Abstract
Introduction
Rotator cuff (RC) tears result in muscle atrophy and fat infiltration within the RC muscles. An estimation of muscle quality and deformation, or extensibility, is useful in selecting the most appropriate surgical procedure. We determined if non-invasive quantitative assessment of intramuscular fat using MRI could be used to predict extensibility of the supraspinatus (SSP) muscle.
Methods
Seventeen cadaveric shoulders were imaged to assess intramuscular fat infiltration. Extensibility and histological evaluations were then performed.
Results
Quantitative fat infiltration positively correlated with histological findings and presented a positive correlation with muscle extensibility (r = 0.69; p = 0.002). Extensibility was not significantly different between shoulders graded with a higher fat content vs. those with low fat when implementing qualitative methods.
Discussion
A non-invasive prediction of whole-muscle extensibility may directly guide pre-operative planning to determine if the torn edge could efficiently cover the original footprint while aiding in postoperative evaluation of RC repair.
Keywords: Rotator Cuff Tear, Supraspinatus Muscle, Fat Infiltration, Tendon Retraction, Rotator Cuff Repair, Muscle Extensibility
Introduction
Rotator cuff tears are the most common cause of shoulder pain and related disability with a prevalence of 80%, especially in the middle-aged and elderly population 1,2. Rotator cuff tears result in muscle/tendon retraction over time, deterioration of the length-tension relationship of the musculotendinous unit, muscle atrophy, increased stiffness, and fat tissue infiltration within the rotator cuff muscles 3–5. These abnormal changes lead to a decrease in the deformation, or extensibility, of the muscle-tendon structure, requiring larger tensile loads to repair the tendons 6,7. Upon failure of conservative treatment, patients are required to undergo highly individualized surgical treatments to repair the torn tendon to restore mechanical function and reduce pain.
Fatty infiltration in the rotator cuff muscles increases with age and is a progressive and irreversible result of rotator cuff tears 8,9,5. Fat tissue build-up in the muscles correlates with poor functional outcome after repair, including decreased muscle strength, poor shoulder mobility, suboptimal healing after repair, and an increase in the rate of rupture 8,10. A decrease in the extensibility of the musculotendinous unit can lead to incomplete reconstruction and may require additional surgical procedures. More importantly, excessive tensile forces on the abnormal tissue during surgery, required to bring torn tendons onto the footprint region for repair, can eventually lead to gap formation and failure at the repair site.
Individualized assessment of muscle quality, together with an estimation of the supraspinatus (SSP) muscle-tendon extensibility, is necessary to assist in pre-surgical planning and monitoring recovery throughout the rehabilitation process. Magnetic resonance imaging (MRI) has been utilized in the past for rotator cuff assessment, though primarily to simply assess gross muscle and tear size. Imaged-based qualitative grading systems are also often used to assess fatty streaks in the muscle and estimate the age-related decrease in the regenerative potential of rotator cuff tendons 10,11. However, these 2-dimensional methods are unable to estimate the extensibility of the musculotendinous unit. Furthermore, there is no quantitative approach describing volumetric fat infiltration in the SSP muscle. The aim of this study was to quantify volumetric fat infiltration in the SSP muscle using MRI and evaluate the extensibility of this muscle. We hypothesized that volumetric fat infiltration could be correlated to the extensibility of the SSP musculotendinous unit. This approach could potentially provide a non-invasive methodology for prediction of whole-muscle extensibility, directly estimating muscle quality and aiding in prognosis, pre-operative planning, and post-surgical rehabilitation.
Materials and Methods
Seventeen fresh-frozen cadaveric shoulders (median age 78; range, 25 to 94 years; 3 females and 14 males) were obtained from the Mayo Clinic Anatomy Department after institutional review board approval from the Mayo Clinic bio-specimens sub-committee. The shoulders were kept frozen at −20°C until the day of testing.
Magnetic Resonance Imaging
The specimens were thawed for 24 hours at room temperature before imaging. Magnetic resonance imaging (MRI) examinations were performed on a 70cm bore 3.0T clinical MRI scanner (GE Healthcare, DV 25.0 R02 software) using a 3-channel shoulder surface coil (GE Healthcare: 3.0T HD Shoulder Array, model 2414331). Soft tissues, including skin, fat and muscles in the shoulders were preserved during the scanning process. The total acquisition time for the qualitative and quantitative imaging protocols was approximately 4 minutes.
