Abstract
Identification of alterations in the cellular composition of the human immune system is key to understanding the autoimmune process. Recently, a subset of FOXP3+ cells with low CD25 expression was found to be increased in peripheral blood from systemic lupus erythematosus (SLE) patients, although its functional significance remains controversial. Here we find in comparisons with healthy donors that the frequency of FOXP3+ cells within CD127lowCD25low CD4+ T cells (here defined as CD25lowFOXP3+ T cells) is increased in patients affected by autoimmune disease of varying severity, from combined immunodeficiency with active autoimmunity, SLE to type 1 diabetes. We show that CD25lowFOXP3+ T cells share phenotypic features resembling conventional CD127lowCD25highFOXP3+ Tregs, including demethylation of the Treg-specific epigenetic control region in FOXP3, HELIOS expression, and lack of IL-2 production. As compared to conventional Tregs, more CD25lowFOXP3+HELIOS+ T cells are in cell cycle (33.0% vs 20.7% Ki-67+; P = 1.3 × 10−9) and express the late-stage inhibitory receptor PD-1 (67.2% vs 35.5%; P = 4.0 × 10−18), while having reduced expression of the early-stage inhibitory receptor CTLA-4, as well as other Treg markers, such as FOXP3 and CD15s. The number of CD25lowFOXP3+ T cells is correlated (P = 3.1 × 10−7) with the proportion of CD25highFOXP3+ T cells in cell cycle (Ki-67+). These findings suggest that CD25lowFOXP3+ T cells represent a subset of Tregs that are derived from CD25highFOXP3+ T cells, and are a peripheral marker of recent Treg expansion in response to an autoimmune reaction in tissues.
Keywords: Regulatory T cells (Tregs), Autoimmunity, FOXP3, Treg-specific demethylated region (TSDR), CD25
Highlights
-
•
FOXP3+ compartment within CD127lowCD25low T cells is expanded in autoimmune patients.
-
•
Increased numbers of CD25lowFOXP3+ T cells are a circulating marker of autoimmunity.
-
•
CD25lowFOXP3+ HELIOS+ T cells are fully demethylated at the FOXP3 TSDR.
-
•
CD25lowFOXP3+ T cells could represent a terminal differentiation stage of regulatory T cells.
1. Introduction
FOXP3+ regulatory T cells (Tregs) are produced in the thymus as a specific T cell lineage following high affinity TCR engagement that results in the demethylation of the Treg-specific demethylated region (TSDR) in FOXP3 and stable FOXP3 expression [1]. Following emigration from the thymus and activation, naïve Tregs proliferate and differentiate into memory Tregs that are actively recruited to peripheral compartments to suppress immune responses against self-antigen and maintain tissue integrity [2]. It is becoming increasingly apparent that there is considerable heterogeneity in memory Treg subsets in humans [3], [4]. One major challenge for studying human Tregs is that in general peripheral blood cells from patients are more readily available, rather than the effector T cells and Tregs present in the inflamed tissue and associated lymph nodes. A better understanding of the composition of the Treg compartment in peripheral blood is therefore needed to investigate the potential contribution to disease made by Tregs and to identify cellular alterations of the peripheral compartment associated with the onset of pathogenic autoimmune destruction of the targeted tissue.
Recently, a subset of FOXP3+ CD4+ T cells with low expression of CD25 was reported to be increased in peripheral blood of autoimmune systemic lupus erythematosus (SLE) patients [5], [6], [7], [8], [9], a finding that was later expanded to the peripheral blood of multiple sclerosis [10] and rheumatoid arthritis [11] patients. The frequency of this cell subset has been demonstrated to be associated with increased SLE disease activity in one study [7] but not in another [5]. Nevertheless, in this second study the frequency of CD25low FOXP3+ T cells was correlated with dsDNA antibodies levels [5], [7] suggesting that these cells may be directly pathogenic or biomarkers of autoimmunity in these patients. However, their origin and function in SLE patients and healthy individuals remain ambiguous [12], [13]. In the present study, we characterise these CD127lowCD25lowFOXP3+ CD4+ T cells (henceforth designated as CD25lowFOXP3+ cells), and demonstrate that they share phenotypic features with Tregs, including demethylation of the FOXP3 TSDR and constitutive expression of the transcription factor HELIOS in a majority of the cells, and an inability to produce IL-2 compared to FOXP3− Teffs. However, compared to conventional CD127lowCD25highFOXP3+ Tregs, CD25lowFOXP3+ cells showed increased expression of activation and proliferation markers such as PD-1 and Ki-67, and reduced expression of Treg-associated molecules, including FOXP3 and CTLA-4. We suggest that these cells represent the last stage of the natural life-cycle of TSDR-demethylated Tregs in vivo and that chronic stimulation in the form of active autoimmunity increases their prevalence.
2. Methods
2.1. Subjects
Study participants included 34 SLE patients recruited from Guy's and St Thomas' NHS Foundation Trust. All patients satisfied ACR SLE classification criteria and were allocated a disease activity using SLEDAI-2K at the time of sampling. SLE patients were recruited from a clinic in which the severity of disease was such that none the patients were on high dose oral corticosteroids (>15 mg/day) or B-cell depleting therapy. SLE patients were compared to a cohort of 24 age- and sex-matched healthy donors from the Cambridge BioResource (CBR). A second cohort of 112 healthy donors from the CBR was used for the analysis of Ki-67 expression within the assessed T cell subsets.
Combined immunodeficiency patients (CID; N = 7) were recruited from Cambridge University Hospitals and Papworth Hospital NHS Foundation Trusts, and compared to six age- and sex-matched healthy donors from the CBR. Patients were selected on the presentation of immune infiltration in the lungs and active autoimmunity in the absence of a known genetic cause, although the clinical symptoms were consistent with those associated with recently characterised CTLA4 germline mutations [14]. All CID patients were treated with immunoglobulin replacement therapy and prophylactic antibiotics.
Adult long-standing T1D patients (N = 15) and healthy controls (HC; N = 15) were recruited from the CBR. Newly diagnosed T1D patients (ND; N = 49) and unaffected siblings of other T1D probands (N = 40) were collected from the JDRF Diabetes–Genes, Autoimmunity and Prevention (D-GAP) study (http://paediatrics.medschl.cam.ac.uk/research/clinical-trials/). ND patients were characterised as having been diagnosed with T1D less than two years prior to their blood donation (with one exception of 42 months). Unaffected siblings were islet autoantibody-negative (IAA, IA2, GAD and ZnT8), and were not related to any T1D patient included in this study. All donors were of white ethnicity and all healthy controls and unaffected siblings were individuals without autoimmune disease (self-reported). Baseline characteristics for all participating subjects are summarised in Table 1.
Table 1.
Baseline characteristics of study participants included in the association analyses.
| Cohort | N | Age (years) |
Male N (%) | |
|---|---|---|---|---|
| Median | Range | |||
| SLE | 34 | 36 | 20–72 | 2 (5.9%) |
| Healthy controls (CBR) - cohort 1 | 24 | 42 | 22–62 | 1 (4.2%) |
| Healthy controls (CBR) - cohort 2 | 112 | 49 | 26–78 | 30 (26.8%) |
| CID | 7 | 23 | 13–45 | 5 (71.4%) |
| Healthy controls (CBR) | 6 | 34 | 17–47 | 4 (66.7%) |
| T1D discovery cohort | ||||
| T1D (D-GAP)a | 49 | 13 | 6–34 | 32 (65.3%) |
| T1D (CBR)b | 15 | 32 | 22–32 | 5 (33.3%) |
| T1D (combined) | 64 | 14 | 6–42 | 37 (58.0%) |
| Unaffected Siblings (D-GAP)c | 40 | 13 | 6–31 | 21 (52.5%) |
| Healthy Controls (CBR) | 15 | 27 | 18–37 | 4 (26.7%) |
| Healthy controls (combined) | 55 | 15 | 6–37 | 28 (45.9%) |
| T1D replication cohort | ||||
| T1D (CBR) | 15 | 37 | 17–52 | 5 (33.3%) |
| Healthy Controls (CBR) | 15 | 37 | 22–47 | 4 (26.7%) |
Baseline characteristics for the study participants stratified by the study cohorts.
Newly diagnosed T1D patients (duration of disease ≤ 3 years) enrolled in the Diabetes - Genes, Autoimmunity and Prevention (D-GAP) study.
Long-standing adult T1D patients enrolled from the Cambridge BioResource (CBR).
First-degree sibling of a T1D patient, reporting no autoimmune disease and determined to be negative for the following T1D-associated autoantibodies: IAA, IA2, GAD and ZnT8. CID, Combined immunodeficiency; T1D, type 1 diabetes; SLE; systemic lupus erythematosus.
2.2. Ethics
All samples and information were collected with written and signed informed consent. The D-GAP study was approved by the Royal Free Hospital & Medical School research ethics committee; REC (08/H0720/25). Adult long-standing T1D patients and healthy volunteers were enrolled in the CBR. The study was approved by the local Peterborough and Fenland research ethics committee (05/Q0106/20). Informed consent was obtained from CID patients, parents, or both (R&D Ref: P01685, REC Ref: 12/WA/0148) and from SLE patients (REC Ref: 07/H0718/49). The study conformed to the Declaration of Helsinki and all local ethical requirements.
