Abstract
Introduction
Multiple pathways may exist for age-related tongue muscle degeneration. Cell death is one mechanism contributing to muscle atrophy and decreased function. We hypothesized with aging, apoptosis and apoptotic regulators would be increased, and muscle fiber size and number would be reduced in extrinsic tongue muscles.
Methods
Cell death indices, expression of caspase-3 and Bcl-2, and measures of muscle morphology and number were determined in extrinsic tongue muscles of young and old rats.
Results
Significant increases in cell death, caspase-3, and Bcl-2 were observed in all extrinsic tongue muscles along with reductions in muscle fiber number in old rats.
Discussion
We demonstrated that apoptosis indices increase with age in lingual muscles and that alterations in apoptotic regulators may be associated with age-related degeneration in muscle fiber size and number. These observed apoptotic processes may be detrimental to muscle function, and may contribute to degradation of cranial functions with age.
Keywords: age-related pathology, biology of aging, apoptosis, muscle, animal model, speech, swallowing
Introduction
In the next 30 years, the number of individuals 60 years and older will increase to more than 2 billion, representing 22% of the population.1 Sarcopenia, the age-related degeneration of skeletal muscle that culminates in reductions in muscle mass, strength, and function,2 may contribute to communication and swallowing deficits in elderly people, negatively impacting health, nutrition, respiratory function, and quality of life.3-8 Processes primary to the age-related decline in the cranial muscles have been understudied. Knowledge of underlying cellular mechanisms contributing to age-related muscular degeneration in the cranial muscles is a clinical priority due to the high incidence of communication and swallowing disorders in the aging population.
The tongue has an important role in speech, respiration, and swallowing. During speech, tongue position and movement is crucial to the production of vowels, consonants, and constant-vowel combinations.9-11 For respiratory actions, the tongue's primary function is to maintain patency of the upper airway through the dilation and/or narrowing of the pharynx during breathing.9,12-14 Additionally, the tongue is active during the swallow, contributing to the formation, transport, and propulsion of the bolus.9,15,16 Due to the cross-system role of the tongue musculature, age-associated reductions in tongue muscle structure and function may have deleterious effects on these critical actions.
Multiple causal pathways may exist for age-related degeneration of the tongue musculature that manifest as alterations in muscle fiber biochemistry and a transition toward more slowly contracting muscle fibers,17-19 reductions in neuromuscular junction size and number,20 decreased strength and force production,17,21,22 and increased fatigue.17,22 However, mechanisms underlying these morphological and physiological changes remain elusive. Possible causal pathways suggested by recent findings in limb muscles include cell apoptosis or programmed cell death.23-26 Apoptosis of single myonuclei along the length of the muscle fiber has been implicated as a cause of age-related reductions in total muscle fiber number and in muscle fiber diameter.23,27-33 It has been well established in limb musculature that apoptosis of myonuclei increases with age.26,33-35 In addition, resulting imbalances and/or misregulation in the capability of aged muscle to replace nuclei eliminated via apoptosis may be primary processes leading to muscle atrophy and ultimately to reductions in muscle mass, strength and function.23,25,34,36,37 Mechanisms that regulate apoptotic processes, such as mitochondrial dysfunction, mitochondrial damage, and increased caspase activity in the cell cytosol, may contribute to age-related muscle degeneration.38-40 The protein family Bcl-2 has a major role in regulating mitochondrial mediated cell apoptosis, and activation of caspase-3 by mitochondrial and/or the death receptor mediated apoptotic pathways execute cell death.39-43 However, processes identified in limb skeletal muscles may not be found in cranial skeletal muscles due to distinctions in muscle fiber types and geometric arrangements.44-48 Age-induced apoptosis has never been characterized in muscles of tongue and may represent a converging mechanism through which age-related cranial muscle degeneration ensues.
The purpose of this study was to examine the effect of age on cell apoptosis, protein regulators of cell apoptosis, and muscle fiber size and number in extrinsic tongue muscles of young adult and old rats. We hypothesized that with aging, cell death would be increased, protein expression of apoptotic regulators involved in intrinsic and extrinsic pathways of cell death would be altered, and that there would be a reduction in muscle fiber size and number in the genioglossus (GG), styloglossus (SG), and hyoglossus (HG) muscles of old compared to young adult rats.
Materials and Methods
Animals
This study was performed in accordance with the NIH Guide for Care and Use of Laboratory Animals, 8th Edition, 2011. The animal care and use protocol was approved by University of Wisconsin School of Medicine and Public Health Animal Care and Use Committee. A total of 8 young adult (9 months old) and 8 old (32 months old) male Fischer 344/Brown Norway rats were obtained from the National Institute on Aging colony (Harlan Laboratories, Indianapolis, IN). The Fischer 344/Brown Norway rat median life span is approximately 36 months of age.49 Rats were housed in standard polycarbonate cages in pairs in a light controlled environment with a 12:12-hour light-dark reversed light cycle with food and water provided ad libitum. Animals were obtained 2 weeks before experimentation to allow for acclimation to the vivarium and light cycle reversal.
Tissue collection
Rats were anesthetized with isoflurane (4%), and euthanized by an overdose of Beuthanasia (0.3 mL, IP injection). The genioglossus (GG), styloglossus (SG), and hyoglossus (HG) muscles were extracted and rinsed in 0.9% saline solution. Muscles from the right or left side of the tongue were either individually embedded in optimum cutting temperature compound (OCT) and snap-frozen in 2-methylbutane cooled by liquid nitrogen or immediately frozen in liquid nitrogen, and stored at -80°C until future use.
TUNEL assay and laminin staining analysis
DNA fragmentation was determined using a standard TUNEL assay (TdT-mediated dUTP nick end labeling; In Situ Cell Death Detection Kit, Roche). OCT embedded tissue was cut into serial 10 μm cross-sections using a -16°C cryostat (Leica CM 1850, Leica Biosystems) and mounted on slides. Tissue was then fixed with 4% paraformaldehyde and washed with phosphate-buffered saline (PBS). Tissue sections were permeabilized for 2 minutes on ice in a solution consisting of 0.1% Triton X-100 and 0.1% Triton, and washed with PBS. The TUNEL reaction mixture was then prepared per manufacturer's recommendations and was applied to cross-sections. Tissue sections were then incubated for 60 minutes at 37°C. Positive-controls were incubated for 10 minutes with DNase I recombinant (1000 U/ml in 50 mM Tris-HCl pH 7.5, 10 mM MgCl2, and 1 mg/ml BSA) to induce DNA strand breaks prior to TUNEL labeling. Negative-controls were incubated for 60 minutes at 37°C with label solution.
Following TUNEL labeling, tissue sections were washed with PBS and blocked in a solution of 10% normal goat serum and 0.3% Triton X-100 suspended in PBS for 20 minutes. Sections were then incubated for 1 hour at room temperature in diluted primary antibody (polyclonal rabbit anti-laminin IgG, Sigma) for cross-sectional area analysis and washed in PBS. Slides were then incubated with the fluorescent secondary antibody (Cy3 conjugated goat anti-rabbit IgG, Roche) for 1 hour at room temperature. Following PBS wash, slides were mounted with ProLong Gold Antifade with DAPI (Life Technologies) to visualize nuclei and stored at 4 °C to preserve fluorescence until microscopy was performed. Negative control slides were stained following the same procedure with omission of the primary antibody.
