Abstract
Objective
In previous studies using oesophageal squamous cells from patients with Barrett's oesophagus (NES-B cells) and from patients without Barrett's oesophagus (NES-G cells), we showed that acid and bile salts induced CDX2 expression only in NES-B cells. CDX2, a transcription factor required to form intestinal epithelium, is a target of NF-κB signaling, which can be inhibited by aspirin. We explored mechanisms underlying differences between NES-B and NES-G cells in CDX2 expression, and effects of aspirin on that CDX2 expression.
Design
We exposed NES-B and NES-G cells to acid and bile salts, with and without aspirin, and evaluated effects on IκB-NF-κB-PKAc complex activation, p65 NF-κB subunit function, and CDX2 expression.
Results
In both NES-B and NES-G cells, acid and bile salts activated NADPH oxidase to generate H2O2, which activated the IκB-NF-κB-PKAc complex. NES-B cells exhibited higher levels of phosphorylated IκB and p65 and greater NF-κB transcriptional activity than NES-G cells, indicating greater IκB-NF-κB-PKAc complex activation by acid and bile salts in NES-B cells, and p65 siRNA prevented their increased expression of CDX2. Aspirin blocked IκB phosphorylation, p65 nuclear translocation, CDX2 promoter activation, and CDX2 expression induced by acid and bile salts in NES-B cells.
Conclusions
Differences between NES-B and NES-G cells in NF-κB activation by acid and bile salts can account for their differences in CDX2 expression, and their CDX2 expression can be blocked by aspirin. These findings might explain why some GORD patients develop Barrett's oesophagus while others do not, and why aspirin might protect against development of Barrett's oesophagus.
Keywords: Oesophageal Disease, Inflammation, Gastro-Oesophageal Reflux Disease
Introduction
It has been estimated that 2% to 7% of adults in Western countries have Barrett's oesophagus, the condition in which an intestinal-type metaplastic mucosa at risk for malignancy replaces oesophageal squamous mucosa damaged by gastro-oesophageal reflux disease (GORD) 1, 2. Up to 40% of Western adults report symptoms of GORD, and it is not clear why only a minority of those individuals develop Barrett's oesophagus 3. We have previously demonstrated that there are differences among individuals in molecular pathways activated when oesophageal squamous epithelial cells are exposed to acid and bile salts, the noxious agents in refluxed gastric juice, and we have proposed that those differences might determine whether the GORD-damaged oesophagus heals through the process of squamous cell regeneration or through metaplasia with the development of Barrett's oesophagus 4-8.
A number of epidemiologic studies have suggested that the use of aspirin and other nonsteroidal anti-inflammatory drugs (NSAIDs) protects against the development of oesophageal adenocarcinoma, the cancer that develops from Barrett's metaplasia 9, 10. Interestingly, some recent case-control studies have found that aspirin protects against the development of Barrett's metaplasia among individuals with symptoms of GORD, while other NSAIDS do not 11, 12. It is not clear why protection against Barrett's metaplasia should be limited to aspirin, but no other NSAID. Both aspirin and non-aspirin NSAIDs exert some of their beneficial effects by inhibiting the cyclooxygenase enzymes that generate prostaglandins from arachidonic acid 13, 14. Unlike other NSAIDs, however, aspirin also inhibits the pro-inflammatory NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) pathway 15. In the pancreas, NF-κB signaling has been shown to play a key role in the molecular reprogramming process that underlies pancreatic acinar-to-ductal metaplasia 16. Therefore, the putative, unique protective effect of aspirin against the development of Barrett's metaplasia might be attributable to aspirin's inhibitory effect on NF-κB that is not shared by other NSAIDs.
NF-κB is not activated in normal oesophageal squamous epithelium, but this transcription factor is activated in oesophageal epithelium inflamed by GORD 17. In earlier studies, we showed that acid and bile salts induce NF-κB pathway signaling in oesophageal squamous cells 6, 18. Another important transcription factor that might be influenced by NF-κB signaling is caudal-related homeobox transcription factor 2 (CDX2), a key developmental transcription factor that directs the formation of intestinal epithelium 19, 20. CDX2 mRNA and protein are found frequently in biopsy specimens of the intestinal-type metaplasia of Barrett's oesophagus 21-24.
The CDX2 promoter contains two putative NF-κB binding sites 25. A number of studies have demonstrated that acid and bile salts can increase activity of the Cdx2 promoter and increase Cdx2 mRNA expression in cultured oesophageal squamous cells from rats and some human subjects 26-29. Like NF-κB, CDX2 expression has been found in biopsy specimens of human oesophagus that is inflamed by GORD, but not in biopsies of the uninflamed oesophagus 24. In animal models of reflux oesophagitis, the reflux-damaged oesophageal epithelium exhibits increased Cdx2 expression before the appearance of a Barrett's-like metaplasia 30-32. These studies suggest that CDX2 expression by inflamed oesophageal squamous epithelium might herald the development of Barrett's oesophagus, and that CDX2 expression might be a consequence of NF-κB pathway signaling.
In earlier studies, we found that acid and bile salts induced CDX2 expression in oesophageal squamous cells from patients with Barrett's oesophagus, but not from GORD patients without Barrett's oesophagus 6. In squamous cells from patients with Barrett's oesophagus, furthermore, we found that acid and bile salts stimulated the p50 and p65 NF-κB subunits to translocate to the nucleus, but only the p50 subunit bound the CDX2 promoter and induced CDX2 expression 6. In the present study, we explored mechanisms underlying our observation that acid and bile salts induce CDX2 expression only in oesophageal squamous cells from patients with Barrett's oesophagus, and whether aspirin might inhibit that expression.
Materials and Methods
Culture of Primary Oesophageal Squamous Cells and Oesophageal Squamous Cell Lines
We used 4 non-neoplastic, telomerase-immortalized oesophageal squamous (NES) cell lines that were created from endoscopic biopsy specimens of squamous epithelium in the distal oesophagus of GORD patients with Barrett's oesophagus (NES-B3T and NES-B10T) and GORD patients without Barrett's oesophagus (NES-G2T and NES-G4T) 5, 6. For brevity, in the remainder of the report we will refer to the two squamous cell lines from GORD patients with Barrett's oesophagus simply as NES-B cells, and the two squamous cell lines from GORD patients without Barrett's oesophagus simply as NES-G cells. We also established primary cultures of oesophageal squamous epithelial cells (NES-B3P, NES-B6P, and NES-B7P) from the distal oesophagus of 3 GORD patients with Barrett's oesophagus using techniques as previously described 6. These studies were approved by the institutional review board of the Dallas VA Medical Center. Primary cell cultures and cell lines were co-cultured with a fibroblast feeder layer and maintained in growth medium as previously described 33, 34. Primary cell cultures and cell lines were maintained at 37°C in a 5% CO2 incubator. For individual experiments, primary cell cultures and cell lines were seeded equally into collagen IV-coated wells (BD Biosciences, San Jose, CA) in the absence of fibroblast feeder layers, and maintained in growth medium.
Acid and Bile Salt Exposure
Cells were exposed to one of three different experimental media: 1) Acidic full growth medium (brought to a pH of 4.0 with 1M HCl), 2) Neutral bile salt medium (containing conjugated bile acids with a total concentration of 400 μM at pH 7.2 as previously described by us 6), or 3) Acidic bile salt medium (the same bile acid solution at pH 4.0). Neutral full growth medium (pH 7.2) served as the control medium. For the 24-hour exposures, the pH of the acidic full-growth medium and the acidic bile salt medium was adjusted to pH 5.5.
