Abstract
BACKGROUND
Increased β-secretase (BACE1) activity has consistently been detected in the brain tissue and cerebrospinal fluid (CSF) of subjects with mild cognitive impairment (MCI) and probable Alzheimer’s disease (AD) compared to controls. The collection of CSF by lumbar puncture is invasive. We sought to identify the presence of plasma BACE1 activity and determine potential alterations in MCI subjects with clinical follow-ups for 3 years using patients with diagnosed probable AD dementia compared to healthy controls (HC).
METHODS
75 probable AD patients, 96 MCI individuals and 53 age- and sex-matched HC were recruited from three independent international academic memory clinics and AD research expert centers. Plasma BACE1 activity was measured by a synthetic fluorescence substrate enzyme-linked immunosorbent assay. BACE1 protein expression was assessed by Western blotting using three different antibodies that recognize the epitopes of the N-, C-, and full-length BACE1.
RESULTS
Compared to HC, plasma BACE1 activity (Vmax) significantly increased by 53.2% in MCI subjects and by 68.9% in probable AD patients. Interestingly, MCI subjects who converted to probable AD dementia at follow-ups exhibited significantly higher BACE1 activity compared to cognitively stable MCI non-converters and also showed higher levels of BACE1 activity than AD patients.
CONCLUSIONS
The plasma BACE1 activity is significantly increased in MCI converters and probable AD patients. The sensitivities of BACE1 activity for the patients were 84% and the specificities were 88%. Our results indicate that plasma BACE1 activity may be a biomarker for AD risk and could predict progression from prodromal to probable AD dementia.
Keywords: β-secretase, BACE1, mild cognitive impairment, Alzheimer’s disease dementia, biomarker diagnosis, prediction
Alzheimer’s disease (AD) is the most common cause of dementia in populations older than 60 years (1–3). The progressive formation of amyloid plaques and vascular deposits consisting of the amyloid beta peptide (Aβ) is a pathological hallmark of AD (4–6). In particular, the accumulation of Aβ in the brain is an early pathophysiological event that occurs a decade or more before symptom onset (7,8). Aβ is generated from amyloid precursor protein (APP) by enzymatic digestion involving β- and γ-secretase activities (9,10). The β-site APP-cleaving enzyme 1 (BACE1) is a 501 amino acid-long glycosylated type I transmembrane endoprotease (11–13). We have demonstrated that BACE1 activity significantly increased in brains of patients with sporadic AD (sAD) and mild cognitive impairment (MCI) (14–18). More elevated BACE1-cleaved APP products were found by examining the Swedish mutation compared to wild-type substrate (11,12,17,19). The familial AD was caused by the APP Swedish mutation that enhances APP cleavage by BACE1 (19,20) and suggests that elevated BACE1 activity in the brain can induce AD (14–17). Moreover, a rare mutation close to the BACE1 cleavage site in the APP gene that protects against cognitive decline and the risk of developing AD substantially supports the hypothesis that BACE1 plays a key role in AD pathogenesis (21).
BACE1 is the rate-limiting enzyme in amyloidogenesis (22). Measurements of its concentration and activity have been proposed as surrogate biomarkers for AD (23). In previous studies, we have found a significant increase of both BACE1 enzymatic activity and protein concentrations in the cerebrospinal fluid (CSF) of individuals with MCI (24). BACE1 inhibitors have been shown to have therapeutic effects in AD animal models (25–28) and their potential role in lowering risk in developing AD has been investigated in clinical trials (29,30). Early initiation of treatment requires the early detection of disease, including accurate prediction at the asymptomatic or presymptomatic stages. We have also shown (24) that early detection of elevated BACE1 concentrations in CSF may be indicative of AD pathology in prodromal individuals with a higher risk of developing AD (31–33). The increased BACE1 enzymatic activity and protein concentrations in CSF provide biological evidence of identifying preclinical stages of AD versus age- and sex-matched healthy controls (HC) (24). The interaction of CSF BACE1 activity with established core CSF biomarkers, Aβ1-42, Aβ1-40, total tau (t-tau), and tau phosphorylated at threonine 181 (p-tau181), has been previously investigated.34 Moreover, BACE1 activity was significantly elevated in APOE ε4 carriers compared with APOE ε4 non-carriers and correlated with CSF concentrations of Aβ1-40, t-tau, and p-tau181, thus indicating that greater BACE1 activity in CSF is dynamically linked to underlying AD brain pathology and disease severity.24,34,35 Furthermore, CSF BACE1 activity is one of the strongest predictors of AD risk compared with other biomarker candidates such as brain atrophy (revealed via magnetic resonance imaging (MRI)-based hippocampal volume reduction), CSF concentrations of t-tau, p-tau, Aβ1-42, as well as APOE status or age (36).
In addition to CSF biomarkers, which necessitate invasive lumbar puncture procedures, (37,38) potential biomarkers for AD risk that can be obtained from more accessible sources such as blood (plasma/serum) are eagerly required. An ideal biomarker will need to be directly related to the disease pathogenesis in the brain. From this viewpoint, BACE1 is supposed to be a relevant biomarker. To our knowledge, no data has been published on peripheral BACE1 expression and activity in cognitively HC, MCI individuals, and AD patients. Therefore, in the present study, we sought to investigate BACE1 activity and protein expression in plasma samples of HC, MCI individuals (converters versus non-converters), and probable AD dementia patients.
We found that plasma concentrations of BACE1 are able to stratify the clinically relevant diagnostic subgroups mentioned above at baseline and predict the progression and conversion of MCI to overt AD dementia.
MATERIALS AND METHODS
Participants
A total of 224 individuals were recruited from three independent international academic AD research centers and memory clinics: (n=131) individuals from the Department of Psychiatry and Psychotherapy, Alzheimer Memorial Center, Ludwig-Maximilian University, Munich, Germany; (n=68) individuals from the Department of Neuroscience and Physiology, University of Gothenburg, Sahlgrenska University Hospital, Sweden; (n=25) individuals from the Memory Center, Roskamp Institute, USA). Three age-matched multi-site study cohorts were assembled: 75 probable AD patients, 96 MCI individuals and 53 age- and sex-matched HC. No age differences were found among the three groups using a generalized linear model. In accordance with previously published BACE1 studies (24,35), the diagnosis of probable AD was made according to the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) criteria, including the MMSE score (39,40) MCI was diagnosed according to the Petersen criteria (33). MCI individuals performed 1.5 standard deviation below the age-adjusted reference average in memory scales using the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) cognitive battery (41) HC individuals were represented by age-matched cognitively and physically healthy individuals. Patients with psychiatric comorbidity were excluded by means of history, clinical examination, and Composite International Diagnostic Interview (CIDI) (42). We obtained the clinical data from three independent centers where individuals with probable AD and MCI, including cognitively stable MCI and MCI who converted to AD, were enrolled. In all clinical centers, the stable MCI group was followed clinically for 2–3 years, while follow-up of MCI converters continued until conversion to AD dementia.