TUNEL- and DAPI-stained nuclei and laminin staining were examined under a fluorescent microscope (Nikon N-STORM) equipped with a digital camera (Andor iXon 897 EMCCD). The microscopist (HK) was masked to the animal age group. Photographs of four random non-overlapping images at an objective magnification of 20× were taken.50 FIJI (LOCI, University of Wisconsin Madison) was used to analyze images for quantification of indices of apoptosis. The number of TUNEL- and DAPI-positive nuclei were counted for each image. The TUNEL index was calculated by counting the number of TUNEL-positive nuclei divided by the total number of nuclei (DAPI-positive nuclei) multiplied by 100 for each of the four images from each muscle (GG, SG, and HG).50
The MATLAB application SMASH (Semi-automatic Muscle Analysis using Segmentation of Histology) was used to calculate GG and SG muscle fiber cross-sectional area (CSA; μm2), minimal Feret's diameter (minimum distance of parallel tangents at opposing borders of the muscle fiber; μm), and the coefficient of variance ((standard deviation/mean)ˆ103; %) in four non-overlapping images.51-55 The HG was excluded from muscle fiber morphometric analyses due to the high number of elongated fibers in each tissue cross-section which would not permit and confound accurate calculation of muscle fiber cross sectional area. True cross-sections were difficult to obtain because of the geometry of the HG muscle. Data analysis was constrained to muscle fibers between 150 and 2,500 μm2 to allow exclusion of non-muscle connective tissue bodies and vasculature.52 Elongated and elliptically shaped regions were also excluded.
Western Blot Analysis
Western blot analyses were performed to determine the protein expression of caspase-3 and Bcl-2 in the GG, SG, and HG muscles.56 Following tissue homogenization, the protein concentration was determined (DC Protein Assay; Bio-Rad), 50 μg of protein from each tissue sample was loaded into a 4-20% precast gradient gel (Criterion TGX, Bio-Rad), and gel electrophoresis was performed. Proteins were then transferred to a nitrocellulose membrane and washed in TBST (Tris buffered saline, 0.15% Tween 20). Membranes were blocked in 5% non-fat dry milk in TBST for one hour at room temperature, and then incubated with caspase-3 (Cell Signaling Technology) or Bcl-2 (BioLegend) antibodies overnight at 4°C. Following primary antibody incubation, membranes were washed in TBST and incubated for 1 hour in horseradish peroxidase-conjugated secondary antibodies (Cell Signaling Technology). After final TBST washes, membranes were developed (SuperSignal West Femto Chemiluminescent Substrate, Thermo Scientific) for 5 minutes using a ChemiDoc-It2 Imaging System (UVP). Following immunodetection, membranes were washed with TBST and stained with SimplyBlue SafeStain (Invitrogen) to determine the total amount of protein transferred for data normalization.57-59 Blots were analyzed with FIJI to quantify caspase-3 and Bcl-2 protein expression; data are represented as a ratio of protein band relative to the total protein in lieu of loading controls.
Statistical Analysis
T-tests were used to compare TUNEL indices, protein expression, the number of muscle fibers per unit area between each age group, and body weights between age groups. Pearson correlation coefficients were used to evaluate the relationship between body weight and all muscle fiber morphometric measures. Since weight is often associated with age, we used a one-way analysis of covariance (ANCOVA) with a body weight covariate to compare dependent variables between groups. The critical value of obtaining statistical significance was set at α=0.05. Data were presented as means ± SE. SPSS 23 was used for all analyses (IBM SPSS Statistics, IBM Corp., Armonk, NY).
Results
Body Weight
Body weights were significantly greater in the old rat group (t14=-9.82, p<.001). Because muscle fiber morphometric measures can be highly correlated with body size,60,61 Pearson correlation statistics were used to evaluate the relationship between body weight and all muscle fiber morphometric measures. We found moderate-to-high correlations and thus used a one-way analysis of covariance (ANCOVA) with a body weight covariate to compare dependent variables between groups (cross-sectional area, R = 0.576; minimal Feret's diameter, R = 0.617; coefficient of variance, R = -0.877).
TUNEL Index
In the old rat group, the number of nuclei staining positively for DNA fragmentation, an indication of cell apoptosis or programmed cell death, was significantly greater in the GG, SG, and HG muscles than in the young adult rat group (Fig. 1a-c: t14=-3.018, p=0.009; t14=-6.028, p=0.008; t14=-8.389; p<0.001; respectively). That is, with increasing age there was a significant increase in the index of cell death for all muscles studied in the extrinsic tongue. Representative images of immunostained cross-sections from young adult and old HG muscles are found in Figure 2.
Figure 1.
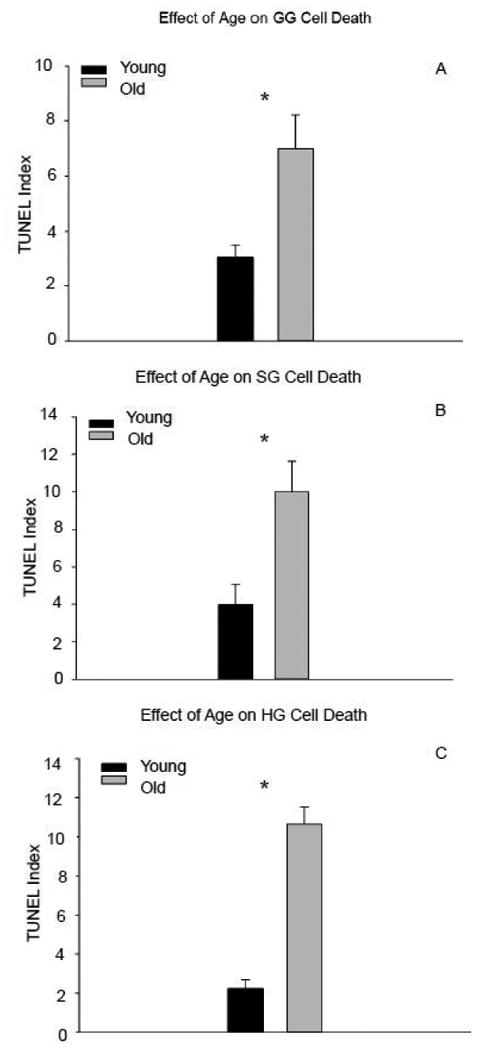
Cell death indices were significantly increased in the genioglossus (A), styloglossus (B), and hyoglossus (C) muscles of old versus young adult rats. (* signifies p<0.05)
Figure 2.