2.3. PBMC sample preparation
PBMCs were isolated by Ficoll gradient centrifugation and cryopreserved in 10% heat-inactivated human AB serum, as described previously [15]. T1D patients and healthy controls were recruited contemporaneously and samples were processed and stored by the same investigators to prevent spurious findings caused by differential sample preparation.
Cryopreserved PBMCs (10 × 106 per donor) were thawed at 37 °C and resuspended in X-VIVO (Lonza) + 1% heat-inactivated, filtered human AB serum (Sigma). Cell viability following resuscitation was assessed in a subset of 40 donors using the Fixable Viability Dye eFluor 780 (eBioscience) and was found to be consistently very high (95.6%; min = 86.8%, max = 98.2%) for all samples analysed in this study.
2.4. Cell culture and in vitro stimulation
To reduce the effects of experimental variation and other potential covariates, PBMC samples were processed in batches of a minimum of ten samples per day. T1D patients and healthy controls were matched as closely as possible for age (within 5 year age-bands), sex and time of sample preparation.
After thawing, PBMCs were resuspended in RPMI medium (Gibco) supplemented with 10% FBS, 2 mM l-Glutamine and 100 μg/mL Pen-Strep and cultured (106 PBMCs/well) in 24-well flat-bottom cell culture plate (BD). For cytokine production assays, cells were initially rested for 30 min at 37 °C and then cultured in the presence or absence of 5 ng/mL PMA, 100 ng/mL ionomycin and 0.67 μl/mL Monensin GolgiStop (BD Biosciences) for 4 h at 37 °C. For a subset of 66 donors, 106 cells were cultured with medium alone and 0.67 μl/mL Monensin to determine background levels of cytokine production in unstimulated cells.
2.5. Intracellular immunostainings
After activation, PBMCs were harvested, and stained with Fixable Viability Dye eFluor 780 for 20 min at 4 °C. Cells were then stained with fluorochrome-conjugated antibodies against surface receptors (see Supplementary Table 1) for 1 h at 4 °C. Fixation and permeabilisation was performed using FOXP3 Fix/Perm Buffer Set (BioLegend) and cells were then stained with intracellular antibodies for 1 h at 4 °C (see Supplementary Table 1). All experiments were performed in an anonymised, blinded manner without prior knowledge of disease state.
2.6. Flow cytometry
Immunostained samples were acquired using a BD Fortessa (BD Biosciences) flow cytometer with FACSDiva software (BD Biosciences) and analysed using FlowJo (Tree Star, Inc.). Dead-cell exclusion based on the Fixable Viability Dye was performed for the intracellular immunostainings.
2.7. Analysis of the epigenetic demethylation profile by next-generation sequencing
Total PBMCs from seven healthy CBR donors (three males and four females) were stained with fluorophore-conjugated antibodies (see Supplementary Table 1) and sorted using a BD Aria Fusion flow cytometer (BD Biosciences). Methylation of the FOXP3 TSDR was performed using a next-generation sequencing method, as described previously [16].
2.8. Statistical analyses
Statistical analyses were performed using Prism software (GraphPad) and Stata (www.stata.com). Association of the frequency of CD25lowFOXP3+ T cells with T1D, SLE and CID was calculated using two-tailed unpaired student's t-tests. The effects of age, sex and time of collection were controlled by the experimental design used in this study and, therefore, not included as additional covariates. Given that most immune phenotypes showed moderate to strong right skew that violated the assumption of normality, the phenotypes were log-transformed before statistical testing.
Comparison of the expression of the interrogated immune markers between CD127lowCD25lowFOXP3+ CD4+ T cells and: (i) CD25highFOXP3+, (ii) CD25highFOXP3− and (iii) CD25lowFOXP3− CD4+ T cells was performed within individuals using two-tailed paired student's t-tests. The correlations between immune subsets were calculated using linear regression analysis.
To account for the issue of multiple testing we applied a conservative Bonferroni correction to determine the thresholds for significant results: (i) for the comparison of the frequency of CD25lowFOXP3+ T cells between healthy donors and patients from three different autoimmune diseases, we considered P values < 0.0167 significant (Bonferroni correction for three independent tests); (ii) for the comparison of the ten assessed immune markers between the CD25low and CD25high Treg subsets, we considered P values < 0.005 significant (Bonferroni correction for ten independent tests).
3. Results
3.1. Frequency of CD25lowFOXP3+ T cells is increased in blood from patients with active autoimmunity
To investigate the peripheral alterations in FOXP3+ T cell subsets, we performed a detailed immunophenotyping characterisation of cryopreserved peripheral blood mononuclear cells (PBMCs) of different cohorts of patients with autoimmune disease (summarised in Table 1). Analysis of the flow cytometry profile of patients with systemic autoimmunity as compared to healthy donors revealed that the frequency of FOXP3+ CD4+ T cells is highly increased in CD127low cells of some patients. We found that among SLE and CID patients with increased CD127low FOXP3-expressing cells there is a notable loss of CD25 expression, which results in an extremely high frequency of CD127lowCD25lowFOXP3+ cells (Fig. 1A). These findings suggest that the frequency of FOXP3+ cells in the CD127lowCD25low T cell subset (CD25lowFOXP3+ cells; depicted in red in Fig. 1B) is increased as a result of an active autoimmune response and could be a specific marker of Treg activation. Given the lack of peripheral markers that reflect chronic immune activation, we therefore decided to focus our analysis on this population of CD25lowFOXP3+ cells, and investigate their frequency in the peripheral blood of autoimmune patients.
Fig. 1.
Frequency of CD25lowFOXP3+cells is increased in patients with autoimmune disease. (A) Patterns of CD25 and FOXP3 expression among CD127low CD4+ T cells from healthy donors and patients with autoimmune manifestations. (B) Gating strategy for the delineation of the T-cell subsets characterised in this study. Distribution of FOXP3+ cells among: (i) CD127lowCD25high conventional Tregs (depicted in blue); and (ii) CD127lowCD25low T cells (depicted in red). The vertical dotted line represents the threshold for the gating of FOXP3+ cells (histograms). (C, D) Scatter plots depict the frequency (geometric mean ± 95% CI) of FOXP3+ cells among CD127lowCD25low T cells in SLE patients (N = 34 patients vs 24 healthy donors) and combined immunodeficiency patients with active autoimmunity (N = 7 patients vs 6 healthy donors) (C); or in a cohort of T1D patients (N = 62; depicted by red circles) and healthy donors (N = 54; depicted by black squares) (D). P values were calculated using two-tailed unpaired t-tests. P values < 0.0167 were considered significant (Bonferronni correction for the comparison in three different diseases). The initial CD4+ T cell gate (CD4 versus dead cell exclusion dye) was derived from a lymphocyte gate (defined on forward and side scatter) followed by single-cell discrimination. HC, healthy controls; T1D, type 1 diabetes patients; SLE, systemic lupus erythematosus patients; CID, combined immunodeficiency patients.
Consistent with previous findings [7], [8], we confirmed that the frequency of FOXP3+ cells among CD127lowCD25low T cells (gating strategy Fig. 1B) was markedly increased in SLE patients (geometric mean (GeoM) = 13.5%) compared to age- and sex-matched healthy controls (5.5%, P = 2.1 × 10−5, N = 24, Fig. 1C), which likely reflects the systemic immune activation in SLE patients. In support of this hypothesis, we also detected a high frequency of CD25lowFOXP3+ cells in a small cohort of seven CID patients, characterised by severe active autoimmunity compared to age- and sex-matched healthy controls (12.1% and 4.0%, respectively, P = 6.5 × 10−3; Fig. 1C).
We also found that the frequency of FOXP3+ cells among CD127lowCD25low T cells was significantly increased in T1D patients (6.8%) compared to age- and sex-matched healthy controls (4.6%; P = 2.7 × 10−6; Fig. 1D). This association was also observed when comparing the frequency of CD25lowFOXP3+ cells within total CD4+ T cells (0.32% vs 0.23% in T1D patients and controls, respectively; P = 1.1 × 10−3; Supplementary Fig. 1A). We replicated the finding of increased FOXP3+ cells among CD127lowCD25low T cells in an independent cohort of 15 long-standing T1D patients (10.39%) and 15 age- and sex-matched healthy controls (6.3%; P = 7.7 × 10−3; Supplementary Fig. 1B). Furthermore, we noted that the increased frequency of FOXP3+ cells was mainly restricted to the CD127lowCD25low T cell subset, as we observed only a small increased frequency of conventional CD127lowCD25highFOXP3+ Tregs in T1D patients (5.6%) compared to healthy donors (4.8%; P = 8.0 × 10−3; Supplementary Fig. 2).