BACE1 Enzymatic Activity Assay
All blood samples were handled in an identical fashion, including coding, centrifugation, plasma extraction, and storage conditions. The maximum delay between blood drawing and centrifugation was one hour; each plasma sample was stored in a −80°C freezer until assayed in duplicate for BACE1 activity. All measures were performed blinded to the clinical status of the study participants.
BACE1 activity assays were performed as previously described with minor modifications (24). In brief, synthetic peptide substrates containing the β-cleavage site (Calbiochem, EMD, Gibbstown, NJ, USA) at a 10mM concentration in reaction buffer (50mM acetic acid buffer, pH 4.5, 100mM NaCl) were used for BACE1 activity assay. Ten microliters of plasma were mixed with 100 microliters of buffer with the final pH of ~4.5, which is optimal for the BACE1 activity assay. The fluorescence was measured at 430 nm (excitation wavelength) and 520 nm (emission wavelength). BACE1 activity was corrected by plasma total protein content and calculated through Vmax and Vmean as previously described (24) and expressed in fluorescence units (FU)/time. Plasma BACE1 activity was tested in the presence of the β-Secretase Inhibitor IV (Calbiochem, EMD, Gibbstown, NJ, USA), while recombinant BACE1 peptide (Sigma, St Louis, MO, USA) was used as positive control during the assay. The inhibition ratio was obtained by the following equation: Inhibition (%)= (1-S/C) ×100, where C indicates the plasma BACE1 activity in the absence of β-Secretase Inhibitor IV, and S indicates the plasma BACE1 activity in the presence of different concentration of β-Secretase Inhibitor IV. The IC50 value was calculated using the GraphPad Prism 5 software (GraphPad Inc., San Diego, CA, USA). To confirm the specificity of plasma BACE1 activity, we coated a 96-well plate with the BACE1 specific antibody MAB5308 (1:4000, EMD Millipore, Billerica, MA, USA) to capture the BACE1 protein and, then, we measured BACE1 activity.
BACE1 Western Blot Assay
For Western blot analysis, plasma samples were mixed with an equal volume of sodium dodecyl sulphate (SDS) sample buffer and separated using a 10% SDS polyacrylamide gel. The protein was then transferred to the nitrocellulose membrane. The membrane was stained with 1% Ponceau S staining solution as loading control and AD patients’ brain lysates were utilized as positive controls. The membrane was then probed with anti-BACE1 N-terminus (B0681, Sigma-Aldrich, St Louis, MO, USA), C-terminus (B0806, Sigma-Aldrich, St Louis, MO, USA) or ectodomain monoclonal antibody (Mab931, R&D Systems, Minneapolis, MN, USA). Ponceau S staining solution (Sigma, St Louis, MO, USA) was used as loading control. Intensities of protein bands were determined using Quality One 1-D Analysis Software (Bio-Rad Life Science, Hercules, CA, USA).
Statistical Analysis
Appropriate descriptive statistics including mean, standard deviation, median, and range were computed in presenting the overall data. Generalized linear models were fitted with multiple comparison tests to compare diagnostic groups and other categorical predictors for each of the outcome measures including BACE1 protein expression, BACE1 activities, total protein concentrations, and BACE1 activity/MMSE values. These models allowed us to account for age effects without inflating the type I error level while simultaneously adjusting for key factors. The data, including correlation in each diagnostic group, was analyzed using the statistical package SPSS (version 11.5.1; SPSS Inc, Chicago, IL, USA).
RESULTS
Detection of BACE1 protein expression and enzymatic activity in human plasma
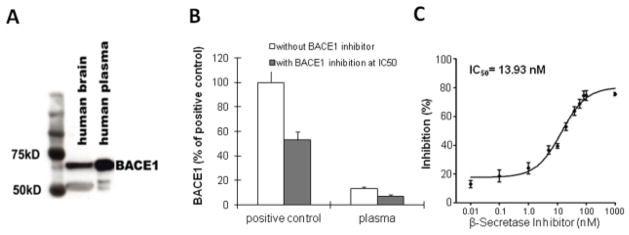
To investigate whether BACE1 protein could be detected in plasma, a specific anti-BACE1 N-terminus antibody was utilized. We found that plasma samples contained BACE1 protein at the expected size, approximately 70kD (Figure 1A), similar to that found in the human brain and CSF (16,17,24). A fluorogenic APP-derived peptide was employed as a BACE1 substrate to examine BACE1 specific activity, with BACE1 APP-derived peptide substrate used as a positive control. Plasma BACE1 enzymatic activity was inhibited by a specific BACE1 inhibitor (IC50 dose at ~14nM) (Figure 1B &1C). In our study, the IC50 dose of 14nM necessary for inhibiting BACE1 is significantly different from the IC50 of both BACE2 and cathepsin D (43). These findings indicate that BACE1 protein is detectable via ELISA methods and its enzymatic activity is abundant in human plasma.
Figure 1.
Plasma β-site amyloid precursor protein-cleaving enzyme1 (BACE1) protein and activity. This figure shows the presence of BACE1 activity in human plasma samples as assessed by Western blot analysis. An ectodomain monoclonal antibody (R&D Systems) was utilized, about 70-kD BACE1 proteins were detected in human brain lysates and plasma samples (A). The results indicated that plasma samples express BACE1 with functional enzymatic activity as reported in the brain. (B). When recombinant BACE1 (Sigma) was used as a positive control, the BACE1 enzymatic activity in plasma was specifically inhibited nearly in half at 15 nM (IC50) of the inhibitor concentration, and the error bars stand for S.D. (C) Dose-dependent reaction of β-Secretase Inhibitor IV on plasma BACE1 enzymatic activity. The IC50 value was calculated (n = 3) using software GraphPad Prism 5. The results are expressed as mean ± SD.