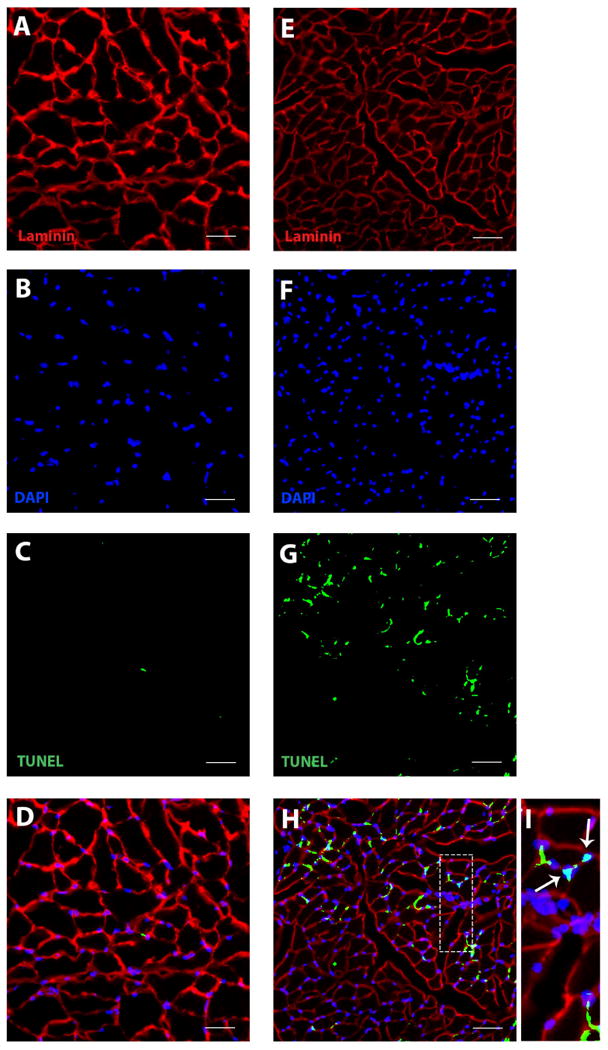
Representative images for TUNEL labeling for young adult (A, B, C, D) and old rat (E, F, G, H) hyoglossus muscles. Immunostaining of laminin (A, E) and myonuclei (B, F) was performed following TUNEL staining (C, G) to identify co-localization of TUNEL-positive myonuclei with regard to the basal lamina. Images were super imposed to allow for the identification of apoptotic muscle nuclei. The enlarged box demonstrates TUNEL-positive nuclei (arrow) with myonuclei in relation to the sarcolemma (I). (scale bar = 100 μm)
Protein Expression
The expression of full-length caspase-3 was significantly higher in all extrinsic tongue muscles of old compared to young adult rats (Fig 3a-c: GG, t14=-3.93, p<0.001; SG, t13=-6.38, p<0.001; and HG t14=-9.037, p<0.001; respectively). There was no evidence of caspase-3 cleavage in the extrinsic tongue muscles. With aging, there was also significantly increased expression of Bcl-2 in the GG (t14=-6.819, p<0.001; Fig. 4a), SG (t13=-6.494, p<0.001; Fig. 4b), and HG muscles (t14=-8.07, p<0.001; Fig. 4c). As demonstrated in Figure 5, protein expression of both caspase-3 and Bcl-2 increased in the extrinsic tongue musculature of old rats compared to young adult rats.
Figure 3.
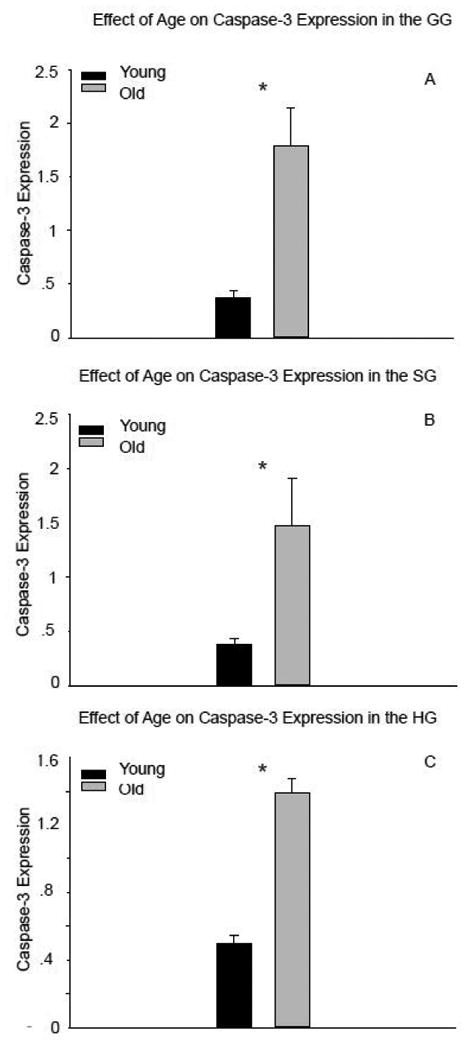
Caspase-3 protein expression was significantly elevated in all extrinsic muscles of old rats compared to young adult rats (genioglossus, A; styloglossus, B; hyoglossus, C). (* signifies p<0.05)
Figure 4.
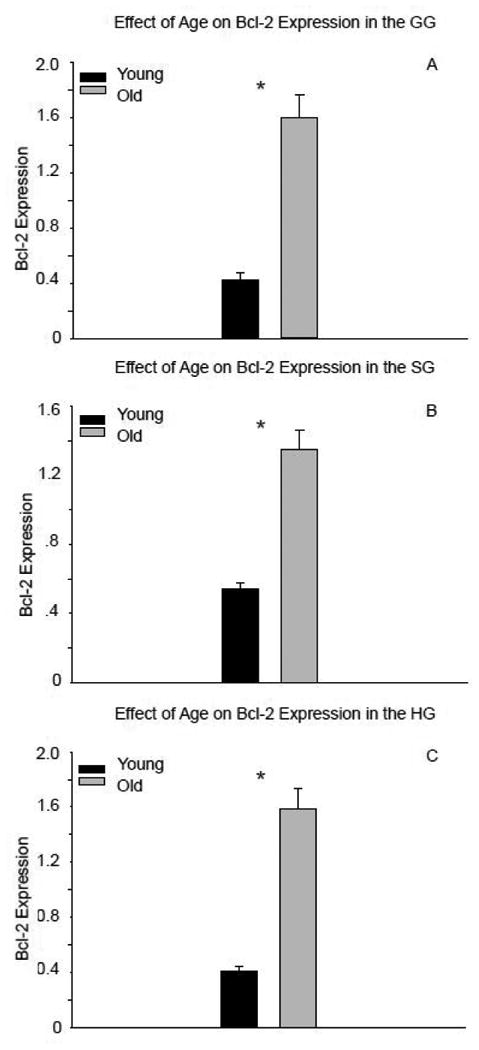
Expression of Bcl-2 significantly increased in the genioglossus (A), styloglossus (B), and hyoglossus (C) with age. (* signifies p<0.05)
Figure 5.
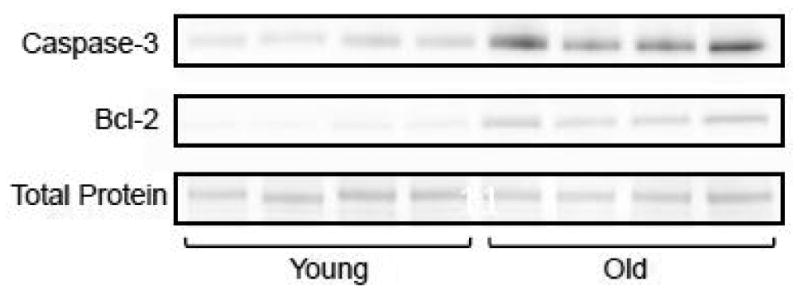
Representative immunoblots from the genioglossus muscle of 4 young adult and 4 old rats.
