cells) [62, 82]. Derived from normal men or premenopausal women, hPMs express hERα36 and GPER but not hERα66 or ERβ [62]. Exposure to lipopolysaccharides (LPS) induces expression of pro-inflammatory genes IL-6 and TNF-α, and this effect is blocked by pre-incubation with estradiol [62]. Interestingly, both ICI182,780 (ICI), an ER antagonist/GPER agonist, and G15, a GPER antagonist, block the effects of estradiol. If ICI is acting predominantly as a GPER agonist in this situation, then it is unclear how opposing actions on GPER – either inhibiting GPER with G15 or activating GPER with ICI — could block the effects of estradiol. Alternatively, if ICI is acting predominantly as an ER antagonist, then this suggests that both hERα36 and GPER are required for estradiol to block LPS-dependent IL-6 and TNF-α expression. To explore this further, the authors found that G1 pretreatment (GPER agonist) failed to reduce LPS-dependent IL-6 expression, suggesting that ICI182,780 acts predominantly as an ER antagonist rather than as a GPER agonist in this context [62]. These results also suggest that both hERα36 and GPER must be activated to block LPS-dependent IL-6 expression.
To test the hypothesis that hERα36 and GPER are physically associated to regulate the anti-inflammatory response in hPMs, Pelekanou and colleagues used proximity ligation assay, a histological method to visualize protein colocalization [75], and found that LPS treatment caused an association between hERα36 and GPER [62]. Additionally, hERα36 and GPER were associated with each other in the area of atherosclerosis plaques from human samples with coronary artery disease [62], suggesting that hERα36 and GPER inhabit the same membrane complex in vivo.
In Ishikawa cells, estradiol increases proliferation. Estradiol-dependent proliferation was blocked following silencing of GPR30 or ERα by RNA interference [82]. This suggests that both proteins are involved, but whether they interact or are parts of two independent pathways was not known. To test for a direct interaction, Vivacqua and colleagues used co-immunoprecipitation and found that ERα and GPER interact directly [82], supporting the hypothesis that GPER and ERα interact to regulate proliferation in Ishikawa cells.
Using the BG-1 ovarian cancer cell line, Albanito and colleagues demonstrated that both ERα and GPER are required for estradiol-dependent proliferation [3]. In BG-1 cells, estradiol treatment upregulates c-fos expression, increases levels of phosphorylated ERK1/2 and cell proliferation. These effects were blocked by antisense oligonucleotides targeting either ERα or GPER, demonstrating that ERα and GPER are necessary for estradiol-dependent increase in cfos, phosphorylated-ERK1/2 and proliferation, similar to what was reported in Ishikawa cells [82]. In contrast to studies in Ishikawa and hPM cells, it is not known whether ERα and GPER interact in BG-1 cells.
EVIDENCE FOR GPER ACTING AUTONOMOUSLY
GPER activity influences physiology throughout the body, including metabolic functions [4, 36, 71] and reproductive [11, 21] and cardiovascular systems [32, 40, 41, 48, 49]. A majority of these studies rely solely on pharmacologic approaches to implicate GPER signaling. Pharmacologic approaches, while useful, are limited by non-specific effects. For example, G-1, developed as a selective GPER agonist that would not activate nuclear estrogen receptors [5], was later shown to influence nuclear estrogen receptor activity [74]. Without complementary genetic approaches, such as the use of estrogen receptor mutant animals or cell lines, it is difficult to investigate the degree to which GPER acts autonomously.
Comparing phenotypes among estrogen receptor deficient animals
The use of targeted, loss-of-function mutations in estrogen receptor genes is a powerful approach for identifying the function of estrogen receptors in animals. If estradiol administration causes a phenotype in wildtype animals and in animals deficient in ERα or ERβ, but estradiol administration fails to cause the same phenotype in animals deficient in GPER, then this suggests that estradiol acts via GPER but independently of nuclear estrogen receptors. This paradigm was tested using the cardiac ischemia-reperfusion injury model: ischemia causes cardiac damage, but estradiol administration reduces cardiac damage following ischemia [26, 53, 76]. To identify the receptor required for estradiol’s protective effects, Kabir and colleagues subjected hearts from male mutant mice lacking either GPER, ERα or ERβ to ischemia-reperfusion injury in the presence of estradiol or vehicle. Estradiol treatment protected wildtype and ERα and ERβ mutant mice from injury, but had no effect on GPER mutant mice [33]. These results indicate that GPER, but not nuclear estrogen receptors, are required for estradiol-dependent protection of cardiac injury following ischemia in male mice. Furthermore, these results also support the hypothesis that GPER acts as an autonomous estrogen receptor in vivo.
Crucially, Kabir and colleagues used ERα knockout mice that have no detectable expression of ERα splice variants ERα36 and ERα46 [14], splice variants that encode membrane-associated ERα proteins [39, 84, 85]. Thus, it is difficult to argue that the protective effects of estradiol following cardiac ischemia are due to membrane-associated ERα signaling.
A second example comes from zebrafish embryos. Acute exposure to estradiol increased heart rate in zebrafish embryos. This effect was absent in GPER mutant zebrafish. In contrast, estradiol exposure increased heart rate in ERα and ERβ mutant embryos [Romano & Gorelick, bioRxiv preprint doi: https://doi.org/10.1101/088955], demonstrating that GPER, but not nuclear estrogen receptors, are required for estradiol-dependent increase of heart rate in zebrafish embryos.