Investigation of plasma BACE1 activity in MCI individuals and AD patients at different stages
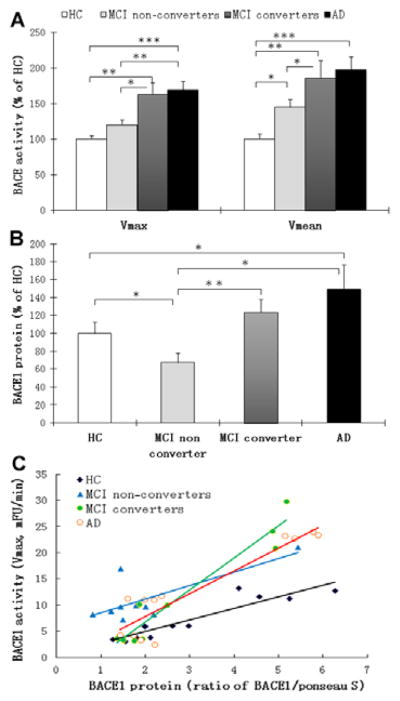
MCI converters (n=71) and AD patients (n=75) showed significantly higher BACE1 activity compared to HC (n=53) and MCI non-converters, which increased Vmax by 62.8% (p=0.001) and 68.9% (p<0.001) in MCI converters and AD patients, respectively (Figure 2A). Compared to HC, the Vmean of BACE1 activity in MCI non-converters, MCI converters, and AD patients increased by 45.0% (p<0.05), 85.4% (p=0.001), and 97.3% (p<0.001), respectively (Figure 2A). The results of the generalized linear model supported the fact that the association of increased peripheral BACE1 activity with both AD and MCI converters is valid. There was no significant difference in BACE1 activity between MCI individuals and AD patients for Vmax (p=0.756) and for Vmean (p=0.8). To validate the increased plasma BACE1 activity in MCI converters and AD patients (Figure 2A) compared to HC, we used an anti-BACE1 antibody to precipitate the protein and measured the enzymatic activity of the antibody-captured protein. Again, there was elevated enzymatic activity of the antibody-captured BACE1 in plasma of MCI individuals (p=0.0004) and AD patients (p=0.0001) compared to that of HC (Supplementary Figures 1&2).
Figure 2.
BACE1 enzymatic activity and protein expression in plasma samples from healthy control (HC) individuals, mild cognitive impaired (MCI) individuals and Alzheimer’s disease (AD) patients. The activity of BACE1 was measured by using synthetic peptide substrates containing the BACE1 cleavage site as we reported 17 with modifications. (A) BACE1 activity as percentage of HC group. The BACE1 activity of plasma samples (both expressed as Vmax or Vmean) exhibited an increase from cognitively HC to MCI non-converters, MCI converters and finally to AD. *P< 0.05, **P< 0.01 and ***P< 0.001 compared to HC, respectively. (B) Density scanning Western blot analysis of BACE1 protein expression in human plasma samples, and bars represent the relative protein expression as percentages of HC (arbitrary unit of BACE1/Ponceau S). (C) Association between plasma BACE1 activity (Vmax, mFU/min) and BACE1 protein expression (Ratio of BACE1/Ponceau S).
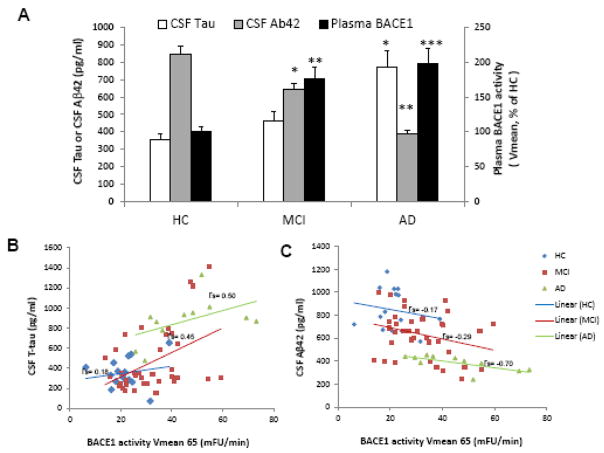
In addition to measuring plasma BACE1 activity in HC, MCI non-converters and converters, and AD patients (Figure 2A), we also detected a significantly increased BACE1 protein expression in both AD patients and MCI converters compared to those detected in HC and MCI non-converters (Figure 2B). There was also a significant correlation between activity and protein expression of BACE1 in HC and MCI non-converters versus MCI converters and AD (Figure 2C) detected. Elevated BACE1 activities in both MCI converters and AD patients are also highly associated with disease-specific proteins Tau and Aβ1-42 concentrations in CSF (Figure 3A–3C). Moreover, BACE1 activities were associated with disease progression as measured by MMSE (Figure 4A).
Figure 3.
Plasma BACE1 activity comparisons among different diagnostic groups: aged-matched HC and MCI non-converters, MCI converters and AD patients and validation of plasma BACE1 activity with core AD biomarkers.
To validate BACE1 activity association with AD diagnosis, we compared with the core AD biomarkers, CSF tau and CSF Aβ1-42 (A). There are significant correlations between BACE1 activity and CSF tau (**P<0.01) or CSF Aβ(*P<0.05) in MCI individuals and AD patients (***P< 0.001, BACE1 vs CSF tau or CSF Aβ1-42). After medial correlation analyses, we have found a significant correlation between BACE1 activities and CSF Tau concentrations (B): rs = 0.45; P<0.05 and a significant inverse correlation between BACE1 activities and CSF Aβ1-42 concentrations (C): rs = −0.29; P<0.05 in the MCI group. In the AD group, we also found a significant correlation between BACE1 activities and CSF Tau concentrations (B): rs = 0.50; P<0.05 and a inverse significant correlation between BACE1 activities and CSF Aβ1-42 concentrations (C): rs = −0.70; P<0.01 whereas, in the HC group, there was no significant correlation either between BACE1 activities and CSF Tau concentrations (B): rs = 0.18 or between BACE1 activities and CSF Aβ1-42 concentrations (C): rs = −0.17.
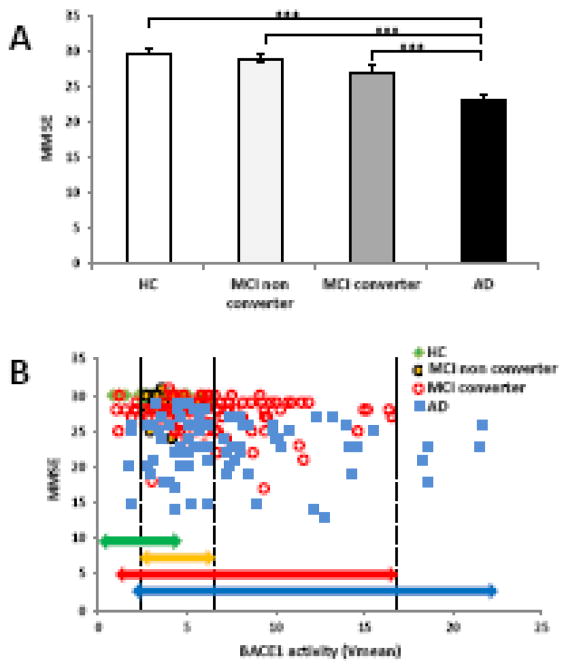
Figure 4.
