Abstract
Large-conductance Ca2+-activated potassium (BKCa) channels are key determinants of vascular smooth muscle excitability. Impaired BKCa channel function through remodeling of BKCa β1 expression and function contributes to vascular complications in animal models of diabetes. Yet, whether similar alterations occur in native vascular smooth muscle from humans with type 2 diabetes is unclear. In this study, we evaluated BKCa function in vascular smooth muscle from small resistance adipose arteries of non-diabetic and clinically diagnosed type 2 diabetic patients. We found that BKCa channel activity opposes pressure-induced constriction in human small resistance adipose arteries, and this is compromised in arteries from diabetic patients. Consistent with impairment of BKCa channel function, the amplitude and frequency of spontaneous BKCa currents, but not Ca2+ sparks were lower in cells from diabetic patients. BKCa channels in diabetic cells exhibited reduced Ca2+ sensitivity, single-channel open probability and tamoxifen sensitivity. These effects were associated with decreased functional coupling between BKCa α and β1 subunits, but no change in total protein abundance. Overall, results suggest impairment in BKCa channel function in vascular smooth muscle from diabetic patients through unique mechanisms, which may contribute to vascular complications in humans with type 2 diabetes.
Introduction
The World Health Organization estimates that ~350 million people worldwide have non-insulin-dependent type 2 diabetes, a number that is expected to double by 20301. Vascular complications (e.g. hypertension, coronary heart disease, stroke) are among the most prominent causes of morbidity and mortality in type 2 diabetic patients1–3. While endothelial dysfunction has long been recognized as a key link in the pathogenesis of vascular complications during diabetes, emerging data in human and animal models also implicate vascular smooth muscle dysfunction in this process3–11. At present however, there is limited information about the mechanisms underlying changes in vascular smooth muscle function in native cells from type 2 diabetic patients.
The contractile state of vascular smooth muscle in small resistance arteries is controlled by multiple ion channels12. Among them, large-conductance Ca2+-activated potassium (BKCa) channels play a key role in control of vascular smooth muscle contractility via tonic regulation of membrane potential13. Physiologically, these channels are activated by membrane depolarization and localized Ca2+ release events through ryanodine receptors (e.g. Ca2+ sparks) located in the sarcoplasmic reticulum13–15. The opening of BKCa channels by Ca2+ sparks results in the occurrence of BKCa-mediated spontaneous transient outward currents (STOCs), leading to vascular smooth muscle hyperpolarization and consequently vasodilation. Conversely, their inhibition causes membrane potential depolarization and vasoconstriction. BKCa channels are composed of four pore-forming α subunits in association with accessory β subunits16. Four distinct BKCa β subunits (β1-β4) have been identified, with BKCa β1 being predominantly expressed in vascular smooth muscle16. The BKCa β1 subunit regulates BKCa channel activity by modulating its apparent Ca2+ sensitivity as well as its biophysical properties17. Impairment in BKCa β1 expression and function has been implicated in aberrant vascular BKCa channel activity in animal models of diabetes and hypertension, and in immortalized cell lines from human diabetic subjects9,18–25. However, whether similar alterations in BKCa channel activity occur in native, freshly dissociated vascular smooth muscle cells from humans with type 2 diabetes has not been established.
Here, we investigated BKCa channel function in freshly isolated, small resistance adipose arteries and corresponding native vascular smooth muscle cells from obese non-diabetic and clinically diagnosed type 2 diabetic patients. Data revealed a reduction in iberiotoxin (IbTx) sensitivity of intact arteries from diabetic patients. This was associated with impaired BKCa channel activity due to decreased BKCa β1 function in diabetic vascular smooth muscle cells. In contrast to results observed in animal models9,21–23,25, impaired BKCa β1 function was not due to changes in BKCa β1 abundance, but to a reduced functional association between BKCa α and β1 subunits in diabetic cells. Thus, results suggest a mechanism for altered vascular BKCa channel function in patients with type 2 diabetes with similar, but not identical features to those observed in animal models of diabetes.
Results
To establish whether BKCa channel function is impaired in native vascular smooth muscle cells during type 2 diabetes, small diameter arteries were dissected from adipose tissue obtained from obese non-diabetic and clinically diagnosed type 2 diabetic patients undergoing surgical sleeve gastrectomy (see Methods section). Available patient information is included in Supplementary Table S1.
