Abstract
Lead (Pb) free non-toxic perovskite solar cells have become more important in the commercialization of the photovoltaic devices. In this study the structural, electronic, optical and mechanical properties of Pb-free inorganic metal halide cubic perovskites CsBX3 (B = Sn, Ge; X = I, Br, Cl) for perovskite solar cells are simulated using first-principles Density Functional Theory (DFT). These compounds are semiconductors with direct band gap energy and mechanically stable. Results suggest that the materials have high absorption coefficient, low reflectivity and high optical conductivity with potential application in solar cells and other optoelectronic energy devices. On the basis of the optical properties, one can expect that the Germanium (Ge) would be a better replacement of Pb as Ge containing compounds have higher optical absorption and optical conductivity than that of Pb containing compounds. A combinational analysis of the electronic, optical and mechanical properties of the compounds suggests that CsGeI3 based perovskite is the best Pb-free inorganic metal halide semiconductor for the solar cell application. However, the compound with solid solution of CsGe(I0.7Br0.3)3 is found to be mechanically more ductile than CsGeI3. This study will also guide to obtain Pb-free organic perovskites for optoelectronic devices.
Introduction
Recently, metal halide perovskites have drawn a great attention in the scientific community1–11 due to their remarkable performance in solar cells thanks to their outstanding opto-electronic properties such as tunable bandgap, high optical absorption, broad absorption spectrum, small carrier effective masses, dominant point defect, long charge diffusion lengths and high charge carrier mobility1,3. In addition to this, these materials are abundant in nature and inexpensive. As a result solar cells based on these materials would be cheaper and more efficient than silicon-based photovoltaic (PV) technology1. Because of these extraordinary properties, this group of semiconductors have the potential to be used in a wide range of electronic devices beyond solar cells such as light emitting diodes, photodetector and solar-to-fuel energy conversion devices8–11. To predict a specific device application and improvements, a deeper and fundamental understanding the properties of the semiconductors is necessary. Therefore, studying the electronic, optical and mechanical properties as well as understanding the overall characteristics of the system is utmost important.
Metal halide perovskite materials can be described by the general formula ABX3, where A is a cation, B is a metal ion and X is a halogen anion. In the last few years, different properties of metal halide perovskites have been reported for single or group of compounds (see Supplementary Section “Literature Review”) to be used in solar cells after the discovery of organic perovskites12. Most of the perovskite compounds with high performance contain lead (Pb) which is toxic and undesirable. Herein, we have considered Pb-free inorganic metal halide perovskite compounds to understand their general characteristics using first-principles Density Functional Theory (DFT). This work focuses on investigation of the structural, electronic, optical and mechanical properties of Pb-free metal halide perovskites CsBX3 (B = Sn, Ge; X = I, Br, Cl) and compared with Pb-containing compounds CsPbX3 (X = I, Br, Cl). The atoms Sn and Ge have been chosen to replace Pb because of their similarity in chemical composition and valency from the periodic table.
The investigation of the optical properties of solids is important for better understanding the electronic properties of the materials. The study of the optical functions also reveals the response of a material to light. Therefore, deep knowledge on the optical parameters is essential for the practical applications of a material in optoelectronic devices like solar cell, diode, laser, etc. However, less study has been carried out on the optical properties of the considered perovskite compounds. In particular the optical properties of CsGeX3 (X = I, Br, Cl) materials are still unexplored in detail. Also understanding the mechanical properties of these materials including mechanical stability, rigidity and ductility are useful to predict the importance of the materials for industrial applications. To the best of our knowledge, the elastic constants and moduli of the Pb-free CsSnI3, CsSnCl3, CsGeI3 and CsGeCl3 compounds are still not reported. Therefore, we have considered selected metal halide perovskite compounds and investigated their structural, electronic, optical and mechanical properties using first-principles Density Functional Theory (DFT) to obtain suitable Pb-free perovskites for solar cells and other optoelectronic devices.