Correlations of clinical MMSE scores with plasma BACE1 activity in HC, MCI non-converters, MCI converters, and AD subgroups. (A) The MMSE score alone in HC, MCI non-converters, MCI converters, and AD resulted in a significant difference between HC and MCI (***P<0.001). (B) Distributions of BACE1 activity and correlations with MMSE scores in different diagnostic groups. The distributions of BACE1 activity among HC, MCI non-converters, MCI converters, and AD patients were plotted with MMSE scores. The green line shows the area of HC BACE1 activity; the yellow line shows the area of MCI non-converter BACE1 activity. HC and MCI non-converters areas were significantly overlapped. The red line shows MCI converter BACE1 activity and the blue line shows the area of AD patient BACE1 activity. Both MCI converters and AD patients share highly overlapped BACE1 activity and MMSE scores.
Effects of sex on plasma BACE1 activity in MCI individuals and AD patients
Based on the generalized linear model for Vmax, we found that BACE1 activity belonging to MCI non-converters, MCI converters, and AD increased in females by 67.2% (P>0.05), 105.7% (P=0.012) and 100.2% (P<0.001), respectively, compared with females of the HC group (Supplementary Figure 3A). In males, plasma BACE1 Vmax activity in MCI non-converters, MCI converters, and AD patients was increased by 10.8% (P>0.05), 40.8% (P=0.033), and 59.3% (P=0.012), respectively, compared with males of the HC group (Supplementary Figure 3A). The Vmean values of plasma BACE1 activity showed similar sex differences; in females, Vmean BACE1 activity in MCI non-converters, MCI converters, and AD patients increased by 67.2 (P>0.05), 105.7 (P=0.021), and 100.24% (P=0.001), respectively, versus females of the HC group. When considering the males, BACE1 activity in the MCI non-converters, MCI converters, and AD subgroups was increased by 26.2% (P>0.05), 59.5% (P=0.007), and 85.5% (P=0.013), respectively, by setting the enzymatic activity as a reference in the corresponding male HC group (Supplementary Figure 3B). No significant sex differences were found in terms of BACE1 activity between HC, MCI, and AD (Supplementary Figure 3C). The generalized linear model showed an association of plasma BACE1 activity with the clinical diagnosis, after adjusting for sex (p=0.0002) (Table 1). No statistically significant associations were observed between BACE1 activity and age (p=0.6258) or sex (p=0.1582) (Table 1), as well as centers, (p=0.7670).
Table 1. General Demographics of Subject and Multivariate Regression Analysis.
The general demographics of subject populations and multivariate regression analysis of association between plasma BACE1 activity (MaxV_Prot) and the clinical diagnosis from three independent clinical centers. While BACE1 activity is highly different between diagnoses, the generalized linear model showed no statistically significant associations between BACE1 activity and age or sex, as well as centers.
| Variable | HC n = 53 | MCI n = 96 | AD n = 75 |
|---|---|---|---|
| Subjects by Site (DEU/USA/SWE), n | 30/7/16 | 49/9/38 | 50/10/15 |
| Sex, Male/Female, n | 25/28 | 45/51 | 25/50 |
| Age, years, Mean + S.E. | 68+0.1 | 69.5+0.1 | 73.84+0.12 |
| MMSE (0–30): Mean + S.E. Range |
29.65+0.03 28–30 |
27.14+0.03 17–30 |
22.80+0.05 13–29 |
| Parameter | Estimate | Standard Error | p-value |
| Clinical Center | |||
| DEU (versus SWE) | −0.0063 | 0.0324 | 0.8463 |
| USA (versus SWE) | 0.0161 | 0.0544 | 0.7670 |
| Diagnosis | |||
| AD (versus MCI) | −0.0405 | 0.0270 | 0.1337 |
| HC (versus MCI) | 0.2294 | 0.0621 | 0.0002 |
| Sex, Female (versus Male) | −0.0424 | 0.0300 | 0.1582 |
| Age, All Groups | −0.0008 | 0.0016 | 0.6258 |
Abbreviations:
HC = Age- and sex-matched healthy controls; MCI = mild cognitive impairment;
AD = Alzheimer’s disease; MMSE = Mini-Mental State
Examination DEU = Germany; USA = United State; SWE = Sweden
Validation of plasma BACE1 activity in comparison with the established core CSF AD biomarkers and cognition
As plasma activity of BACE1 increased in MCI individuals and AD patients versus HC, MCI non-converters, MCI converters, and AD (Figure 2A), we validated BACE1 activity as a plasma biomarker candidate by comparing the established CSF predictors for progression or conversion to dementia, tau protein and Aβ1-42 peptide (54, 55), with plasma BACE1 activity, and found a significant correlation (Figure 3A). In the MCI group, correlational analysis further revealed a significant positive correlation between BACE1 activity and CSF Tau concentrations (Figure 3B: rs= 0.45; P<0.05) and an inverse correlation between BACE1 activity and CSF Aβ1-42 concentrations (Figure 3C: rs= −0.29; P<0.05). Similarly, in the AD group, we observed a significant correlation between BACE1 activity and CSF Tau concentrations (Figure 3C: rs= 0.50; P<0.05) and an inverse correlation between BACE1 activity and CSF Aβ1-42 concentrations (Figure 3C: rs= −0.70; P<0.01). In contrast, in the HC group, there was no significant correlation found between BACE1 activity and CSF Tau concentrations (Figure 3B: rs= 0.18) as well as CSF Aβ1-42 concentrations (Figure 3C: rs= −0.17).
We also examined the association between BACE1 activity and cognition. Mini-Mental state examination (MMSE) scores were significantly lower in MCI converters and AD patients compared to those found in healthy age-matched controls and MCI non-converters (Figure 4A; p<0.001). In order to assess the link between BACE1 activity and MMSE, we performed correlation analysis between BACE1 activity and MMSE across diagnoses (Figure 4B). In particular, we discovered a high overlap BACE1 activity between values 2–6 mFu/min/μg Vmean between HC and MCI non-converters, as shown in Figure 4B (green and yellow lines). Moreover, MCI converters (red line) and AD patients (blue line) exhibited a substantial overlap in BACE1 activity in the same range as shown in Figure 4B. We further found that all AD patients and MCI converters had values of BACE1 activity (Vmean) ≥ 2.6; while those with values 2–6 mFu/min/μg of BACE1 activity were HC (Vmean <2) and MCI (Vmean <6) non-converters. These findings demonstrate that plasma BACE1 enzymatic activity is highly correlated with cognitive functions.