SLE disease activity index (SLEDAI) scores were available from 33 SLE patients evaluated for the frequency of CD127lowCD25lowFOXP3+ Tregs. We noted that there was a trend towards increased frequency of CD25lowFOXP3+ cells with increased SLEDAI at the time of sampling, although it did not reach statistical significance (Supplementary Fig. 3A). Previously a correlation between disease activity and frequency of CD25lowFOXP3+ cells was reported [7], whereas another study did not [5]. These apparently conflicting results could be due to disease heterogeneity and relatively small sample sizes. Future longitudinal studies assessing the frequency of CD25lowFOXP3+ cells in SLE and other autoimmune patients with high levels of this Treg subset before and following drug treatments will aid in resolving this uncertainty. In the T1D patients, the frequency of CD25lowFOXP3+ cells was not associated with duration of disease indicating that the increased level of CD25low Tregs in T1D is not restricted to the time of disease diagnosis (Supplementary Fig. 3B). The analysis was restricted to the 49 recently diagnosed T1D patients (median 11 months, range 2–42 months) from the D-GAP cohort, which was a much larger cohort and displayed a more homogeneous distribution of time since diagnosis compared to the cohort of long-standing diabetics.
3.2. CD25lowFOXP3+ cells are demethylated at the FOXP3 TSDR
In humans FOXP3 is not exclusively expressed in Tregs, but can also be transiently up-regulated in activated Teffs. However, in thymically-derived Tregs constitutive expression of FOXP3 is known to require a demethylated TSDR [2]. To assess the TSDR methylation profile of CD25lowFOXP3+ cells we sorted these cells from four healthy donors, and compared the methylation of the TSDR in CD25lowFOXP3+ cells, conventional CD25highFOXP3+ Tregs and the respective FOXP3− subsets (Fig. 2A). We found that the majority of CD25lowFOXP3+ cells were demethylated at the TSDR (Fig. 2B and C). The epigenetic demethylation pattern in CD25lowFOXP3+ cells was similar to CD25highFOXP3+ Tregs at all nine interrogated CpG sites in the FOXP3 TSDR (mean = 57.1% and 80.5% demethylation, respectively; Fig. 2B); in contrast, <7% of CD25lowFOXP3− and CD25highFOXP3− cells had demethylated TSDRs. These findings indicate that the majority of CD25lowFOXP3+ cells are bona fide Tregs, and are not Teffs transiently upregulating FOXP3 expression as a result of immune activation.
Fig. 2.
CD25lowFOXP3+cells are demethylated at the FOXP3 Treg-specific demethylated region (TSDR). (A) Gating strategy for FACS sorting of four CD4+ T-cell subsets: (i) CD127lowCD25lowFOXP3− (depicted in green), (ii) CD127lowCD25lowFOXP3+ (depicted in red), (iii) CD127lowCD25highFOXP3− (depicted in grey), and (iv) CD127lowCD25highFOXP3+ (depicted in blue). (B) Frequency (mean ± SEM) of reads demethylated at eight or nine of the nine interrogated CpG sites in the FOXP3 TSDR. The data were obtained from sorted cells from four independent healthy donors. (C) Graphic depicts the proportion of demethylated reads at the nine interrogated CpG sites from the FOXP3 TSDR in one illustrative donor. Each horizontal line represents one sequencing read, with light green representing a methylated read (C) and dark green representing a demethylated read (T). Note that the plot is representative of a male donor. For female donors, X-chromosome inactivation causes half of the reads to be methylated and a correction factor of two was applied to obtain the frequency of demethylated reads.
3.3. CD25lowFOXP3+ cells express the Treg-specific HELIOS transcription factor and exhibit features of an activated phenotype
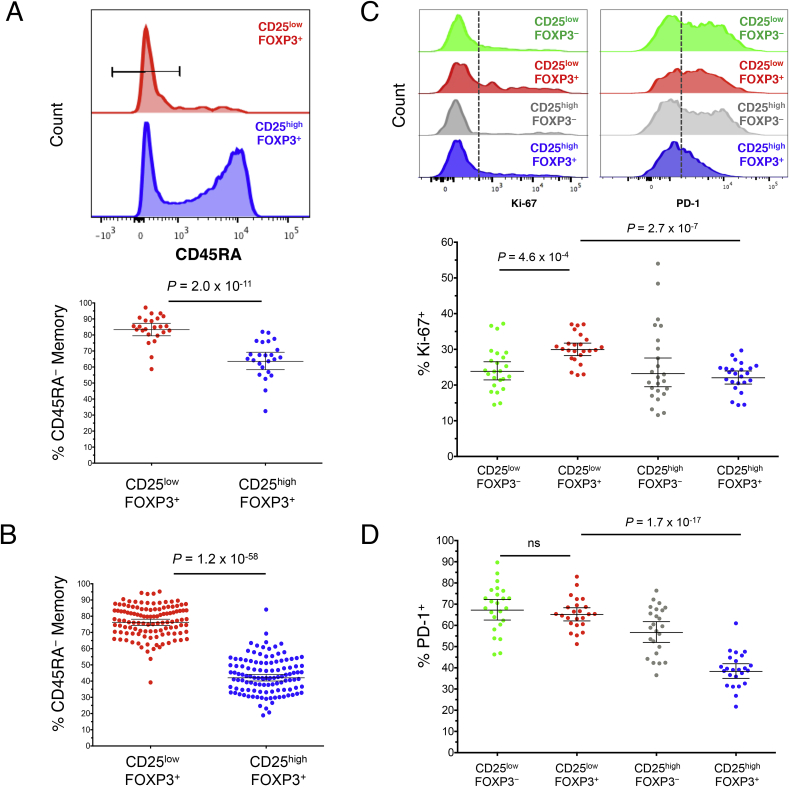
Having established that a majority of CD25lowFOXP3+ cells are stably demethylated at the FOXP3 TSDR, we next performed a detailed phenotypic characterisation of this subset by flow cytometry in 24 healthy adult donors to investigate phenotypic similarities and differences between CD25lowFOXP3+ and classical Tregs (CD25highFOXP3+). One distinguishing feature of these cells was the higher frequency of memory phenotype (CD45RA−) cells compared to their CD25highFOXP3+ counterparts (83.8% and 64.7% CD45RA− cells, respectively; P = 2.0 × 10−11; Fig. 3A). This difference was particularly noticeable among the younger cohort (median age = 14 years) of 116 T1D patients and unaffected siblings (76.9% and 43.4%, respectively; P = 1.2 × 10−58; Fig. 3B), which have a higher proportion of CD45RA+ naïve cells amongst their CD25highFOXP3+ conventional Tregs compared to adult donors, suggesting that the majority of CD25lowFOXP3+ cells have responded previously to antigen, or have expanded in an antigen-independent manner, following their emigration from the thymus. Since the majority of CD25lowFOXP3+ cells are CD45RA−, we focused further analyses on memory FOXP3+ cells.
Fig. 3.
CD25lowFOXP3+T cells display a memory phenotype. (A, B) Representative histograms and summary scatter plots depict the frequency (geometric mean ± 95% CI) of CD45RA− memory T cells amongst the CD25lowFOXP3- and CD25lowFOXP3+ subsets in a population of 24 adult (median age = 42 years) healthy donors (A) or in a population of 116 younger (median age = 14 years) T1D patients (N = 62) and healthy donors (N = 54) (B). (C, D) Representative histograms and the frequency distribution (geometric mean ± 95% CI) of Ki-67+ (C) and PD-1+ (D) cells in the CD45RA− compartment of the four assessed immune subsets. P values were calculated using two-tailed paired t-tests comparing the frequency of the assessed immune subsets from the same individual. P values < 0.005 were considered significant (Bonferronni correction for the comparison of ten independent immune markers between CD25lowFOXP3+ and CD25highFOXP3+ T cells). Gating strategy to delineate: (i) CD127lowCD25lowFOXP3− (highlighted in green), (ii) CD127lowCD25lowFOXP3+ (highlighted in red), (iii) CD127lowCD25highFOXP3− (highlighted in grey), and (iv) CD127lowCD25highFOXP3+ (highlighted in blue) CD4+ T cells is depicted in Fig. 2A.
We found that an increased frequency of CD45RA− CD25lowFOXP3+ cells express the proliferation marker Ki-67 compared to their CD25highFOXP3+ counterparts (29.9% and 22.0%, respectively; P = 2.7 × 10−7; Fig. 3C). In addition to Ki-67, CD45RA− CD25lowFOXP3+ cells were also characterised by a marked increased frequency of PD-1+ cells compared to CD25highFOXP3+ Tregs (65.1% and 38.3%, respectively; P = 1.7 × 10−17; Fig. 3D), and had a frequency of PD-1+ cells more similar to their CD25lowFOXP3− counterparts (67.2%; Fig. 3D).
Furthermore, we demonstrated that, similarly to CD25highFOXP3+ CD45RA− memory Tregs, the majority of CD25lowFOXP3+ CD45RA− memory cells also express the transcription factor HELIOS, although the proportion of HELIOS+ cells was significantly lower (51.1%) compared to CD25highFOXP3+ CD45RA− memory Tregs (77.8%; P = 3.0 × 10−12; Fig. 4A and B). Consistent with the reduction in CD25 and HELIOS, CD25lowFOXP3+ cells also showed a significantly lower expression of other classical Treg markers compared to CD25highFOXP3+ Tregs, such as TIGIT (65.1% vs 78.0%; P = 3.7 × 10−8), CD15s (20.7% vs 33.5%; P = 5.8 × 10−12) and most notably, CTLA-4 (60.0% vs 84.7%; P = 9.4 × 10−11; Fig. 4A and B). Furthermore, we found that the expression of FOXP3 was markedly lower in CD25lowFOXP3+ cells compared to CD25highFOXP3+ Tregs (MFI = 1171 and 2160 respectively, P = 3.6 × 10−11; Fig. 4C), suggesting that CD25lowFOXP3+ cells show a decreased expression of classical Treg-associated molecules.