Results and Discussion
Structural properties
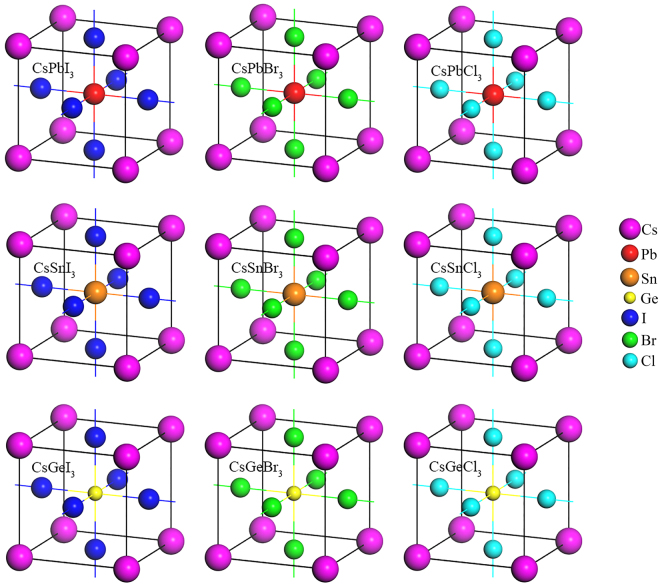
The cubic metal halide perovskites CsBX3 (B = Pb, Sn, Ge; X = I, Br, Cl) have space group (no. 221). The unit cell of the material contains five atoms with one formula unit shown in Fig. 1. In the unit cell, the Cs atoms occupy the corner positions with 1a Wyckoff site and (0, 0, 0) fractional coordinates, the B atoms occupy the body centred position with 1b Wyckoff site and () fractional coordinates, and the X atoms occupy the face centred positions with 3c Wyckoff site and () fractional coordinates.
Figure 1.
Unit cells of the considered cubic metal halide perovskites CsBX3 (B = Sn, Ge; X = I, Br, Cl) as compared with Pb-based compounds CsPbX3 (X = I, Br, Cl). Replacement of halogen atoms is showing from left to right while the replacement of Pb is showing from top to bottom. The molecular models are optimized by DFT calculations.
The calculated equilibrium lattice parameter a and the volume of the optimized cells are in good agreement with the experimental results as well as with the available theoretical results (see Supplementary Table 1). We have also found that the replacement of halogen atom (X) by a lighter and smaller halogen atom reduces the lattice parameter (Fig. 2) and the unit cell volume (Supplementary Figure 1). Similarly, the replacement of Pb by tin (Sn) or germanium (Ge) causes the reduction of lattice parameter of perovskites as shown in Fig. 2. A clean periodic behaviour in term of the change in lattice parameter as well as the unit cell volume is observed with the considered metal halide perovskite materials when changing the size of halides. This is a general features for the most compounds and it is because of the change in atomic size and charge.
Figure 2.
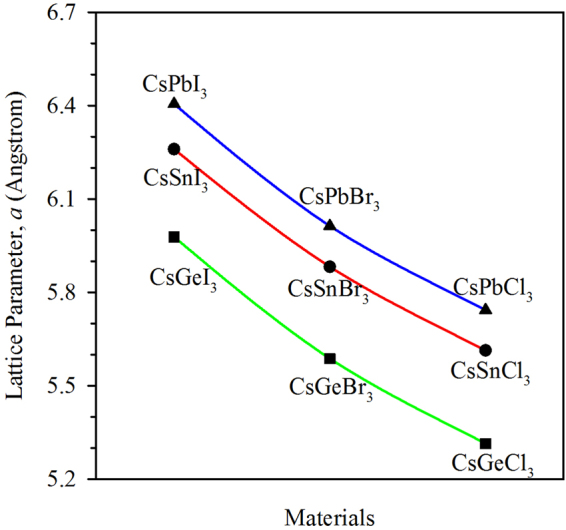
Variation of lattice parameter due to the replacement of atoms by similar atoms. The lattice parameter is seen to change periodically depending upon the size of atoms in the unit cell.
The replacement of one type of atoms by a similar atom from a compound can affect the structural parameters of the compound. As a consequence, the material properties like electronic, optical and mechanical properties are also affected. Thus, one can expect to tune the properties of a material by replacing the constituent atoms with the atoms which are in similar groups in the periodic table.
Electronic properties
We have calculated the electronic band gap using generalized gradient approximation (GGA) of Perdew-Berke-Ernzerhof (PBE). The calculated electronic band gap values are found to be in good agreement with the available results in the literature using the same theory (see Supplementary Table 2) while some diversity has been found with other results. Sometimes hybrid potential like HSE (Heyd-Scuseria-Ernzerhof)13 may give better estimation14. But it is not the perfect one as diversity has been found for some compounds (see Supplementary Table 2). Thus, it is still a challenge to find the appropriate potential to predict the theoretical electronic band gap for this system of materials. Similar to the unit cell volume, the electronic band gap is seen to change periodically for changing the halogen atoms as shown in Fig. 3. Here, higher band gap is observed for the compounds containing lighter halogen atoms. However, exception is observed in case of replacement of Pb by Sn and Ge. The Ge containing compounds have higher electronic band gaps than Sn containing compounds as for Ge, the electronic states of conduction band slightly shift towards the high energy and this is very common for this group of compounds14.
Figure 3.