To our knowledge, this is the first study that measures BACE1 enzymatic activity and protein expression in plasma samples of HC, MCI converters to AD, MCI non-converters, and probable AD. First, we demonstrated that BACE1 exists as a functional enzyme in human plasma. Second, we found that BACE1 activity significantly increased in the plasma of patients with probable AD and MCI (including both MCI converters and MCI non-converters) compared to HC individuals after adjusting for sex, age, and recruiting center. Third, MCI individuals who converted to probable AD at clinical follow-ups exhibited significantly higher BACE1 activity than MCI non-converters. Moreover, we found that both MCI-AD converters and probable AD patients exhibit significantly elevated BACE1 activity relative to that of HC and MCI non-converter groups. These findings suggest that plasma BACE1 activity may not only be associated with AD risk and disease activity, but may also help predict progression from prodromal AD to probable AD dementia. Increased BACE1 activity presents a novel, pathophysiologically relevant and easily accessible candidate biomarker (currently entering Phase III of diagnostic development and validation) to support the detection of underlying AD pathophysiology and to predict conversion to AD. Such a novel dynamic plasma biomarker may represent a reliable indicator that improves early detection of the disease and facilitates the early initiation of disease-modifying treatments. We also identified cut-point values for BACE1 activity distinguishing HC individuals (Vmean < 2 mFu/min/μg) from MCI converters as well as AD patients (Vmean > 2.6 mFu/min/μg). Finally, we observed a correlation between plasma BACE1 enzymatic activity and cognitive function. This association has not been reported for other biomarker candidates previously tested and may be a potential target for the development of a surrogate biomarker (indicating clinical outcome in trials and early treatment).
Our data suggests a potential role for plasma BACE1 activity as a useful peripheral diagnostic and mechanistic biomarker in the prodromal cognitive decline to syndromal AD dementia continuum. Plasma BACE1 activity might be employed in disease-modifying clinical trials for subject screening, selection, and stratification, as well as for validating target engagement and mechanism of actions.
DISCUSSION
AD is characterized by a decade(s)-long asymptomatic stage and an initial prodromal symptomatic phase preceding the manifestation of overt AD dementia syndrome (3,44,45). Due to the disappointing results of Phase III trials of anti-amyloid compounds tested in mild-to-moderately impaired AD dementia patients (44,46,47), the asymptomatic to prodromal stages are increasingly recognized as the most promising therapeutic window for interventions aimed at effectively modulating the underlying pathophysiological and early symptomatic progression of AD (3,44,46). Although no disease-modifying treatment for AD is currently available, searching for disease-related biomarkers reflecting key molecular mechanisms and predicting the progression and conversion to AD dementia becomes urgent as new therapeutic interventions are being developed and tested earlier in target-populations (44,48).
Despite enormous efforts performed at an international level to standardize methods, CSF biomarkers have not yet accomplished the widespread approval and availability necessary for diagnostic use in clinical practice. However, advanced blood (plasma/serum)-based technologies and biomarkers promise to provide a new class of non-invasive and easy-to-use tests suitable for global application, such as acting as a screening tool in large numbers of asymptomatic or prodromal individuals at risk for developing AD (39). In this respect, BACE1 is emerging as one of the most encouraging biomarker candidates owing to its crucial enzymatic activity involved in processing APP to produce Aβ peptides; in addition, BACE1 appears to be associated with synaptic function (49,50).
We developed and optimized a plasma-based assay to detect BACE1 enzymatic activity in human plasma samples characterized by high sensitivity and specificity (Figure 1). Moreover, we used both logistics and ROC analyses for the BACE1 activity for AD, MCI converters, MCI non-converter compared to HC. Data showed that the sensitivities of BACE1 activity for AD, MCI converters and MCI converters are 64–84%, 66–70%, 64–84%, respectively. For the specificities, there are 86–88%, 86–88%, 64–88% for AD, MCI-converters, and MCI non-converters, respectively. Besides our tests, several other BACE1 activity-based methods utilizing the combination of ELISA assays with substrate cleavage analysis have been established for CSF. However, some of these methodological approaches may not be suitable for plasma BACE1 activity assays due to the existence of high-abundance proteins in plasma that might interfere with the antibodies used to recognize BACE1, thus limiting the assay sensitivity. Here, we optimized the enzymatic assay conditions not only to detect human plasma BACE1 activity, but also to quantify BACE1 protein expression. We were able to “capture” plasma BACE1 protein by using a specific BACE1 antibody and confirmed the increase in captured BACE1 enzymatic activity both in MCI and AD cases (Supplementary Figure 1). Moreover, we investigated the combination of enzymatic activity and clinical psychometric data (MMSE) to attain a better understanding of the role of BACE1 activity in plasma. Our method is relatively simple, rapid and shows little loss or interference with enzymatic activities. In addition, there were no significant differences in the activity of plasma BACE1 measured across the three academic memory clinics. As a result, the assay reported in the present study is highly sensitive, reliable, robust, and the findings are validated across multiple sites. There are also some overlaps in BACE1 activity among different groups, suggesting that we might need to further epitomize the conditions for plasma BACE1 activity assays. Nonetheless, our results need to be replicated and independently validated.
The potential roles of peripheral BACE1 protein expression and activity as candidate biomarkers of AD have not yet been investigated. To date, several studies examined the potential significance of BACE1 in AD brain and in CSF. In particular, such analyses reported the association between CSF BACE1 activity and hippocampus atrophy in AD (36), between elevated BACE1 activity in sAD and the intensity of axonal degeneration (51) as well as between significantly elevated BACE1 concentrations and activity in CSF of MCI individuals.52 Compared with a recent study using human protein microarrays identifying a panel of 50 multi-antibodies as biomarkers in blood for detecting MCI (53), our study showed BACE1 as a single target biomarker with promising significance and specificity as a biomarker for early diagnosis of AD. Our previous studies have established BACE1 activity assay in brain tissue and CSF and have also been replicated and reported by many other independent laboratories (16, 17, 24). However, in this study we used human plasma samples (not recombinant protein) to study the dose-dependent reaction of β-Secretase Inhibitor IV on BACE1 enzymatic activity and showed the concentration at which the curve passes through the 50% inhibition level as IC50 (~14nM), not the maximum inhibition of BACE1. Our maximum inhibition of BACE1 activity in the human plasma is ~80% instead of 100% as seen in the recombinant protein, suggesting a small portion of nonspecific proteins in human plasma may interfere with the BACE1 activity assay.