Fig. 4.
CD25lowFOXP3+cells show reduced expression of several conventional Treg markers. (A) Representative histograms depict the distribution of the expression of the conventional Treg markers HELIOS, TIGIT, CD15s and CTLA-4 amongst: (i) CD127lowCD25lowFOXP3− (highlighted in green), (ii) CD127lowCD25lowFOXP3+ (highlighted in red), (iii) CD127lowCD25highFOXP3- (highlighted in grey), and (iv) CD127lowCD25highFOXP3+ (highlighted in blue) memory CD4+ T cells. (B) Scatter plots depict the distribution (geometric mean ± 95% CI) of HELIOS (n = 24), TIGIT (n = 24), CD15s (n = 24) and CTLA-4 (n = 13) in the CD45RA− compartment of the four assessed immune subsets. (C) Expression of FOXP3 (geometric mean ± 95% CI) was measured in the CD25lowFOXP3+ (depicted by red squares) and CD25highFOXP3+ (depicted by blue circles) subsets from 24 healthy donors. P values were calculated using two-tailed paired t-tests comparing the assessed immunophenotypes between CD25lowFOXP3+ and the other three delineated subsets from the same individual. P values < 0.005 were considered significant (Bonferronni correction for the comparison of ten independent immune markers between CD25lowFOXP3+ and CD25highFOXP3+ T cells). MFI, mean fluorescence intensity.
3.4. HELIOS+CD45RA− CD25lowFOXP3+ cells are demethylated at the FOXP3 TSDR to the same degree as conventional HELIOS+CD45RA− CD25high FOXP3+ Tregs
To further investigate the methylation profile of FOXP3+ cells, we next assessed the TSDR methylation in the HELIOS+ and HELIOS− subsets in three additional healthy donors. In agreement with their putative Treg lineage, we confirmed that the HELIOS+ subsets of both CD25lowFOXP3+ cells and conventional CD25highFOXP3+ Tregs are virtually completely demethylated at the FOXP3 TSDR (>95%; Fig. 5A). In contrast, the HELIOS− subsets of CD25lowFOXP3+ cells and conventional CD25highFOXP3+ Tregs contained a much lower proportion of cells demethylated at the TSDR (21% and 64%, respectively). Furthermore, to investigate if the FOXP3 methylation status of CD25lowFOXP3+ cells was maintained in autoimmune patients, we also assessed the TSDR methylation in three SLE patients recalled based on an increased frequency of CD25lowFOXP3+ cells. Consistent with the results obtained from healthy donors, we found that HELIOS+ CD25lowFOXP3+ cells were fully demethylated at the FOXP3 TSDR (>95%; Supplementary Fig. 4).
Fig. 5.
HELIOS+CD45RA−CD25lowFOXP3+cells are demethylated at TSDR as much as conventional HELIOS+CD45RA−CD25highFOXP3+Tregs. Frequency (mean ± SEM) of reads demethylated at eight or nine of the nine interrogated CpG sites in the FOXP3 TSDR in CD45RA− CD25lowFOXP3+ cells and CD45RA− CD25highFOXP3+ Tregs stratified by the expression of HELIOS. The data were obtained from sorted cells from three independent healthy donors.
Since HELIOS expression is highly enriched in FOXP3+ cells demethylated at the TSDR, we examined other phenotypes within the FOXP3+ cells stratified by HELIOS expression. CD25lowFOXP3+ cells demethylated at the TSDR as defined by HELIOS expression had a higher proportion in cell cycle as compared to CD25highFOXP3+ cells expressing HELIOS (33.0% and 20.7%, respectively; Fig. 5B and C). In addition, the proportion of cells expressing PD-1 and the per cell level of PD-1 were both increased on CD25lowFOXP3+ demethylated at the TSDR as compared to their CD25high counterparts (Fig. 5B and C). Expression of TIGIT, CTLA-4 and CD15s were also compared (Supplementary Figs. 5A–D) with HELIOS stratification revealing a high percentage (>89%) of TIGIT+ cells in both populations of demethylated FOXP3+ cells, but a reduced number expressing CD15s and CTLA-4 in the CD25lowFOXP3+ cells demethylated at the TSDR as compared to their CD25high counterparts. High expression of CTLA-4 was present on CD25highFOXP3+HELIOS− cells, a population with >50% of the cells having a demethylated TSDR (Fig. 5A). Expression of FOXP3 was found to be significantly higher within both conventional CD25high Tregs (MFI > 2100) compared to CD25lowFOXP3+ HELIOS+ T cells (MFI = 1590), despite their demethylated TSDR. Notably, the expression of FOXP3 was markedly lower in CD25lowFOXP3+ HELIOS− T cells (MFI = 952), which is consistent with their methylated TSDR (Supplementary Fig. 5E). Furthermore, analysis of CD45RA+ expression revealed a significantly lower frequency of CD45RA+ cells within total CD25lowFOXP3+ HELIOS+ cells (4.7%) compared to their CD25high counterparts (20.8%; P = 2.0 × 10−12; Supplementary Fig. 5F). These data suggest that most of the CD45RA+ cells observed within CD25lowFOXP3+ T cells are memory effector T cells that have re-expressed CD45RA on their surface and are characterised by being HELIOS− and expressing lower levels of FOXP3. Finally, since it was possible that the expansion of CD25lowFOXP3+ HELIOS− cells (most of which lack a demethylated TSDR and might be activated effector cells) could have been responsible for the increase of CD25lowFOXP3+ cells in autoimmune patients (Fig. 1B and C), we examined the distribution of HELIOS+ and HELIOS− cells within the CD25lowFOXP3+ subset. We determined that HELIOS+ CD25lowFOXP3+ cells were increased in SLE, CID and T1D patients as compared to their healthy control cohorts (Supplementary Figs. 6A and B) similar to the findings with CD25lowFOXP3+ cells (Fig. 1C and D) and that HELIOS+ cells contributed significantly to all cohorts examined (Supplementary Figs. 6C and D).
3.5. Low IL-2 production from HELIOS+CD45RA− CD25lowFOXP3+ cells
To characterise the function of HELIOS+CD45RA− CD25lowFOXP3+ cells, we assessed the production of two key cytokines, IL-2 and IFN-γ, in ten donors (five T1D patients and five healthy controls) following in vitro stimulation (Fig. 6). Consistent with their Treg-like phenotype, we found that both the HELIOS+CD45RA− CD25lowFOXP3+ and CD25highFOXP3+ subsets, which are highly demethylated at the TSDR (Fig. 5A), showed a low frequency of IL-2+ (2.0% and 1.0%, respectively) and IFN-γ+ cells (5.1% and 1.4%, respectively; Fig. 6B and C). This was in marked contrast with the HELIOS+CD25lowFOXP3− subset, which was found to secrete significantly higher levels of both IL-2 (21.3%; P = 2.8 × 10−5; Fig. 6B) and IFN-γ (27.9%; P = 1.1 × 10−3; Fig. 6C), compared their FOXP3+ counterparts (2.0% and 5.1% for IL-2 and IFN-γ, respectively). In agreement with their regulatory phenotype, we found a strong reduction of IL-2+ and IFN-γ+ cells (2.0% and 5.1%, respectively) in HELIOS+CD45RA− CD127lowCD25lowFOXP3+ cells compared to conventional Teffs (59.8%, P = 5.3 × 10−9 and 63.5%, P = 2.0 × 10−7 for IL-2+ and IFN-γ+ cells, respectively; Fig. 6B and C).
Fig. 6.
HELIOS+CD45RA−CD25lowFOXP3+cells show impaired production of IL-2 and IFN-γ. (A) Gating strategy to delineate the CD45RA−HELIOS+ subset of: (i) CD127lowCD25lowFOXP3+ (highlighted in red), (ii) CD127lowCD25highFOXP3+ (highlighted in blue), and (iii) CD127+CD25low/+FOXP3− HELIOS− conventional (Conv) effector (highlighted in black) subsets of CD4+ T cells. (B, C) Bar graphs depict the frequency (mean ± 95% CI) of IL-2+ and IFN-γ+ cells in the CD45RA−HELIOS+ compartment (or the CD45RA−HELIOS− compartment in the case of the conventional effector T cells) of the five assessed immune subsets depicted in panel A. Cytokine production was assessed in one single batch of ten donors. P values were calculated using two-tailed paired t-tests. P values < 0.005 were considered significant (Bonferronni correction for the comparison of ten independent immune markers between CD25lowFOXP3+ and CD25highFOXP3+ T cells). FACS gating plots depict data from one illustrative donor. *P < 0.05; **P < 0.01; ***P < 0.001.