Ethinyl estradiol (EE2) can reduce severity of established disease in experimental autoimmune encephalomyelitis (EAE), an animal model of multiple sclerosis, when administered following the onset of disease symptoms [80]. EE2 treatment reduced disease severity in ERα −/− mice, but was not effective in GPER −/− mice [89], suggesting that EE2 acts via GPER, not ERα, to improve EAE symptoms. This protective effect was associated with increased production of the anti-inflammatory cytokine IL-10. Following treatment with EE2, IL-10 was increased in both wildtype and ERα −/− mice, but not in GPER −/− mice [89]. Though the complete story of how GPER mediates immune function is still unclear, this study suggests that GPER functions autonomously to increase IL-10 production and improves disease outcome in EAE. Note that ERβ function was not explored in the context of EE2-induced reduction in EAE severity, thus it is possible that GPER and ERβ interact, or influence parallel pathways, to reduce EAE symptoms. To make matters more complex, the preceding model examined estrogen ability to reduce symptoms in established EAE. Other studies using genetic approaches have shown that estrogens, when administered prior to EAE onset, exhibit neuroprotective effects and that this is dependent on ERα [77, 78]. The degree to which GPER is involved in neuroprotection of EAE onset is not well understood.
GPER and ER signaling influence parallel signaling pathways to achieve the same result
Two examples of GPER and ER acting in parallel signaling pathways to achieve the same result come from studies investigating apoptosis in two distinct cell types: thymocytes and islet cells. Prolonged treatment with E2 can lead to thymic atrophy. This effect can only be partially attributed to ERα signaling [79] and subsequent studies identified an insignificant contribution from ERβ [15]. Using a genetic approach, E2-induced thymic atrophy was attenuated in both ERα −/− mice and GPER −/− mice [83]. However, further investigation demonstrated that ERα activity blocked thymocyte maturation, which triggered cell death, whereas GPER activity induced mature thymocytes to undergo apoptosis [83]. Consistent with these results, the GPER agonist G-1 induced thymic atrophy via apoptosis but did not block thymocyte maturation [83]. Together, these results indicate that GPER acts autonomously to induce thymocyte apoptosis, while ERα acts to block thymocyte maturation. Activation of either receptor, potentially acting via independent pathways, leads to thymic atrophy.
Treating mice with streptozotocin (STZ) causes pancreatic islet cell apoptosis and is commonly used to generate animal models of type 1 diabetes [38]. Estradiol (E2) can protect islet cells from STZ-dependent apoptosis, however this protection is only partially mediated by nuclear estrogen receptors. In ERα −/− or ERβ −/− mice, STZ + E2 exhibited partial protection of pancreatic β-cell apoptosis compared to STZ + E2 in wildtype mice [38], suggesting that multiple estrogen receptors cooperate to mediate the protective effects of E2 in the pancreas. Consistent with this idea, in ERα/ERβ double knockout mice, E2 only partially protected islet cells from STZ-dependent apoptosis [43], implicating a third estrogen receptor, likely to be GPER. E2 failed to protect GPER deficient female mice from STZ-dependent apoptosis [43]. Further, G1 protected cultured wildtype islet cells from STZ-induced oxidative stress [43]. Islet cells cultured from GPER mutant mice were no longer protected by G1, but the protective effects of E2 were still present in GPER deficient islets [43]. These results suggest that ERα, ERβ and GPER respond to E2 to protect pancreatic islet cells from oxidative stress, but that GPER plays the dominant role. It is possible that GPER acts via a separate, independent pathway from ERα and ERβ to protect islet cells from STZ-dependent apoptosis. This idea is supported by the fact that in ERα/ERβ double knockout mice, E2 is only partially protective.
Comparing gene expression following differential estrogen receptor activity
Notas and colleagues used cDNA microarrays to compare gene expression following pharmacologic inhibition of ERα versus GPER in multiple cells lines [54]. Identifying transcripts uniquely regulated by GPER would support the hypothesis that GPER can act independently of ERα. Cell lines were incubated with a membrane-impermeable estradiol-BSA conjugate (E2-BSA), in the presence or absence of ICI182,780 (ER antagonist/GPER agonist) or G15 (GPER antagonist). In T47D and SKBR3 cell lines, which express both GPER and hERα36, 17 out of 403 transcripts (4%) and 33 out of 393 transcripts (8%) were significantly and uniquely inhibited by G15. These transcripts were upregulated at least 1.5x by E2-BSA and downregulated by E2-BSA + G15 but not downregulated by E2-BSA + ICI182,780. The majority of transcripts were inhibited by both ICI and G15, suggesting some interplay between GPER and ERα. However, it is important to note that the presence of transcripts uniquely downregulated by G15 suggests that GPER can act as an autonomous estrogen receptor. The fact that this response is different between different tumor cell lines supports the idea that the degree to which GPER acts autonomously of ERα depends on the cell type.
This genomic approach has several limitations. G15 may also have some activity against ERα [12], meaning that the few transcripts uniquely regulated by G15 could be GPER-independent. The use of microarrays to compare gene expression is more biased than using RNAseq, which has the potential to reveal not only additional differentially expressed genes but also differences in expression of noncoding RNAs. Ultimately, comparing gene expression among ER and GPER mutant cells may be a powerful approach to identify genes uniquely regulated by a specific estrogen receptor.
Is cross-talk between GPER and ER signaling influenced by cell type, developmental stage or pathology?
Evidence from knockout mice and cultured cells suggests that GPER can act as an autonomous receptor and can also interact with nuclear estrogen receptors (Figure 2). However, the degree to which GPER acts autonomously likely depends on cell type, differentiation status and pathology, i.e. whether the cell is quiescent, proliferative or cancerous.