Aspirin Treatment
For Western blotting experiments, cells were pretreated with 100 μM aspirin (Sigma) for 2 hours, after which acid and bile salt medium containing aspirin was added for 30 minutes. Cell lysates were then collected. For RT-PCR experiments, cells were pretreated with 100 μM aspirin (Sigma) for 2 hours, after which acid and bile salt medium containing aspirin was added for 24 hours. Total RNAs were then collected. Following transfection with the CDX2 promoter, cell lines and primary cell cultures were pretreated with 100 μM aspirin (Sigma) for 2 hours. For experiments in the cell lines, acid and bile salt medium containing aspirin was added for 60 minutes, then removed and replaced with neutral pH medium for 6 hours; for experiments in primary cell cultures, acid and bile salt medium containing aspirin was added for 24 hours. Cell extracts were then assayed for luciferase activities. Neutral full growth medium (pH 7.2), with or without aspirin, served as controls.
Additional details are provided in the Supplemental Material and Methods section and Supplemental Table 1.
Results
Nuclear translocation of p50 and p65 NF-κB subunits is greater in NES-B cells than in NES-G cells following acid and bile salt exposure
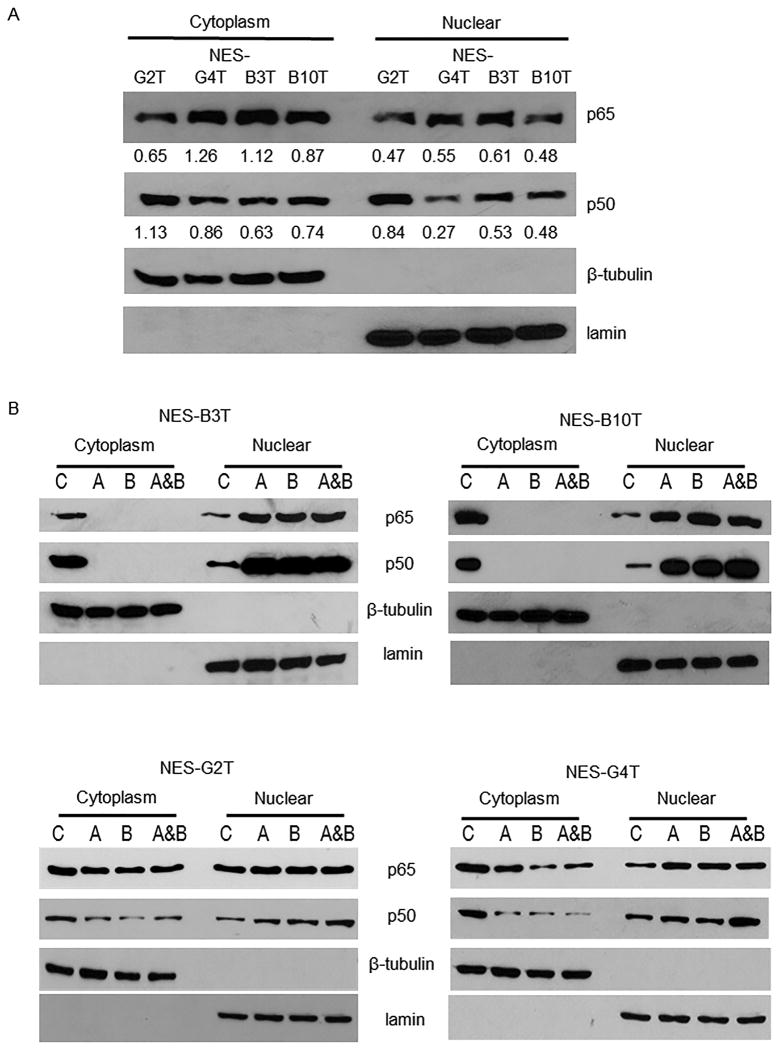
At baseline, we found that NES-B cells and NES-G cells have similar levels of cytoplasmic and nuclear p65 and p50 proteins (Figure 1A). In agreement with our earlier studies6, treatment with acid, bile salts, or a combination of both eliminated any detectable cytoplasmic levels of p65 and p50, and resulted in markedly increased nuclear levels of both p50 and p65 NES-B cells (Figure 1B). In contrast, treatment of NES-G cells with acid and bile salts reduced, but did not eliminate, cytoplasmic levels of p50 and p65, and resulted in increased nuclear levels of p50 with only a slight increase in nuclear levels of p65 (Figure 1 B&C and Supplemental Figure 1).
Figure 1.
Acid, bile salts and acidic bile salts markedly increase nuclear levels of p50 and p65 in NES-B cells. Representative experiments of Western blotting for cytoplasmic and nuclear p65 and p50 protein in NES-G and NES-B cell lines (A) at baseline (numbers depict semi-quantitative analyses with respect to loading controls shown beneath individual gels), and B) after exposure to acid, bile salts, and acidic bile salts. C) Representative experiments of immunofluorescence for cytoplasmic and nuclear p65 and p50 proteins in NES-G2T cells (scale bar = 50 μm). D) Acid, bile salts, and acidic bile salts do not increase DNA binding of either p50 or p65 to the CDX2 promoter in NES-G cell lines. Representative experiments of chromatin immune-precipitation (ChIP) assays for p50 and p65; IgG served as a control. Input demonstrates sheared chromatin without immune-precipitation added to the assays. Immunofluorescence images and Western blots shown are representative of the results from 2 independent experiments. C, untreated control; A, acid; B, bile salts; A&B, acidic bile salts; M, marker.
In earlier studies using NES-B cells, we found that, although acid and bile salt exposure caused nuclear translocation of both p50 and p65, only the p50 subunit bound to the CDX2 gene promoter and increased CDX2 expression 6. In the present study using NES-G cells, ChIP assay showed only minimal binding of p50 and p65 to CDX2 promoter DNA in untreated NES-G cells at baseline (Figure 1D). Although acid and bile salt exposure caused nuclear translocation of p50 in NES-G cells, that nuclear translocation was not accompanied by increased binding of p50 to the CDX2 promoter (Figure 1D). These findings reveal considerable differences between oesophageal squamous cells from GORD patients with and without Barrett's oesophagus in how acid and bile salts activate NF-κB, and provide a molecular explanation for our earlier observation that acid and bile salts did not increase CDX2 expression in NES-G cells 6.
In addition to p50, increased nuclear levels of p65 are required for acid and bile salts to induce CDX2 promoter activation in oesophageal squamous cells
As discussed, in NES-B cells, exposure to acid and bile salts causes nuclear translocation of both the p50 and p65 NF-κB subunits and activation of the CDX2 promoter, but only the p50 subunit binds the promoter. In NES-G cells, in contrast, acid exposure causes nuclear translocation of the p50 subunit, and that p50 does not bind the CDX2 promoter. One potential explanation for p50 activation of the CDX2 promoter in NES-B cells is that the increase in nuclear levels of p65 are greater than that observed in NES-G cells. Even though p65 does not bind the promoter directly, perhaps such a large increase in nuclear levels are required for promoter activation. To explore this possibility, we knocked down p65 using a specific siRNA in one NES-B cell line (NES-B10T) in which we had previously demonstrated an increase in CDX2 promoter activity with acidic bile salt exposure 6. Figure 2A demonstrates that transfection with p65 siRNA indeed knocked down p65 protein in NES-B10T whole cell lysates at baseline and following exposure to acid, bile salts, and acidic bile salts. Compared to control cells, the NES-B10T cells with p65 knockdown showed only a slight reduction in p50 nuclear translocation after treatment with acid, bile salts, or acidic bile salts (Figure 2B). However, the acid and bile salt-induced increase in CDX2 promoter activity seen in control cells was virtually abolished by p65 knockdown (Figure 2C). These findings demonstrate that increased nuclear levels of p65 as well as p50 are essential for acid and bile salts to activate the CDX2 promoter in oesophageal squamous cells.
Figure 2.