Qualitative MR Imaging and Fat Infiltration
A standard clinical MRI acquisition protocol, which included coronal and sagittal T1 weighted fast spin echo (FSE) (FSE-XL; TR/TE, 700–900/min Full; echo train length, 4; FOV, 14cm; matrix 384×256; slice/space, 4/0; bandwidth, 32kHz; NEX, 2), was used to classify the shoulders according to the Goutallier 12 and Fuchs 13 classification systems. Briefly, assessment was made using the T1-weighted MR images in which the image slice immediately lateral to the scapular spine’s attachment to the body of the scapula was evaluated. Grading for the Goutallier classification was as follows: stage 0 (normal muscle); stage 1 (some fatty streaks); stage 2 (less fat than muscle); stage 3 (muscle is equal to fatty infiltration); stage 4 (fatty infiltration is greater than muscle). Fuchs grading system consisted of the following: grade 0 (no fat to minimal fat); grade 1 (more muscle than fat); stage 2 (amount of fat is equal/greater than muscle). Inter-rater agreement was assessed for the Goutallier and Fuchs classification systems between two musculoskeletal (MSK) radiologists.
Quantitative MR Imaging and Fat Infiltration
Image datasets acquired with a 3-dimensional, isotropic 2-point Dixon MRI sequence (LAVA FLEX, TR/TE, 3.9/1.2; FOV, 30cm; matrix, 200×200; slice, 1.5mm; flip angle, 10º; bandwidth, 142.86kHz; NEX, 1) were used for volumetric intramuscular fat content quantification. A 2-point Dixon acquisition exploits 3.5ppm difference between resonance frequencies of hydrogen nuclei in water and fat molecules to produce four sets of images: water only, fat only, in-phase, and out-of-phase. Since the water and fat images obtained from the scanner present magnitude images with absolute values, fat fraction will be overestimated in areas with low fat content. To prevent this overestimation, the water, fat, in-phase and out-of-phase image datasets were imported into MATLAB (Rev. 2015b; The MathWorks, Natick, MA) where real-valued fat and water images were obtained as previously described 14. These new water and fat images were corrected for intensity by the CIIC-method, as previously described 15, and exported as vtk-files to ITK-SNAP for segmentation of the SSP muscle 16. The whole SSP muscle was manually segmented from both the water and the fat images making sure not to include any macroscopic fat around the muscle (Fig. 1). The muscle volume mask was then imported back into MATLAB together with the associated water and fat datasets, and the mean fat fraction of the muscle was calculated within the segmentation. Mean volumetric intramuscular fat fraction values were obtained from all masks based on the fat-water fraction maps according to equation 1.
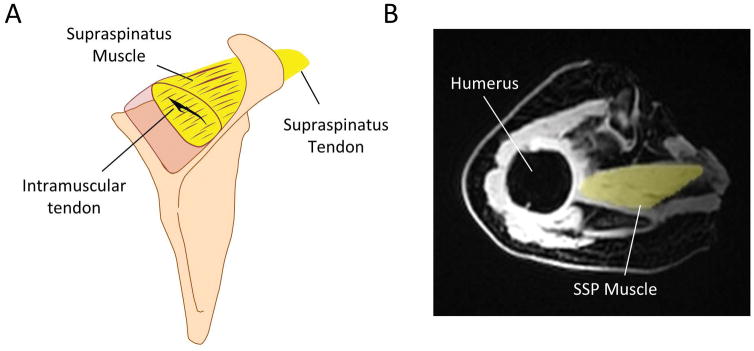
Fig. 1.
A) Schematic of supraspinatus (SSP) muscle and tendon. B) Volumetric masks were generated from both the fat and water magnetic resonance (MR) images (MR fat-image with masked SSP muscle is shown). Intramuscular fat fraction values were obtained from all masks based on the fat-water image datasets.
| (1) |
Supraspinatus Tendon Extensibility
After MR imaging and in preparation for mechanical testing, each scapula was disarticulated from the humerus, all soft tissue removed, and the supraspinatus muscle exposed for mechanical testing. Distribution of rotator cuff tears were also classified according to the classification established by Post et al. during dissection of the shoulders 17. The supraspinatus tendon was detached from the humeral head insertion and separated from the other rotator cuff tissues. The scapula was secured in a custom-designed shoulder experimental set-up and the free edge of the tendon was then sutured for preparation for tensile testing. The tendon suture was secured to a load cell for measuring loads, and a linear potentiometer was implemented to acquire displacement data (Fig. 2). The tendon was axially retracted up to 60 Newtons (N) using a pneumatic system for two cycles to precondition the muscle, and data from a third cycle was analyzed. Load and muscle displacement data were used to measure extensibility at 60N in mm. Extensibility was considered as the measured displacement, or deformation, of the musculotendinous unit with the applied load. Musculotendinous stiffness (N/mm) was calculated from the linear portion of the load vs. displacement curve.
Fig. 2.