Figure 2. Models for autonomous GPER signaling versus GPER-ER signaling interactions.
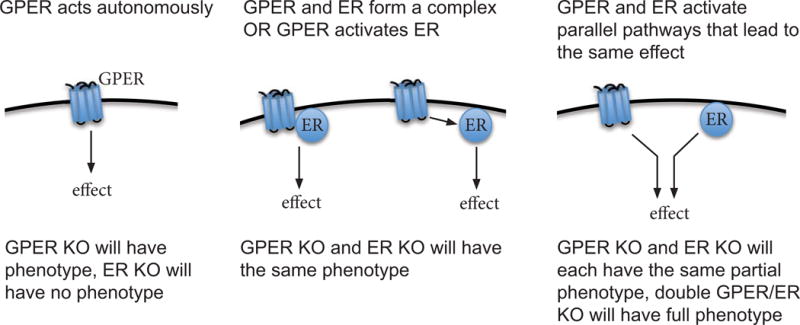
KO, knockout animal; GPER, G protein-coupled estrogen receptor; ER, nuclear estrogen receptor alpha or beta
Comparing the function of GPER in different cancer cell lines, we see that GPER requires ERα for the estradiol-dependent proliferation of ovarian cancer cells (BG-1) and endometrial adenocarcinoma cells (Ishikawa)[82], but not to induce cell proliferation in breast cancer cells (SKBR3) [3]. This suggests that GPER/ER interactions and crosstalk may depend on the cellular context of the tumor or cell type. In contrast, GPER signals autonomously to induce apoptosis in thymocytes [83] and to protect pancreatic islet cells from oxidative stress-induced apoptosis [38], both in vivo and in cultured cells. This suggests that GPER acts autonomously in different cell types, and that GPER can act to induce opposite effects depending on the cell type in which it is acting. In these examples, nuclear estrogen receptors activated signaling in a parallel pathway to achieve a similar effect. This indicates that GPER and nuclear ERs are sometimes present in the same cell or tissue type, yet signal independently of each other.
FUTURE DIRECTIONS: MERGING PHARMACOLOGIC AND GENETIC APPROACHES
With existing pharmacologic and genetic tools, it is possible to investigate the degree to which GPER acts autonomously of nuclear estrogen receptors in vivo. Comparing phenotypes among ER- and GPER-deficient mice, as Kabir and colleagues did for cardiac ischemia [33], is a powerful approach that can be applied to any phenotype of interest exhibited by GPER-deficient mice. To control for genetic compensation, one could compare phenotypes between GPER-deficient mice [45] and mice with homozygous mutations in both nuclear estrogen receptors alpha and beta [14]. Additionally, because estrogen signaling is conserved among vertebrates, many of these approaches could be carried out in zebrafish, where CRISPR-Cas technology allows rapid and straightforward generation of embryos with multiple loss-of-function mutations [31]. Comparing gene expression between wildtype, GPER mutant, ERα mutants, ERβ mutants and compound mutant animals will be a powerful approach to identify genes regulated by specific estrogen receptors in vivo and to identify the degree to which GPER and nuclear ER signaling overlap.
Small molecules that specifically target GPER, with low or no affinity for nuclear estrogen receptors, can be used to identify genes specifically regulated by GPER in cultured cells, isolated tissues and whole animals. Pharmacologic approaches avoid genetic compensation, which can be induced by deleterious mutations but are less likely to be induced by gene knockdown or temporary perturbation of receptor activity [70]. Comparing gene expression in a whole animal, tissue or cell type of interest following exposure to GPER-, ERα- or ERβ-specific agonists (such as G1, PPT and DPN [5, 28]) could provide important insights into GPER- versus ERα- versus ERβ-dependent gene expression. For example, administering such chemicals to different groups of mice, and then comparing gene expression in the liver, would generate an interesting list of candidate genes regulated by specific estrogen receptor types in vivo. Varying the dose and duration of treatment could be used to infer whether gene expression changes are regulated by a specific estrogen receptor directly or indirectly. Such gene expression studies could be performed on multiple different organisms in parallel, to compare the degree to which estrogen receptor regulate unique or overlapping genes in different species [27].
Pharmacologic approaches are limited by non-specific effects. However, non-specific effects could be controlled by combining pharmacologic and genetic approaches. In the previous example, a specific GPER agonist, such as G1, could be administered to wildtype and to GPER mutant animals. By comparing gene expression in the liver between wildtype and GPER-deficient animals following G1 exposure, it should be possible to identify genes non-specifically regulated by G1 and exclude them from future analyses. Analogous approaches could be used to identify genes non-specifically regulated by ERα- and ERβ-specific agonists and antagonists.
Biochemistry and cell biology should also be used to explore associations between GPER and ER proteins in vivo. Immunoprecipitation approaches can be used to test GPER and ER interactions in vivo, while newer histologic methods, such as proximity ligation, can be used to detect small amounts of GPER and ER proteins in fixed tissue and determine whether they interact. While these approaches were used to show GPER-ERα interactions in human primary monocytes and in cultured Ishikawa cells [62, 82], there are many more cell types that express both GPER and nuclear estrogen receptors that have yet to be assayed for interactions among estrogen receptors. It will be interesting to explore how cell type and ligand regulate interactions between GPER and nuclear estrogen receptors.
Highlights.