Increases in nuclear levels of p65 are required for acid and bile salts to induce CDX2 promoter activation in oesophageal squamous cells. A) Western blot demonstrating p65 protein levels in whole cell lysates of NES-B10T cells transfected with control siRNA or p65 siRNA, untreated and following exposure to acid, bile salts and acidic bile salts. B) Representative Western blot demonstrating p50 nuclear protein levels in NES-B10T cells transfected with control siRNA or p65 siRNA, untreated and following exposure to acid, bile salts, and acidic bile salts. U, untreated; A, acid; B, bile salts; A&B, acidic bile salts. Western blots shown are representative of the results from 2 independent experiments. C) CDX2 promoter activation induced by acid and bile salts in NES-B10T containing control siRNA or p65 siRNA. Bar graphs represent the mean ±SEM. ***, p ≤ 0.001 compared with non-acidic bile salt treated controls.
Acid and bile salts cause a slight increase in p50/p65 heterodimer formation and a strong decrease in IκB- and PKAc-bound p65 in NES-B cells, but not in NES-G cells
The p50 and p65 NF-κB subunits form a heterodimer that, in unstimulated cells, is held inactive in the cytoplasm by being bound to a member of the inhibitory IκB protein family 35, 36. This IκB-NF-κB complex can be activated through phosphorylation of the IκB protein, which is then degraded, releasing the p50/p65 heterodimer and enabling it to translocate to the nucleus. We performed immunoprecipitation for p50 followed by Western blotting for p65 to confirm that these NF-κB proteins form heterodimers in NES-G2T and NES-B10T cell lines (Figure 3A). Unstimulated, both cell lines exhibited p50/p65 heterodimer formation. Exposure to acid, bile salts, and acidic bile salts caused a minimal increase in heterodimer formation in NES-G2T cells, and a slight increase in heterodimer formation in NES-B10T cells (Figure 3A). Next, we performed immunoprecipitation for p65 followed by Western blotting for IκB to determine the amount of p65 bound to IκB in the cytoplasm. In both NES-G cell lines, exposure to acid, bile salts and acidic bile salts caused only a minimal decrease in IκB-bound p65 (Figure 3B). In contrast, these same exposures caused a marked decrease in the amount of p65 that remained bound to IκB in the NES-B cells (Figure 3B). The heterodimer formation and transcriptional activity of the p65 subunit can be regulated by phosphorylation of p65 through the catalytic subunit of protein kinase A (PKAc), another component of the IκB-NF-κB complex 36, 37. Using immunoprecipitation for p65, we found that PKAc is indeed a component of the IκB-NF-κB complex in NES cells (Figure 3B). In both NES-G cell lines, exposure to acid and bile salts caused only a minimal change in PKAc-bound p65 (Figure 3B). In NES-B cells, in contrast, exposure to the combination of acid and bile salts virtually eliminated PKAc binding to p65 (Figure 3B).
Figure 3.
NES-B cells exposed to acid, bile salts and acidic bile salts show a greater increase in p50/p65 heterodimer formation and a greater decrease in IκB- and PKAc-bound p65 in the cytoplasm than NES-G cells. Representative Western blots demonstrating A) immunoprecipitation (IP) for p50 and immunoblotting (IB) for p65 proteins and B) IP for p65 and IB for IκB and PKAc. C, untreated control; A, acid; B, bile salts; A&B, acidic bile salts. Western blots shown are representative of the results from 2 independent experiments.
Acid and bile salts increase NF-kB/p65 transcriptional activity in NES-B cells, but not in NES-G cells
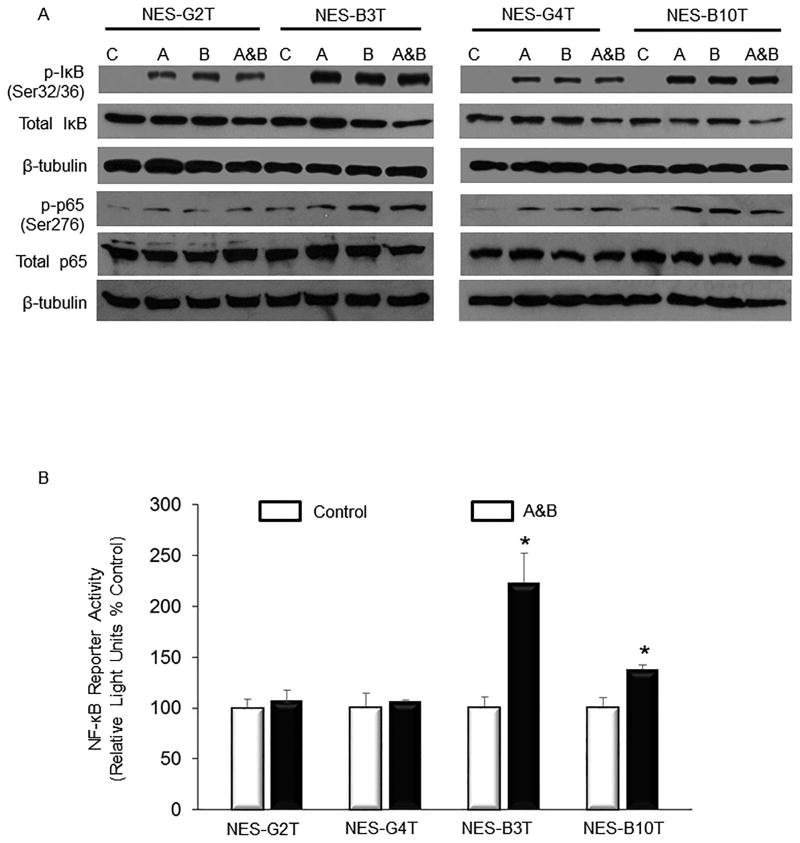
Phosphorylation of IκB at Ser32 and Ser36 leads to its ubiquitination and rapid proteasomal degradation, releasing the p50/p65 heterodimer complex and enabling it to translocate to the nucleus 35. Signals that cause degradation of IκB also can result in activation of PKAc, which phosphorylates p65 at Ser276, stimulating its transcriptional activity 35, 37. We found that exposure to acid, bile salts, and acidic bile salts markedly increased phosphorylation of both IκB (Ser32/36) and p65 (Ser276) in NES-B cells (Figure 4A). In contrast, these same exposures caused a smaller increase in IκB phosphorylation, with only a minimal increase in p65 phosphorylation in NES-G cells (Figure 4A). Using an NF-κB reporter construct, we found that treatment with acidic bile salts significantly increased NF-κB/p65 transcriptional activity in NES-B cells, but not in NES-G cells (Figure 4B).
Figure 4.