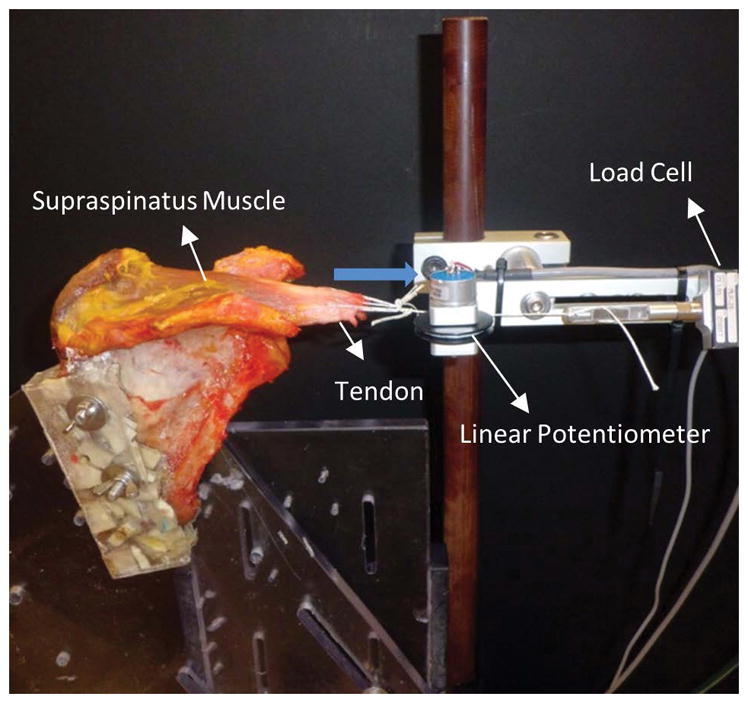
The scapula was secured in a custom-designed experimental set-up and the tendon rigidly fixed and axially retracted (blue arrow) to obtain loads and displacements (extensibility). Loads were measured using a single-axis load cell and displacements were obtained using a linear potentiometer.
Histology
After mechanical testing of the shoulders, the muscle specimens were processed from frozen sections and stained with hematoxylin and eosin (H&E). Briefly, sections of each muscle at the belly and close to the muscle-tendon interface were cut for staining. Specimens were sectioned in the longitudinal (coronal) plane at 8μm thickness and intramuscular adipocytes (fat) were evaluated. The amount of intramuscular fat in each section was graded as previously described using a semi-quantitative approach 18. A 5-point scale (0, 1, 2, 3, or 4) was used, where a grade of 0 represented no fat and a grade of 4 represented numerous fat deposits.
Statistical Analysis
Kappa statistics were used to estimate and describe the inter-rater reliability and agreement measures of fatty infiltration for all specimens between two raters for the Goutallier and Fuchs classification systems. Agreement of intramuscular fat in the supraspinatus muscle belly and muscle-tendon junction via histology was also evaluated for two additional independent raters. Agreement was classified as follows: poor: <0.01, slight: 0.01–0.2, fair: 0.21–0.4, moderate: 0.41–0.6, substantial: 0.61–0.8, almost perfect: 0.81–1. Since all scores are measured on an ordinal scale with more than two levels, weighted kappa statistics were used to take into account the magnitude of the difference between the raters. Pearson correlation coefficients were used to assess the association between extensibility, stiffness, fat fraction and histology. T-test was used to evaluate differences between extensibility, stiffness and fat fraction quantified with MRI, with qualitative classification methods.
Results
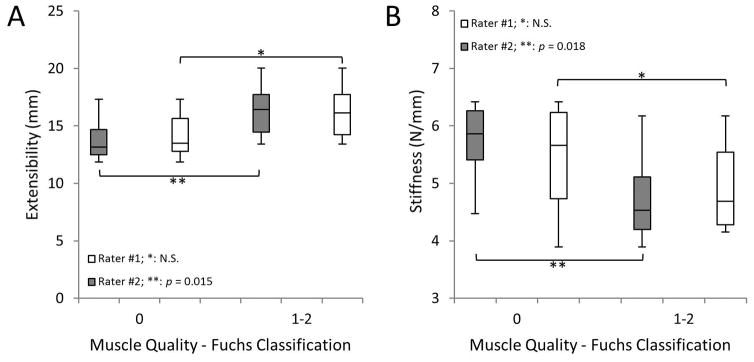
Comparison of inter-observer results demonstrated moderate to substantial agreement when evaluating the shoulders using the 5-tiered system of Goutallier (weighted κ value of 0.54; 95% confidence limits: 0.30–0.77) and the 3-tiered system of Fuchs (weighted κ value of 0.64; 95% confidence limits: 0.40–0.89), respectively. Histological evaluation using a 5-tiered system showed substantial agreement between raters for both sections of the muscle (Muscle Belly: weighted κ value of 0.78; 95% confidence limits: 0.54–1.00; Distal Muscle: weighted κ value of 0.66; 95% confidence limits: 0.42–0.92). A summary of the histological and qualitative classifications from each rater is presented in Table 1. Dissection and visual observation across the seventeen specimens revealed a distribution of rotator cuff tears as follows: small (3), medium (1), large (3), massive (2), and intact/no tear (8). Load vs. displacement curves for all specimens were created to obtain musculotendinous extensibility (mm) and stiffness (N/mm). There was a significant negative correlation between muscle-tendon extensibility and muscle stiffness (Fig. 3). Interestingly, rater specificity was observed on extensibility and stiffness outcomes between shoulders graded with a higher fat content vs. those with a low fat content when implementing the clinical qualitative grading methods of Goutallier and Fuchs (Fig. 4, Goutallier data not shown).