Decreased IBTx sensitivity in arteries from diabetic subjects
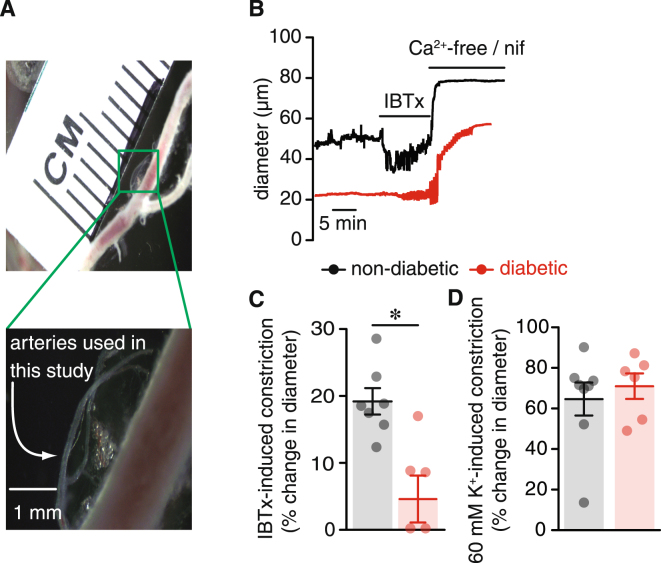
Small diameter adipose arteries (average passive diameter between 50–90 μm; see Fig. 1A) from non-diabetic and clinically diagnosed diabetic patients were pressurized to 80 mmHg, and allowed to develop stable myogenic tone. Mean basal myogenic tone was 44 ± 9% in non-diabetic arteries, and was modestly, although not significantly, elevated to 49 ± 3% in arteries from diabetic patients (P = 0.325). To assess the contribution of BKCa channels to vascular function, adipose arteries from non-diabetic and diabetic patients were challenged with the selective BKCa channel inhibitor iberiotoxin (IBTx; 100 nM). IBTx caused robust vasoconstriction in non-diabetic arteries (24% decrease in diameter) (Fig. 1B and C; Supplementary Table S2). This is consistent with BKCa channel activity opposing vasoconstriction. In contrast, IBTx had little effect on pressurized adipose arteries from diabetic patients (4% decrease in diameter; Fig. 1B and C; Supplementary Table S2; P < 0.05). Arteries from both groups responded to external application of a 60 mM K+ solution with robust constriction (Fig. 1D; Supplementary Table S2), suggesting that differences in the IBTx response were not simply due to the inability of diabetic arteries to respond to a contractile stimulus. This decrease in IBTx sensitivity in diabetic arteries suggests impairment in BKCa channel function in arteries from diabetic patients.
Figure 1.
Adipose arteries from diabetic subjects exhibit blunted response to IBTx. (A) Exemplary image of small resistance adipose arteries used in this study. The image below shows an expanded view of the area in the green square. (B) Representative diameter recordings of pressurized (80 mmHg) adipose arteries from non-diabetic and diabetic patients before and after perfusion of 100 nM iberiotoxin or a Ca2+ free + nifedipine (1 μM) solution. (C) Summary of iberiotoxin-induced constriction in non-diabetic (n = 7 arteries, 4 subjects) and diabetic (n = 5 arteries, 5 subjects) adipose arteries pressurized to 80 mmHg. (D) Scatter/bar plot summarizing vascular tone at 80 mmHg in response to 60 mM extracellular K+ in non-diabetic (n = 8 arteries, 4 subjects) and diabetic (n = 6 arteries, 5 subjects) adipose arteries. *P < 0.05, Mann-Whitney test. Significance was compared between non-diabetic and diabetic datasets.
Decreased STOC amplitude and frequency in vascular smooth muscle from diabetic patients
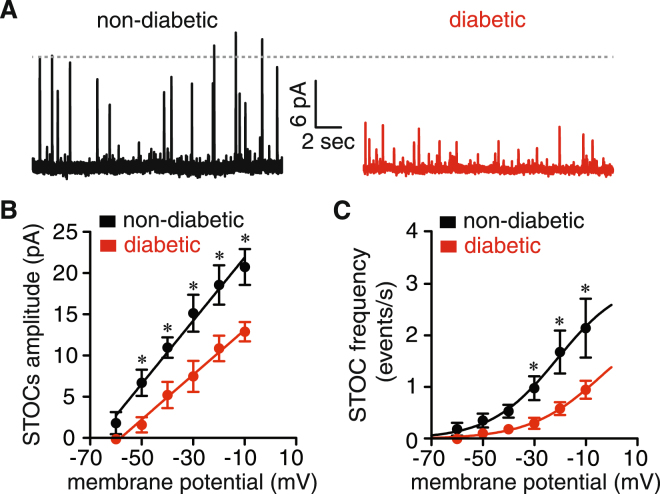
BKCa-mediated STOCs were recorded at membrane potential ranging from −60 mV to −10 mV. Figure 2A displays representative STOCs recordings from freshly dissociated vascular smooth muscle obtained from small diameter adipose arteries from non-diabetic and diabetic patients at −40 mV. STOCs amplitude and frequency increased with membrane depolarization in cells of both groups. However, a significant reduction in STOC amplitude was evident at several membrane potentials examined in cells from diabetic compared to non-diabetic patients (Fig. 2A and B). The slope of the STOC amplitude-voltage relationship was significantly smaller in diabetic (0.27 ± 0.01 pA/mV) compared to non-diabetic cells (0.38 ± 0.02 pA/mV; P < 0.05; F test) (Fig. 1B). STOC frequency was also significantly decreased in diabetic vascular smooth muscle cells at voltages from −30 to −10 mV (Fig. 1C; P < 0.05). The voltage dependency of STOC frequency was shifted toward more depolarized potentials in diabetic cells (V50 = −4 ± 4 mV) compared to non-diabetic cells (V50 = −21 ± 7 mV; Fig. 2C). These results suggest that BKCa channel activity is altered in vascular smooth muscle from diabetic patients.