Muscle Morphology
Muscle fiber CSA and the minimum Feret's diameter were measured in a total of 1678 muscle fibers in the GG and 1604 muscle fibers in the SG. For each rat on average, 104 muscle fibers from each GG image and 100 muscle fibers from each SG image were measured. A coefficient of variance was determined for both GG and SG muscle fiber CSA and minimum Feret's diameter.
Genioglossus
With increasing age, muscle fiber diameter and CSA were not significantly reduced (respectively, F1, 15 = 4.58, p = 0.052; F1, 15 = 3.857, p = 0.072; Fig. 6a-b). Variability in muscle fiber diameter and size did not significantly change with increased age (F1, 15 = 0.028, p = 0.87; F1, 15 = 0.016, p = 0.90, respectively; Fig. 6c-d). However, there was a significant reduction in muscle fiber number in the old group versus the young adult group (t61= 2.12, p=0.038; Figure 7a).
Figure 6.
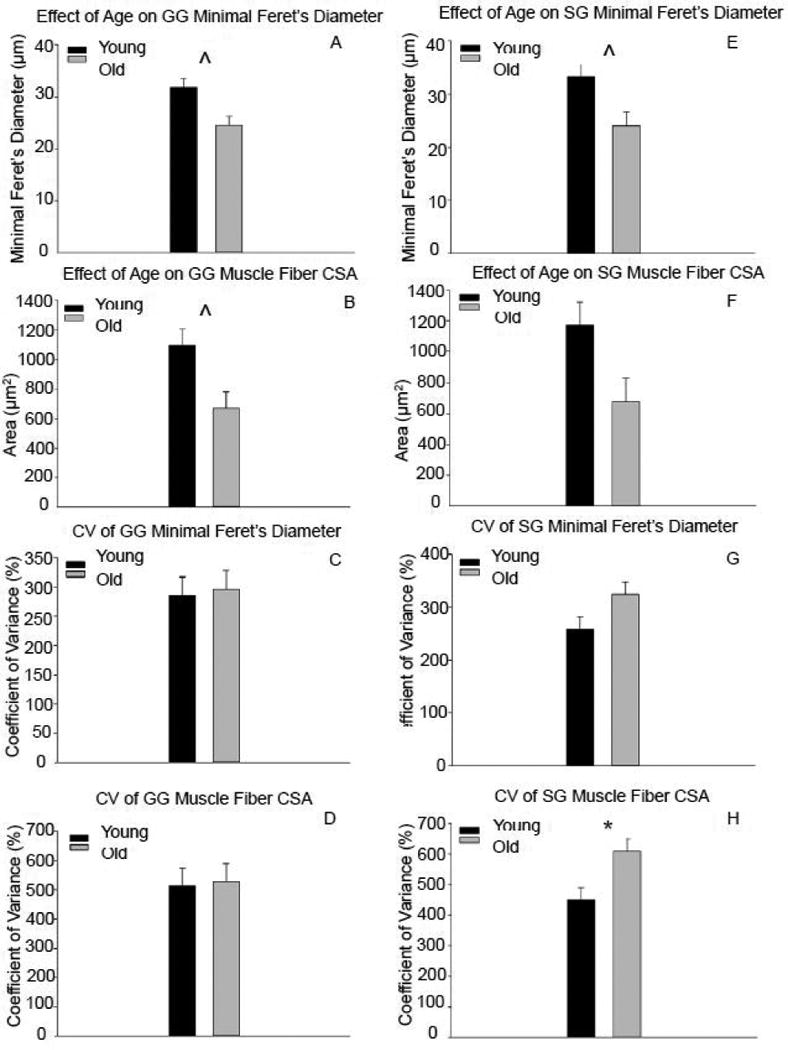
With increasing age, reductions in genioglossus muscle diameter and cross-sectional area were observed, but were not significantly different (A, B). In the styloglossus muscle, muscle fiber diameter was not significantly reduced with increasing age (E), and significantly more variability in muscle fiber cross-sectional area (H) was observed in the old rat group. (* signifies p<0.05; ˆ signifies p<0.08)
Figure 7.
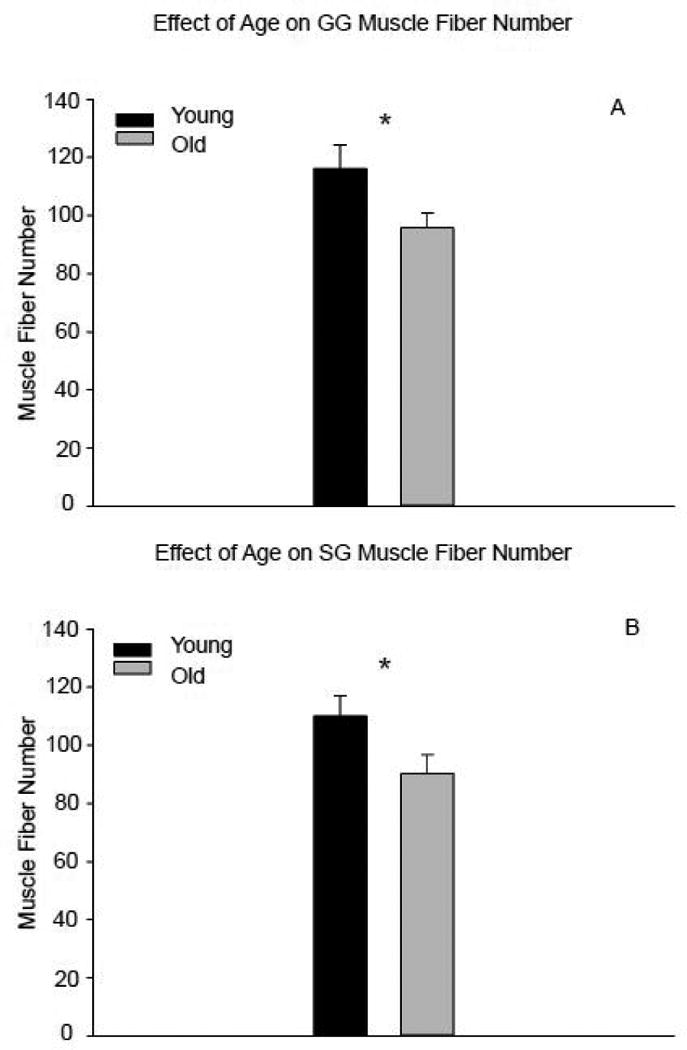
There was a significant reduction in the number of muscle fibers in the old versus young adult rats in the genioglossus (A) and styloglossus muscles (B). (* signifies p<0.05)
Styloglossus
Muscle fiber diameter was not significantly decreased with age (F1, 15 = 3.66, p = 0.078; Fig. 6e). There was no difference in muscle fiber CSA with increasing age (F1, 15 = 2.913, p = 0.11; Fig. 6f). The old group had more variability in muscle fiber CSA compared to the young adult group (F1, 15 = 4.59, p = 0.052; Fig. 6h). However, variations in muscle fiber diameter were not observed with increased age (F1, 15 = 2.22, p = 0.16; Fig. 6g]. The old group had a significant reduction in muscle fiber number compared with the young adult group (t62= 2.05, p=0.044; Fig. 7b).