Table 1.
Summary of qualitative evaluations
| Classification | Rater #1 | Rater #2 | |||
|---|---|---|---|---|---|
| †Goutallier | |||||
|
| |||||
| Grade 0–1 (low fat) | 11 | 9 | |||
|
| |||||
| Grade 2–4 (high fat) | 6 | 8 | |||
|
| |||||
| †Fuchs | |||||
|
| |||||
| Grade 0 (low fat) | 11 | 9 | |||
|
| |||||
| Grade 1–2 (high fat) | 6 | 8 | |||
|
| |||||
| Histology | |||||
|
| |||||
| Muscle Belly | Muscle/Tendon Junction | ||||
|
|
|
||||
| Rater #1 | Rater #2 | Rater #1 | Rater #2 | ||
|
| |||||
| Grade 0 | 1 | 1 | 1 | 4 | |
|
| |||||
| Grade 1 | 6 | 8 | 6 | 6 | |
|
| |||||
| Grade 2 | 3 | 3 | 6 | 4 | |
|
| |||||
| Grade 3 | 7 | 5 | 3 | 2 | |
|
| |||||
| Grade 4 | - | - | 1 | 1 | |
Data shown represents the number of specimens in each category for all raters.
Grading for both classification systems were grouped as low and high fat specimens.
Fig. 3.
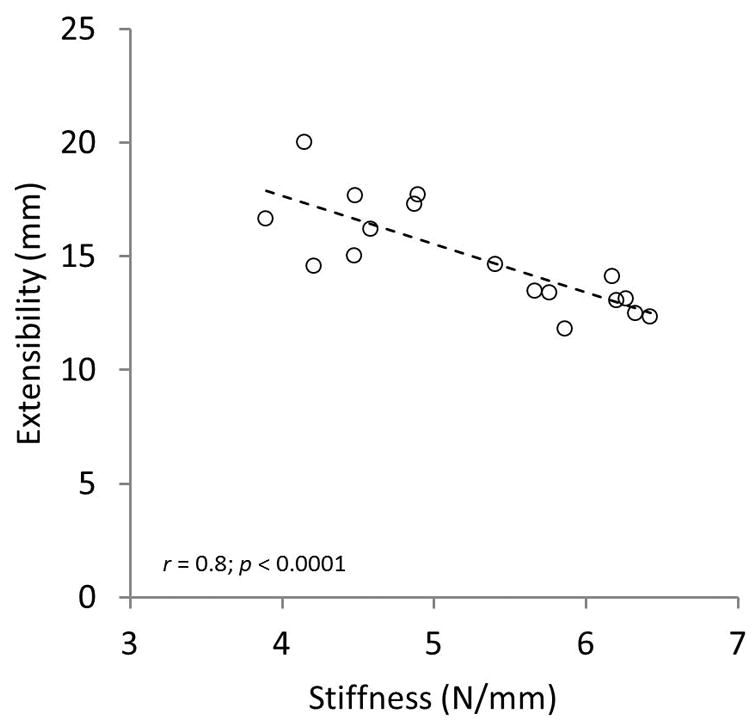
Supraspinatus (SSP) muscle extensibility outcomes showed a significant negative correlation with the experimental measured stiffness.
Fig. 4.
SSP muscle quality classified using the clinical qualitative grading system of Fuchs for two independent raters vs. A) SSP muscle extensibility and B) stiffness. Statistical significance between muscles with high vs. low grades was rater-dependent. Similar outcomes were observed when implementing the Goutallier classification system (data not shown).
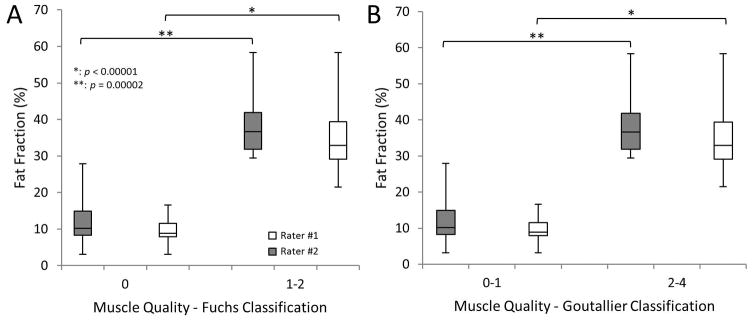
Volumetric fat fraction quantified with MRI positively correlated with histological findings at the muscle belly and muscle-tendon sections (Fig. 5); although a higher correlation was observed at the muscle belly. Interobserver fatty infiltration (Goutallier and Fuchs) and volumetric fat fraction (%) are shown in Figure 6. Shoulders that were qualitatively graded with a higher fat content, using both classification systems, showed a significant increase in fat fraction, for both raters, compared to shoulders with a lower grading. Fat fraction negatively correlated with experimentally measured muscle stiffness and positively correlated with musculotendinous extensibility (Fig. 7).