GPER acts as an autonomous estrogen receptor in vivo
GPER also interacts with nuclear estrogen receptors
Whether GPER influences nuclear estrogen receptor signaling may depend on cell type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Acconcia F, Ascenzi P, Fabozzi G, Visca P, Marino M. S-palmitoylation modulates human estrogen receptor-alpha functions. Biochemical and biophysical research communications. 2004;316:878–883. doi: 10.1016/j.bbrc.2004.02.129. [DOI] [PubMed] [Google Scholar]
- 2.Akama KT, Thompson LI, Milner TA, McEwen BS. Post-synaptic density-95 (PSD-95) binding capacity of G-protein-coupled receptor 30 (GPR30), an estrogen receptor that can be identified in hippocampal dendritic spines. J Biol Chem. 2013;288:6438–6450. doi: 10.1074/jbc.M112.412478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albanito L, Madeo A, Lappano R, Vivacqua A, Rago V, Carpino A, et al. G Protein-Coupled Receptor 30 (GPR30) Mediates Gene Expression Changes and Growth Response to 17β-Estradiol and Selective GPR30 Ligand G-1 in Ovarian Cancer Cells. Cancer Research. 2007;67:1859–1866. doi: 10.1158/0008-5472.CAN-06-2909. [DOI] [PubMed] [Google Scholar]
- 4.Balhuizen A, Kumar R, Amisten S, Lundquist I, Salehi A. Activation of G protein-coupled receptor 30 modulates hormone secretion and counteracts cytokine-induced apoptosis in pancreatic islets of female mice. Molecular and cellular endocrinology. 2010;320:16–24. doi: 10.1016/j.mce.2010.01.030. [DOI] [PubMed] [Google Scholar]
- 5.Bologa CG, Revankar CM, Young SM, Edwards BS, Arterburn JB, Kiselyov AS, et al. Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat Chem Biol. 2006;2:207–212. doi: 10.1038/nchembio775. [DOI] [PubMed] [Google Scholar]
- 6.Chambon P. How I became one of the fathers of a superfamily. Nat Med. 2004;10:1027–1031. doi: 10.1038/nm1004-1027. [DOI] [PubMed] [Google Scholar]
- 7.Chen Z, Yuhanna IS, Galcheva-Gargova Z, Karas RH, Mendelsohn ME, Shaul PW. Estrogen receptor alpha mediates the nongenomic activation of endothelial nitric oxide synthase by estrogen. The Journal of clinical investigation. 1999;103:401–406. doi: 10.1172/JCI5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng SB, Graeber CT, Quinn JA, Filardo EJ. Retrograde transport of the transmembrane estrogen receptor, G-protein-coupled-receptor-30 (GPR30/GPER) from the plasma membrane towards the nucleus. Steroids. 2011;76:892–896. doi: 10.1016/j.steroids.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 9.Cheng SB, Quinn JA, Graeber CT, Filardo EJ. Down-modulation of the G-protein-coupled estrogen receptor, GPER, from the cell surface occurs via a trans-Golgi-proteasome pathway. J Biol Chem. 2011;286:22441–22455. doi: 10.1074/jbc.M111.224071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cotter KA, Yershov A, Novillo A, Callard GV. Multiple structurally distinct ERalpha mRNA variants in zebrafish are differentially expressed by tissue type, stage of development and estrogen exposure. Gen Comp Endocrinol. 2013;194:217–229. doi: 10.1016/j.ygcen.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Das SK, Tan J, Raja S, Halder J, Paria BC, Dey SK. Estrogen targets genes involved in protein processing, calcium homeostasis, and Wnt signaling in the mouse uterus independent of estrogen receptor-alpha and -beta. The Journal of biological chemistry. 2000;275:28834–28842. doi: 10.1074/jbc.M003827200. [DOI] [PubMed] [Google Scholar]
- 12.Dennis MK, Field AS, Burai R, Ramesh C, Petrie WK, Bologa CG, et al. Identification of a GPER/GPR30 antagonist with improved estrogen receptor counterselectivity. The Journal of steroid biochemistry and molecular biology. 2011;127:358–366. doi: 10.1016/j.jsbmb.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dewing P, Boulware MI, Sinchak K, Christensen A, Mermelstein PG, Micevych P. Membrane estrogen receptor-alpha interactions with metabotropic glutamate receptor 1a modulate female sexual receptivity in rats. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:9294–9300. doi: 10.1523/JNEUROSCI.0592-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M. Effect of single and compound knockouts of estrogen receptors alpha (ERalpha) and beta (ERbeta) on mouse reproductive phenotypes. Development. 2000;127:4277–4291. doi: 10.1242/dev.127.19.4277. [DOI] [PubMed] [Google Scholar]
- 15.Erlandsson MC, Ohlsson C, Gustafsson JA, Carlsten H. Role of oestrogen receptors alpha and beta in immune organ development and in oestrogen-mediated effects on thymus. Immunology. 2001;103:17–25. doi: 10.1046/j.1365-2567.2001.01212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evans R. A transcriptional basis for physiology. Nat Med. 2004;10:1022–1026. doi: 10.1038/nm1004-1022. [DOI] [PubMed] [Google Scholar]
- 17.Filardo E, Quinn J, Pang Y, Graeber C, Shaw S, Dong J, et al. Activation of the novel estrogen receptor G protein-coupled receptor 30 (GPR30) at the plasma membrane. Endocrinology. 2007;148:3236–3245. doi: 10.1210/en.2006-1605. [DOI] [PubMed] [Google Scholar]