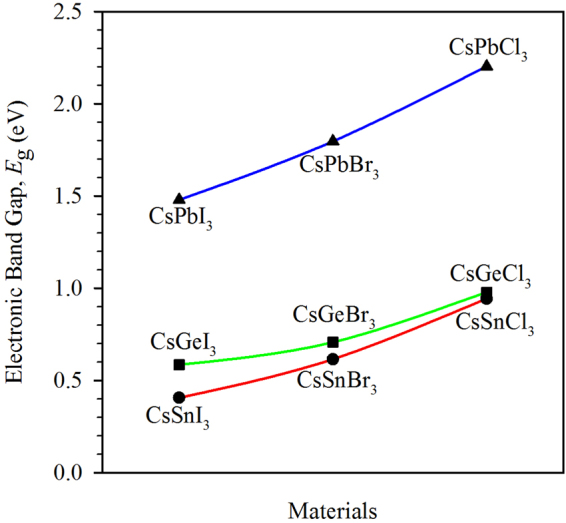
Variation of the electronic band gap due to the replacement of atoms by similar atoms.
The study of the electronic band structure (Supplementary Figure 3) suggests that the pattern of the band structure is almost not affected by replacement of a halogen atom with other halogen atoms. Due to this characteristics, metal halide perovskites are suitable to be used in light emitting diodes (LED) as the band gap of these materials can easily be tuned by changing the halogen contents while the other properties remain almost same. Also, from the total and partial densities of states (Supplementary Figure 4) one can expect that the replacement of I by Br and Cl increases the number of electronic states in valence band just below the Fermi level. But the opposite trend is observed during the replacement of Pb by Sn or Ge. Moreover, the total DOS below the Fermi level mainly comes from the p orbital of halogen atoms. In addition to this the total DOS above the Fermi level is mainly contributed by p orbital of B atoms as from Pb-6p, Sn-5p and Ge-4p.
Optical properties
The detail optical properties including real and imaginary part of dielectric functions, refractive index, absorption spectra, reflectivity and photoconductivity of our considered compounds are investigated up to the photon energy of 30 eV to reveal the response of the materials in solar and high energy radiation (see Supplementary Section “Optical Functions”).
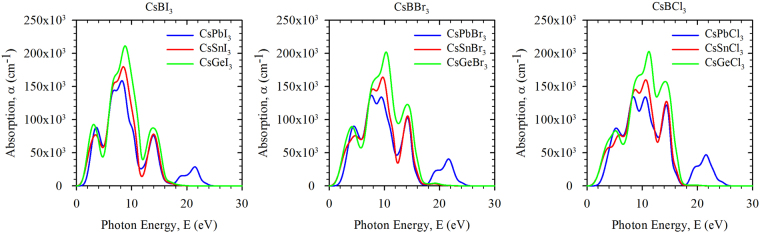
The optical absorption coefficient of a material is a measure of the penetration of light at specific wavelength (energy) into the material before it gets absorbed15. It also gives information about the optimum solar energy conversion efficiency which is important for the practical application of a material in solar cell. The calculated optical absorption spectra of the considered compounds under the simulated illumination is presented in Fig. 4. In general, there are mainly three light absorption peaks observed for all compounds except those containing Pb for which an additional peak is observed in high energy region. The replacement of Pb by Sn or Ge affects the magnitude of the absorption spectra, but the position of the peaks remain at the same energy. However, the replacement of I by Br and Br by Cl shift the position of the peaks towards the higher energy. Most of the considered compounds show high absorption between the energy ranges from 2–16 eV with first peak at about 4 eV, second broad peak centred at about 10 eV and third peak at about 14 eV. It is also found that the maximum absorption is obtained with Ge based compounds compared to Pb and Sn based counterpart. Therefore, Ge would be a better substitute of Pb in the inorganic perovskites for application in solar cells10. The efficiency of the Ge based inorganic perovskite solar cells (CsGeX3) is, however, to be investigated if it can be competitive with the existing organic-inorganic hybrid perovskite solar cells, such as (CH3NH3)PbX3.
Figure 4.
Calculated optical absorption of perovskites CsBX3 (B = Pb, Sn, Ge; X = I, Br, Cl) as a function of incident photon energies.