Fig. 5.
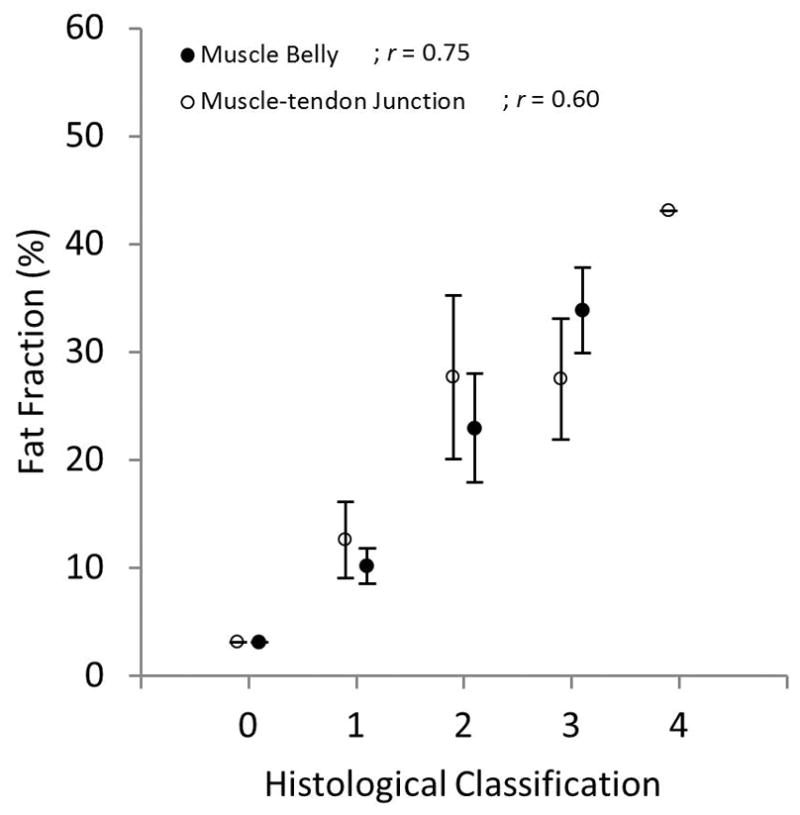
Fat fraction quantified using MRI vs. histological classifications for the muscle belly and muscle-tendon junction of the SSP muscle. Qualitative histological classifications for both muscle sections positively correlated with quantitative fat fraction.
Fig. 6.
Muscle quality classified using the qualitative grading systems of A) Fuchs and B) Goutallier vs. fat fraction quantified using MRI for two raters. Tissue classified with high grades using both qualitative classification systems presented a significantly larger fat fraction component compared to muscle with minimal fat.
Fig. 7.
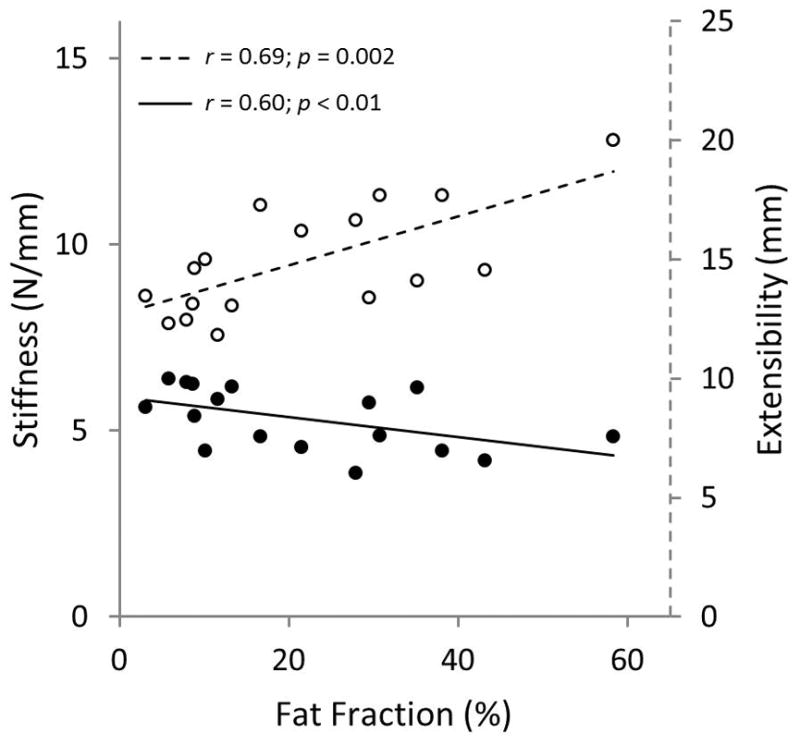
Measured experimental stiffness (solid circles) negatively correlated with fat fraction outcomes from MRI, while musculotendinous extensibility (open circles) positively correlated with fat fraction.