Figure 2.
STOCs amplitude and frequency are reduced in vascular smooth muscle from diabetic patients. (A) Representative traces of spontaneous whole-cell BKCa currents (e.g. STOCs) at −40 mV in vascular smooth muscle from non-diabetic and diabetic patients. (B,C) Voltage dependency of STOC amplitude (B) and frequency (C) in cells from non-diabetic (n = 10 cells, 4 subjects) and diabetic (n = 13 cells, 4 subjects) patients. Solid lines represent the best fit of the amplitude data using a linear regression function and of the frequency data using a Boltzmann sigmoidal function. *P < 0.05, unpaired t test. Significance was compared between corresponding non-diabetic and diabetic datasets.
Ca2+ spark activity is similar in vascular smooth muscle from non-diabetic and diabetic patients
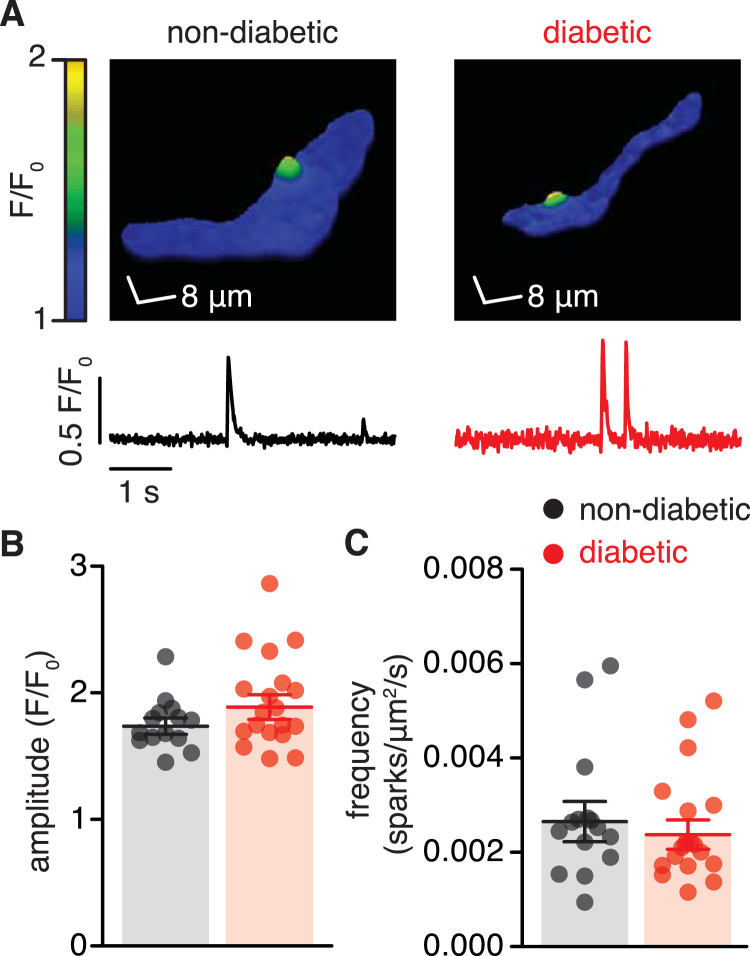
STOC activity in vascular smooth muscle is tightly coupled to Ca2+ sparks13,15,26. These events were recorded from non-diabetic and diabetic vascular smooth muscle loaded with the fluorescent Ca2+ indicator fluo-4 AM to evaluate whether changes in Ca2+ spark activity could account for impairment in BKCa channel function in vascular smooth muscle from diabetic patients (Fig. 3A). Figure 3B and C shows that the amplitude and frequency of Ca2+ sparks were similar in non-diabetic and diabetic cells. These results suggest that impaired BKCa channel function is not due to changes in Ca2+ spark activity in vascular smooth muscle from diabetic patients.
Figure 3.
The frequency and amplitude of Ca2+ sparks is similar in non-diabetic and diabetic vascular smooth muscle. (A) Representative three-dimensional pseudo-color images of Ca2+ sparks (upper panels) and fractional fluorescence traces (F/F0; lower panels) from those sites in fluo-4 AM-loaded vascular smooth muscle cells from non-diabetic and diabetic patients. Scatter/bar plots summarizing Ca2+ spark amplitude (B) and frequency (C) in cells from non-diabetic (n = 254 events, 14 cells, 3 subjects) and diabetic (n = 333 events, 19 cells, 5 subjects) patients. *P < 0.05, Mann-Whitney test. Significance was compared between non-diabetic and diabetic datasets.