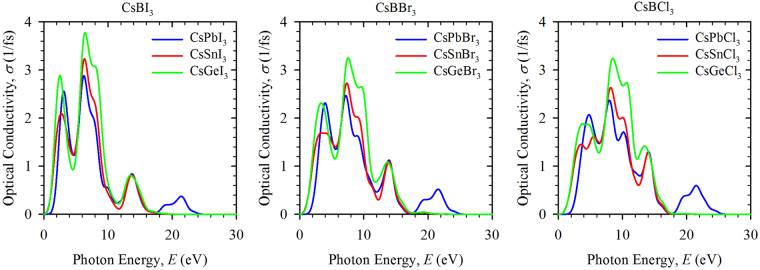
Reflectivity is another important optical property for the solar cell and other applications of the perovskite. All the investigated perovskite materials show a low reflectivity in the IR-Visible-UV region of the spectra (see Supplementary Figure 7). This low reflectivity suggests that the materials have high absorptivity and/or transmitivity. However, our study of the dielectric function (see Supplementary Section “Optical Functions”) suggests that the materials have high transmitivity at high energy region and low transmittance in low energy region. Therefore, the results of the reflectivity and dielectric function confirm that the perovskite compounds have high absorption at low energy (<10 eV) region of the energy spectrum. On the other hand, the optical conductivity is a good reflection of the photoconductivity16. The real part of the optical conductivity is shown in Fig. 5. As can be seen, most of the perovskite compounds have high optical conductivity at low energy region. Similar to the phenomenon observed in absorption, Ge based compounds have higher optical conductivity than other metal cations based materials. Moreover, the magnitude of the spectra is higher for I containing compounds, while the spectra is seen to broaden for Br and Cl. In addition, the higher value of the extinction coefficient (Supplementary Figure 9) in the low energy region of the spectra also reveals that Ge is found to be a better replacement of Pb for solar cells.
Figure 5.
Calculated optical conductivity of perovskites CsBX3 (B = Pb, Sn, Ge; X = I, Br, Cl) as a function of incident photon energies.
Mechanical properties
The elastic constants are essential to predict the mechanical stability of a crystal. The well-known Born stability criteria17 for cubic crystal is given by: . The three independent elastic constants (C 11, C 12 and C 44) of the cubic perovskite compounds are calculated using the finite strain theory18 implemented in CASTEP module and presented in Table 1 with available theoretical and experiment results. The calculated elastic constants for CsPbCl3 are in good agreement with the only available theoretical results reported in literature19. Also, the calculated results for CsSnBr3 and CsGeBr3 are in good agreement with the available theoretical results reported by Brik20. The calculated elastic constants are positive and satisfy the Born stability criteria, which implies that the compounds are mechanically stable.
Table 1.
The calculated and the available theoretical and experimental values of elastic constants C ij (GPa), bulk modulus B (GPa), shear modulus G (GPa), Young’s modulus Y (GPa), Pugh’s ratio B/G and Poisson ratio υ of cubic perovskites CsBX3 (B = Pb, Sn, Ge; X = I, Br, Cl).
| Phase | C 11 | C 12 | C 44 | B | G | Y | B/G | v | References |
|---|---|---|---|---|---|---|---|---|---|
| CsPbI3 | 34.23 | 4.46 | 3.24 | 14.38 | 6.30 | 14.49 | 2.283 | 0.32 | This Work |
| 23 | Cal.21 | ||||||||
| 19.8 | Cal.22 | ||||||||
| 14.4 | Cal.23 | ||||||||
| 7.9 | 20.1 | 2.544 | 0.33 | Exp.24 | |||||
| CsPbBr3 | 42.32 | 6.51 | 4.24 | 18.45 | 7.90 | 20.74 | 2.335 | 0.31 | This Work |
| 27 | Cal.21 | ||||||||
| 23.5 | Cal.22 | ||||||||
| CsPbCl3 | 48.97 | 7.83 | 5.08 | 21.54 | 9.27 | 24.32 | 2.324 | 0.31 | This Work |
| 46.04 | 6.10 | 4.91 | 22.59 | 8.98 | 23.34 | 2.16 | 0.30 | Cal.19 | |
| 31 | Cal.21 | ||||||||
| 25.8 | Cal.22 | ||||||||
| CsSnI3 | 21.34 | 1.22 | 5.74 | 6.30 | 7.55 | 16.18 | 0.83 | 0.07 | This Work |
| CsSnBr3 | 43.89 | 6.69 | 5.21 | 19.09 | 8.94 | 23.19 | 2.135 | 0.30 | This Work |
| 44.65 | 3.93 | 6.57 | 17.50 | 10.55 | 0.08 | Cal.20 | |||