Discussion
The amount of fat content within the muscle and the extent of muscle atrophy are main factors in clinical decision-making affecting surgical procedures, post-surgical outcomes, healing, pain and rehabilitation. Although muscle degeneration can heavily influence the surgical approach, the extent and interplay between muscle mechanical properties, such as elasticity or stiffness, and fatty degeneration are still unknown. Pulling the retracted SSP musculotendinous unit to the greater tuberosity can occasionally be challenging during a surgical procedure due to either a large gap size or less tendon extensibility, sometimes changing the course of surgery.
Clinical qualitative classifications based on computed tomography (CT) and MRI have been established by Goutallier 12 and Fuchs 13, respectively, to assess the amount of fat in the muscle, though primarily to estimate the regenerative potential for healing of the repair. One cause of poor muscle quality is fatty infiltration; this has been considered an important prognostic factor for healing, as its presence may indicate a muscle with loss of strength 19,20 and poor vascularization 21,22. Although clinical qualitative classification systems can provide stages of fatty degeneration, these are qualitative in nature and with vast variability, overestimate muscle fat, lack validation 23 and more importantly, do not provide an estimate of muscle/tendon extensibility. While inter-observer results from this study demonstrated moderate to substantial agreement in both classification systems, results showed a significant rater-dependency when evaluating musculotendinous extensibility and stiffness in shoulders classified with high grades compared to those with a lower grade. These shortcomings and results confirm the need for additional methods that can more accurately evaluate muscle quality and muscle properties.
Quantitative methods using MRI have been developed to quantify fat infiltration in the rotator cuff muscles. Nardo et al. found a more consistent and higher correlation between fat fraction and clinical parameters, such as pain and range of motion, compared to the Goutallier classification system 24. In the current study, histological findings at the muscle belly and muscle-tendon junction of the supraspinatus muscle positively correlated with quantitative measurements obtained using MRI. Furthermore, quantitative fat fraction measurements correlated with the qualitative systems of Goutallier and Fuchs, demonstrating high fat fraction outcomes on shoulders classified as being in advanced stages of degeneration, compared to shoulders classified as normal or with minimal fatty streaks, which showed a significant lower fat content.
Shoulders with high degenerative grades resulted in higher extensibility outcomes when compared to muscles with low grading, while shoulders with high grading resulted in low stiffness values when compared to muscles with minimal degeneration. In the clinical setting, rotator cuff tears with a retracted SSP muscle generally present poor muscle quality and lower musculotendinous extensibility when compared to normal muscles. Nakagaki et al. showed no correlation between 2D fatty streaks and rotator cuff defects, with SSP muscle/tendon retraction 25. On the other hand, compared to the initial muscle/tendon tear properties, the force needed to pull a tendon considerably increases after a repair due to changes in muscle quality, intramuscular fat infiltration and an increase in inter- and intra- muscular connective tissues 3. The current study showed that quantitative volumetric fat fraction of the SSP muscle evaluated using MRI resulted in a significant and positive correlation with the extensibility of the isolated musculotendinous unit. This difference from clinical observations might be due to the condition of testing of the muscle. In this study, a controlled experiment was performed in which the supraspinatus muscle was isolated from other tissues and extensibility measurements were obtained. Inter- connective tissue, superficial adipose tissue, and other superficial muscles could have a role in the overall extensibility of the whole-muscle structure. Although an increase in fat fraction allowed for more extensibility of the muscle which might be beneficial for repair of the tear during surgery, this abnormal muscle quality and environment could eventually lead to poor healing and a reduction of muscle fiber force production 11.
There are several limitations in this study. First, the small number of specimen with various rotator cuff tears (small, medium, large and massive) prevented us from obtaining correlations between tendon extensibility, tear grade and volumetric fat infiltration. However, the purpose of the study was to assess the extensibility of the muscle-tendon and its correlation to fat content measured with MRI, independent of the tear classification. Second, experimental outcomes may be different from those observed in whole supraspinatus muscle structures adjacent to superficial fat, superficial connective tissues and additional muscles. Additionally, these outcomes may be different between in-vitro and in-vivo conditions and future studies should be conducted to evaluate the effect of fatty infiltration in the extensibility of the musculotendinous unit. It is important to note that histological evaluation was qualitative and two-dimensional, and that intramuscular fat content may vary within the specimens’ muscle length. Studies have previously described that in the presence of a tear and retraction of the muscle belly, sarcomere length and pennation angle between muscle fibers change 26,27. Future studies with a larger specimen number should investigate the effect of these changes and fatty infiltration on the extensibility of the musculotendinous unit.