Discussion
Our results supported our hypotheses in part. We observed increased cell death and expression of apoptotic regulator proteins in all muscles of the extrinsic tongue with age, a reduction in muscle fiber number in the genioglossus and styloglossus, and higher variability of myofiber size in the styloglossus muscle. The results are indicative of an underlying cellular mechanism that may contribute to age-related lingual muscle degeneration, and, if also found in humans, may be associated with the development of oromotor decline evidenced in elderly people.62-64
Cell apoptosis, or programmed cell death, is characterized by a series of molecular, biochemical, and morphological changes that result in cellular self-destruction.34,65-67 Activated by two main pathways, intrinsic (mitochondrial mediated) or extrinsic (death receptor mediated), apoptosis is a conserved molecular process that occurs normally during development and aging, and is an important homeostatic mechanism that maintains cellular populations in a variety of tissues.34,67,68 The mitochondrial mediated pathway to cell death is highly regulated by the Bcl-2 protein family.38,40,42,43 Cytotoxic stress results in the imbalance of apoptotic regulator proteins, such as Bcl-2, and initiates a cascade of events that lead to cell death through the release of apoptogenic factors from the mitochondria into the cell cytosol. Mitochondrial and death receptor mediated pathways of cell death intersect at the activation of caspase-3 in the cell cytosol.40 The main executioner of cell death, caspase-3 coordinates the destruction of key cellular proteins and initiates apoptotic DNA fragmentation.39,41,42 Our findings in muscles of the tongue were consistent with cell death pathways proposed in the limb literature.24,69 With aging, we also observed upregulated expression of apoptotic regulator proteins, Bcl-2 and caspase-3, suggesting that both extrinsic and intrinsic mediated pathways of cell death may contribute to the increased DNA fragmentation we detected in extrinsic tongue muscles of old rats.
Age-related increases in muscle cell apoptosis have been found in the soleus, extensor digitorum, and gastrocnemius muscles in aging Fischer 344/Brown Norway rats,23-25 and in the vastus lateralis muscle of elderly humans.27,28 In our study, significant increases in nuclear DNA fragmentation, indicative of cell apoptosis, were observed in the genioglossus, styloglossus, and hyoglosuss muscles of old rats compared to young adult rats. We observed elevated Bcl-2 and caspase-3 protein expression with increasing age, that is comparable to previously reported data in aged rat limb muscle.24,69 The increase in Bcl-2, an anti-apoptotic regulator, may be a compensatory measure to combat an increase in pro-apoptotic events occurring during mitochondrial mediated cell death, in order to prevent the age-related loss of muscle mass and size.70-72 However, an alternative explanation for elevated Bcl-2 may be related to the protein's loss of function as an anti-apoptotic regulator.69,71 Previous studies have reported that phosphorylation of Bcl-2 inactivates the anti-apoptotic function of the protein, and that Bcl-2 phosphorylation increases with age in skeletal muscles in conjunction with increased Bcl-2 expression.69 This suggests that Bcl-2 expression may be upregulated to counteract the increase in the biologically inactive phosphorylated form of the protein. To further elucidate the mechanism of increased Bcl-2 elevation, it would be helpful in the future to examine the ratio of pro- and anti-apoptotic regulators of the Bcl-2 family of proteins, and determine the level of Bcl-2 phosphorylation. With aging, we also observed increased expression of full length caspase-3, without a concomitant increase of the activated and cleaved form, in the GG, SG, and HG muscles. This is consistent with previous reports from the limb literature.24 An age-related increase in the full length caspase-3 protein may suggest that extrinsic tongue muscles are more susceptible to apoptosis.70,71,73,74 The absence of the activated, cleaved form of caspase-3, may mean that few muscles fibers are actively undergoing apoptosis at the 32 month time-point24 or may, in fact, be indicative of a caspase-independent pathway of age-related myonuclear loss.38,70,71,73,74 Misregulation of these apoptotic processes may have deleterious consequences in the affected tissue.36,66
In aging tongue muscle, alterations in the apoptotic regulator proteins, Bcl-2 and caspase-3, resulting in increased activation of apoptotic processes, may have led to the removal of individual myonuclei, the associated sarcoplasm, 65,66 and contributed to the eventual atrophy and loss of muscle fibers we observed in the genioglossus and styloglossus muscles of old rats. When an imbalance exists between mechanisms of muscle regeneration and degeneration, an increase in apoptotic signaling may lead to protein degradation, fiber atrophy, myofiber loss, and decreased function in that muscle.32,34-37,68,75-77 As such, the morphological changes in muscle fiber size and reductions in myofiber number, consistent with age-related sarcopenia, that we observed in GG and SG muscles of our study may be associated with increased muscle cell death.24,29 Consistent with limb literature, we observed reductions in muscle fiber cross-sectional area (CSA), minimal Feret's diameter, a robust measure of muscle fiber size due to its insensitivity to deviations from an optimal cross-section, and a significant reduction in muscle fiber number with increasing age in the GG and SG muscles. Because of the high variability of muscle fiber size in the cranial musculature, we also determined the variance coefficient for muscle fiber CSA and minimal Feret's diameter.53,54,78 We identified significantly greater variability of muscle fiber CSA in the SG of old rats, which could be indicative of atrophic muscle fibers and a disruption in the ability of the muscle to replace and/or repair damaged or degenerating fibers.52-54 Our results suggest that age-related muscle atrophy may be associated with increased activation or the misregulation of apoptotic processes in the extrinsic tongue muscles. The ramifications of age-related myonuclear loss and reductions in myofiber size and number may be detrimental to muscle function, leading to losses in muscle mass and contractile function, and must be investigated further.
Apoptosis may be an underlying cellular mechanism contributing to age-related cranial muscle degeneration. Understanding how age-related processes contribute to other manifestations of aging, the destruction of myonuclei and myofibers in tongue muscles, and functional deficits is crucial to the development of potential pharmacological and exercise-based therapies for communication and swallowing disorders.
Acknowledgments
This work was supported by the National Institute of Health: the National Institute on Deafness and Other Communication Disorders, grants R01DC005935, R01DC008149, R01DC01458, and T32 DC009401, and by the National Institute on Aging, grant F31AG054315. The authors gratefully acknowledge the assistance of Dr. Miranda J. Cullins, Dr. Tiffany J. Glass, Kellie Bowen, and Dr. John A. Russell for their support and critical analysis of the study.
Abbreviations
- ANCOVA
analysis of covariance
- CSA
cross-sectional area
- GG
genioglossus
- HG
hyoglossus
- MHC
myosin heavy chain
- OCT
optimum cutting temperature compound
- PBS
phosphate-buffered saline
- SG
styloglossus
- SMASH
Semi-automatic Muscle Analysis using Segmentation of Histology
- TUNEL
TdT-mediated dUTP nick end labeling
- TBST
Tris buffered saline
Footnotes
Ethical Publication Statement: We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Disclosure of Conflicts of Interest: None of the authors has any conflict of interest to disclose.