| CsSnCl3 | 50.66 | 8.71 | 6.01 | 22.70 | 10.20 | 26.61 | 2.221 | 0.30 | This Work |
| CsGeI3 | 40.32 | 8.18 | 8.87 | 18.89 | 11.28 | 28.22 | 1.674 | 0.25 | This Work |
| CsGeBr3 | 48.08 | 10.82 | 10.07 | 23.24 | 12.92 | 32.70 | 1.799 | 0.27 | This Work |
| 49.52 | 11.63 | 11.52 | 24.26 | 14.08 | 0.19 | Cal.20 | |||
| CsGeCl3 | 54.93 | 13.08 | 11.99 | 27.03 | 15.02 | 38.02 | 1.800 | 0.27 | This Work |
The results of bulk modulus, shear modulus, Young’s modulus, Pugh’s ratio and Poisson ratio of the perovskite compounds were calculated using the second order elastic constants and summarized in Table 1. We also compare our results with available theoretical and experimental results previously reported. The results of bulk modulus for CsPbX3 (X = I, Br, Cl) calculated in this work are found to be in reasonable agreement with previous theoretical calculations21–23. The only experimental result of elastic moduli for CsPbI3 reported by Rakita et al.24 also agrees well with our simulation. In addition, calculated moduli for CsSnBr3 and CsGeBr3 match very well with literature20. The bulk modulus is an indicator of the stiffness of a volume of material. The calculated values of bulk modulus of the perovskite lie between 6.30 GPa (for CsSnI3) and 27.03 GPa (for CsGeCl3), indicating that these perovskite compounds are flexible. Because of their flexibility and softness, the metal halide perovskites can easily be developed into thin film and make them more useful in applications such as solar cells. By changing the halogen atom from I to Br then to Cl, the value of bulk modulus is seen to increase. Similarly, the replacement of Pb by Sn and then Sn by Ge increases the bulk modulus of the perovskite compounds except CsSnI3 which shows extremely low value of bulk modulus. Similar trend is observed for shear and Young’s modulus with no exception.
The ratio of the bulk modulus to shear modulus (B/G) also known as Pugh’s ratio and the Poisson ratio are considered as a measure to predict the failure mode, i.e., ductility and brittleness of a material25. The critical value of Pugh’s ratio to separate the ductile materials from brittle is found to be 1.75, while the critical value of Poisson ratio for the separation of ductile from brittle is ~0.2625. If the value of Pugh’s ratio is greater than 1.75 or the value of Poisson ratio is greater than 0.26, then the material is considered as ductile otherwise it is brittle. Also, the higher value of Pugh’s ratio and the Poisson ratio indicate more ductility of a material. According to the criteria, CsSnI3 is brittle, CsGeI3 is found near brittle-ductile boarder line and the rest of the compounds are ductile in nature. Figure 6 presents the variation of Pugh’s ratio for different compounds. The same characteristics are observed for Poisson ratio (Supplementary Figure 11). The smaller values of Pugh’s ratio and the Poisson ratio of CsSnI3 indicate that the material is very brittle and it would not be convenient to handle for applications in a device. The higher ductility is observed in compounds containing Pb. Among them, CsPbBr3 shows the highest ductility.
Figure 6.
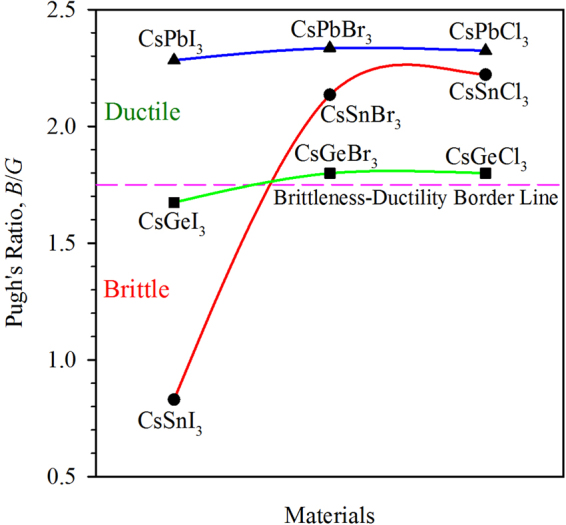
Variation of the Pugh’s ratio of the perovskite with different composition. The pink dashed line separates the ductile materials from brittle.
Lead Free Perovskites
The summary of the key properties of the metal halide perovskites are presented in Table 2. In comparison, Ge based compounds have a higher optical absorption and optical conductivity than Pb containing compounds, which suggests that Ge would be a better replacement of lead.
Table 2.