In conclusion, 2D classification systems routinely used in the clinical setting to assess muscle quality are qualitative in nature, rater-dependent, and unable to predict the extensibility of the musculotendinous unit. Our results show a volumetric (3D) quantitative assessment of the quality of the supraspinatus muscle using magnetic resonance imaging, and demonstrated that intramuscular fat was positively correlated with the extensibility of the detached supraspinatus musculotendinous unit. Quantitative estimations of the extensibility and volumetric fat infiltration of rotator cuff muscles are novel and important factors that may be able to guide in surgical planning and post-surgical evaluation of rotator cuff muscle repairs.
Acknowledgments
This study was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number R21 AR065550, and an internal award from the Mayo Clinic. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations
- RC
Rotator Cuff
- SSP
Supraspinatus
- MRI
Magnetic Resonance Imaging
- CT
Computed Tomography
- N
Newton
- MSK
Musculoskeletal
Footnotes
Ethical Publication Statement: We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines
Disclosure of Conflicts of Interest: ODL owns stocks or stock options at Advanced MR Analytics (AMRA), receives research support from AMRA and is on the advisory board of AMRA; AK owns stocks or stock options at AMRA; MA is a consultant for AMRA. The authors declare that no competing interests exist that would have bias the work, results, interpretation and conclusions of the submitted manuscript.
References
- 1.Milgrom C, Schaffler M, Gilbert S, van Holsbeeck M. Rotator-cuff changes in asymptomatic adults. The effect of age, hand dominance and gender. J Bone Joint Surg Br. 1995;77(2):296–298. [PubMed] [Google Scholar]
- 2.Yamamoto A, Takagishi K, Osawa T, Yanagawa T, Nakajima D, Shitara H, Kobayashi T. Prevalence and risk factors of a rotator cuff tear in the general population. J Shoulder Elbow Surg. 2010;19(1):116–120. doi: 10.1016/j.jse.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 3.Gerber C, Meyer DC, Schneeberger AG, Hoppeler H, von Rechenberg B. Effect of tendon release and delayed repair on the structure of the muscles of the rotator cuff: an experimental study in sheep. J Bone Joint Surg Am. 2004;86-A(9):1973–1982. doi: 10.2106/00004623-200409000-00016. [DOI] [PubMed] [Google Scholar]
- 4.Safran O, Schroeder J, Bloom R, Weil Y, Milgrom C. Natural history of nonoperatively treated symptomatic rotator cuff tears in patients 60 years old or younger. Am J Sports Med. 2011;39(4):710–714. doi: 10.1177/0363546510393944. [DOI] [PubMed] [Google Scholar]
- 5.Williams MD, Ladermann A, Melis B, Barthelemy R, Walch G. Fatty infiltration of the supraspinatus: a reliability study. J Shoulder Elbow Surg. 2009;18(4):581–587. doi: 10.1016/j.jse.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 6.Berth A, Neumann W, Awiszus F, Pap G. Massive rotator cuff tears: functional outcome after debridement or arthroscopic partial repair. J Orthop Traumatol. 2010;11(1):13–20. doi: 10.1007/s10195-010-0084-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ward SR, Hentzen ER, Smallwood LH, Eastlack RK, Burns KA, Fithian DC, Friden J, Lieber RL. Rotator cuff muscle architecture: implications for glenohumeral stability. Clin Orthop Relat Res. 2006;448:157–163. doi: 10.1097/01.blo.0000194680.94882.d3. [DOI] [PubMed] [Google Scholar]
- 8.Farshad M, Wurgler-Hauri CC, Kohler T, Gerber C, Rothenfluh DA. Effect of age on fatty infiltration of supraspinatus muscle after experimental tendon release in rats. BMC Res Notes. 2011;4:530. doi: 10.1186/1756-0500-4-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Melis B, Nemoz C, Walch G. Muscle fatty infiltration in rotator cuff tears: descriptive analysis of 1688 cases. Orthop Traumatol Surg Res. 2009;95(5):319–324. doi: 10.1016/j.otsr.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Gladstone JN, Bishop JY, Lo IK, Flatow EL. Fatty infiltration and atrophy of the rotator cuff do not improve after rotator cuff repair and correlate with poor functional outcome. Am J Sports Med. 2007;35(5):719–728. doi: 10.1177/0363546506297539. [DOI] [PubMed] [Google Scholar]