Impaired BKCa activity in vascular smooth muscle from diabetic patients
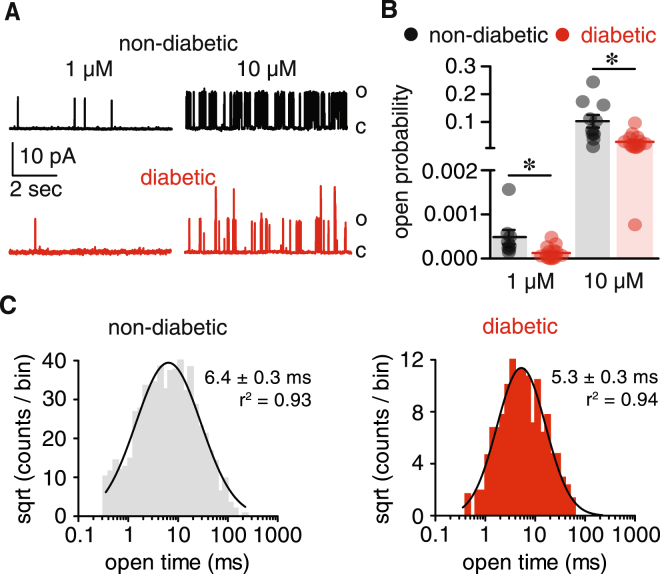
Single BKCa currents were recorded in excised membrane patches to evaluate whether differences in the functional properties of BKCa channels account for compromised channel activity during diabetes. BKCa currents were monitored at a physiological membrane potential (−40 mV) with bath solution containing either 1 μM or 10 μM free Ca2+. Increasing free Ca2+ from 1 μM to 10 μM augmented the open probability (P o) of BKCa channels in both non-diabetic and diabetic cells (Fig. 4A and B). However, BKCa channel P o was significantly lower in diabetic cells compared to non-diabetic cells at each Ca2+ concentration tested (Fig. 4A and B). In addition, open time histograms revealed a shift toward shorter openings in diabetic cells (Fig. 4C; P < 0.05; F test). These results suggest a reduction in apparent Ca2+ sensitivity and dwell open time of BKCa channels in vascular smooth muscle cells from diabetic patients.
Figure 4.
Decreased BKCa channel activity in vascular smooth muscle from diabetic patients. (A) Representative single BKCa channel traces at −40 mV from excised membrane patches of isolated human non-diabetic and diabetic vascular smooth muscle cells in the presence of 1 μM and 10 μM free Ca2+ bath solution (C: closed; O: open). (B) Scatter/bar plot summarizing BKCa channel open probability (Po) at the indicated free Ca2+ concentration from human non-diabetic (n = 18 cells, 9 subjects) and diabetic (n = 27 cells, 15 subjects) cells. *P < 0.05, Mann-Whitney test. (C) Open dwell time histograms of BKCa channels in non-diabetic and diabetic vascular smooth muscle. The goodness of the fit was assessed with R2 and the F test was used for comparison between open time fits. Significance was compared between non-diabetic and diabetic datasets.
Altered BKCa β1 subunit function in vascular smooth muscle from diabetic patients
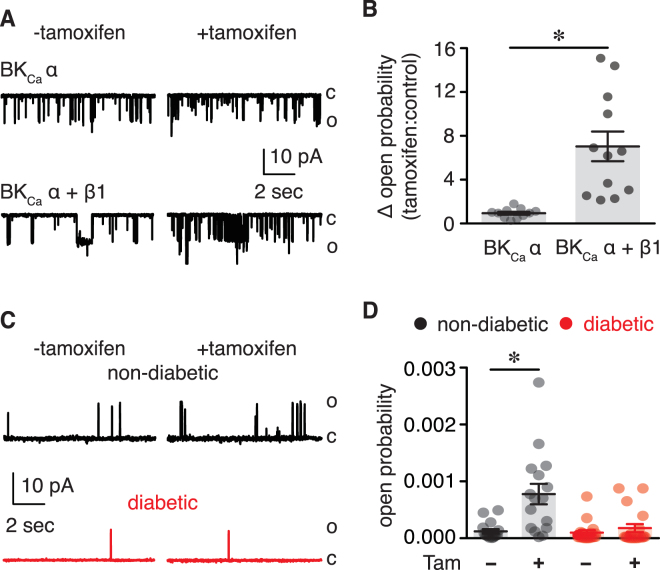
The BKCa channel function is highly dependent on expression and function of the accessory BKCa β subunit17,27. To examine whether impaired BKCa channel activity in vascular smooth muscle from diabetic patients is due to changes in BKCa β subunit function, single BKCa currents were recorded in the absence or presence of tamoxifen (1 μM). This drug increases BKCa channel P o by engaging the BKCa β subunit as shown in Fig. 5A and B, and as previously reported by our group and others9,19,28. Whereas tamoxifen significantly increased the P o of BKCa in non-diabetic cells, it had a minimal effect in vascular smooth muscle from diabetic subjects (Fig. 5C and D). These results suggest that a reduction in BKCa β subunit function may contribute to impaired BKCa channel activity in vascular smooth muscle cells from diabetic patients.