As compared to the HELIOS+ fraction, we found that a higher portion of HELIOS− CD45RA− CD25lowFOXP3+ cells produced IFN-γ (Fig. 6A, Supplementary Fig. 7A). These findings are consistent with a previous study, showing that HELIOS−FOXP3+ T cells produced IFN-γ, and were increased among T1D patients [17]. Although we found no evidence for differential IFN-γ production in T1D patients compared to healthy controls among HELIOS− CD45RA−CD127lowCD25lowFOXP3+ cells, on a per cell basis (Supplementary Fig. 7B), the higher frequency of the CD25lowFOXP3+ subset among patients resulted in a significant increase in the frequency of circulating FOXP3+ cells with the capability to produce IFN-γ following stimulation among total CD4+ T cells (P = 2.5 × 10−3; Supplementary Fig. 7C). These data suggest that HELIOS− CD45RA− CD127lowCD25lowFOXP3+ cells contributed to the increased frequency of IFN-γ+ cells reported among FOXP3+ cells from T1D patients [17].
3.6. CD25lowFOXP3+ T cells are highly correlated with proliferating CD25highFOXP3+ Tregs
To investigate the possible relationship between CD25high and CD25low FOXP3+HELIOS+ Tregs, we hypothesized that if CD25high FOXP3+HELIOS+ Tregs are the precursors of the CD25low FOXP3+HELIOS+ T cell subset, the numbers of CD25high Tregs in cycle (Ki-67+) and CD25low FOXP3+HELIOS+ T cells should be correlated. This correlation would be required to maintain homeostasis of Treg numbers such that as memory CD25high Tregs are required to increase in peripheral compartments to respond to inflammatory conditions, a higher Treg turnover would lead to more CD25high Tregs moving into the CD25low compartment and ultimately to cell death. We assessed the total numbers of CD45RA− Ki-67+ Tregs both in the cohort of 24 healthy volunteers (cohort 1) and in an independent replication cohort (cohort 2) of 112 healthy volunteers. We found that the frequency of CD45RA− CD4+ Ki-67+ CD25highFOXP3+HELIOS+ Tregs was significantly correlated with the frequency of CD25lowFOXP3+HELIOS+ T cells within total memory CD4+ T cells (r2 = 0.18, P = 3.1 × 10−7; Fig. 7A). Similarly, we observed a strong correlation between the numbers of in-cycle Ki-67+ Tregs and the CD25highFOXP3+HELIOS+ Treg compartment (r2 = 0.46, P = 1.2 × 10−19; Fig. 7B), as well as a significant correlation between both the total CD25low and CD25high FOXP3+HELIOS+ T cell compartments (r2 = 0.15, P = 3.9 × 10−6; Fig. 7C). These observed correlations were very consistent within both cohorts of healthy volunteers, and suggest that proliferation of conventional CD25high FOXP3+HELIOS+ T cells is critical to promote the homeostatic repopulation of the CD25high Treg subset, which is maintained at a steady state frequency through the progression of a proportion of CD25high Tregs to the CD25low FOXP3+HELIOS+ compartment.
Fig. 7.
Proliferating Ki-67+CD25highFOXP3+HELIOS+Tregs correlate with the frequencies of the CD25lowand CD25highCD127lowFOXP3+HELIOS+subsets. (A, B) Data shown depict the correlation between the frequency within CD45RA− CD4+ T cells of in-cycle (Ki-67+) CD4+CD45RA− CD127lowCD25high Tregs (FOXP3+HELIOS+) and the frequency of either CD25low FOXP3+HELIOS+ T cells (A) or conventional CD25high FOXP3+HELIOS+ Tregs (B). (C) Data shown depict the correlation between the frequencies of circulating CD4+CD45RA− CD25low FOXP3+HELIOS+ and CD25high FOXP3+HELIOS+ T cells. Frequencies of the assessed immune subsets were measured in PBMCs from healthy volunteers from two independent cohorts: cohort 1 containing 24 donors (depicted in red) and cohort 2 containing 112 donors (depicted in black). The r2 values represent the coefficient of determination of the linear regression in the combined cohorts, and the P values correspond to the F statistic testing the null hypothesis that the slope of the linear regression analysis is equal to 0.
4. Discussion
The identification of reliable biomarkers of disease activity has been a major challenge of autoimmune diseases, particularly in organ-specific diseases, such as T1D, where there is limited access to the inflamed target tissues. In this study we characterised a subset of FOXP3+ CD127lowCD25low CD4+ T cells, and show that it could be a peripheral biomarker of a recent or chronic autoimmune or cytokine-driven inflammatory reaction in the tissues. We showed that in addition to SLE, where the increase of FOXP3+ CD25low T cells has been observed in multiple studies [5], [6], [7], [8], [9], the proportion of FOXP3+ cells in the CD127lowCD25low subset is increased in CID and T1D patients. Although the frequency of FOXP3+ cells in the CD127lowCD25low subset was compared in T1D patients versus controls in one previous study with no difference observed [13], we note that the number of participants was small: 10 healthy control individuals and 16 patients. In contrast, Zoka et al. [18] observed that the proportion of CD25low cells among FOXP3+CD4+ T cells is higher in T1D patients than in controls, a phenotype consistent with our observations.
A major strength of this study is that we were able to use a recently developed assay [16] to precisely assess the methylation status of the FOXP3 TSDR of CD25lowFOXP3+ cells, a feature that was lacking in the previous SLE studies [7], [8] or studies of this subset from healthy individuals [13]. This method provides a more quantitative assessment of the methylation pattern of the FOXP3 locus [16] in the different immune subsets, which allowed us to demonstrate that the epigenetic profile of CD25lowFOXP3+ cells was remarkably similar to conventional CD25high FOXP3+ Tregs (57.1% and 80.5% demethylated at the TSDR, respectively). We went on to define that this epigenetic similarity was caused primarily by the TSDR methylation status of HELIOS+ cells: virtually all HELIOS+ cells were found to be demethylated in both the CD25high and CD25low FOXP3+ subsets. This finding is consistent with a previous study assessing the FOXP3 TSDR methylation profile in different CD4+CD127low T-cell subsets discriminated by their expression of FOXP3 and CD25 [19]. The majority of CD25lowFOXP3+ cells sorted from synovial fluid mononuclear cells of juvenile idiopathic arthritis patients were shown to have a demethylated FOXP3 TSDR, suggesting that this subset may be enriched at inflammatory sites [19]. In the current study we also found that the proportion of cells expressing TIGIT was elevated over 2-fold in both FOXP3+HELIOS+ subsets as compared to their FOXP3+HELIOS− counterparts. Stable demethylation of the FOXP3 TSDR occurs in the thymus upon strong T-cell receptor stimulation [1], therefore suggesting that CD25low FOXP3+HELIOS+ cells are bona-fide thymically-derived Tregs that have lost the expression of CD25.
In further support of the hypothesis that CD25lowFOXP3+ HELIOS+ T cells could be a subset of the classical FOXP3+ Treg subset, these cells were unable to produce IL-2 following in vitro activation. This finding was in contrast with the report by Yang et al [8] that CD25lowFOXP3+ T cells from new-onset SLE patients were able to secrete IL-2. However, we note that the IL-2 production reported in Yang et al was much lower compared to CD25highFOXP3− Teffs, and immune subsets were not stratified based on the expression of CD127, CD45RA and HELIOS. It is therefore likely that the residual production of IL-2 observed by Yang et al in CD25lowFOXP3+ T cells was due primarily to HELIOS− T cells. In contrast, in our study we demonstrate that CD45RA− CD25lowCD127low HELIOS+FOXP3+ cells have a profound inability to produce IL-2 as compared to CD127+CD25high CD45RA− HELIOS−FOXP3− Teffs. We also noted the overall heterogeneity in the CD127low subset in regard to IL-2 and IFN-γ secretion (Fig. 6). A similar proportion of CD127low cells lacking both FOXP3 and HELIOS expression secrete IL-2 and IFN-γ as compared to their CD127+ counterparts and are likely effector T cells. In healthy individuals we observed that these putative effector cells are the largest portion of the CD45RA− CD25lowCD127low gate (Fig. 1B), consistent with previous observations [13].
In addition to reduced levels of CD25, CD25low HELIOS+FOXP3+ Tregs had lower expression of CTLA-4, CD15s and FOXP3 as compared to CD25high HELIOS+FOXP3+ Tregs, suggesting that CD25low Tregs could have decreased suppressive function. One limitation of our study is that we are not able to directly assess the suppressive capacity of CD25low HELIOS+FOXP3+ cells, as sorting on the intracellular transcription factors precludes the use of these cells for functional assays and surrogate surface markers are not yet defined. Also, as described above, the CD45RA− CD25lowCD127low gate has a high proportion of effector cells present, making the results of suppression experiments using populations of cells gated as CD25lowCD127low difficult to interpret. Notably, despite the cellular heterogeneity inherent in the CD25lowCD127low subset, two studies did test the suppressive capacity of sorted CD25lowCD127low CD4+ T cells [7], [13]. Suppression of proliferation by Teffs was mediated by CD25lowCD127low CD4+ T cells in both studies; however IFN-γ secretion by Teffs was not suppressed in the one study that examined this parameter [7]. The reduced suppression mediated by CD25lowCD127low CD4+ T cells could be due to the fact that a larger proportion of effector cells are present in this subset as compared with their CD25+ counterparts. Overall the observation of suppression by CD25lowCD127low CD4+ T cells supports the conclusion that the CD25low HELIOS+FOXP3+ Tregs present in the heterogeneous CD25lowCD127low CD4+ T cell population are functionally suppressive. Future studies are needed to unravel the heterogeneity present in both the CD25low and CD25high CD127lowCD4+ T cell subsets.