- 11.Gumucio JP, Korn MA, Saripalli AL, Flood MD, Phan AC, Roche SM, Lynch EB, Claflin DR, Bedi A, Mendias CL. Aging-associated exacerbation in fatty degeneration and infiltration after rotator cuff tear. Journal of shoulder and elbow surgery. 2014;23(1):99–108. doi: 10.1016/j.jse.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goutallier D, Postel JM, Bernageau J, Lavau L, Voisin MC. Fatty muscle degeneration in cuff ruptures. Pre- and postoperative evaluation by CT scan. Clin Orthop Relat Res. 1994;(304):78–83. [PubMed] [Google Scholar]
- 13.Fuchs B, Weishaupt D, Zanetti M, Hodler J, Gerber C. Fatty degeneration of the muscles of the rotator cuff: assessment by computed tomography versus magnetic resonance imaging. J Shoulder Elbow Surg. 1999;8(6):599–605. doi: 10.1016/s1058-2746(99)90097-6. [DOI] [PubMed] [Google Scholar]
- 14.Rydell J, Knutsson H, Pettersson J, Johansson A, Farneback G, Dahlqvist O, Lundberg P, Nystrom F, Borga M. Phase sensitive reconstruction for water/fat separation in MR imaging using inverse gradient. Med Image Comput Comput Assist Interv. 2007;10(Pt 1):210–218. doi: 10.1007/978-3-540-75757-3_26. [DOI] [PubMed] [Google Scholar]
- 15.Andersson T, Romu T, Karlsson A, Noren B, Forsgren MF, Smedby O, Kechagias S, Almer S, Lundberg P, Borga M, Leinhard OD. Consistent intensity inhomogeneity correction in water-fat MRI. J Magn Reson Imaging. 2015;42(2):468–476. doi: 10.1002/jmri.24778. [DOI] [PubMed] [Google Scholar]
- 16.Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage. 2006;31(3):1116–1128. doi: 10.1016/j.neuroimage.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 17.Post M, Silver R, Singh M. Rotator cuff tear. Diagnosis and treatment. Clin Orthop Relat Res. 1983;(173):78–91. [PubMed] [Google Scholar]
- 18.Kim HM, Galatz LM, Lim C, Havlioglu N, Thomopoulos S. The effect of tear size and nerve injury on rotator cuff muscle fatty degeneration in a rodent animal model. J Shoulder Elbow Surg. 2012;21(7):847–858. doi: 10.1016/j.jse.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerber C, Schneeberger AG, Hoppeler H, Meyer DC. Correlation of atrophy and fatty infiltration on strength and integrity of rotator cuff repairs: a study in thirteen patients. J Shoulder Elbow Surg. 2007;16(6):691–696. doi: 10.1016/j.jse.2007.02.122. [DOI] [PubMed] [Google Scholar]
- 20.Goutallier D, Postel JM, Gleyze P, Leguilloux P, Van Driessche S. Influence of cuff muscle fatty degeneration on anatomic and functional outcomes after simple suture of full-thickness tears. Journal of shoulder and elbow surgery. 2003;12(6):550–554. doi: 10.1016/s1058-2746(03)00211-8. [DOI] [PubMed] [Google Scholar]
- 21.Lohr JF, Uhthoff HK. The microvascular pattern of the supraspinatus tendon. Clin Orthop Relat Res. 1990;(254):35–38. [PubMed] [Google Scholar]
- 22.Rathbun JB, Macnab I. The microvascular pattern of the rotator cuff. J Bone Joint Surg Br. 1970;52(3):540–553. [PubMed] [Google Scholar]
- 23.Alizai H, Nardo L, Karampinos DC, Joseph GB, Yap SP, Baum T, Krug R, Majumdar S, Link TM. Comparison of clinical semi-quantitative assessment of muscle fat infiltration with quantitative assessment using chemical shift-based water/fat separation in MR studies of the calf of post-menopausal women. Eur Radiol. 2012;22(7):1592–1600. doi: 10.1007/s00330-012-2404-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nardo L, Karampinos DC, Lansdown DA, Carballido-Gamio J, Lee S, Maroldi R, Ma CB, Link TM, Krug R. Quantitative assessment of fat infiltration in the rotator cuff muscles using water-fat MRI. J Magn Reson Imaging. 2014;39(5):1178–1185. doi: 10.1002/jmri.24278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakagaki K, Ozaki J, Tomita Y, Tamai S. Extensibility of the supraspinatus muscle with a rotator cuff tear evaluated by magnetic resonance imaging. Mod Rheumatol. 2000;10(3):150–154. doi: 10.3109/s101650070022. [DOI] [PubMed] [Google Scholar]
- 26.Beeler S, Ek ET, Gerber C. A comparative analysis of fatty infiltration and muscle atrophy in patients with chronic rotator cuff tears and suprascapular neuropathy. J Shoulder Elbow Surg. 2013;22(11):1537–1546. doi: 10.1016/j.jse.2013.01.028. [DOI] [PubMed] [Google Scholar]
- 27.Melis B, DeFranco MJ, Chuinard C, Walch G. Natural history of fatty infiltration and atrophy of the supraspinatus muscle in rotator cuff tears. Clin Orthop Relat Res. 2010;468(6):1498–1505. doi: 10.1007/s11999-009-1207-x. [DOI] [PMC free article] [PubMed] [Google Scholar]