Part of the material contained within this manuscript was presented at the 24th Annual Meeting of the Dysphagia Research Society in Tucson, AZ February 25-27, 2016.
References
- 1.Nations U. World population ageing 2013. Department of Economic and Social Affairs PD. 2013 [Google Scholar]
- 2.Rosenberg IH. Epidemiologic and methodologic problems in determing nutritional status of older persons - Proceedings of a conference held in Albuquerque, New Mexico, October 19-21, 1988 - Summary Comments. American Journal of Clinical Nutrition. 1989;50(5):1231–1233. [PubMed] [Google Scholar]
- 3.Martin BJ, Corlew MM, Wood H, Olson D, Golopol LA, Wingo M, Kirmani N. The association of swallowing dysfunction and aspiration pneumonia. Dysphagia. 1994;9(1):1–6. doi: 10.1007/BF00262751. [DOI] [PubMed] [Google Scholar]
- 4.Odderson IR, Keaton JC, McKenna BS. Swallow Management in Patients on an Acute Stroke Pathway - Quality is Cost-Effective. Archives of Physical Medicine and Rehabilitation. 1995;76(12):1130–1133. doi: 10.1016/s0003-9993(95)80121-9. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt J, Holas M, Halvorson K, Reding M. Videofluoroscopic evidence of aspiration predicts pneumonia and death but not dehydration following stroke. Dysphagia. 1994;9(1):7–11. doi: 10.1007/BF00262752. [DOI] [PubMed] [Google Scholar]
- 6.Baum BJ, Bodner L. Aging and Oral Motor Function - Evidence for Altered Performance among Older Persons. Journal of dental research. 1983;62(1):2–6. doi: 10.1177/00220345830620010401. [DOI] [PubMed] [Google Scholar]
- 7.Clark HM, Henson PA, Barber WD, Stierwalt JA, Sherrill M. Relationships among subjective and objective measures of tongue strength and oral phase swallowing impairments. American journal of speech-language pathology / American Speech-Language-Hearing Association. 2003;12(1):40–50. doi: 10.1044/1058-0360(2003/051). [DOI] [PubMed] [Google Scholar]
- 8.Roy N, Stemple J, Merrill RM, Thomas L. Dysphagia in the elderly: Preliminary evidence of prevalence, risk factors, and socioemotional effects. Annals of Otology Rhinology and Laryngology. 2007;116(11):858–865. doi: 10.1177/000348940711601112. [DOI] [PubMed] [Google Scholar]
- 9.Hiiemae KM, Palmer JB. Tongue movements in feeding and speech. Critical Reviews in Oral Biology & Medicine. 2003;14(6):413–429. doi: 10.1177/154411130301400604. [DOI] [PubMed] [Google Scholar]
- 10.Holstege G, Subramanian HH. Two different motor systems are needed to generate human speech. Journal of Comparative Neurology. 2016;524(8):1558–1577. doi: 10.1002/cne.23898. [DOI] [PubMed] [Google Scholar]
- 11.Stone M, Epstein MA, Iskarous K. Functional segments in tongue movement. Clinical Linguistics & Phonetics. 2004;18(6-8):507–521. doi: 10.1080/02699200410003583. [DOI] [PubMed] [Google Scholar]
- 12.Fregosi RF. Influence of tongue muscle contraction and transmural pressure on nasopharyngeal geometry in the rat. Journal of applied physiology. 2011;111(3):766–774. doi: 10.1152/japplphysiol.01501.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fregosi RF, Fuller DD. Respiratory-related control of extrinsic tongue muscle activity. Respiration physiology. 1997;110(2-3):295–306. doi: 10.1016/s0034-5687(97)00095-9. [DOI] [PubMed] [Google Scholar]
- 14.Fuller DD, Williams JS, Janssen PL, Fregosi RF. Effect of co-activation of tongue protrudor and retractor muscles on tongue movements and pharyngeal airflow mechanics in the rat. Journal of Physiology-London. 1999;519(2):601–613. doi: 10.1111/j.1469-7793.1999.0601m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palmer JB, Hiiemae KM. Eating and breathing: Interactions between respiration and feeding on solid food. Dysphagia. 2003;18(3):169–178. doi: 10.1007/s00455-002-0097-9. [DOI] [PubMed] [Google Scholar]
- 16.Van Daele DJ, McCulloch TM, Palmer PM, Langmore SE. Timing of glottic closure during swallowing: A combined electromyographic and endoscopic analysis. Annals of Otology Rhinology and Laryngology. 2005;114(6):478–487. doi: 10.1177/000348940511400610. [DOI] [PubMed] [Google Scholar]
- 17.Kletzien H, Russell JA, Leverson GE, Connor NP. Differential effects of targeted tongue exercise and treadmill running on aging tongue muscle structure and contractile properties. Journal of Applied Physiology. 2013;114(4):472–481. doi: 10.1152/japplphysiol.01370.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Connor NP, Russell JA, Jackson MA, Kletzien H, Wang H, Schaser AJ, Leverson GE, Zealear DL. Tongue muscle plasticity following hypoglossal nerve stimulation in aged rats. Muscle & nerve. 2012 doi: 10.1002/mus.23499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schaser AJ, Wang H, Volz LM, Connor NP. Biochemistry of the Anterior, Medial, and Posterior Genioglossus in the Aged Rat. Dysphagia. 2011;26(3):256–263. doi: 10.1007/s00455-010-9297-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson AM, Connor NP. Effects of Electrical Stimulation on Neuromuscular Junction Morphology in the Aging Rat Tongue. Muscle & nerve. 2011;43(2):203–211. doi: 10.1002/mus.21819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Russell JA, Connor NP. Effects of age and radiation treatment on function of extrinsic tongue muscles. Radiation Oncology. 2014;9 doi: 10.1186/s13014-014-0254-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Becker BJ, Russell JA, Connor NP. Effects of aging on evoked retrusive tongue actions. Archives of oral biology. 2015;60(6):966–971. doi: 10.1016/j.archoralbio.2015.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dirks A, Leeuwenburgh C. Apoptosis in skeletal muscle with aging. American Journal of Physiology-Regulatory Integrative and Comparative Physiology. 2002;282(2):R519–R527. doi: 10.1152/ajpregu.00458.2001. [DOI] [PubMed] [Google Scholar]
- 24.Rice KM, Blough ER. Sarcopenia-related apoptosis is regulated differently in fast- and slow-twitch muscles of the aging F344/N × BN rat model. Mechanisms of Ageing and Development. 2006;127(8):670–679. doi: 10.1016/j.mad.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 25.Dirks AJ, Leeuwenburgh C. The role of apoptosis in age-related skeletal muscle atrophy. Sports Medicine. 2005;35(6):473–483. doi: 10.2165/00007256-200535060-00002. [DOI] [PubMed] [Google Scholar]