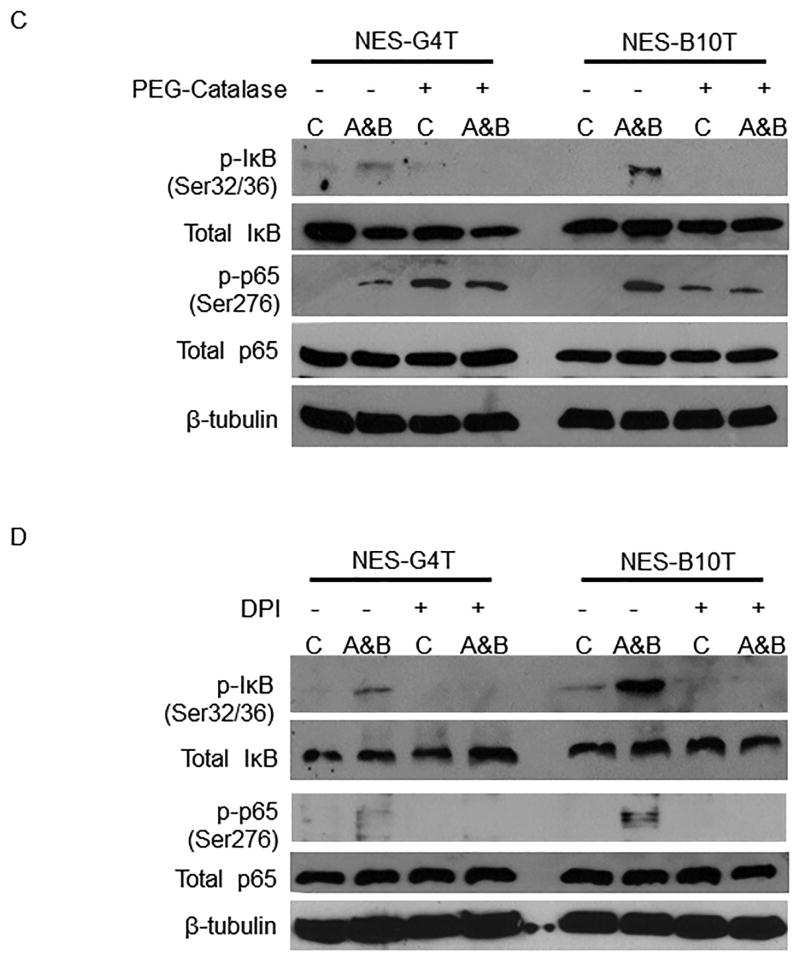
Exposure to acid, bile salts and acidic bile salts causes a greater increase in IκB-NF-κB-PKAc complex activation in NES-B cells than in NES-G cells. A) Representative Western blots demonstrating acid and bile salt-induced phospho-IkB (Ser32/36) and phospho-p65 (Ser276) in NES cells. B) Luciferase reporter assay for NF-κB/p65 activity in response to acidic bile salts in NES cells. *, p < 0.05 compared with non-acidic bile salt treated controls. C&D) Acidic bile salts cause oesophageal squamous cells to generate H2O2 through NADPH oxidase, thereby activating the IκB-NF-κB-PKAc complex. Representative Western blots demonstrating inhibition of acidic bile salt-induced increases in phospho-IκB and phospho-p65 by C) PEG-catalase, a H2O2 scavenger, and D) DPI, an NADPH oxidase inhibitor. C, untreated controls; A, acid; B, bile salts; A&B, acidic bile salts. Western blots shown are representative of the results from 2 independent experiments.
Oesophageal squamous cells exposed to acidic bile salts generate H2O2 via the NADPH oxidase system to cause phosphorylation of IκB and p65
Both IκB degradation and PKAc activation have been linked with generation of reactive oxygen species (ROS) and, in earlier studies, we demonstrated that oesophageal squamous cells exposed to acidic bile salts generate ROS via the NADPH oxidase system 38. Since H202 is the principal ROS produced by the NADPH oxidase system, we treated NES cells with PEG-catalase (a H2O2 scavenger) or DPI (an NADPH oxidase inhibitor), exposed them to acidic bile salts, and performed Western blots for phospho-IκB (Ser32/36) and phospho-p65 (Ser276). Both PEG-catalase (Figure 4C) and DPI (Figure 4D) prevented acidic bile salts from increasing phosphorylation of IκB and p65 in NES-G4T and NES-B10T cells. This suggests that acidic bile salts activate the IκB-NF-κB-PKAc complex by generating intracellular H202 via the NAPH oxidase system.
Aspirin blocks phosphorylation of IκB, nuclear translocation of p65, activation of CDX2 promoter, and expression of CDX2 mRNA induced by acid and bile salts in NES-B cells
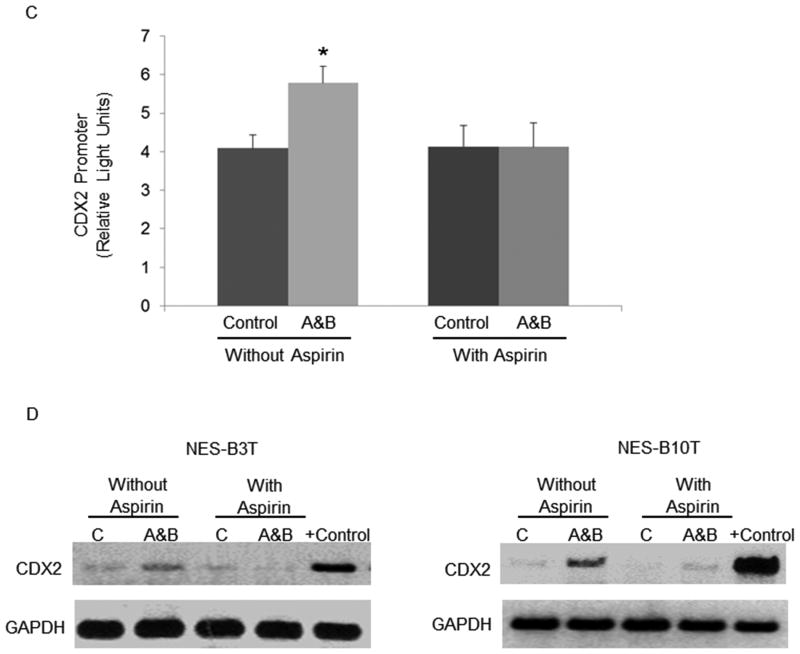
Phosphorylation of IκB (Ser32/36), a critical step in NF-κB activation, is the major function of the IκB kinase (IKK)β protein 35. In an earlier study, we showed that acidic bile salts activate IKKβ, which in turn phosphorylates IκB in NES-B10T cells 39. To prevent NF-κB activation, therefore, IKKβ is the logical target. IKKβ is a target of aspirin, but not of non-aspirin NSAIDs 15. In earlier studies, we found that acid and bile salts induced CDX2 expression in NES-B cells, but not in NES-G cells 6. Therefore, we treated only the NES-B cells with acidic bile salts and aspirin (100 μM), and assessed IκB and p65 phosphorylation as well as CDX2 promoter activity and mRNA expression. Aspirin treatment abolished the acidic bile salt-induced increase in phosphorylation of IκB but not p65 (Figure 5A), and blocked the increase in p65 nuclear translocation (Figure 5B). Furthermore, aspirin eliminated the increase in CDX2 promoter activity induced by acidic bile salts in NES-B10T cells (Figure 5C), and aspirin abolished the increase in CDX2 mRNA expression induced by acidic bile salts in NES-B3T and NES-B10T cells (Figure 5D).
Figure 5.
Aspirin prevents the increase in phosphorylation of IκB, nuclear levels of p65 nuclear, CDX2 promoter activation, and CDX2 mRNA expression induced by acid and bile salt in NES-B cells. A) Representative Western blots demonstrating phospho- and total IkB serine 32/36 and phospho- and total p65 (Ser276) in NES-B10T cells. B) Immunofluorescence of nuclear p65 protein induced by acidic bile salts with and without treatment with aspirin in NES-B10T cells; DAPI demonstrates the number of cell nuclei in the same field (scale bar = 50 μm). C) CDX2 promoter activation induced by acidic bile salts with and without treatment with aspirin in NES-B10T cells. Bar graphs represent the mean ±SEM. *, p ≤ 0.05 compared with non-acidic bile salt treated controls D) PCR analysis of CDX2 mRNA expression induced by acidic bile salts in NES-B3T and NES-B10T cells with and without treatment with aspirin. C, untreated controls; A&B, acidic bile salts; the Barrett's epithelial cell line BAR-T served as a positive control for CDX2 mRNA expression. Western blots shown are representative of the results from 2 independent experiments.