Figure 5.
Impaired BKCa β1 subunit function in human diabetic vascular smooth muscle. (A) Representative single BKCa channel records at +40 mV and 1 μM free Ca2+ obtained from excised membrane patches of HEK293 cells expressing only BKCa α subunit or coexpressing BKCa α + BKCa β1 subunits in the absence and presence of tamoxifen (1 μM). (B) Bar plot summarizing the change in open probability (tamoxifen: control) for HEK293 cells expressing BKCa α (n = 11 cells) or BKCa α + BKCa β1 (n = 12 cells) subunits. *P < 0.05, unpaired t test C) Representative single BKCa channel traces recorded from excised membrane patches of vascular smooth muscle from non-diabetic and diabetic patients with 1 μM free Ca2+ in the bath before (−) and after (+) application of 1 μM tamoxifen. (D) Amalgamated data summarizing BKCa open probability in non-diabetic (n = 17 cells, 9 subjects) and diabetic (n = 19 cells, 8 subjects) cells in the presence or absence of tamoxifen. (C: closed; O: open) *P < 0.05, Wilcoxon test. Significance for Fig. 5B was compared between cells co-expressing BKCa α + BKCa β1 vs. BKCa α only. Significance for Fig. 5D was compared to corresponding - tamoxifen datasets.
Reduced association between BKCa α and BKCa β1 subunits in vascular smooth muscle from diabetic patients
To examine the molecular mechanisms underlying altered BKCa channel activity and BKCa β subunit function during diabetes, protein expression of the pore forming BKCa α subunit and BKCa β1 subunit were examined using Western blot analysis. Antibodies were validated for specificity using HEK293 cells expressing either BKCa α or BKCa β1 subunits (Fig. 6A and Supplementary Fig. S1). Bands of similar molecular weight for the BKCa α subunit were found in lysates from HEK293 cells stably expressing this subunit and human adipose arteries (Fig. 6A). The molecular weight of the BKCa β1 subunit was slightly higher in lysates from human adipose arteries compared to HEK293 cell transfected with a BKCa β1 construct (Fig. 6A), perhaps reflecting differences in post-translational modifications as recently observed29. A non-specific band of ~40 kDa was observed in lysates from HEK cells, but only cells expressing BKCa β1 showed the expected immunoreactive band at 25 kDa (Fig. 6A and Supplementary Figs S1A and S2A). Also note that only one immunoreactive band of expected molecular weight for the BKCa β1 was detected in human adipose arterial lysates (Supplementary Figs S1B and S2A). Subsequent examination of total protein abundance for BKCa α and BKCa β1 subunits in human non-diabetic and diabetic arteries revealed no differences between groups (Fig. 6B and C).
Figure 6.
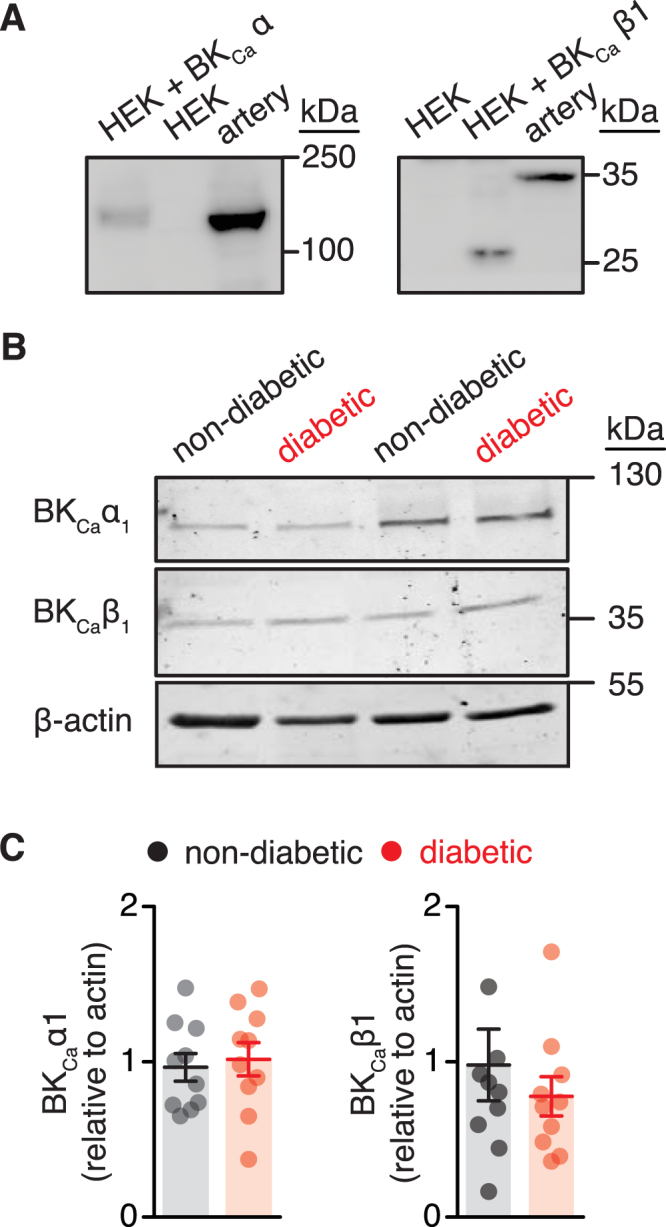
No change in BKCa subunit total protein levels in arterial lysates from non-diabetic and diabetic patients. (A) Representative immunoreactive bands corresponding to BKCa α1 and BKCa β1 subunits in lysates from untransfected HEK293 cells (HEK), HEK293 cells transfected with either BKCa α1 or BKCa β1 subunit and human arteries from non-diabetic patients (n = 3 lysates per condition). Full-length blots are shown in Supplementary Fig. S2A. (B) Representative immunoreactive bands corresponding to BKCa α1 and BKCa β1 subunits, and β-actin as a normalization control. Full-length blots are shown in Supplementary Fig. S2B. (C) Corresponding densitometric summary data for each subunit obtained using arterial lysates from non-diabetic (n = 10 lysates) and diabetic (n = 10 lysates) patients. *P < 0.05, Mann-Whitney test. Significance was compared between non-diabetic and diabetic datasets.