- 26.Dirks-Naylor AJ, Lennon-Edwards S. Cellular and molecular mechanisms of apoptosis in age-related muscle atrophy. Current aging science. 2011;4(3):269–278. [PubMed] [Google Scholar]
- 27.Lexell J, Taylor CC, Sjostrom M. What is the cuase of the aging atrophy-total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15-year-old to 83-year-old men. Journal of the Neurological Sciences. 1988;84(2-3):275–294. doi: 10.1016/0022-510x(88)90132-3. [DOI] [PubMed] [Google Scholar]
- 28.Lexell J. Human aging, muscle mass, and fiber-type composition. Journals of Gerontology Series a-Biological Sciences and Medical Sciences. 1995;50:11–16. doi: 10.1093/gerona/50a.special_issue.11. [DOI] [PubMed] [Google Scholar]
- 29.Brunner F, Schmid A, Sheikhzadeh A, Nordin M, Yoon J, Frankel V. Effects of aging on type II muscle fibers: A systematic review of the literature. Journal of Aging and Physical Activity. 2007;15(3):336–348. doi: 10.1123/japa.15.3.336. [DOI] [PubMed] [Google Scholar]
- 30.Deschenes MR. Effects of aging on muscle fibre type and size. Sports Medicine. 2004;34(12):809–824. doi: 10.2165/00007256-200434120-00002. [DOI] [PubMed] [Google Scholar]
- 31.Fulle S, Centurione L, Mancinelli R, Sancilio S, Manzoli FA, Di Pietro R. Stem Cell Ageing and Apoptosis. Current Pharmaceutical Design. 2012;18(13):1694–1717. doi: 10.2174/138161212799859657. [DOI] [PubMed] [Google Scholar]
- 32.Alway SE, Siu PM. Nuclear apoptosis contributes to sarcopenia. Exercise and Sport Sciences Reviews. 2008;36(2):51–57. doi: 10.1097/JES.0b013e318168e9dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Warner HR. Aging and regulation of apoptosis. Current Topics in Cellular Regulation, Vol 35. 1997;35:107–121. doi: 10.1016/s0070-2137(97)80004-0. [DOI] [PubMed] [Google Scholar]
- 34.Tower J. Programmed cell death in aging. Ageing Research Reviews. 2015;23:90–100. doi: 10.1016/j.arr.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adhihetty PJ, Hood DA. Mechanisms of apoptosis in skeletal muscle. BAM-PADOVA- 2003;13(4):171–180. [Google Scholar]
- 36.Marzetti E, Calvani R, Bernabei R, Leeuwenburgh C. Apoptosis in Skeletal Myocytes: A Potential Target for Interventions against Sarcopenia and Physical Frailty - A Mini-Review. Gerontology. 2012;58(2):99–106. doi: 10.1159/000330064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marzetti E, Calvani R, Cesari M, Buford TW, Lorenzi M, Behnke BJ, Leeuwenburgh C. Mitochondrial dysfunction and sarcopenia of aging: From signaling pathways to clinical trials. International Journal of Biochemistry & Cell Biology. 2013;45(10):2288–2301. doi: 10.1016/j.biocel.2013.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peterson CM, Johannsen DL, Ravussin E. Skeletal muscle mitochondria and aging: a review. Journal of aging research. 2012;2012:194821. doi: 10.1155/2012/194821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kumar S. Caspase function in programmed cell death. Cell Death and Differentiation. 2007;14(1):32–43. doi: 10.1038/sj.cdd.4402060. [DOI] [PubMed] [Google Scholar]
- 40.Czabotar PE, Lessene G, Strasser A, Adams JM. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nature Reviews Molecular Cell Biology. 2014;15(1):49–63. doi: 10.1038/nrm3722. [DOI] [PubMed] [Google Scholar]
- 41.McIlwain DR, Berger T, Mak TW. Caspase Functions in Cell Death and Disease (vol 5, a008656, 2013) Cold Spring Harbor Perspectives in Biology. 2015;7(4) doi: 10.1101/cshperspect.a008656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wolf BB, Schuler M, Echeverri F, Green DR. Caspase-3 is the primary activator of apoptotic DNA fragmentation via DNA fragmentation factor-45/inhibitor of caspase-activated DNase inactivation. Journal of Biological Chemistry. 1999;274(43):30651–30656. doi: 10.1074/jbc.274.43.30651. [DOI] [PubMed] [Google Scholar]
- 43.Cory S, Huang DCS, Adams JM. The Bcl-2 family: roles in cell survival and oncogenesis. Oncogene. 2003;22(53):8590–8607. doi: 10.1038/sj.onc.1207102. [DOI] [PubMed] [Google Scholar]
- 44.Connor NP, Ota F, Nagai H, Russell JA, Leverson G. Differences in age-related alterations in muscle contraction properties in rat tongue and hindlimb. Journal of Speech Language and Hearing Research. 2008;51(4):818–827. doi: 10.1044/1092-4388(2008/059). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller JL, Watkin KL, Chen MF. Muscle, adipose, and connective tissue variations in intrinsic musculature of the adult human tongue. Journal of Speech Language and Hearing Research. 2002;45(1):51–65. doi: 10.1044/1092-4388(2002/004). [DOI] [PubMed] [Google Scholar]
- 46.Mu LC, Sanders I. Neuromuscular organization of the canine tongue. Anatomical Record. 1999;256(4):412–424. doi: 10.1002/(SICI)1097-0185(19991201)256:4<412::AID-AR8>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 47.Gilbert RJ, Napadow VJ. Three-dimensional muscular architecture of the human tongue determined in vivo with diffusion tensor magnetic resonance imaging. Dysphagia. 2005;20(1):1–7. doi: 10.1007/s00455-003-0505-9. [DOI] [PubMed] [Google Scholar]
- 48.Gilbert RJ, Napadow VJ, Gaige TA, Wedeen VJ. Anatomical basis of lingual hydrostatic deformation. The Journal of experimental biology. 2007;210(Pt 23):4069–4082. doi: 10.1242/jeb.007096. [DOI] [PubMed] [Google Scholar]
- 49.Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. Growth curves and survival characteristics of the animals used in the biomarkers of aging program. Journals of Gerontology Series A-Biological Sciences and Medical Sciences. 1999;54(11):B492–B501. doi: 10.1093/gerona/54.11.b492. [DOI] [PubMed] [Google Scholar]
- 50.Siu PM, Tam EW, Teng BT, Pei XM, Ng JW, Benzie IF, Mak AF. Muscle apoptosis is induced in pressure-induced deep tissue injury. Journal of Applied Physiology. 2009;107(4):1266–1275. doi: 10.1152/japplphysiol.90897.2008. [DOI] [PubMed] [Google Scholar]
- 51.Ceglia L, Niramitmahapanya S, Price LL, Harris SS, Fielding RA, Dawson-Hughes B. An evaluation of the reliability of muscle fiber cross-sectional area and fiber number measurements in rat skeletal muscle. Biological Procedures Online. 2013;15 doi: 10.1186/1480-9222-15-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith LR, Barton ER. SMASH - semi-automatic muscle analysis using segmentation of histology: a MATLAB application. Skeletal Muscle. 2014;4 doi: 10.1186/2044-5040-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Coulton GR, Morgan JE, Partridge TA, Sloper JC. The mdx mouse skeletal-muscle myopathy.1. A hisological, morphometric and biochemical investigation. Neuropathology and Applied Neurobiology. 1988;14(1):53–70. doi: 10.1111/j.1365-2990.1988.tb00866.x. [DOI] [PubMed] [Google Scholar]