In summary, our findings provide intriguing evidence that plasma BACE1 activity is significantly increased in individuals with MC. The use of peripheral plasma-based BACE1 as a biomarker in MCI and AD cases has numerous advantages: (1) is minimally invasive, (2) is widely accessible, available and generalizable, (3) reflects a disease-relevant pathophysiology biomarker involved in amyloid production, (4) low costs, (5) is amenable to repeated sampling for time-course analyses, and (6) may be combined with other biomarker candidates for optimization of sensitivity and specificity. Measurement of plasma BACE1 activity also has promising potential as a clinical diagnostic test for wide screenings of subjects at risk for AD. Future clinical development and validation in additional independent clinical populations to define appropriate cut-off points and relationships to other core pathophysiological and topographical AD biomarkers is warranted. Plasma and CSF BACE1, as mechanism of action biomarker, also has great promise to potentially identify responders in trials with BACE-inhibitors and other anti-amyloid targeted therapies.
Supplementary Material
Acknowledgments
This work was supported by the National Key Research and Development Program, Ministry of Science and Technology of China 2016YFC1300500-03 (YS), the National Institute on Aging (NIHR01AG032441-01, YS, NIHR21 AG049237, RL and RO1AG025888, YS); Alzheimer’s Association (Zenith Award and IIRG-07-59510, YS); the American Health Assistance Foundation (G2006-118, RL); HH is supported by the AXA Research Fund, the Fondation Université Pierre et Marie Curie and the Fondation pour la Recherche sur Alzheimer, Paris, France. Ce travail a beneficie d’une aide de l’Etat «Investissements d’avenir » ANR-10-IAIHU-06 (HH). The research leading to these results has received funding from the program “Investissements d’avenir” ANR-10-IAIHU-06 (Agence Nationale de la Recherche-10-IA Agence Institut Hospitalo-Universitaire-6) (HH). We would like to thank Dr. Michael Ewers from Ludwig-Maximilian University, Munich, Germany, for the critical discussion of the study concept. We would also like to thank Juliet Shen for her English editing services.
Footnotes
DISCLOSURES
The authors report no biomedical financial interests or potential conflicts of interest.
ARTICLE INFORMATION
From Neurodegenerative Disorder Research Center and Brain Bank, Material Science at Microscale National Laboratory, School of Life Sciences, Key Laboratory of Brain Function and Disease, Chinese Academy of Sciences, University of Science and Technology of China, Hefei, China (YS). Center for Advanced, Therapeutic Strategies for Brain Disorders (YS). Center for Hormone Advanced Science and Education (RL). Memory Center (AK, MM), Roskamp Institute, Sarasota, FL34203 USA. Department of Economics, Arizona State University, Tempe, AZ, USA (JW). Department of Psychiatry and Psychotherapy, University Hospital of Tübingen, Tübingen, Germany (TL). Center of Old Age Psychiatry, Psychiatric University Hospital, Wilhelm Klein-Strasse 27, CH-4012Basel, Switzerland (TL). Department of Psychiatry and Psychotherapy, Alzheimer Memorial Center, Ludwig-Maximilian University (SL, HH), Munich, Germany. Department of Neurology and Alzheimer’s Disease Research Center (AL), Emory University School of Medicine, Atlanta, GA, USA. Department of Neuroscience and Physiology, University of Gothenburg, Sahlgren’s University Hospital, Mölndal, Sweden (KB, AW). IHU-A-ICM – Paris Institute of Translational Neurosciences, Pitié-Salpêtrière University Hospital, Paris, France (HH, SL). Beijing Institute for Brain Disorders RL), Beijing, China 100069. Department of Neurology, University of Florida College of Medicine (YS), Gainesville, FL31620, USA. AXA Research Fund & UPMC Chair, Paris, France (HH). Sorbonne Universités, Université Pierre et Marie Curie, Paris 06, Institut de la Memoiré et de la Maladie d’Alzheimer (IM2A) & Institut du Cerveau et de la Moelle épinière (ICM), Département de Neurologie (HH, SL), Hôpital de la Pitié-Salpêtrière, Paris, France
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ballard C, Gauthier S, Corbett A, Brayne C, Aarsland D, Jones E. Alzheimer’s disease. Lancet. 2011;377:1019–1031. doi: 10.1016/S0140-6736(10)61349-9. [DOI] [PubMed] [Google Scholar]
- 2.Blennow K, Hampel H, Weiner M, Zetterberg H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat Rev Neurol. 2010;6:131–144. doi: 10.1038/nrneurol.2010.4. [DOI] [PubMed] [Google Scholar]
- 3.Hampel H, Frank R, Broich K, Teipel SJ, Katz RG, Hardy J, et al. Biomarkers for Alzheimer’s disease: academic, industry and regulatory perspectives. Nat Rev Drug Discov. 2010;9:560–574. doi: 10.1038/nrd3115. [DOI] [PubMed] [Google Scholar]
- 4.Masters CL, Selkoe DJ. Biochemistry of Amyloid β-Protein and Amyloid Deposits in Alzheimer Disease. Cold Spring Harb Perspect Med. 2012;2:a006262. doi: 10.1101/cshperspect.a006262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanzi RE, Bertram L. Twenty years of the Alzheimer’s disease amyloid hypothesis: a genetic perspective. Cell. 2005;120:545–555. doi: 10.1016/j.cell.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 6.Perrin RJ, Fagan AM, Holtzman DM. Multimodal techniques for diagnosis and prognosis of Alzheimer’s disease. Nature. 2009;461:916–922. doi: 10.1038/nature08538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bastin ME, Muñoz Maniega S, Ferguson KJ, Brown LJ, Wardlaw JM, et al. Quantifying the effects of normal ageing on white matter structure using unsupervised tract shape modelling. Neuroimage. 2010;51:1–10. doi: 10.1016/j.neuroimage.2010.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blennow K, de Leon MJ, Zetterberg H. Alzheimer’s disease. Lancet. 2006;368:387–403. doi: 10.1016/S0140-6736(06)69113-7. [DOI] [PubMed] [Google Scholar]