Summary of the key properties to predict lead free perovskites.
| CsPbI 3 Less Optical Absorption Medium Photoconductivity Highly Ductile | CsPbBr 3 Less Optical Absorption Less Photoconductivity Highly Ductile | CsPbCl 3 Less Optical Absorption Less Photoconductivity Highly Ductile |
| CsSnI 3 Medium Optical Absorption Medium Photoconductivity Highly Brittle | CsSnBr 3 Medium Optical Absorption Less Photoconductivity Ductile | CsSnCl 3 Medium Optical Absorption Less Photoconductivity Ductile |
| CsGeI 3 High Optical Absorption High Photoconductivity Less Brittle | CsGeBr 3 High Optical Absorption Medium Photoconductivity Ductile | CsGeCl 3 High Optical Absorption Medium Photoconductivity Ductile |
On the other hand, CsSnI3 is not suitable to use in any device as it is highly brittle. Furthermore, the Sn based compounds CsSnX3 (X = I, Br, Cl) have low optical absorption and conductivity than that of Ge based compounds. Considering all these properties, we can conclude that CsGeI3 would be a better lead-free perovskite material for solar cell application as it has highest absorption and photoconductivity in the solar spectrum. However, the problem with CsGeI3 is that it is slightly brittle and also having poor film forming ability10. Nevertheless the issue of brittleness should be improved by incorporation of Br with CsGeI3 in solid solution26, and consequently the film forming ability may also be improved.
To find a ductile lead free perovskite, the mechanical properties of solid solutions CsGe(I1−xBrx)3 have been calculated and the variation of the Pugh’s ratio for different combinations of I and Br is presented in Fig. 7. According to the results, the maximum ductility is found at x = 0.3 in CsGe(I1−xBrx)3. Therefore, CsGe(I0.7Br0.3)3 is more ductile than CsGeI3 also the material is found to be mechanically stable and soft (see Supplementary Table 3) and can easily be developed into thin film. The electronic band gap of CsGe(I0.7Br0.3)3 is found as 0.83 eV, also the absorption and the optical conductivity is expected between that of CsGeI3 and CsGeBr3 as the optical properties is seen to change periodically (Supplementary Figure 10). Therefore CsGe(I0.7Br0.3)3 would be the best Pb-free perovskite for those applications in which ductility is required.
Figure 7.
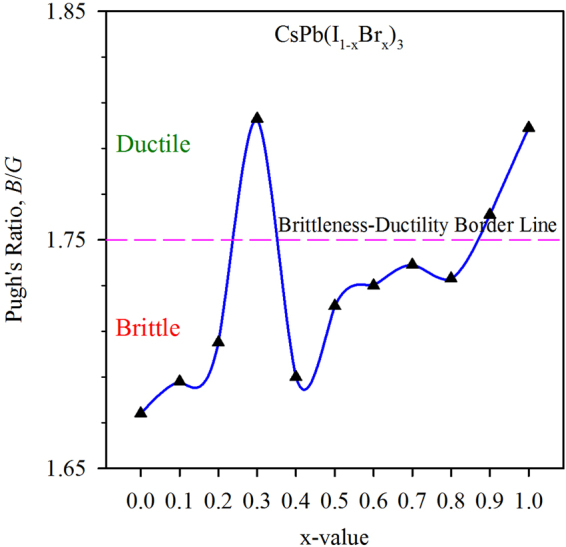
Variation of Pugh’s ratio for different combination of I and Br in CsGe(I1−xBrx)3. CsGe(I0.7Br0.3)3, CsGe(I0.1Br0.9)3 and CsGeBr3 are ductile while the others are brittle and the maximum ductility is found in CsGe(I0.7Br0.3)3.
Conclusion
First-principles DFT calculations have been performed to investigate the structural, electronic, optical and mechanical properties of Pb-free inorganic metal halide cubic perovskites CsBX3 (B = Sn, Ge; X = I, Br, Cl) and compared with Pb based compounds. This study suggests that the electronic band gap value is affected due to the replacement of halogen atom while the other properties remain almost same. This is a key feature of the materials to be used in light emitting diodes (LED) as the band gap of these materials can easily be tuned by changing the halogen contents. The investigated perovskite compounds are mechanically stable and can easily be developed into thin films as the materials have low bulk modulus. According to the optical properties analysis, Ge atom appears superior to Pb for the considered inorganic perovskites, as Ge based compounds have higher optical absorption and optical conductivity than the Pb based compounds. By analysing the combined effects of the electronic, optical as well as mechanical properties of CsGeX3 (X = I, Br, Cl), it can be concluded that CsGeI3 is a better candidate as a Pb- free perovskite for solar cell application. However, CsGeI3 is slightly brittle and may not be superior in applications that require ductility. This issue has been resolved by partially replacing iodine with solid solution of Br atom CsGe(I0.7Br0.3)3.
Computational Methods
The first-principles density functional theory (DFT)27,28 calculations have been performed using the plane wave pseudopotential method as implemented in CASTEP29 module of Materials Studio 8.0. Vanderbilt type ultrasoft pseudopotential30 was used to treat electron ion interaction for all calculations. The exchange-correlation energy was evaluated by using the generalized gradient approximation (GGA) of Perdew-Berke-Ernzerhof (PBE)31. To optimize the crystal structure and find the ground state energy the Broyden–Fletcher–Goldfarb–Shanno (BFGS)32 minimization technique was used. Elastic constants were calculated by using the finite strain theory18 which was implemented within CASTEP module.