An increased frequency of CD25low FOXP3+ Tregs has now been reported in a growing number autoimmune diseases, including SLE, rheumatoid arthritis and multiple sclerosis [5], [6], [7], [8], [9], [10], [11]. In this study we expanded this observation to patients with CID and T1D, suggesting a common mechanism of CD25low FOXP3+ Tregs in countering effector T cells as well as other cells promoting inflammation. Further characterisation of CD25low FOXP3+ Tregs in longitudinal studies correlating their frequencies with disease status and in response to therapeutic interventions would help determine their value as biomarkers. One study has shown in a small group of patients that treatment with glucocorticoids and cyclophosphamide decreased the frequency of CD25low FOXP3+ Tregs in SLE patients [5].
In our study we have provided a detailed phenotypic characterisation of CD25low as compared to conventional CD25high FOXP3+ Tregs. In contrast to the reduced expression of several molecules that are abundant in CD25high FOXP3+ Tregs such as CTLA-4, CD15s and FOXP3 itself, the frequency of cells expressing PD-1 and Ki-67 in CD25low Tregs was higher than in conventional CD25high Tregs, suggesting that the CD25low HELIOS+FOXP3+ population may represent the consequences of CD25high Tregs attempting to suppress ongoing inflammatory responses in tissues. The progression of CD25high Tregs to the CD25low Treg subset is supported by our observation of the strong correlation between the frequency of CD25high Tregs in cell cycle (Ki-67+) with the number of CD25low Tregs. The high proportion (15–40%) of memory FOXP3+ Tregs in cycle is consistent with their shorter half-lives as compared to other T cell subsets [20], [21]. Thus, given the fact that Treg percentages normally remain constant in an individual through time [21], and a high proportion of the cells are replicating, Treg cell death must be a common outcome following cell division. We propose that the decreased expression of CD25 on Tregs, most likely caused by exposure to inflammatory conditions, causes less responsiveness to IL-2, reduced expression of FOXP3 and other Treg-associated molecules, and an increased probability of cell death. Despite the reduced IL-2 responsiveness in CD25low Tregs, it is possible that the Tregs remain functional and that the upregulation of PD-1 could compensate for reductions in FOXP3 and CTLA-4 levels [22]. Consistent with this hypothesis, previous studies have reported that PD-1 is a critical inhibitory molecule that is upregulated on T cells after activation [23], [24], [25]. In contrast, chronic PD-1 signaling within peripheral compartments has been reported to lead to reduced STAT5 phosphorylation, decreased expression of CD25, FOXP3 and CTLA-4, and decreased Treg suppressive function [26], [27], [28]. Additional functional studies are required to resolve these apparently contradictory mechanisms.
5. Conclusions
We hypothesize that the presence of a low frequency of CD25low HELIOS+FOXP3+ cells in peripheral blood from healthy individuals reflects a normal physiological mechanism to maintain, genetically-regulated, Treg levels. Their increased frequency in peripheral blood from autoimmune patients, which is particularly noteworthy in patients with chronic systemic inflammation, is indicative of an inflammatory insult that drives the expansion of the Treg population, which can be transient or chronic, in an attempt to regulate an overt autoimmune Teff response. Given the paucity of reliable peripheral biomarkers of disease activity, our findings suggest that the frequency of CD25low HELIOS+FOXP3+ Tregs could provide valuable information about recent or ongoing tissue inflammation and could have a clinical application for the stratification of patients with autoimmunity.
Author contributions
R.C.F., J.A.T., L.S.W. and M.L.P. designed experiments and interpreted data. R.C.F., H.Z.S., W.S.T., D.B.R., A.J.C., J.O., X.C.D., D.J.S., N.S., M.M. and M.L.P. performed experiments. X.Y. analysed the data. C.W. supervised the statistical analysis of the data. T.J.V., D.B.D., H.B and A.C. provided samples and clinical outcome data. R.C.F., J.A.T., L.S.W. and M.L.P. conceived the study and wrote the paper.
Acknowledgements
This work was supported by the JDRF UK Centre for Diabetes - Genes, Autoimmunity and Prevention (D-GAP; 4-2007-1003) in collaboration with M. Peakman and T. Tree at Kings College London, a strategic award to the Diabetes and Inflammation Laboratory from the JDRF (9-2011-253) and the Wellcome Trust (WT; WT061858/091157), and the National Institute for Health Research Cambridge Biomedical Research Centre. RCF is funded by an advanced JDRF post-doctoral fellowship (2-APF-2017-420-A-N). CW is funded by the Wellcome Trust (088998).
We thank staff of the National Institute for Health Research (NIHR) Cambridge BioResource recruitment team for assistance with volunteer recruitment and K. Beer, T. Cook, S. Hall and J. Rice of the Cambridge BioResource for blood sample collection. We thank C. Guy from the Department of Paediatrics, University of Cambridge for D-GAP sample recruitment. We thank M. Woodburn and T. Attwood from the Diabetes and Inflammation Laboratory, University of Cambridge for their contribution to sample management and N. Walker and H. Schuilenburg from the Diabetes and Inflammation Laboratory, University of Cambridge for data management. This research was supported by the Cambridge NIHR BRC Cell Phenotyping Hub. In particular, we wish to thank Anna Petrunkina Harrison, Simon McCullum, Christopher Bowman and Esther Perez from the Cambridge NIHR BRC Cell Phenotyping Hub for their advice and support in cell sorting. We thank Howard Martin, Fay Rodger and Ruth Littleboy for running the Illumina MiSeq in the Molecular Genetics Laboratories, Addenbrooke's Hospital, Cambridge. We thank members of the NIHR Cambridge BioResource SAB and management committee for their support and the NIHR Cambridge Biomedical Research Centre for funding. Access to NIHR Cambridge BioResource volunteers and their data and samples is governed by the NIHR Cambridge BioResource SAB. Documents describing access arrangements and contact details are available at http://www.cambridgebioresource.org.uk/. We also thank H. Stevens, P. Clarke, G. Coleman, S. Dawson, S. Duley, M. Maisuria-Armer and T. Mistry from the Diabetes and Inflammation Laboratory, University of Cambridge for preparation of PBMC samples.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.jaut.2017.07.009.
Contributor Information
Linda S. Wicker, Email: linda.wicker@well.ox.ac.uk.
Marcin L. Pekalski, Email: marcin.pekalski@well.ox.ac.uk.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
Frequency of CD127lowCD25lowFOXP3+T cells is increased in T1D patients. (A) Scatter plot depicts the total frequency (geometric mean ± 95% CI) of CD25lowFOXP3+ cells out of CD4+ T cells in our discovery cohort of 62 T1D patients (depicted by red circles) and 54 healthy controls (depicted by black squares) (B) Scatter plots depict the frequency (geometric mean ± 95% CI) of FOXP3+ cells from CD127lowCD25low T cells in: (i) an independent replication cohort consisting of 15 T1D patients and 15 healthy controls. P values were calculated using two-tailed unpaired t-tests comparing the geometric mean of the assessed immune subsets between T1D patients and healthy controls (HC).
Minimal increase in the frequency of CD127lowCD25highFOXP3+T cells in T1D patients. Scatter plot depicts the total frequency (geometric mean ± 95% CI) of CD25highFOXP3+ cells (classical Tregs) out of CD4+ T cells in our discovery cohort of 62 T1D patients (depicted by red circles) and 54 healthy controls (depicted by black squares). P values were calculated using two-tailed unpaired t-tests comparing the geometric mean of CD25highFOXP3+ Tregs between T1D patients and healthy controls (HC).
Association of the frequency of CD127lowCD25lowFOXP3+T cells with disease activity. (A) Data shown depicts the correlation between the frequency of FOXP3+ cells among CD127lowCD25low T cells and the SLE disease activity index (SLEDAI) at the time of sampling in SLE patients. (B) Scatter plot depicts the correlation between the frequency of FOXP3+ cells among CD127lowCD25low T cells and the time since diagnosis in 49 recently diagnosed T1D patients (median 11 months, range 2–42 months) from the D-GAP cohort. P values were obtained by linear regression analysis.
TSDR methylation profile of HELIOS+CD45RA−CD25lowFOXP3+cells is maintained in SLE patients. Frequency (mean ± SEM) of reads demethylated at eight or nine of the nine interrogated CpG sites in the FOXP3 TSDR in CD45RA− HELIOS+ CD25lowFOXP3+ cells and CD45RA− HELIOS− CD25lowFOXP3− Teffs. The data were obtained from sorted cells from three independent SLE donors.