- 9.De Strooper B, Vassar R, Golde T. The secretases: enzymes with therapeutic potential in Alzheimer disease. Nat Rev Neurol. 2010;6:99–107. doi: 10.1038/nrneurol.2009.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vassar R, Kovacs DM, Yan R, Wong PC. The beta-secretase enzyme BACE in health and Alzheimer’s disease: regulation, cell biology, function, and therapeutic potential. J Neurosci. 2009;29:12787–12794. doi: 10.1523/JNEUROSCI.3657-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, et al. Beta-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286:735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- 12.Yan R, Bienkowski MJ, Shuck ME, Miao H, Tory MC, Pauley AM, et al. Membrane-anchored aspartyl protease with Alzheimer’s disease beta-secretase activity. Nature. 1999;402:533–537. doi: 10.1038/990107. [DOI] [PubMed] [Google Scholar]
- 13.Sinha S, Anderson JP, Barbour R, Basi GS, Caccavello R, Davis D, et al. Purification and cloning of amyloid precursor protein beta-secretase from human brain. Nature. 1999;402:537–540. doi: 10.1038/990114. [DOI] [PubMed] [Google Scholar]
- 14.Fukumoto H, Cheung BS, Hyman BT, Irizarry MC. Beta-secretase protein and activity are increased in the neocortex in Alzheimer disease. Arch Neurol. 2002;59:1381–1389. doi: 10.1001/archneur.59.9.1381. [DOI] [PubMed] [Google Scholar]
- 15.Holsinger RM, McLean CA, Beyreuther K, Masters CL, Evin G. Increased expression of the amyloid precursor beta-secretase in Alzheimer’s disease. Ann Neurol. 2002;51:783–786. doi: 10.1002/ana.10208. [DOI] [PubMed] [Google Scholar]
- 16.Li R, Lindholm K, Yang LB, Yue X, Citron M, Yan R, et al. Amyloid beta peptide load is correlated with increased beta-secretase activity in sporadic Alzheimer’s disease patients. Proc Natl Acad Sci U S A. 2004;101:3632–3637. doi: 10.1073/pnas.0205689101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang LB, Lindholm K, Yan R, Citron M, Xia W, Yang XL, et al. Elevated beta-secretase expression and enzymatic activity detected in sporadic Alzheimer disease. Nat Med. 2003;9:3–4. doi: 10.1038/nm0103-3. [DOI] [PubMed] [Google Scholar]
- 18.Cheng X, He P, Lee T, Yao H, Li R, Shen Y. High activities of BACE1 in brains with mild cognitive impairment. American Journal of Pathology. 2014;184:1–7. doi: 10.1016/j.ajpath.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rabe S, Reichwald J, Ammaturo D, de Strooper B, Saftig P, Neumann U, et al. The Swedish APP mutation alters the effect of genetically reduced BACE1 expression on the APP processing. J Neurochem. 2011;119:231–239. doi: 10.1111/j.1471-4159.2011.07412.x. [DOI] [PubMed] [Google Scholar]
- 20.Mullan M, Crawford F, Axelman K, Houlden H, Lilius L, Winblad B, et al. A pathogenic mutation for probable Alzheimer’s disease in the APP gene at the N-terminus of beta-amyloid. Nat Genet. 1992;1:345–347. doi: 10.1038/ng0892-345. [DOI] [PubMed] [Google Scholar]
- 21.Jonsson T, Atwal JK, Steinberg S, Snaedal J, Jonsson PV, Bjornsson S, et al. A mutation in APP protects against Alzheimer’s disease and age-related cognitive decline. Nature. 2012;488:96–99. doi: 10.1038/nature11283. [DOI] [PubMed] [Google Scholar]
- 22.Wang H, Li R, Shen Y. β-Secretase: its biology as a therapeutic target in diseases. Trends Pharmacol Sci. 2013;34(4):215–25. doi: 10.1016/j.tips.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hampel H, Shen Y. Beta-site amyloid precursor protein cleaving enzyme 1 (BACE1) as a biological candidate marker of Alzheimer’s disease. Scand J Clin Lab Invest. 2009;69:8–12. doi: 10.1080/00365510701864610. [DOI] [PubMed] [Google Scholar]
- 24.Zhong Z, Ewers M, Teipel S, Bürger K, Wallin A, Blennow K, et al. Levels of beta-secretase (BACE1) in cerebrospinal fluid as a predictor of risk in mild cognitive impairment. Arch Gen Psychiatry. 2007;64:718–726. doi: 10.1001/archpsyc.64.6.718. [DOI] [PubMed] [Google Scholar]
- 25.Dineen TA, Weiss MM, Williamson T, Acton P, Babu-Khan S, Bartberger MD, et al. Design and Synthesis of Potent, Orally Efficacious Hydroxyethylamine Derived β-Site Amyloid Precursor Protein Cleaving Enzyme (BACE1) Inhibitors. J Med Chem. 2012;55:9025–9044. doi: 10.1021/jm300118s. [DOI] [PubMed] [Google Scholar]
- 26.Weiss MM, Williamson T, Babu-Khan S, Bartberger MD, Brown J, Chen K, et al. Design and Preparation of a Potent Series of Hydroxyethylamine Containing β-Secretase Inhibitors That Demonstrate Robust Reduction of Central β-Amyloid. J Med Chem. 2012;55:9009–9024. doi: 10.1021/jm300119p. [DOI] [PubMed] [Google Scholar]
- 27.May PC, Dean RA, Lowe SL, Martenyi F, Sheehan SM, Boggs LN, et al. Robust central reduction of amyloid-β in humans with an orally available, non-peptidic β-secretase inhibitor. J Neurosci. 2011;31:16507–16516. doi: 10.1523/JNEUROSCI.3647-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lahiri DK, Maloney B, Long JM, Greig NH. Lessons from a BACE1 inhibitor trial: Off-site but not off base. Alzheimers Dement. 2014 doi: 10.1016/j.jalz.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yan R, Vassar R. Targeting the β secretase BACE1 for Alzheimer’s disease therapy. Lancet Neurol. 2014;13:319–329. doi: 10.1016/S1474-4422(13)70276-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghosh AK, Osswald HL. BACE1 (β-secretase) inhibitors for the treatment of Alzheimer’s disease. Chem Soc Rev. 2014 doi: 10.1039/C3CS60460H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morris JC, Cummings J. Mild cognitive impairment (MCI) represents early-stage Alzheimer’s disease. J Alzheimers Dis. 2005;7:235–239. doi: 10.3233/jad-2005-7306. [DOI] [PubMed] [Google Scholar]
- 32.Petersen RC, Bennett D. Mild cognitive impairment: is it Alzheimer’s disease or not? J Alzheimers Dis. 2005;7:241–245. doi: 10.3233/jad-2005-7307. [DOI] [PubMed] [Google Scholar]