We have used the plane-wave cutoff energy as 550 eV through the calculations, and for the sampling of the Brillouin zone the Monkhorst-Pack scheme33 k-points of 12 × 12 × 12 has been taken. The convergence thresholds of 5 × 10−6 eV/atom for the total energy, 0.01 eV/Å for the maximum force, 0.02 GPa for maximum stress, and 5 × 10−4 Å for the maximum displacements were used to optimize the geometry of crystal structure.
Electronic supplementary material
Author Contributions
M.R. and K.K.O. conceived the study. M.R. carried the numerical calculations, analysed the data, and prepared the manuscript. K.K.O., A.D., H.W., and T.T. contributed to data analysis and results interpretation. All the authors contributed to the manuscript preparation and discussed the results.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-13172-y.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhang W, Eperon GE, Snaith HJ. Metal halide perovskites for energy applications. Nature Energy. 2016;1:16048. doi: 10.1038/nenergy.2016.48. [DOI] [Google Scholar]
- 2.Swarnkar A, et al. Quantum dot–induced phase stabilization of α-CsPbI3 perovskite for high-efficiency photovoltaics. Science. 2016;354:92–95. doi: 10.1126/science.aag2700. [DOI] [PubMed] [Google Scholar]
- 3.Yin WJ, Shi T, Yan Y. Unique properties of halide perovskites as possible origins of the superior solar cell performance. Advanced Materials. 2014;26:4653–4658. doi: 10.1002/adma.201306281. [DOI] [PubMed] [Google Scholar]
- 4.Marshall K, et al. Enhanced stability and efficiency in hole-transport-layer-free CsSnI3 perovskite photovoltaics. Nature Energy. 2016;1:16178. doi: 10.1038/nenergy.2016.178. [DOI] [Google Scholar]
- 5.Sutton RJ, et al. Bandgap‐tunable cesium lead halide perovskites with high thermal stability for efficient solar cells. Advanced Energy Materials. 2016;6:1502458. doi: 10.1002/aenm.201502458. [DOI] [Google Scholar]
- 6.Hoffman JB, Schleper AL, Kamat PV. Transformation of sintered CsPbBr3 nanocrystals to cubic CsPbI3 and gradient CsPbBrxI3–x through halide exchange. Journal of the American Chemical Society. 2016;138:8603–8611. doi: 10.1021/jacs.6b04661. [DOI] [PubMed] [Google Scholar]
- 7.Huang, L.-y. & Lambrecht, W. R. Electronic band structure trends of perovskite halides: Beyond Pb and Sn to Ge and Si. Physical Review B93, 195211 (2016).
- 8.Akkerman QA, et al. Strongly emissive perovskite nanocrystal inks for high-voltage solar cells. Nature Energy. 2016;2:16194. doi: 10.1038/nenergy.2016.194. [DOI] [Google Scholar]
- 9.Eperon GE, et al. Inorganic caesium lead iodide perovskite solar cells. Journal of Materials Chemistry A. 2015;3:19688–19695. doi: 10.1039/C5TA06398A. [DOI] [Google Scholar]
- 10.Krishnamoorthy T, et al. Lead-free germanium iodide perovskite materials for photovoltaic applications. Journal of Materials Chemistry A. 2015;3:23829–23832. doi: 10.1039/C5TA05741H. [DOI] [Google Scholar]
- 11.Ramasamy P, et al. All-inorganic cesium lead halide perovskite nanocrystals for photodetector applications. Chemical communications. 2016;52:2067–2070. doi: 10.1039/C5CC08643D. [DOI] [PubMed] [Google Scholar]
- 12.Kojima A, et al. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. Journal of the American Chemical Society. 2009;131:6050–6051. doi: 10.1021/ja809598r. [DOI] [PubMed] [Google Scholar]
- 13.Heyd J, Scuseria GE, Ernzerhof M. Hybrid functionals based on a screened Coulomb potential. The Journal of Chemical Physics. 2003;118:8207–8215. doi: 10.1063/1.1564060. [DOI] [Google Scholar]
- 14.Körbel S, Marques MA, Botti S. Stability and electronic properties of new inorganic perovskites from high-throughput ab initio calculations. Journal of Materials Chemistry C. 2016;4:3157–3167. doi: 10.1039/C5TC04172D. [DOI] [Google Scholar]
- 15.Roknuzzaman M, et al. Physical properties of predicted Ti2CdN versus existing Ti2CdC MAX phase: An ab initio study. Computational Materials Science. 2016;113:148–153. doi: 10.1016/j.commatsci.2015.11.039. [DOI] [Google Scholar]