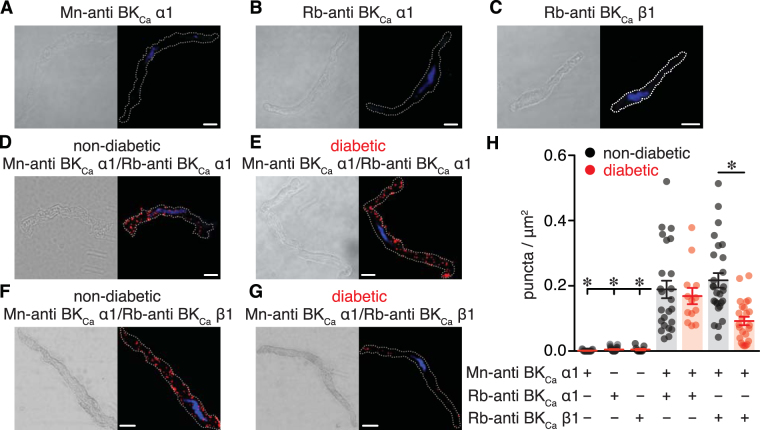
To further explore mechanisms that may contribute to compromised BKCa channel activity and BKCa β1 subunit function during diabetes, the coupling between BKCa α and BKCa β1 was examined using Proximity Ligation Assay (PLA)30 and immunofluorescence analysis. PLA fluorescent puncta are generated when proteins of interest are 40 nm or less apart. PLA signals were negligible when primary antibodies for BKCa α and/or BKCa β1 subunits were omitted from the preparation (Fig. 7A,B,C and H). As a positive control, vascular smooth muscle from non-diabetic and diabetic subjects stained with two different BKCa α subunit antibodies demonstrated robust PLA signals with similar number of PLA puncta (Fig. 7D,E and H). In vascular smooth muscle co-labeled for BKCa α and BKCa β1, data revealed a significant reduction in PLA puncta in diabetic cells compared to non-diabetic cells (Fig. 7F,G and H). Immunofluorescence analysis showed strong BKCa α-associated immunofluorescence of similar intensity (normalized to cytosol) along the plasma membrane of non-diabetic (1.7 ± 0.1) and diabetic (1.6 ± 0.1) vascular smooth muscle (P = 0.460; Mann Whitney Test; Supplementary Fig. S3). Conversely, the intensity of the BKCa β1-associated fluorescence along the plasma membrane was markedly reduced in diabetic cells (0.8 ± 0.1) compared to non-diabetic cells (1.5 ± 0.1; P < 0.05; Mann Whitney Test; Supplementary Fig. S3). Altogether, these results suggest that BKCa β1-mediated regulation of BKCa channels may be compromised in vascular smooth muscle from adipose arteries of diabetic patients due to suppressed functional coupling between BKCa α and BKCa β1 subunits, rather than a reduction in cellular subunit abundance.
Figure 7.