Aspirin blocks acid and bile salt-induced CDX2 promoter activation in primary oesophageal squamous cells from patients with Barrett's oesophagus
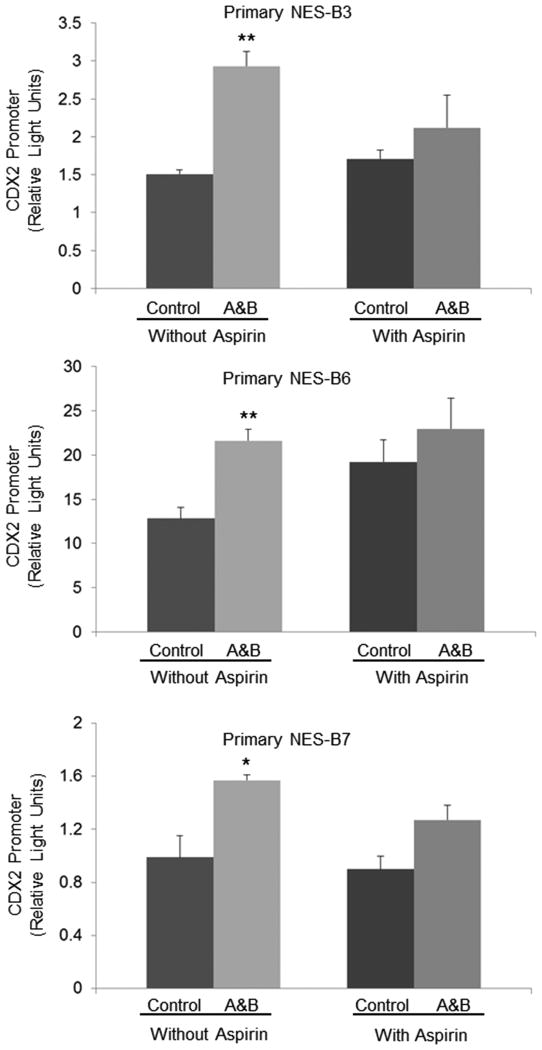
Using primary oesophageal squamous cell cultures established from endoscopic biopsies obtained from three patients with Barrett's oesophagus (primary NES-B3, NES-B6, and NES-B7), we confirmed the effects of aspirin on CDX2 promoter activation induced by acidic bile salts. As in the oesophageal squamous cell lines, treatment with acidic bile salts caused a significant increase in CDX2 promoter activity that was blocked by aspirin treatment in all three primary cell cultures (Figure 6). A schematic model summarizing mechanisms elucidated by our study is provided in Figure 7.
Figure 6.
Aspirin blocks CDX2 promoter activation by acidic bile salts in primary cultures of oesophageal squamous epithelial cells from patients with Barrett's oesophagus (NES-B3, NES-B6, NES-B7). Bar graphs represent the mean ±SEM. A&B, acidic bile salts; *, p<0.05 compared to non-acidic bile salt treated controls; **, p<0.01 compared to non-acidic bile salt treated controls.
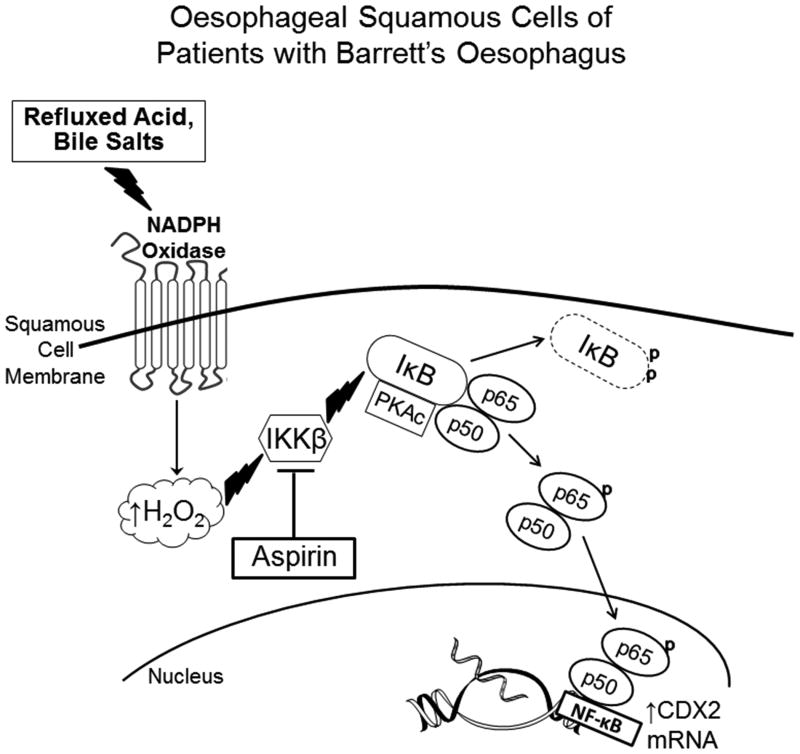
Figure 7.
Schematic demonstrating mechanism whereby refluxed acid and bile salts cause NF-κB pathway activation resulting in CDX2 expression in oesophageal squamous cells of patients with Barrett's oesophagus. Refluxed acid and bile salts stimulate NADPH oxidase to generate H2O2, which activates IKKβ, which in turn activates the IκB-NF-κB-PKAc complex through phosphorylation of IκB. This leads to the degradation of IκB, which releases the p50/p65 heterodimer. PKAc in the activated IκB-NF-κB-PKAc complex phosphorylates p65, inducing further formation of p50/p65 heterodimers, which translocate to the nucleus to stimulate transcription of NF-κB p50 target genes (e.g.CDX2) that might play a role in columnar metaplasia. By blocking the activity of IKKβ, aspirin can stop NF-κB signaling and CDX2 expression. Thus, aspirin might protect against the development of Barrett's oesophagus in GORD patients whose squamous epithelium is predisposed to heal reflux esophagitis through metaplasia rather than squamous cell regeneration.
Discussion
Our exploration of mechanisms whereby acid and bile salts induce CDX2 expression in oesophageal squamous cells from patients with Barrett's oesophagus (NES-B cells), but not in those from GORD patients without Barrett's oesophagus (NES-G cells) has yielded a number of novel findings. In both NES-B cells and NES-G cells, we have shown that acid and bile salts activate the NADPH oxidase system to generate H2O2, which activates the IκB-NF-κB-PKAc complex through a series of phosphorylations. However, there is far greater activation of this complex in NES-B cells, as evidenced by our finding of much higher levels of phosphorylated IκB and phosphorylated p65 in NES-B cells than in NES-G cells exposed to the same concentrations of acid and bile salts. As a result of the limited IκB-NF-κB-PKAc complex activation in NES-G cells, the p65 NF-κB subunit remains bound to IκB in the cytoplasm and, unlike the p50 subunit, p65 does not translocate to the nucleus. This is important because we also have shown that increased nuclear p65 protein levels are required for CDX2 promoter activation by acid and bile salts, even though it is only p50 that binds the promoter. Finally, we have demonstrated that aspirin, which can prevent IKKβ from activating IκB, blocks the phosphorylation of IκB, nuclear translocation of p65, activation of CDX2 promoter, and expression of CDX2 mRNA induced by acid and bile salts in NES-B cells.
In earlier reports, we proposed that differences among individuals in the oesophageal molecular pathways activated by refluxed acid and bile salts might determine whether reflux oesophagitis heals through squamous cell regeneration or through the process of columnar metaplasia 4-8. Our present study has elucidated major differences between oesophageal cells from GORD patients with and without Barrett's oesophagus in the degree of IκB-NF-κB-PKAc complex activation by acid and bile salts. Those differences can explain our earlier observation that acid and bile salts induce CDX2 expression in NES-B cells, but not in NES-G cells. CDX2 is a key homeotic gene required for development of intestinal-type columnar epithelium and, in animal models of reflux esophagitis, expression of CDX2 in oesophageal squamous cells heralds the development of intestinal metaplasia 30-32. Thus, differences among patients in the degree to which reflux induces oesophageal activation of the IκB-NF-κB-PKAc complex (and hence NF-κB signaling leading to the expression of CDX2) might determine whether GORD results in the development of Barrett's intestinal-type metaplasia. It remains unclear whether the progenitor cells for Barrett's metaplasia reside in the oesophagus (in squamous epithelial basal cells or in cells of the oesophageal gland ducts) or in the gastric cardia 40. In any case, the ability to express CDX2 (as do NES-B cells) appears to be a prerequisite for the development of intestinal-type metaplasia 41.