- 18.Filardo EJ, Quinn JA, Bland KI, Frackelton AR. Estrogen-Induced Activation of Erk-1 and Erk-2 Requires the G Protein-Coupled Receptor Homolog, GPR30, and Occurs via Trans-Activation of the Epidermal Growth Factor Receptor through Release of HB-EGF. Mol Endocrinol. 2000;14:1649–1660. doi: 10.1210/mend.14.10.0532. [DOI] [PubMed] [Google Scholar]
- 19.Flouriot G, Brand H, Denger S, Metivier R, Kos M, Reid G, et al. Identification of a new isoform of the human estrogen receptor-alpha (hERα) that is encoded by disInct transcripts and that is able to repress hERα acIvaIon funcIon 1. The EMBO Journal. 2000;19:4688. doi: 10.1093/emboj/19.17.4688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Funakoshi T, Yanai A, Shinoda K, Kawano MM, Mizukami Y. G protein-coupled receptor 30 is an estrogen receptor in the plasma membrane. Biochemical and biophysical research communications. 2006;346:904–910. doi: 10.1016/j.bbrc.2006.05.191. [DOI] [PubMed] [Google Scholar]
- 21.Gao F, Ma X, Ostmann AB, Das SK. GPR30 activation opposes estrogen-dependent uterine growth via inhibition of stromal ERK1/2 and estrogen receptor alpha (ERalpha) phosphorylation signals. Endocrinology. 2011;152:1434–1447. doi: 10.1210/en.2010-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Green S, Walter P, Kumar V, Krust A, Bornert JM, Argos P, et al. Human oestrogen receptor cDNA: sequence, expression and homology to v-erb-A. Nature. 1986;320:134–139. doi: 10.1038/320134a0. [DOI] [PubMed] [Google Scholar]
- 23.Greene GL, Gilna P, Waterfield M, Baker A, Hort Y, Shine J. Sequence and expression of human estrogen receptor complementary DNA. Science. 1986;231:1150–1154. doi: 10.1126/science.3753802. [DOI] [PubMed] [Google Scholar]
- 24.Greytak SR, Callard GV. Cloning of three estrogen receptors (ER) from killifish (Fundulus heteroclitus): differences in populations from polluted and reference environments. Gen Comp Endocrinol. 2007;150:174–188. doi: 10.1016/j.ygcen.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 25.Haas E, Bhattacharya I, Brailoiu E, Damjanovic M, Brailoiu GC, Gao X, et al. Regulatory role of G protein-coupled estrogen receptor for vascular function and obesity. Circ Res. 2009;104:288–291. doi: 10.1161/CIRCRESAHA.108.190892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hale SL, Birnbaum Y, Kloner RA. beta-Estradiol, but not alpha-estradiol, reduced myocardial necrosis in rabbits after ischemia and reperfusion. American heart journal. 1996;132:258–262. doi: 10.1016/s0002-8703(96)90419-6. [DOI] [PubMed] [Google Scholar]
- 27.Hao R, Bondesson M, Singh AV, Riu A, McCollum CW, Knudsen TB, et al. Identification of Estrogen Target Genes during Zebrafish Embryonic Development through Transcriptomic Analysis. PLoS ONE. 2013;8:e79020. doi: 10.1371/journal.pone.0079020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harrington WR, Sheng S, Barnett DH, Petz LN, Katzenellenbogen JA, Katzenellenbogen BS. Activities of estrogen receptor alpha- and beta-selective ligands at diverse estrogen responsive gene sites mediating transactivation or transrepression. Mol Cell Endocrinol. 2003;206:13–22. doi: 10.1016/s0303-7207(03)00255-7. [DOI] [PubMed] [Google Scholar]
- 29.Irsik DL, Carmines PK, Lane PH. Classical estrogen receptors and ERalpha splice variants in the mouse. PLoS One. 2013;8:e70926. doi: 10.1371/journal.pone.0070926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Isensee J, Meoli L, Zazzu V, Nabzdyk C, Witt H, Soewarto D, et al. Expression pattern of G protein-coupled receptor 30 in LacZ reporter mice. Endocrinology. 2009;150:1722–1730. doi: 10.1210/en.2008-1488. [DOI] [PubMed] [Google Scholar]
- 31.Jao LE, Wente SR, Chen W. Efficient multiplex biallelic zebrafish genome editing using a CRISPR nuclease system. Proc Natl Acad Sci U S A. 2013;110:13904–13909. doi: 10.1073/pnas.1308335110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jessup JA, Lindsey SH, Wang H, Chappell MC, Groban L. Attenuation of salt-induced cardiac remodeling and diastolic dysfunction by the GPER agonist G-1 in female mRen2.Lewis rats. PloS one. 2010;5:e15433. doi: 10.1371/journal.pone.0015433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kabir ME, Singh H, Lu R, Olde B, Leeb-Lundberg LMF, Bopassa JC. G Protein-Coupled Estrogen Receptor 1 Mediates Acute Estrogen-Induced Cardioprotection via MEK/ERK/GSK-3Î2 Pathway after Ischemia/Reperfusion. PLoS ONE. 2015;10:e0135988. doi: 10.1371/journal.pone.0135988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kang L, Zhang X, Xie Y, Tu Y, Wang D, Liu Z, et al. Involvement of estrogen receptor variant ER-alpha36, not GPR30, in nongenomic estrogen signaling. Mol Endocrinol. 2010;24:709–721. doi: 10.1210/me.2009-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar P, Wu Q, Chambliss KL, Yuhanna IS, Mumby SM, Mineo C, et al. Direct interactions with G alpha i and G betagamma mediate nongenomic signaling by estrogen receptor alpha. Mol Endocrinol. 2007;21:1370–1380. doi: 10.1210/me.2006-0360. [DOI] [PubMed] [Google Scholar]
- 36.Kumar R, Balhuizen A, Amisten S, Lundquist I, Salehi A. Insulinotropic and antidiabetic effects of 17beta-estradiol and the GPR30 agonist G-1 on human pancreatic islets. Endocrinology. 2011;152:2568–2579. doi: 10.1210/en.2010-1361. [DOI] [PubMed] [Google Scholar]