HELIOS expression defines distinct FOXP3+subsets. Scatter plots depict the distribution (geometric mean ± 95% CI) of TIGIT (n = 24) (A), CD15s (n = 24) (B), CTLA-4 (both frequency and MFI of the positive fraction; n = 13) (C, D), FOXP3 MFI (n = 24) (E) and CD45RA (n = 24) (F) in the HELIOS+ and HELIOS− fractions of the (i) CD25lowFOXP3+ T cells (depicted in red) and (ii) conventional CD25lowFOXP3+ Tregs (depicted in blue). P values were calculated using two-tailed paired t-tests.
The frequency of HELIOS+CD25lowFOXP3+cells is increased in patients with autoimmune disease. (A, B) Scatter plots depict the distribution (geometric mean ± 95% CI) of HELIOS+FOXP3+ cells among CD127lowCD25low T cells in SLE patients (N = 34 patients vs 24 healthy donors) and combined immunodeficiency (CID) patients with active autoimmunity (N = 7 patients vs 6 healthy donors) (A); and in a cohort of T1D patients (N = 62; depicted by red circles) and healthy donors (N = 54; depicted by black squares) (B). (C, D) Scatter plots depict the distribution (geometric mean ± 95% CI) of HELIOS+ cells within CD25lowFOXP3+ T cells in the cohort of SLE and CID patients (C) and in the cohort of T1D patients (D). P values were calculated using two-tailed unpaired t-tests comparing the geometric mean of the assessed immune subsets between patients and the respective healthy control groups. .HC, healthy controls; T1D, type 1 diabetes patients; SLE, systemic lupus erythematosus patients; CID, combined immunodeficiency patients; ns = non-significant.
Production of IFN-γ from HELIOS−CD45RA−CD127lowCD25lowFOXP3+T cells is not altered in T1D patients. (A) Gating strategy illustrating the production of IFN-γ in the HELIOS− and HELIOS+ CD45RA− fractions of CD127lowCD25lowFOXP3+ cells. FACS gating plot is a representative example. (B) Plot depicts the distribution of the frequency (geometric mean ± 95% CI) of IFN-γ+ HELIOS− T cells in the CD45RA− CD127lowCD25lowFOXP3+ population. Frequency of IFN-γ+ cells was compared between T1D patients (N = 62; depicted by red circles) and healthy donors (N = 54; depicted by black squares) following in vitro stimulation with phorbol-12-myristate-13-acetate (PMA) and ionomycin. (C) Plot depicts the distribution of the frequency (geometric mean ± 95% CI) of IFN-γ+ HELIOS− T cells in the CD45RA− CD127lowCD25lowFOXP3+ population out of total CD4 T cells from the same donors as in (B). P values were calculated by linear regression of the log-transformed data, including batch as a covariate. HC, healthy controls; T1D, type 1 diabetic patients.
Antibodies and immunostaining panels used for flow cytometry. Detailed description of the fluorochrome-conjugated antibodies and immunostaining panels used in this study.
References
- 1.Ohkura N., Hamaguchi M., Morikawa H., Sugimura K., Tanaka A., Ito Y., Osaki M., Tanaka Y., Yamashita R., Nakano N., Huehn J., Fehling H.J., Sparwasser T., Nakai K., Sakaguchi S. T cell receptor stimulation-induced epigenetic changes and Foxp3 expression are independent and complementary events required for Treg cell development. Immunity. 2012;37:785–799. doi: 10.1016/j.immuni.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 2.Miyara M., Yoshioka Y., Kitoh A., Shima T., Wing K., Niwa A., Parizot C., Taflin C., Heike T., Valeyre D., Mathian A., Nakahata T., Yamaguchi T., Nomura T., Ono M., Amoura Z., Gorochov G., Sakaguchi S. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30:899–911. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 3.Bin Dhuban K., D'Hennezel E., Nashi E., Bar-Or A., Rieder S., Shevach E.M., Nagata S., Piccirillo C.A. Coexpression of TIGIT and FCRL3 identifies Helios+ human memory regulatory T cells. J. Immunol. 2015;194:3687–3696. doi: 10.4049/jimmunol.1401803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fuhrman C.A., Yeh W.-I., Seay H.R., Saikumar Lakshmi P., Chopra G., Zhang L., Perry D.J., McClymont S.A., Yadav M., Lopez M.-C., V Baker H., Zhang Y., Li Y., Whitley M., von Schack D., Atkinson M.A., Bluestone J.A., Brusko T.M. Divergent phenotypes of human regulatory T cells expressing the receptors TIGIT and CD226. J. Immunol. 2015;195:145–155. doi: 10.4049/jimmunol.1402381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang B., Zhang X., Tang F.L., Zhu L.P., Liu Y., Lipsky P.E. Clinical significance of increased CD4+CD25−Foxp3+ T cells in patients with new-onset systemic lupus erythematosus. Ann. Rheum. Dis. 2008;67:1037–1040. doi: 10.1136/ard.2007.083543. [DOI] [PubMed] [Google Scholar]
- 6.Chowdary Venigalla R.K., Tretter T., Krienke S., Max R., Eckstein V., Blank N., Fiehn C., Dick Ho A., Lorenz H.-M. Reduced CD4+,CD25− T cell sensitivity to the suppressive function of CD4+,CD25high,CD127−/low regulatory T cells in patients with active systemic lupus erythematosus. Arthritis Rheum. 2008;58:2120–2130. doi: 10.1002/art.23556. [DOI] [PubMed] [Google Scholar]
- 7.Bonelli M., Savitskaya A., Steiner C.-W., Rath E., Smolen J.S., Scheinecker C. Phenotypic and functional analysis of CD4+CD25−Foxp3+ T cells in patients with systemic lupus erythematosus. J. Immunol. 2009;182:1689–1695. doi: 10.4049/jimmunol.182.3.1689. [DOI] [PubMed] [Google Scholar]
- 8.Yang H., Zhang W., Zhao L., Li Y., Zhang F., Tang F., He W., Zhang X. Are CD4+CD25-Foxp3+cells in untreated new-onset lupus patients regulatory T cells? Arthritis Res. Ther. 2009;11:1–9. doi: 10.1186/ar2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suen J.-L., Li H.-T., Jong Y.-J., Chiang B.-L., Yen J.-H. Altered homeostasis of CD4+ FoxP3+ regulatory T-cell subpopulations in systemic lupus erythematosus. Immunology. 2009;127:196–205. doi: 10.1111/j.1365-2567.2008.02937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fransson M., Burman J., Lindqvist C., Atterby C., Fagius J., Loskog A. T regulatory cells lacking CD25 are increased in MS during relapse. Autoimmunity. 2010;43:590–597. doi: 10.3109/08916930903541190. [DOI] [PubMed] [Google Scholar]
- 11.de Paz B., Prado C., Alperi-López M., Ballina-García F.J., Rodriguez-Carrio J., López P., Suárez A. Effects of glucocorticoid treatment on CD25−FOXP3+ population and cytokine-producing cells in rheumatoid arthritis. Rheumatology. 2012;51:1198–1207. doi: 10.1093/rheumatology/kes039. [DOI] [PubMed] [Google Scholar]
- 12.Horwitz D.A. Identity of mysterious CD4(+)CD25(-)Foxp3(+) cells in SLE. Arthritis Res. Ther. 2010;12:101. doi: 10.1186/ar2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu W., Putnam A.L., Xu-yu Z., Szot G.L., Lee M.R., Zhu S., Gottlieb P.A., Kapranov P., Gingeras T.R., de St. Groth B.F., Clayberger C., Soper D.M., Ziegler S.F., Bluestone J.A. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J. Exp. Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuehn H.S., Ouyang W., Lo B., Deenick E.K., Niemela J.E., Avery D.T., Schickel J.-N., Tran D.Q., Stoddard J., Zhang Y., Frucht D.M., Dumitriu B., Scheinberg P., Folio L.R., Frein C.A., Price S., Koh C., Heller T., Seroogy C.M., Huttenlocher A., Rao V.K., Su H.C., Kleiner D., Notarangelo L.D., Rampertaap Y., Olivier K.N., McElwee J., Hughes J., Pittaluga S., Oliveira J.B., Meffre E., Fleisher T.A., Holland S.M., Lenardo M.J., Tangye S.G., Uzel G. Immune dysregulation in human subjects with heterozygous germline mutations in CTLA4. Science. 2014;345:1623–1627. doi: 10.1126/science.1255904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thompson W.S., Pekalski M.L., Simons H.Z., Smyth D.J., Castro-Dopico X., Guo H., Guy C., Dunger D.B., Arif S., Peakman M., Wallace C., Wicker L.S., Todd J.A., Ferreira R.C. Multi-parametric flow cytometric and genetic investigation of the peripheral B cell compartment in human type 1 diabetes. Clin. Exp. Immunol. 2014;177:571–585. doi: 10.1111/cei.12362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rainbow D.B., Yang X., Burren O., Pekalski M.L., Smyth D.J., Klarqvist M.D.R., Penkett C.J., Brugger K., Martin H., Todd J.A., Wallace C., Wicker L.S. Epigenetic analysis of regulatory T cells using multiplex bisulfite sequencing. Eur. J. Immunol. 2015;45:3200–3203. doi: 10.1002/eji.201545646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McClymont S.A., Putnam A.L., Lee M.R., Esensten J.H., Liu W., Hulme M.A., Hoffmüller U., Baron U., Olek S., Bluestone J.A., Brusko T.M. Plasticity of human regulatory T cells in healthy subjects and patients with type 1 diabetes. J. Immunol. 2011;186:3918–3926. doi: 10.4049/jimmunol.1003099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zóka A., Barna G., Somogyi A., Műzes G., Oláh Á., Al-Aissa Z., Hadarits O., Kiss K., Firneisz G. Extension of the CD4+Foxp3+CD25−/low regulatory T-cell subpopulation in type 1 diabetes mellitus. Autoimmunity. 2015;48:289–297. doi: 10.3109/08916934.2014.992518. [DOI] [PubMed] [Google Scholar]