- 54.Briguet A, Courdier-Fruh I, Foster M, Meier T, Magyar JP. Histological parameters for the quantitative assessment of muscular dystrophy in the mdx-mouse. Neuromuscular Disorders. 2004;14(10):675–682. doi: 10.1016/j.nmd.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 55.Dubache-Powell J. Quantitative determination of muscle fiber diameter (minimal Feret's diameter) and percentage of centralized nuclei. TREAT-NMD Neuromuscular Network SOP M. 2008;1 [Google Scholar]
- 56.Janes KA. An analysis of critical factors for quantitative immunoblotting. Science signaling. 2015;8(371):rs2. doi: 10.1126/scisignal.2005966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eaton SL, Roche SL, Hurtado ML, Oldknow KJ, Farquharson C, Gillingwater TH, Wishart TM. Total Protein Analysis as a Reliable Loading Control for Quantitative Fluorescent Western Blotting. Plos One. 2013;8(8) doi: 10.1371/journal.pone.0072457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Welinder C, Ekblad L. Coomassie Staining as Loading Control in Western Blot Analysis. Journal of Proteome Research. 2011;10(3):1416–1419. doi: 10.1021/pr1011476. [DOI] [PubMed] [Google Scholar]
- 59.Piec I, Listrat A, Alliot J, Chambon C, Taylor RG, Bechet D. Differential proteome analysis of aging in rat skeletal muscle. Faseb Journal. 2005;19(6):1143. doi: 10.1096/fj.04-3084fje. -+ [DOI] [PubMed] [Google Scholar]
- 60.Kim GD, Kim BW, Jeong JY, Hur SJ, Cho IC, Lim HT, Joo ST. Relationship of Carcass Weight to Muscle Fiber Characteristics and Pork Quality of Crossbred (Korean Native Black Pig × Landrace) F-2 Pigs. Food and Bioprocess Technology. 2013;6(2):522–529. [Google Scholar]
- 61.Tamaki T, Uchiyama S. Absolute and relative growth of rat skeletal muscle. Physiology & Behavior. 1995;57(5):913–919. doi: 10.1016/0031-9384(94)00359-d. [DOI] [PubMed] [Google Scholar]
- 62.Lirani-Silva C, Mourao LF, Gobbi LT. Dysarthria and Quality of Life in neurologically healthy elderly and patients with Parkinson's disease. Codas. 2015;27(3):248–254. doi: 10.1590/2317-1782/20152014083. [DOI] [PubMed] [Google Scholar]
- 63.Yorkston KM, Bourgeois MS, Baylor CR. Communication and Aging. Phys Med Rehabil Clin N Am. 2010;21(2):309–319. doi: 10.1016/j.pmr.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Caruso AJ, McClowry MT, Max L. Age-related effects on speech fluency. © 1997 by Thieme Medical Publishers, Inc; 1997. pp. 171–180. [DOI] [PubMed] [Google Scholar]
- 65.Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407(6805):770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 66.Hotchkiss RS, Strasser A, McDunn JE, Swanson PE. Mechanisms of disease Cell Death. New England Journal of Medicine. 2009;361(16):1570–1583. doi: 10.1056/NEJMra0901217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Elmore S. Apoptosis: A review of programmed cell death. Toxicologic Pathology. 2007;35(4):495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Narici MV, Maffulli N. Sarcopenia: characteristics, mechanisms and functional significance. British Medical Bulletin. 2010;95(1):139–159. doi: 10.1093/bmb/ldq008. [DOI] [PubMed] [Google Scholar]
- 69.Braga M, Hikim APS, Datta S, Ferrini MG, Brown D, Kovacheva EL, Gonzalez-Cadavid NF, Sinha-Hikim I. Involvement of oxidative stress and caspase 2-mediated intrinsic pathway signaling in age-related increase in muscle cell apoptosis in mice. Apoptosis. 2008;13(6):822–832. doi: 10.1007/s10495-008-0216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Marzetti E, Wohlgemuth SE, Lees HA, Chung HY, Giovannini S, Leeuwenburgh C. Age-related activation of mitochondrial caspase-independent apoptotic signaling in rat gastrocnemius muscle. Mechanisms of Ageing and Development. 2008;129(9):542–549. doi: 10.1016/j.mad.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Marzetti E, Privitera G, Simili V, Wohlgemuth SE, Aulisa L, Pahor M, Leeuwenburgh C. Multiple Pathways to the Same End: Mechanisms of Myonuclear Apoptosis in Sarcopenia of Aging. Thescientificworldjournal. 2010;10:340–349. doi: 10.1100/tsw.2010.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Calvani R, Joseph AM, Adhihetty PJ, Miccheli A, Bossola M, Leeuwenburgh C, Bernabei R, Marzetti E. Mitochondrial pathways in sarcopenia of aging and disuse muscle atrophy. Biological Chemistry. 2013;394(3):393–414. doi: 10.1515/hsz-2012-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dirks AJ, Leeuwenburgh C. Aging and lifelong calorie restriction result in adaptations of skeletal muscle apoptosis repressor, apoptosis-inducing factor, X-linked inhibitor of apoptosis, caspase-3, and caspase-12. Free Radical Biology and Medicine. 2004;36(1):27–39. doi: 10.1016/j.freeradbiomed.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 74.Whitman SA, Wacker MJ, Richmond SR, Godard MP. Contributions of the ubiquitin-proteasome pathway and apoptosis to human skeletal muscle wasting with age. Pflugers Archiv-European Journal of Physiology. 2005;450(6):437–446. doi: 10.1007/s00424-005-1473-8. [DOI] [PubMed] [Google Scholar]
- 75.Carmeli E, Aizenbud D, Rom O. How Do Skeletal Muscles Die? An Overview Advances in experimental medicine and biology. 2015;861:99–111. doi: 10.1007/5584_2015_140. [DOI] [PubMed] [Google Scholar]
- 76.Kandalla PK, Goldspink G, Butler-Browne G, Mouly V. Mechano Growth Factor E peptide (MGF-E), derived from an isoform of IGF-1, activates human muscle progenitor cells and induces an increase in their fusion potential at different ages. Mechanisms of Ageing and Development. 2011;132(4):154–162. doi: 10.1016/j.mad.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 77.Fanzani A, Conraads VM, Penna F, Martinet W. Molecular and cellular mechanisms of skeletal muscle atrophy: an update. Journal of Cachexia Sarcopenia and Muscle. 2012;3(3):163–179. doi: 10.1007/s13539-012-0074-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Baker KK, Ramig LO, Luschei ES, Smith ME. Thyroarytenoid muscle activity associated with hypophonia in Parkinson disease and aging. Neurology. 1998;51(6):1592–1598. doi: 10.1212/wnl.51.6.1592. [DOI] [PubMed] [Google Scholar]