- 37.Kuo J, Hariri OR, Bondar G, Ogi J, Micevych P. Membrane estrogen receptor-alpha interacts with metabotropic glutamate receptor type 1a to mobilize intracellular calcium in hypothalamic astrocytes. Endocrinology. 2009;150:1369–1376. doi: 10.1210/en.2008-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Le May C, Chu K, Hu M, Ortega CS, Simpson ER, Korach KS, et al. Estrogens protect pancreatic beta-cells from apoptosis and prevent insulin-deficient diabetes mellitus in mice. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:9232–9237. doi: 10.1073/pnas.0602956103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li L, Haynes MP, Bender JR. Plasma membrane localization and function of the estrogen receptor α variant (ER46) in human endothelial cells. Proceedings of the National Academy of Sciences. 2003;100:4807–4812. doi: 10.1073/pnas.0831079100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lindsey SH, da Silva AS, Silva MS, Chappell MC. Reduced vasorelaxation to estradiol and G-1 in aged female and adult male rats is associated with GPR30 downregulation. American journal of physiology. Endocrinology and metabolism. 2013;305:E113–118. doi: 10.1152/ajpendo.00649.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lindsey SH, Liu L, Chappell MC. Vasodilation by GPER in mesenteric arteries involves both endothelial nitric oxide and smooth muscle cAMP signaling. Steroids. 2014;81:99–102. doi: 10.1016/j.steroids.2013.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lindsey SH, Yamaleyeva LM, Brosnihan KB, Gallagher PE, Chappell MC. Estrogen receptor GPR30 reduces oxidative stress and proteinuria in the salt-sensitive female mRen2.Lewis rat. Hypertension. 2011;58:665–671. doi: 10.1161/HYPERTENSIONAHA.111.175174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu S, Le May C, Wong WP, Ward RD, Clegg DJ, Marcelli M, et al. Importance of extranuclear estrogen receptor-alpha and membrane G protein-coupled estrogen receptor in pancreatic islet survival. Diabetes. 2009;58:2292–2302. doi: 10.2337/db09-0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Majumder S, Das S, Moulik SR, Mallick B, Pal P, Mukherjee D. G-protein coupled estrogen receptor (GPER) inhibits final oocyte maturation in common carp, Cyprinus carpio. Gen Comp Endocrinol. 2015;211:28–38. doi: 10.1016/j.ygcen.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 45.Martensson UE, Salehi SA, Windahl S, Gomez MF, Sward K, Daszkiewicz-Nilsson J, et al. Deletion of the G protein-coupled receptor 30 impairs glucose tolerance, reduces bone growth, increases blood pressure, and eliminates estradiol-stimulated insulin release in female mice. Endocrinology. 2009;150:687–698. doi: 10.1210/en.2008-0623. [DOI] [PubMed] [Google Scholar]
- 46.Matsuda K, Sakamoto H, Mori H, Hosokawa K, Kawamura A, Itose M, et al. Expression and intracellular distribution of the G protein-coupled receptor 30 in rat hippocampal formation. Neurosci Lett. 2008;441:94–99. doi: 10.1016/j.neulet.2008.05.108. [DOI] [PubMed] [Google Scholar]
- 47.Meitzen J, Luoma JI, Boulware MI, Hedges VL, Peterson BM, Tuomela K, et al. Palmitoylation of estrogen receptors is essential for neuronal membrane signaling. Endocrinology. 2013;154:4293–4304. doi: 10.1210/en.2013-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meyer MR, Amann K, Field AS, Hu C, Hathaway HJ, Kanagy NL, et al. Deletion of G protein-coupled estrogen receptor increases endothelial vasoconstriction. Hypertension. 2012;59:507–512. doi: 10.1161/HYPERTENSIONAHA.111.184606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meyer MR, Field AS, Kanagy NL, Barton M, Prossnitz ER. GPER regulates endothelin-dependent vascular tone and intracellular calcium. Life sciences. 2012;91:623–627. doi: 10.1016/j.lfs.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Migliaccio A, Castoria G, Di Domenico M, de Falco A, Bilancio A, Lombardi M, et al. Steroid-induced androgen receptor-oestradiol receptor beta-Src complex triggers prostate cancer cell proliferation. Embo j. 2000;19:5406–5417. doi: 10.1093/emboj/19.20.5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Migliaccio A, Di Domenico M, Castoria G, de Falco A, Bontempo P, Nola E, et al. Tyrosine kinase/p21ras/MAP-kinase pathway activation by estradiol-receptor complex in MCF-7 cells. Embo j. 1996;15:1292–1300. [PMC free article] [PubMed] [Google Scholar]
- 52.Navarro CE, Saeed S Abdul, Murdock C, Martinez-Fuentes AJ, Arora KK, Krsmanovic LZ, et al. Regulation of cyclic adenosine 3′,5′-monophosphate signaling and pulsatile neurosecretion by Gi-coupled plasma membrane estrogen receptors in immortalized gonadotropin-releasing hormone neurons. Mol Endocrinol. 2003;17:1792–1804. doi: 10.1210/me.2003-0040. [DOI] [PubMed] [Google Scholar]
- 53.Node K, Kitakaze M, Kosaka H, Minamino T, Funaya H, Hori M. Amelioration of ischemia- and reperfusion-induced myocardial injury by 17beta-estradiol: role of nitric oxide and calcium-activated potassium channels. Circulation. 1997;96:1953–1963. doi: 10.1161/01.cir.96.6.1953. [DOI] [PubMed] [Google Scholar]