NF-κB activity can be triggered by a variety of signals, all of which ultimately converge on a common target - the cytoplasmic IκB-NF-κB-PKAc complex 37. Phosphorylation of IκB protein in the complex leads to its degradation, which releases both PKAc and the p65 NF-κB subunit 37. Signals that trigger IκB degradation also can activate PKAc to phosphorylate p65 (Ser276), a post-translational modification that favors p50/p65 heterodimer formation and stimulates p65 transcriptional activity 36, 37, 42. Moreover, the intensity of PKAc activation has been shown to correlate with the loss of PKAc bound to p65 in immunoprecipitation experiments 37. Using immunoprecipitation and Western blotting, we found that PKAc indeed was present in complex with IκB and p65 in our oesophageal squamous cells. We also found that acid and bile salts caused a strong decrease in p65 bound to IκB and to PKAc, which was accompanied by a slight increase in p50/p65 heterodimer formation and a significant increase in NF-kB/p65 transcriptional activity in NES-B cells, but not in NES-G cells.
The association among NSAIDs, Barrett's metaplasia and Barrett's cancers remains a highly controversial topic, and different studies sometimes have arrived at contradictory conclusions. For example, one recent investigation that used meta-analytic methods on pooled data from case-control studies conducted in the BEACON Consortium found no significant association between the use of NSAIDs (including aspirin) and the presence of Barrett's oesophagus 43. Our molecular investigations have yielded a potential explanation for case-control studies finding that aspirin protects against the development of Barrett's oesophagus, while other NSAIDs do not 11, 12. Unlike other NSAIDs, aspirin blocks ATP binding to IKKβ, thus disabling its principal function of phosphorylating IκB 35. We found that aspirin in a concentration of 100 μM abolished the increases in phospho-IκB, CDX2 promoter activation and CDX2 mRNA expression induced by acid and bile salts in NES-B10T cells. We were not surprised that aspirin had no effect on the phosphorylation of p65, because this is an effect of PKAc, an enzyme that is not inhibited by aspirin. Using three primary squamous cell cultures from GORD patients with Barrett's oesophagus, we confirmed that aspirin blocks acid and bile salt-induced CDX2 promoter activation. Patients who take aspirin for chronic inflammatory diseases have serum concentrations in the range of 1000-5000 μM and, therefore, a serum concentration of 100 μM is readily achievable with oral dosing 15. Thus, we have elucidated a molecular mechanism whereby aspirin might protect against the development of Barrett's oesophagus in GORD patients whose squamous epithelium expresses CDX2 in response to the reflux of acid and bile.
Finally, our findings might have important implications for management of Barrett's patients treated with radiofrequency ablation (RFA), which is now the endoscopic procedure of choice for eradicating dysplastic Barrett's oesophagus 3. RFA uses a catheter-based balloon harboring electrodes that deliver radiofrequency energy to the metaplastic mucosa, inflicting an extensive, circumferential thermal injury. Patients are then treated with proton pump inhibitors (PPIs) to control acid reflux and enable healing of the ablated metaplastic columnar mucosa with neo-squamous epithelium. Early studies suggested that Barrett's metaplasia recurrence rates after RFA were low, but recent studies have shown much higher recurrence rates, approaching 50% of patients within 4 years in some reports 44. The origin of these recurrences is not clear, but it seems likely that they result from GORD-induced oesophageal injury that is not entirely prevented by PPI therapy. PPIs, even in high dosages, do not normalize esophageal acid exposure in many patients with Barrett's esophagus, and bile acid reflux continues despite PPI therapy 45. If the pathogenesis of recurrent Barrett's metaplasia involves refluxed acid and bile salts triggering activation of the IκB-NF-κB-PKAc complex, with NF-κB signaling leading to the expression of CDX2, then aspirin treatment might interrupt this process. Thus, our findings provide a molecular rationale for clinical trials of aspirin (in combination with PPIs) to block NF-κB activation in oesophageal epithelial cells so as to prevent recurrent Barrett's metaplasia after RFA.
In conclusion, we have demonstrated that there are substantial differences between oesophageal squamous cells from GORD patients with and without Barrett's oesophagus in the intensity with which acid and bile salts activate the NF-κB pathway. Although acid and bile salts increase the production of H2O2 via the NADPH oxidase system in oesophageal squamous cells of both types of GORD patients, the H2O2 activates the NF-κB pathway sufficiently to cause CDX2 expression only in squamous cells of the Barrett's patients. Furthermore, we found that we could block the CDX2 expression induced by acid and bile salts by using aspirin to inhibit the phosphorylation of IκB. These findings elucidate molecular mechanisms that might explain why some GORD patients develop Barrett's oesophagus while others do not, and why aspirin appears to protect against the development of Barrett's oesophagus in some recent case-control studies. These results also provide a rationale for clinical trials on aspirin for the prevention of recurrent Barrett's metaplasia after RFA.
Supplementary Material
Significance of this study.
What is already known on this subject?
Caudal-related homeobox transcription factor 2 (CDX2) directs the formation of intestinal epithelium, and is judged to play a key role in the pathogenesis of Barrett's intestinal-type metaplasia.
Acid and bile salts induce CDX2 expression in oesophageal squamous cell lines from patients with Barrett's oesophagus (NES-B cells), but not in those from patients who have gastro-oesophageal reflux disease (GORD) without Barrett's oesophagus (NES-G cells).
CDX2 is a target of NF-κB signaling, which can be inhibited by aspirin, but not by other non-steroidal anti-inflammatory drugs (NSAIDs).
Some recent case-control studies have found that aspirin (but no other NSAID) protects against the development of Barrett's oesophagus.
What are the new findings?
In both NES-B and NES-G cells, acid and bile salts activate NADPH oxidase to generate H2O2, which activates the IκB-NF-κB-PKAc complex.
After exposure to acid and bile salts, NES-B cells exhibit much higher levels of phosphorylated IκB, phosphorylated p65, p50/p65 NF-κB heterodimer formation, and NF-κB/p65 transcriptional activity than NES-G cells, indicating far greater activation of the IκB-NF-κB-PKAc complex by acid and bile salts in NES-B cells.
siRNA inhibition of p65 in NES-B cells prevents the increase in expression of CDX2 induced by acid and bile salts.
Aspirin blocks the increases in IκB phosphorylation, p65 nuclear translocation, CDX2 promoter activation, and CDX2 expression induced by acid and bile salts in NES-B cell lines and, in primary cultures of NES-B cells, aspirin blocks the acid and bile salt-induced increase in CDX2 promoter activation.
How might it impact on clinical practice in the foreseeable future?
Our finding of major differences between NES-B cells and NES-G cells in the degree to which acid and bile salts activate NF-κB can account for their differences in CDX2 expression, which might explain why some GORD patients develop Barrett's oesophagus while others do not.
Our finding that aspirin blocks the increase in NF-κB activity induced by acid and bile salts in NES-B cells might explain the finding of some case-control studies that aspirin protects against the development of Barrett's metaplasia while other NSAIDs do not.
Our findings provide a rationale for clinical trials on aspirin for preventing the development of Barrett's oesophagus in GORD patients, and for preventing the recurrence of Barrett's metaplasia in patients treated with radiofrequency ablation.