- 19.Bending D., Pesenacker A.M., Ursu S., Wu Q., Lom H., Thirugnanabalan B., Wedderburn L.R. Hypomethylation at the regulatory T cell–specific demethylated region in CD25hi T cells is decoupled from FOXP3 expression at the inflamed site in childhood arthritis. J. Immunol. 2014;193:2699–2708. doi: 10.4049/jimmunol.1400599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vukmanovic-Stejic M., Zhang Y., Cook J.E., Fletcher J.M., McQuaid A., Masters J.E., Rustin M.H.A., Taams L.S., Beverley P.C.L., Macallan D.C., Akbar A.N. Human CD4+ CD25hi Foxp3+ regulatory T cells are derived by rapid turnover of memory populations in vivo. J. Clin. Investig. 2006;116:2423–2433. doi: 10.1172/JCI28941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bollyky J.B., Long S.A., Fitch M., Bollyky P.L., Rieck M., Rogers R., Samuels P.L., Sanda S., Buckner J.H., Hellerstein M.K., Greenbaum C.J. Evaluation of in vivo T cell kinetics: use of heavy isotope labelling in type 1 diabetes. Clin. Exp. Immunol. 2013;172:363–374. doi: 10.1111/cei.12064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asano T., Meguri Y., Yoshioka T., Kishi Y., Iwamoto M., Nakamura M., Sando Y., Yagita H., Koreth J., Kim H.T., Alyea E.P., Armand P., Cutler C.S., Ho V.T., Antin J.H., Soiffer R.J., Maeda Y., Tanimoto M., Ritz J., Matsuoka K. PD-1 modulates regulatory T cell homeostasis during low-dose IL-2 therapy. Blood. 2017;129:2186–2197. doi: 10.1182/blood-2016-09-741629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharpe A.H., Wherry E.J., Ahmed R., Freeman G.J. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat. Immunol. 2007;8:239–245. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 24.Fife B.T., Bluestone J.A. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol. Rev. 2008;224:166–182. doi: 10.1111/j.1600-065X.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- 25.Francisco L.M., Sage P.T., Sharpe A.H. The PD-1 pathway in tolerance and autoimmunity. Immunol. Rev. 2010;236:219–242. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Franceschini D., Paroli M., Francavilla V., Videtta M., Morrone S., Labbadia G., Cerino A., Mondelli M.U., Barnaba V. PD-L1 negatively regulates CD4+CD25+Foxp3+ Tregs by limiting STAT-5 phosphorylation in patients chronically infected with HCV. J. Clin. Investig. 2009;119:551–564. doi: 10.1172/JCI36604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sage P.T., Francisco L.M., V Carman C., Sharpe A.H. The receptor PD-1 controls follicular regulatory T cells in the lymph nodes and blood. Nat. Immunol. 2013;14:152–161. doi: 10.1038/ni.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong M., La Cava A., Hahn B.H. Blockade of programmed Death-1 in young (New Zealand black × New Zealand White)F1 mice promotes the suppressive capacity of CD4+ regulatory T cells protecting from lupus-like disease. J. Immunol. 2013;190:5402–5410. doi: 10.4049/jimmunol.1202382. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Frequency of CD127lowCD25lowFOXP3+T cells is increased in T1D patients. (A) Scatter plot depicts the total frequency (geometric mean ± 95% CI) of CD25lowFOXP3+ cells out of CD4+ T cells in our discovery cohort of 62 T1D patients (depicted by red circles) and 54 healthy controls (depicted by black squares) (B) Scatter plots depict the frequency (geometric mean ± 95% CI) of FOXP3+ cells from CD127lowCD25low T cells in: (i) an independent replication cohort consisting of 15 T1D patients and 15 healthy controls. P values were calculated using two-tailed unpaired t-tests comparing the geometric mean of the assessed immune subsets between T1D patients and healthy controls (HC).
Minimal increase in the frequency of CD127lowCD25highFOXP3+T cells in T1D patients. Scatter plot depicts the total frequency (geometric mean ± 95% CI) of CD25highFOXP3+ cells (classical Tregs) out of CD4+ T cells in our discovery cohort of 62 T1D patients (depicted by red circles) and 54 healthy controls (depicted by black squares). P values were calculated using two-tailed unpaired t-tests comparing the geometric mean of CD25highFOXP3+ Tregs between T1D patients and healthy controls (HC).
Association of the frequency of CD127lowCD25lowFOXP3+T cells with disease activity. (A) Data shown depicts the correlation between the frequency of FOXP3+ cells among CD127lowCD25low T cells and the SLE disease activity index (SLEDAI) at the time of sampling in SLE patients. (B) Scatter plot depicts the correlation between the frequency of FOXP3+ cells among CD127lowCD25low T cells and the time since diagnosis in 49 recently diagnosed T1D patients (median 11 months, range 2–42 months) from the D-GAP cohort. P values were obtained by linear regression analysis.
TSDR methylation profile of HELIOS+CD45RA−CD25lowFOXP3+cells is maintained in SLE patients. Frequency (mean ± SEM) of reads demethylated at eight or nine of the nine interrogated CpG sites in the FOXP3 TSDR in CD45RA− HELIOS+ CD25lowFOXP3+ cells and CD45RA− HELIOS− CD25lowFOXP3− Teffs. The data were obtained from sorted cells from three independent SLE donors.
HELIOS expression defines distinct FOXP3+subsets. Scatter plots depict the distribution (geometric mean ± 95% CI) of TIGIT (n = 24) (A), CD15s (n = 24) (B), CTLA-4 (both frequency and MFI of the positive fraction; n = 13) (C, D), FOXP3 MFI (n = 24) (E) and CD45RA (n = 24) (F) in the HELIOS+ and HELIOS− fractions of the (i) CD25lowFOXP3+ T cells (depicted in red) and (ii) conventional CD25lowFOXP3+ Tregs (depicted in blue). P values were calculated using two-tailed paired t-tests.
The frequency of HELIOS+CD25lowFOXP3+cells is increased in patients with autoimmune disease. (A, B) Scatter plots depict the distribution (geometric mean ± 95% CI) of HELIOS+FOXP3+ cells among CD127lowCD25low T cells in SLE patients (N = 34 patients vs 24 healthy donors) and combined immunodeficiency (CID) patients with active autoimmunity (N = 7 patients vs 6 healthy donors) (A); and in a cohort of T1D patients (N = 62; depicted by red circles) and healthy donors (N = 54; depicted by black squares) (B). (C, D) Scatter plots depict the distribution (geometric mean ± 95% CI) of HELIOS+ cells within CD25lowFOXP3+ T cells in the cohort of SLE and CID patients (C) and in the cohort of T1D patients (D). P values were calculated using two-tailed unpaired t-tests comparing the geometric mean of the assessed immune subsets between patients and the respective healthy control groups. .HC, healthy controls; T1D, type 1 diabetes patients; SLE, systemic lupus erythematosus patients; CID, combined immunodeficiency patients; ns = non-significant.
Production of IFN-γ from HELIOS−CD45RA−CD127lowCD25lowFOXP3+T cells is not altered in T1D patients. (A) Gating strategy illustrating the production of IFN-γ in the HELIOS− and HELIOS+ CD45RA− fractions of CD127lowCD25lowFOXP3+ cells. FACS gating plot is a representative example. (B) Plot depicts the distribution of the frequency (geometric mean ± 95% CI) of IFN-γ+ HELIOS− T cells in the CD45RA− CD127lowCD25lowFOXP3+ population. Frequency of IFN-γ+ cells was compared between T1D patients (N = 62; depicted by red circles) and healthy donors (N = 54; depicted by black squares) following in vitro stimulation with phorbol-12-myristate-13-acetate (PMA) and ionomycin. (C) Plot depicts the distribution of the frequency (geometric mean ± 95% CI) of IFN-γ+ HELIOS− T cells in the CD45RA− CD127lowCD25lowFOXP3+ population out of total CD4 T cells from the same donors as in (B). P values were calculated by linear regression of the log-transformed data, including batch as a covariate. HC, healthy controls; T1D, type 1 diabetic patients.
Antibodies and immunostaining panels used for flow cytometry. Detailed description of the fluorochrome-conjugated antibodies and immunostaining panels used in this study.