- 33.Petersen RC. Clinical practice. Mild cognitive impairment. N Engl J Med. 2011;364:2227–2234. doi: 10.1056/NEJMcp0910237. [DOI] [PubMed] [Google Scholar]
- 34.Mulder SD, van der Flier WM, Verheijen JH, Mulder C, Scheltens P, Blankenstein MA, et al. BACE1 activity in cerebrospinal fluid and its relation to markers of AD pathology. J Alzheimers Dis. 2010;20:253–260. doi: 10.3233/JAD-2010-1367. [DOI] [PubMed] [Google Scholar]
- 35.Ewers M, Zhong Z, Bürger K, Wallin A, Blennow K, Teipel SJ, et al. Increased CSF-BACE 1 activity is associated with ApoE-epsilon 4 genotype in subjects with mild cognitive impairment and Alzheimer’s disease. Brain. 2008;131:1252–1258. doi: 10.1093/brain/awn034. [DOI] [PubMed] [Google Scholar]
- 36.Ewers M, Cheng X, Zhong Z, Nural HF, Walsh C, Meindl T, et al. Increased CSF-BACE1 activity associated with decreased hippocampus volume in Alzheimer’s disease. J Alzheimers Dis. 2011;25:373–381. doi: 10.3233/JAD-2011-091153. [DOI] [PubMed] [Google Scholar]
- 37.de Almeida SM, Shumaker SD, LeBlanc SK, Delaney P, Marquie-Beck J, Ueland S, et al. Incidence of post-dural puncture headache in research volunteers. Headache. 2011;51:1503–1510. doi: 10.1111/j.1526-4610.2011.01959.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zetterberg H, Tullhög K, Hansson O, Minthon L, Londos E, Blennow K. Low incidence of post-lumbar puncture headache in 1,089 consecutive memory clinic patients. Eur Neurol. 2010;63:326–330. doi: 10.1159/000311703. [DOI] [PubMed] [Google Scholar]
- 39.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 40.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease. recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morris JC, Mohs RC, Rogers H, Fillenbaum G, Heyman A. Consortium to establish a registry for Alzheimer’s disease (CERAD) clinical and neuropsychological assessment of Alzheimer’s disease. Psychopharmacol Bull. 1988;24:641–652. [PubMed] [Google Scholar]
- 42.Robins LN, Wing J, Wittchen HU, Helzer JE, Babor TF, Burke J, et al. The Composite International Diagnostic Interview. An epidemiologic Instrument suitable for use in conjunction with different diagnostic systems and in different cultures. Arch Gen Psychiatry. 1988;45:1069–1077. doi: 10.1001/archpsyc.1988.01800360017003. [DOI] [PubMed] [Google Scholar]
- 43.Andrau D1, Dumanchin-Njock C, Ayral E, Vizzavona J, Farzan M, Boisbrun M, et al. BACE1- and BACE2-expressing human cells: characterization of beta-amyloid precursor protein-derived catabolites, design of a novel fluorimetric assay, and identification of new in vitro inhibitors. J Biol Chem. 2003;278:25859–66. doi: 10.1074/jbc.M302622200. [DOI] [PubMed] [Google Scholar]
- 44.Hampel H, Lista S, Khachaturian ZS. Development of biomarkers to chart all Alzheimer’s disease stages: the royal road to cutting the therapeutic Gordian Knot. Alzheimers Dement. 2012;8:312–336. doi: 10.1016/j.jalz.2012.05.2116. [DOI] [PubMed] [Google Scholar]
- 45.Hampel H, Lista S. Alzheimer disease: From inherited to sporadic AD crossing the biomarker bridge. Nat Rev Neurol. 2012;8:598–600. doi: 10.1038/nrneurol.2012.202. [DOI] [PubMed] [Google Scholar]
- 46.Mullard A. Sting of Alzheimer’s failures offset by upcoming prevention trials. Nat Rev Drug Discov. 2012;11:657–660. doi: 10.1038/nrd3842. [DOI] [PubMed] [Google Scholar]
- 47.Wadman M. US government sets out Alzheimer’s plan. Nature. 2012;485:426–427. doi: 10.1038/485426a. [DOI] [PubMed] [Google Scholar]
- 48.Andreasson U, Portelius E, Andersson ME, Blennow K, Zetterberg H. Aspects of beta-amyloid as a biomarker for Alzheimer’s disease. Biomark Med. 2007;1:59–78. doi: 10.2217/17520363.1.1.59. [DOI] [PubMed] [Google Scholar]
- 49.Giusti-Rodríguez P, Gao J, Gräff J, Rei D, Soda T, Tsai LH. Synaptic deficits are rescued in the p25/Cdk5 model of neurodegeneration by the reduction of β-secretase (BACE1) J Neurosci. 2011;31:15751–15756. doi: 10.1523/JNEUROSCI.3588-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kuhn PH, Koroniak K, Hogl S, Colombo A, Zeitschel U, Willem M, et al. Secretome protein enrichment identifies physiological BACE1 protease substrates in neurons. EMBO J. 2012;31:3157–68. doi: 10.1038/emboj.2012.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shen Y, He P, Cheng X, Ewers M, Sabbagh M, Lehe T, et al. Plasma BACE1 levels are increased in patients of mild cognitive impairment and Alzheimer’s disease. Alzheimer’s Association 13th International Conference on Alzheimer’s Disease and Related Disorders; Paris, France. 2011. [Google Scholar]
- 52.Zetterberg H, Andreasson U, Hansson O, Wu G, Sankaranarayanan S, Andersson ME, et al. Elevated cerebrospinal fluid BACE1 activity in incipient Alzheimer disease. Arch Neurol. 2008;65:1102–1107. doi: 10.1001/archneur.65.8.1102. [DOI] [PubMed] [Google Scholar]
- 53.Winston C, Goetzl E, Akers J, Carter B, Rockenstein E, Galasko D, et al. Prediction of conversion from mild cognitive impairment to dementia with neuronally derived blood exosome protein profile. Alzheimer’s & Dementia: Diagnosis Assessment & Disease Monitoring. 2016 doi: 10.1016/j.dadm.2016.04.001. DOI: http://dx.doi.org/10.1016/j.dadm.2016.04.001. [DOI] [PMC free article] [PubMed]
- 54.van Harten AC, Kester MI, Visser PJ, Blankenstein MA, Pijnenburg YA, van der Flier WM, et al. Tau and p-tau as CSF biomarkers in dementia: a meta-analysis. Clin Chem Lab Med. 2011 Mar;49(3):353–66. doi: 10.1515/CCLM.2011.086. [DOI] [PubMed] [Google Scholar]
- 55.Leuzy A, Chiotis K, Hasselbalch SG, Rinne JO, de Mendonça A, Otto M, et al. Pittsburgh compound B imaging and cerebrospinal fluid amyloid-β in a multicentre European memory clinic study. Brain. 2016;139(Pt 9):2540–53. doi: 10.1093/brain/aww160. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.