Decreased association between BKCa α1 and BKCa β1 subunits in vascular smooth muscle cells from diabetic patients. (A–C) Differential interference contrast (DIC) and confocal fluorescent PLA puncta (red) and DAPI (blue) images of freshly dissociated human vascular smooth muscle labeled with mouse-anti BKCa α1 (A), rabbit-anti BKCa α1 (B), and rabbit-anti BKCa β1 (C) antibodies. (D,E) DIC (left) and fluorescence PLA (red)/DAPI (blue) (right) images of dissociated vascular smooth muscle from non-diabetic (D) and diabetic (E) patients co-labeled with two distinct antibodies for the BKCa α1 subunit. (F,G) DIC (left) and fluorescence PLA (red)/DAPI (blue) (right) images of dissociated human vascular smooth muscle from non-diabetic (F) and diabetic (G) patients co-labeled for BKCa α1 and BKCa β1 subunits. Scale bar = 10 μm. (H) Quantification of PLA fluorescent puncta per μm2 cell area for non-diabetic and diabetic vascular smooth muscle cells labeled for mouse-anti BKCa α1 (n = 19 cells), rabbit-anti BKCa α1 (n = 27 cells), rabbit-anti BKCa β1 (n = 12 cells), mouse-anti BKCa α1 + rabbit-anti BKCa α1 (n = 24 non-diabetic, 14 diabetic cells); mouse-anti BKCa α1 + rabbit-anti BKCa β1 (n = 27 non-diabetic, 24 diabetic cells). *P < 0.05, Mann-Whitney test. Significance for columns 1, 2, 3 and 5 was compared to column 4, and column 7 was compared to column 6.
Discussion
In this study we report three major novel findings related to BKCa channels in native small diameter adipose arteries and vascular smooth muscle cells from non-diabetic and type 2 diabetic patients. First, BKCa channel activity acts to oppose pressure-induced constriction in isolated human resistance adipose arteries, but this is compromised in arteries from diabetic patients. Second, BKCa channel activity is impaired in vascular smooth muscle from diabetic patients, as reflected by reduced apparent Ca2+ sensitivity, dwell open time and BKCa β subunit function. Third, compromised BKCa β subunit function during diabetes is not associated with changes in total protein abundance of this subunit, but rather seems to be the result of a reduction in the association/coupling between BKCa α and BKCa β1 subunits. The implications of these changes are significant as they may impact vascular reactivity and/or contribute to vascular complications in humans with type 2 diabetes, independent of changes in endothelial function6,24,31–33.
Results of experiments examining the effects of IbTx on arterial tone suggest that BKCa channels serve an important physiological role to oppose pressure-induced vasoconstriction in human adipose arteries, but become severely compromised in adipose arteries from diabetic patients (Fig. 1). These results suggest that BKCa channel function is compromised in adipose arteries from humans with diabetes. Consistent with this, BKCa-mediated STOCs amplitude and frequency as well as single BKCa channel function were significantly reduced in dissociated vascular smooth muscle from adipose arteries from diabetic subjects (Figs 2 and 4). Similar results were observed in a number of studies using vascular smooth muscle from several vascular beds and different animal models of diabetes9,21–23,25,34. A reduction in BKCa channel activity and BKCa β1 function may also contribute to vascular complications by aggravating vessel wall remodeling and fibrosis during diabetes35. Thus, a potential common BKCa-mediated mechanism contributing to vascular complications during diabetes may be engaged in a variety of species. In animal models of diabetes, alterations in BKCa channel function have been associated, in part, to changes in the activity of Ca2+ sparks21,22,25. Here, no changes in Ca2+ sparks properties were detected between dissociated vascular smooth muscle from adipose arteries from non-diabetic and diabetic patients (Fig. 3). These results are similar to those found in vascular smooth muscle from high fat diet mice in which Ca2+ spark activity was unaffected compared to cells from low fat fed mice9. Discrepancies with other studies could arise from experimental conditions, use of smooth muscle from different vascular beds, or intrinsic differences between murine and human cells/tissue31,36. Regardless, our results implicate other mechanisms as culprit for comprised BKCa channel activity in human vascular smooth muscle during diabetes.
The accessory BKCa β1 subunit finely tunes the activity of BKCa channels in vascular smooth muscle by increasing their voltage- and calcium-sensitivity27. Impaired BKCa channel function has been associated with suppression of BKCa β1 subunit function in several studies with animal models of diabetes9,21–23,25. Moreover, compromised BKCa channel activity was linked with suppression of BKCa β1 subunit function in human vascular smooth muscle from Han Chinese patients with hypertension24. We found that BKCa β1 subunit function was significantly suppressed in diabetic vascular smooth muscle from adipose tissue as assessed by tamoxifen sensitivity (Figs 4 and 5). Note that consistent with experiments here (Fig. 5), several groups including ours have validated and confirmed that the effects of tamoxifen on BKCa channels require the presence of the BKCa β1 subunit9,19,28. The impairment in BKCa β1 subunit function helps explain the reduced apparent Ca2+ sensitivity and dwell open time of BKCa channels as well as the blunted IBTx-induced constriction in diabetic compared to non-diabetic vascular smooth muscle cells/adipose arteries. The loss of BKCa β1 subunit function in animal models of diabetes9,21–23,25 and in humans with hypertension24 has been mainly attributed to transcriptional and/or post-translational changes in BKCa β1 subunit expression. In contrast with these seemingly consistent molecular mechanisms, no changes in total BKCa α and BKCa β1 protein levels were detected between non-diabetic and diabetic arterial lysates (Fig. 6). Rather, PLA data revealed a reduced association between BKCa α and BKCa β1 subunits in diabetic cells compared to non-diabetic cells (Fig. 7). Furthermore, immunofluorescence imaging provided evidence of a decreased surface expression of BKCa β1, but not BKCa α, in diabetic cells compared to non-diabetic cells (Supplementary Fig. S2). This is important as recent studies have suggested that dynamic trafficking of the BKCa β1 subunit to the plasma membrane is essential for coupling with the mostly membrane bound, pore forming BKCa α subunit in vascular smooth muscle37,38. This dynamic interaction between BKCa subunits contributes to vascular reactivity under basal conditions37 and in response to chronic angiotensin II signaling38. Also note that a recent study using human vascular smooth muscle has shown that changes in KV1.5 surface expression (perhaps due to altered trafficking of the subunit) rather than differences in total protein levels has been associated with impaired KV1.5 channel and vascular reactivity in humans with coronary artery disease39. Thus, it is tempting to speculate, for future studies, that a change in BKCa β1 subunit trafficking may contribute to compromised BKCa channel activity and vascular reactivity in humans with diabetes.