- 54.Notas G, Kampa M, Pelekanou V, Castanas E. Interplay of estrogen receptors and GPR30 for the regulation of early membrane initiated transcriptional effects: A pharmacological approach. Steroids. 2012;77:943–950. doi: 10.1016/j.steroids.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 55.Otto C, Fuchs I, Kauselmann G, Kern H, Zevnik B, Andreasen P, et al. GPR30 does not mediate estrogenic responses in reproductive organs in mice. Biology of reproduction. 2009;80:34–41. doi: 10.1095/biolreprod.108.071175. [DOI] [PubMed] [Google Scholar]
- 56.Pandey DP, Lappano R, Albanito L, Madeo A, Maggiolini M, Picard D. Estrogenic GPR30 signalling induces proliferation and migration of breast cancer cells through CTGF. The EMBO Journal. 2009;28:523–532. doi: 10.1038/emboj.2008.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pang Y, Dong J, Thomas P. Estrogen signaling characteristics of Atlantic croaker G protein-coupled receptor 30 (GPR30) and evidence it is involved in maintenance of oocyte meiotic arrest. Endocrinology. 2008;149:3410–3426. doi: 10.1210/en.2007-1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pang Y, Thomas P. Role of G protein-coupled estrogen receptor 1, GPER, in inhibition of oocyte maturation by endogenous estrogens in zebrafish. Dev Biol. 2010;342:194–206. doi: 10.1016/j.ydbio.2010.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pedram A, Razandi M, Deschenes RJ, Levin ER. DHHC-7 and -21 are palmitoylacyltransferases for sex steroid receptors. Molecular biology of the cell. 2012;23:188–199. doi: 10.1091/mbc.E11-07-0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pedram A, Razandi M, Kim JK, O’Mahony F, Lee EY, Luderer U, et al. Developmental Phenotype of a Membrane Only Estrogen Receptor α (MOER) Mouse. Journal of Biological Chemistry. 2009;284:3488–3495. doi: 10.1074/jbc.M806249200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pedram A, Razandi M, Sainson RC, Kim JK, Hughes CC, Levin ER. A conserved mechanism for steroid receptor translocation to the plasma membrane. J Biol Chem. 2007;282:22278–22288. doi: 10.1074/jbc.M611877200. [DOI] [PubMed] [Google Scholar]
- 62.Pelekanou V, Kampa M, Kiagiadaki F, Deli A, Theodoropoulos P, Agrogiannis G, et al. Estrogen anti-inflammatory activity on human monocytes is mediated through cross-talk between estrogen receptor ERalpha36 and GPR30/GPER1. Journal of leukocyte biology. 2016;99:333–347. doi: 10.1189/jlb.3A0914-430RR. [DOI] [PubMed] [Google Scholar]
- 63.Peyton C, Thomas P. Involvement of epidermal growth factor receptor signaling in estrogen inhibition of oocyte maturation mediated through the G protein-coupled estrogen receptor (Gper) in zebrafish (Danio rerio) Biology of reproduction. 2011;85:42–50. doi: 10.1095/biolreprod.110.088765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pinto PI, Teodosio R, Socorro S, Power DM, Canario AV. Structure, tissue distribution and estrogen regulation of splice variants of the sea bream estrogen receptor alpha gene. Gene. 2012;503:18–24. doi: 10.1016/j.gene.2012.04.081. [DOI] [PubMed] [Google Scholar]
- 65.Prossnitz ER, Arterburn JB. International Union of Basic and Clinical Pharmacology. XCVII. G Protein-Coupled Estrogen Receptor and Its Pharmacologic Modulators. Pharmacol Rev. 2015;67:505–540. doi: 10.1124/pr.114.009712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pupo M, Pisano A, Lappano R, Santolla MF, De Francesco EM, Abonante S, et al. Bisphenol A induces gene expression changes and proliferative effects through GPER in breast cancer cells and cancer-associated fibroblasts. Environmental health perspectives. 2012;120:1177–1182. doi: 10.1289/ehp.1104526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Razandi M, Alton G, Pedram A, Ghonshani S, Webb P, Levin ER. Identification of a structural determinant necessary for the localization and function of estrogen receptor alpha at the plasma membrane. Molecular and cellular biology. 2003;23:1633–1646. doi: 10.1128/MCB.23.5.1633-1646.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Razandi M, Pedram A, Greene GL, Levin ER. Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: studies of ERalpha and ERbeta expressed in Chinese hamster ovary cells. Mol Endocrinol. 1999;13:307–319. doi: 10.1210/mend.13.2.0239. [DOI] [PubMed] [Google Scholar]
- 69.Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A Transmembrane Intracellular Estrogen Receptor Mediates Rapid Cell Signaling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 70.Rossi A, Kontarakis Z, Gerri C, Nolte H, Holper S, Kruger M, et al. Genetic compensation induced by deleterious mutations but not gene knockdowns. Nature. 2015;524:230–233. doi: 10.1038/nature14580. [DOI] [PubMed] [Google Scholar]