Acknowledgments
Funding: This work was supported by Merit Review Award #BX002666 from the U.S. Department of Veterans Affairs Biomedical Laboratory Research Program (S.J.S.), the National Institutes of Health (R01-DK63621and R01-DK103598 to R.F.S. and S.J.S.; K12 HD-068369 and K08-DK099383 to EC; R01-DK097340 to D.H.W.; NCI N01-CN-05014-69 (TO-RFP S-2014 MDA2013-02-02 to R.S.B.; NCI BETRNet program CA163004 and Career Development Award OD 012097 to J.P.L; and the American Gastroenterological Association June and Donald O. Castell Esophageal Clinical Research Award (to K.B.D.);
Abbreviations
- CDX
caudal-related homeobox transcription factor
- ChIP
chromatin immune-precipitation
- DPI
diphenylene iodonium
- GORD
gastro-oesophageal reflux disease
- H2O2
hydrogen peroxide
- IB
immunoblot
- IF
immunofluorescence
- IKK
IκB kinase
- IP
immunoprecipitation
- mRNA
messenger RNA
- NADPH
nicotinamide adenine dinucleotide phosphate
- NES
normal oesophageal squamous
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NSAIDs
nonsteroidal anti-inflammatory drugs
- PKAc
protein kinase A catalytic subunit
- PMSF
phenylmethylsulfonyl fluoride
- siRNA
small inhibitory RNA
- PPI
proton pump inhibitor
- RFA
radiofrequency ablation
- ROS
reactive oxygen species
- RT-PCR
reverse transcription polymerase chain reaction
- SEM
standard error of the mean
Footnotes
Contributorship statement: X.H.: study design; technical and material support; analysis and interpretation of data; critical revision of manuscript; important intellectual content; drafting of manuscript
X.Z.: technical and material support; important intellectual content
C.Y.: technical and material support; important intellectual content
E.C.: technical and material support; important intellectual content
Q.Z.: technical and material support; important intellectual content
K.B.D.: technical and material support; important intellectual content
T.H.P.: technical and material support; important intellectual content
J.P.L.: technical and material support; important intellectual content
D.H.W.: technical and material support; important intellectual content
R.B.: study concept; critical revision of manuscript; important intellectual content
S.J.S.: study concept; analysis and interpretation of data; critical revision of manuscript; important intellectual content
R.F.S.: study concept/design; analysis and interpretation of data; critical revision of manuscript; important intellectual content; drafting of manuscript
Publisher's Disclaimer: VA/US Government Disclaimer: The contents do not represent the views of the U.S. Department of Veterans Affairs or the United State Government.
Competing Interests: None declared
Ethics approval: These studies were approved by the Institutional Review Board of the Dallas VA Medical Center
References
- 1.Ronkainen J, Aro P, Storskrubb T, et al. Prevalence of Barrett's esophagus in the general population: an endoscopic study. Gastroenterology. 2005;129:1825–31. doi: 10.1053/j.gastro.2005.08.053. [DOI] [PubMed] [Google Scholar]
- 2.Rex DK, Cummings OW, Shaw M, et al. Screening for Barrett's esophagus in colonoscopy patients with and without heartburn. Gastroenterology. 2003;125:1670–7. doi: 10.1053/j.gastro.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 3.Spechler SJ, Souza RF. Barrett's esophagus. N Engl J Med. 2014;371:836–45. doi: 10.1056/NEJMra1314704. [DOI] [PubMed] [Google Scholar]
- 4.Souza RF, Shewmake KL, Shen Y, et al. Differences in ERK activation in squamous mucosa in patients who have gastroesophageal reflux disease with and without Barrett's esophagus. Am J Gastroenterol. 2005;100:551–9. doi: 10.1111/j.1572-0241.2005.41122.x. [DOI] [PubMed] [Google Scholar]
- 5.Zhang HY, Zhang X, Chen X, et al. Differences in activity and phosphorylation of MAPK enzymes in esophageal squamous cells of GERD patients with and without Barrett's esophagus. Am J Physiol Gastrointest Liver Physiol. 2008;295:G470–8. doi: 10.1152/ajpgi.90262.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huo X, Zhang HY, Zhang XI, et al. Acid and bile salt-induced CDX2 expression differs in esophageal squamous cells from patients with and without Barrett's esophagus. Gastroenterology. 2010;139:194–203.e1. doi: 10.1053/j.gastro.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang DH, Tiwari A, Kim ME, et al. Hedgehog signaling regulates FOXA2 in esophageal embryogenesis and Barrett's metaplasia. J Clin Invest. 2014;124:3767–80. doi: 10.1172/JCI66603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asanuma K, Huo X, Agoston A, et al. In oesophageal squamous cells, nitric oxide causes S-nitrosylation of Akt and blocks SOX2 (sex determining region Y-box 2) expression. Gut. 2016;65:1416–26. doi: 10.1136/gutjnl-2015-309272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corley DA, Kerlikowske K, Verma R, et al. Protective association of aspirin/NSAIDs and esophageal cancer: a systematic review and meta-analysis. Gastroenterology. 2003;124:47–56. doi: 10.1053/gast.2003.50008. [DOI] [PubMed] [Google Scholar]
- 10.Liao LM, Vaughan TL, Corley DA, et al. Nonsteroidal anti-inflammatory drug use reduces risk of adenocarcinomas of the esophagus and esophagogastric junction in a pooled analysis. Gastroenterology. 2012;142:442–452.e5. doi: 10.1053/j.gastro.2011.11.019. quiz e22-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schneider JL, Zhao WK, Corley DA. Aspirin and nonsteroidal anti-inflammatory drug use and the risk of Barrett's esophagus. Dig Dis Sci. 2015;60:436–43. doi: 10.1007/s10620-014-3349-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Omer ZB, Ananthakrishnan AN, Nattinger KJ, et al. Aspirin protects against Barrett's esophagus in a multivariate logistic regression analysis. Clin Gastroenterol Hepatol. 2012;10:722–7. doi: 10.1016/j.cgh.2012.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Molina MA, Sitja-Arnau M, Lemoine MG, et al. Increased cyclooxygenase-2 expression in human pancreatic carcinomas and cell lines: growth inhibition by nonsteroidal anti-inflammatory drugs. Cancer Res. 1999;59:4356–62. [PubMed] [Google Scholar]
- 14.Piazza GA, Rahm AL, Krutzsch M, et al. Antineoplastic drugs sulindac sulfide and sulfone inhibit cell growth by inducing apoptosis. Cancer Res. 1995;55:3110–6. [PubMed] [Google Scholar]
- 15.Yin MJ, Yamamoto Y, Gaynor RB. The anti-inflammatory agents aspirin and salicylate inhibit the activity of I(kappa)B kinase-beta. Nature. 1998;396:77–80. doi: 10.1038/23948. [DOI] [PubMed] [Google Scholar]
- 16.Liou GY, Doppler H, Necela B, et al. Macrophage-secreted cytokines drive pancreatic acinar-to-ductal metaplasia through NF-kappaB and MMPs. J Cell Biol. 2013;202:563–77. doi: 10.1083/jcb.201301001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Riordan JM, Abdel-latif MM, Ravi N, et al. Proinflammatory cytokine and nuclear factor kappa-B expression along the inflammation-metaplasia-dysplasia-adenocarcinoma sequence in the esophagus. Am J Gastroenterol. 2005;100:1257–64. doi: 10.1111/j.1572-0241.2005.41338.x. [DOI] [PubMed] [Google Scholar]