- 16.Yu G, et al. Photoconductivity and optical conductivity in lightly doped Nd2CuO4−δ. Physica C: Superconductivity. 1992;203:419–425. doi: 10.1016/0921-4534(92)90051-D. [DOI] [Google Scholar]
- 17.Born M. On the stability of crystal lattices. I. Mathematical Proceedings of the Cambridge Philosophical Society. 2008;36:160–172. doi: 10.1017/S0305004100017138. [DOI] [Google Scholar]
- 18.Murnaghan FD. Finite deformations of an elastic solid. American Journal of Mathematics. 1937;59:235–260. doi: 10.2307/2371405. [DOI] [Google Scholar]
- 19.Ghebouli M, Ghebouli B, Fatmi M. First-principles calculations on structural, elastic, electronic, optical and thermal properties of CsPbCl3 perovskite. Physica B: Condensed Matter. 2011;406:1837–1843. doi: 10.1016/j.physb.2011.02.040. [DOI] [Google Scholar]
- 20.Brik M. Comparative first-principles calculations of electronic, optical and elastic anisotropy properties of CsXBr3 (X = Ca, Ge, Sn) crystals. Solid State Communications. 2011;151:1733–1738. doi: 10.1016/j.ssc.2011.08.039. [DOI] [Google Scholar]
- 21.Chang Y, Park C, Matsuishi K. First-principles study of the structural and the electronic properties of the lead-halide-based inorganic-organic perovskites (CH3NH3)PbX3 and CsPbX3 (X = Cl, Br, I) Journal-Korean Physical Society. 2004;44:889–893. doi: 10.3938/jkps.44.638. [DOI] [Google Scholar]
- 22.Murtaza G, Ahmad I. First principle study of the structural and optoelectronic properties of cubic perovskites CsPbM3 (M = Cl, Br, I) Physica B: Condensed Matter. 2011;406:3222–3229. doi: 10.1016/j.physb.2011.05.028. [DOI] [Google Scholar]
- 23.Afsari M, Boochani A, Hantezadeh M. Electronic, optical and elastic properties of cubic perovskite CsPbI3: Using first principles study. Optik - International Journal for Light and Electron Optics. 2016;127:11433–11443. doi: 10.1016/j.ijleo.2016.09.013. [DOI] [Google Scholar]
- 24.Rakita Y, et al. Mechanical properties of APbX3 (A = Cs or CH3NH3; X = I or Br) perovskite single crystals. MRS Communications. 2015;5:623–629. doi: 10.1557/mrc.2015.69. [DOI] [Google Scholar]
- 25.Hadi MA, et al. First-principles prediction of mechanical and bonding characteristics of new T2 superconductor Ta5GeB2. physica status solidi (b) 2016;253:2020–2026. doi: 10.1002/pssb.201600209. [DOI] [Google Scholar]
- 26.Hadi MA, et al. Elastic and thermodynamic properties of new (Zr3−xTix)AlC2 MAX-phase solid solutions. Computational Materials Science. 2017;137:318–326. doi: 10.1016/j.commatsci.2017.06.007. [DOI] [Google Scholar]
- 27.Hohenberg P, Kohn W. Inhomogeneous electron gas. Physical review. 1964;136:B864. doi: 10.1103/PhysRev.136.B864. [DOI] [Google Scholar]
- 28.Kohn W, Sham LJ. Self-consistent equations including exchange and correlation effects. Physical review. 1965;140:A1133. doi: 10.1103/PhysRev.140.A1133. [DOI] [Google Scholar]
- 29.Clark SJ, et al. First principles methods using CASTEP. Zeitschrift für Kristallographie-Crystalline Materials. 2005;220:567–570. doi: 10.1524/zkri.220.5.567.65075. [DOI] [Google Scholar]
- 30.Vanderbilt D. Soft self-consistent pseudopotentials in a generalized eigenvalue formalism. Physical Review B. 1990;41:7892. doi: 10.1103/PhysRevB.41.7892. [DOI] [PubMed] [Google Scholar]
- 31.Perdew JP, Burke K, Ernzerhof M. Generalized gradient approximation made simple. Physical review letters. 1996;77:3865. doi: 10.1103/PhysRevLett.77.3865. [DOI] [PubMed] [Google Scholar]
- 32.Fischer TH, Almlof J. General methods for geometry and wave function optimization. The Journal of Physical Chemistry. 1992;96:9768–9774. doi: 10.1021/j100203a036. [DOI] [Google Scholar]
- 33.Monkhorst HJ, Pack JD. Special points for Brillouin-zone integrations. Physical review B. 1976;13:5188. doi: 10.1103/PhysRevB.13.5188. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.