- 71.Sharma G, Prossnitz ER. Mechanisms of estradiol-induced insulin secretion by the G protein-coupled estrogen receptor GPR30/GPER in pancreatic beta-cells. Endocrinology. 2011;152:3030–3039. doi: 10.1210/en.2011-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Simoncini T, Hafezi-Moghadam A, Brazil DP, Ley K, Chin WW, Liao JK. Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature. 2000;407:538–541. doi: 10.1038/35035131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Singh M, Sétáló G, Guan X, Warren M, Toran-Allerand CD. Estrogen-Induced Activation of Mitogen-Activated Protein Kinase in Cerebral Cortical Explants: Convergence of Estrogen and Neurotrophin Signaling Pathways. The Journal of Neuroscience. 1999;19:1179. doi: 10.1523/JNEUROSCI.19-04-01179.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Smith LC, Ralston-Hooper KJ, Ferguson PL, Sabo-Attwood T. The G Protein-Coupled Estrogen Receptor Agonist G-1 Inhibits Nuclear Estrogen Receptor Activity and Stimulates Novel Phosphoproteomic Signatures. Toxicol Sci. 2016 doi: 10.1093/toxsci/kfw057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Soderberg O, Gullberg M, Jarvius M, Ridderstrale K, Leuchowius KJ, Jarvius J, et al. Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nature methods. 2006;3:995–1000. doi: 10.1038/nmeth947. [DOI] [PubMed] [Google Scholar]
- 76.Sovershaev MA, Egorina EM, Andreasen TV, Jonassen AK, Ytrehus K. Preconditioning by 17beta-estradiol in isolated rat heart depends on PI3-K/PKB pathway, PKC, and ROS. American journal of physiology. Heart and circulatory physiology. 2006;291:H1554–1562. doi: 10.1152/ajpheart.01171.2005. [DOI] [PubMed] [Google Scholar]
- 77.Spence RD, Hamby ME, Umeda E, Itoh N, Du S, Wisdom AJ, et al. Neuroprotection mediated through estrogen receptor-alpha in astrocytes. Proc Natl Acad Sci U S A. 2011;108:8867–8872. doi: 10.1073/pnas.1103833108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Spence RD, Wisdom AJ, Cao Y, Hill HM, Mongerson CR, Stapornkul B, et al. Estrogen mediates neuroprotection and anti-inflammatory effects during EAE through ERalpha signaling on astrocytes but not through ERbeta signaling on astrocytes or neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:10924–10933. doi: 10.1523/JNEUROSCI.0886-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Staples JE, Gasiewicz TA, Fiore NC, Lubahn DB, Korach KS, Silverstone AE. Estrogen receptor alpha is necessary in thymic development and estradiol-induced thymic alterations. Journal of immunology. 1999;163:4168–4174. [PubMed] [Google Scholar]
- 80.Subramanian S, Matejuk A, Zamora A, Vandenbark AA, Offner H. Oral feeding with ethinyl estradiol suppresses and treats experimental autoimmune encephalomyelitis in SJL mice and inhibits the recruitment of inflammatory cells into the central nervous system. Journal of immunology. 2003;170:1548–1555. doi: 10.4049/jimmunol.170.3.1548. [DOI] [PubMed] [Google Scholar]
- 81.Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology. 2005;146:624–632. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- 82.Vivacqua A, Lappano R, De Marco P, Sisci D, Aquila S, De Amicis F, et al. G protein-coupled receptor 30 expression is up-regulated by EGF and TGF alpha in estrogen receptor alpha-positive cancer cells. Mol Endocrinol. 2009;23:1815–1826. doi: 10.1210/me.2009-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang C, Dehghani B, Magrisso IJ, Rick EA, Bonhomme E, Cody DB, et al. GPR30 contributes to estrogen-induced thymic atrophy. Mol Endocrinol. 2008;22:636–648. doi: 10.1210/me.2007-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang Z, Zhang X, Shen P, Loggie BW, Chang Y, Deuel TF. Identification, cloning, and expression of human estrogen receptor-alpha36, a novel variant of human estrogen receptor-alpha66. Biochemical and biophysical research communications. 2005;336:1023–1027. doi: 10.1016/j.bbrc.2005.08.226. [DOI] [PubMed] [Google Scholar]
- 85.Wang Z, Zhang X, Shen P, Loggie BW, Chang Y, Deuel TF. A variant of estrogen receptor-{alpha}, hER-{alpha}36: transduction of estrogen- and antiestrogen-dependent membrane-initiated mitogenic signaling. Proc Natl Acad Sci U S A. 2006;103:9063–9068. doi: 10.1073/pnas.0603339103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Waters EM, Thompson LI, Patel P, Gonzales AD, Ye HZ, Filardo EJ, et al. G-protein-coupled estrogen receptor 1 is anatomically positioned to modulate synaptic plasticity in the mouse hippocampus. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2015;35:2384–2397. doi: 10.1523/JNEUROSCI.1298-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Watson CS, Jeng YJ, Hu G, Wozniak A, Bulayeva N, Guptarak J. Estrogen- and xenoestrogen-induced ERK signaling in pituitary tumor cells involves estrogen receptor-alpha interactions with G protein-alphai and caveolin I. Steroids. 2012;77:424–432. doi: 10.1016/j.steroids.2011.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wu Q, Chambliss K, Lee WR, Yuhanna IS, Mineo C, Shaul PW. Point mutations in the ERalpha Galphai binding domain segregate nonnuclear from nuclear receptor function. Mol Endocrinol. 2013;27:2–11. doi: 10.1210/me.2011-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yates MA, Li Y, Chlebeck PJ, Offner H. GPR30, but not estrogen receptor-alpha, is crucial in the treatment of experimental autoimmune encephalomyelitis by oral ethinyl estradiol. BMC immunology. 2010;11:20. doi: 10.1186/1471-2172-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]