- 18.Huo X, Zhang X, Yu C, et al. In oesophageal squamous cells exposed to acidic bile salt medium, omeprazole inhibits IL-8 expression through effects on nuclear factor-kappaB and activator protein-1. Gut. 2014;63:1042–52. doi: 10.1136/gutjnl-2013-305533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo RJ, Suh ER, Lynch JP. The role of Cdx proteins in intestinal development and cancer. Cancer Biol Ther. 2004;3:593–601. doi: 10.4161/cbt.3.7.913. [DOI] [PubMed] [Google Scholar]
- 20.Beck F. The role of Cdx genes in the mammalian gut. Gut. 2004;53:1394–6. doi: 10.1136/gut.2003.038240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vallbohmer D, DeMeester SR, Peters JH, et al. Cdx-2 expression in squamous and metaplastic columnar epithelia of the esophagus. Dis Esophagus. 2006;19:260–6. doi: 10.1111/j.1442-2050.2006.00586.x. [DOI] [PubMed] [Google Scholar]
- 22.Phillips RW, Frierson HF, Jr, Moskaluk CA. Cdx2 as a marker of epithelial intestinal differentiation in the esophagus. Am J Surg Pathol. 2003;27:1442–7. doi: 10.1097/00000478-200311000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Groisman GM, Amar M, Meir A. Expression of the intestinal marker Cdx2 in the columnar-lined esophagus with and without intestinal (Barrett's) metaplasia. Mod Pathol. 2004;17:1282–8. doi: 10.1038/modpathol.3800182. [DOI] [PubMed] [Google Scholar]
- 24.Eda A, Osawa H, Satoh K, et al. Aberrant expression of CDX2 in Barrett's epithelium and inflammatory esophageal mucosa. J Gastroenterol. 2003;38:14–22. doi: 10.1007/s005350300001. [DOI] [PubMed] [Google Scholar]
- 25.Kim S, Domon-Dell C, Wang Q, et al. PTEN and TNF-alpha regulation of the intestinal-specific Cdx-2 homeobox gene through a PI3K, PKB/Akt, and NF-kappaB-dependent pathway. Gastroenterology. 2002;123:1163–78. doi: 10.1053/gast.2002.36043. [DOI] [PubMed] [Google Scholar]
- 26.Liu T, Zhang X, So CK, et al. Regulation of Cdx2 expression by promoter methylation, and effects of Cdx2 transfection on morphology and gene expression of human esophageal epithelial cells. Carcinogenesis. 2007;28:488–96. doi: 10.1093/carcin/bgl176. [DOI] [PubMed] [Google Scholar]
- 27.Kazumori H, Ishihara S, Rumi MA, et al. Bile acids directly augment caudal related homeobox gene Cdx2 expression in oesophageal keratinocytes in Barrett's epithelium. Gut. 2006;55:16–25. doi: 10.1136/gut.2005.066209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marchetti M, Caliot E, Pringault E. Chronic acid exposure leads to activation of the cdx2 intestinal homeobox gene in a long-term culture of mouse esophageal keratinocytes. J Cell Sci. 2003;116:1429–36. doi: 10.1242/jcs.00338. [DOI] [PubMed] [Google Scholar]
- 29.Hu Y, Williams VA, Gellersen O, et al. The pathogenesis of Barrett's esophagus: secondary bile acids upregulate intestinal differentiation factor CDX2 expression in esophageal cells. J Gastrointest Surg. 2007;11:827–34. doi: 10.1007/s11605-007-0174-3. [DOI] [PubMed] [Google Scholar]
- 30.Tatsuta T, Mukaisho K, Sugihara H, et al. Expression of Cdx2 in early GRCL of Barrett's esophagus induced in rats by duodenal reflux. Dig Dis Sci. 2005;50:425–31. doi: 10.1007/s10620-005-2452-9. [DOI] [PubMed] [Google Scholar]
- 31.Pera M, Pera M, de Bolos C, et al. Duodenal-content reflux into the esophagus leads to expression of Cdx2 and Muc2 in areas of squamous epithelium in rats. J Gastrointest Surg. 2007;11:869–74. doi: 10.1007/s11605-007-0162-7. [DOI] [PubMed] [Google Scholar]
- 32.Ingravallo G, Dall'Olmo L, Segat D, et al. CDX2 hox gene product in a rat model of esophageal cancer. J Exp Clin Cancer Res. 2009;28:108. doi: 10.1186/1756-9966-28-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng E, Zhang X, Huo X, et al. Omeprazole blocks eotaxin-3 expression by oesophageal squamous cells from patients with eosinophilic oesophagitis and GORD. Gut. 2013;62:824–32. doi: 10.1136/gutjnl-2012-302250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jaiswal KR, Morales CP, Feagins LA, et al. Characterization of telomerase-immortalized, non-neoplastic, human Barrett's cell line (BAR-T) Dis Esophagus. 2007;20:256–64. doi: 10.1111/j.1442-2050.2007.00683.x. [DOI] [PubMed] [Google Scholar]
- 35.Perkins ND. Post-translational modifications regulating the activity and function of the nuclear factor kappa B pathway. Oncogene. 2006;25:6717–30. doi: 10.1038/sj.onc.1209937. [DOI] [PubMed] [Google Scholar]
- 36.Gapuzan ME, Pitoc GA, Gilmore TD. Mutations within a conserved protein kinase A recognition sequence confer temperature-sensitive and partially defective activities onto mouse c-Rel. Biochem Biophys Res Commun. 2003;307:92–9. doi: 10.1016/s0006-291x(03)01123-9. [DOI] [PubMed] [Google Scholar]
- 37.Zhong H, SuYang H, Erdjument-Bromage H, et al. The transcriptional activity of NF-kappaB is regulated by the IkappaB-associated PKAc subunit through a cyclic AMP-independent mechanism. Cell. 1997;89:413–24. doi: 10.1016/s0092-8674(00)80222-6. [DOI] [PubMed] [Google Scholar]
- 38.Feagins LA, Zhang HY, Zhang X, et al. Mechanisms of oxidant production in esophageal squamous cell and Barrett's cell lines. Am J Physiol Gastrointest Liver Physiol. 2008;294:G411–7. doi: 10.1152/ajpgi.00373.2007. [DOI] [PubMed] [Google Scholar]
- 39.Huo X, Agoston A, Dunbar KB, et al. Hypoxia-Inducible Factor 2a Plays a Role in Mediating Oesophagitis in Gastro-Oesophageal Reflux Disease. Gut. 2016 doi: 10.1136/gutjnl-2016-312595. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang DH, Souza RF. Transcommitment: Paving the Way to Barrett's Metaplasia. Adv Exp Med Biol. 2016;908:183–212. doi: 10.1007/978-3-319-41388-4_10. [DOI] [PubMed] [Google Scholar]
- 41.Burke ZD, Tosh D. Barrett's metaplasia as a paradigm for understanding the development of cancer. Curr Opin Genet Dev. 2012;22:494–9. doi: 10.1016/j.gde.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 42.Ganchi PA, Sun SC, Greene WC, et al. A novel NF-kappa B complex containing p65 homodimers: implications for transcriptional control at the level of subunit dimerization. Mol Cell Biol. 1993;13:7826–35. doi: 10.1128/mcb.13.12.7826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thrift AP, Anderson LA, Murray LJ, et al. Nonsteroidal Anti-Inflammatory Drug Use is Not Associated With Reduced Risk of Barrett's Esophagus. Am J Gastroenterol. 2016;111:1528–1535. doi: 10.1038/ajg.2016.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Small AJ, Sutherland SE, Hightower JS, et al. Comparative risk of recurrence of dysplasia and carcinoma after endoluminal eradication therapy of high-grade dysplasia versus intramucosal carcinoma in Barrett's esophagus. Gastrointest Endosc. 2015;81:1158–66. doi: 10.1016/j.gie.2014.10.029. e1-4. [DOI] [PubMed] [Google Scholar]
- 45.Spechler SJ, Sharma P, Traxler B, et al. Gastric and esophageal pH in patients with Barrett's esophagus treated with three esomeprazole dosages: a randomized, double-blind, crossover trial. Am J Gastroenterol. 2006;101:1964–71. doi: 10.1111/j.1572-0241.2006.00661.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.