Vascular complications during diabetes may also be associated with impairment in the function of other ion channels in vascular smooth muscle. For example, it was recently reported that L-type Ca2+ channel (LTCC) activity was significantly augmented in vascular smooth muscle from humans with diabetes40. This can stimulate Ca2+ influx to increase global intracellular Ca2+ in vascular smooth muscle. Interestingly, use of a mathematical model of rodent vascular smooth muscle electrophysiology and Ca2+ dynamics showed that increased LTCC activity predominantly contributes to impaired intracellular Ca2+ during diabetic hyperglycemia41. Yet, the model also revealed that this effect on global intracellular Ca2+ is substantially amplified when an increase in LTCC activity is accompanied by a decrease in the activity of potassium (K+) channels41. Indeed, previous studies have shown a selective suppression in voltage-gated K+ (KV) and BKCa channel activity that is associated with downregulation of KV2.1 and BKCa β1 subunit, respectively, in vascular smooth muscle from diabetic mice on a high fat diet9,10. Thus, potentiation of LTCC activation and suppression of K+ channel activity, including that of BKCa channels as in this study, may synergize to impact vascular reactivity and/or contribute to vascular complications in humans with diabetes. Given the role of KV channels in vascular smooth muscle excitability due to their influence on membrane potential, it will be important to examine the function of these channels as well as the individual contributions of different KV subunits in human cells during control conditions and in diabetes. Collectively, these data will critically inform the development of novel computational models specific to human vascular smooth muscle. This computational approach may help identify and predict the relative contribution of many elements (i.e. LTCC, KV channels, BKCa channels, etc.) that interact non-linearly to control vascular smooth muscle excitability in humans during physiological and pathological conditions.
The use of human tissue from obese non-diabetic and clinically diagnosed type 2 diabetic patients provides unparalleled translational significance. Yet, factors such as underlying environmental factors, biological variables such as sex and age, genetic background, disease progression, prescription history, personal habits (e.g. smoking) and comorbidities may confound results. Despite these limitations, data in this study are internally consistent and reproducible across different experimental approaches. Indeed, we also recently demonstrated high reproducibility in biochemical and electrophysiological outcomes using similar tissue samples40. Furthermore, whereas the use of tissue from obese non-diabetic patients is less than ideal as a true “control”, it does highlight the added stress imposed by diabetes in mediating alterations in BKCa channel activity. To conclude, and to the best of our knowledge, this study provides the first direct evidence that compromised BKCa channel activity in native, freshly dissociated vascular smooth muscle may contribute to vascular complications in type 2 diabetic patients. Interestingly, the mechanisms underlying aberrant vascular BKCa channel activity in humans with diabetes follow some, but not all, the molecular features observed in animal models of diabetes. Gathering information from native human cells may be necessary for development of rational strategies to treat vascular complications in humans with type 2 diabetes.
Methods
Human Tissue (Study Approval)
Excised adipose arteries from obese patients undergoing surgical sleeve gastrectomy and that were either non-diabetic or clinically diagnosed with type 2 diabetes were used. Samples were obtained after Institutional Review Board (IRB) approval from the University of Nevada Reno School of Medicine (IRB ID: 2013-019) and in accordance with the guidelines of the Declaration of Helsinki. The need for informed consent was waived by IRBs at the University of Nevada Reno School of Medicine (IRB ID: 2013-019) and the University of California Davis School of Medicine (IRB ID: 597267-1) because the tissue is considered “waste”, has no codification that could be used to identify patients and was determined not to be human subject research in accordance with United States of America federal regulations, as defined by 45 CFR 46.102(f). This precludes the acquisition of detailed clinical profiles other than sex, age and whether the patient was diabetic or not (see Table S1). Therefore, no exclusions were made due to medication history or presence of comorbidities. Patients were considered diabetic if they had a hemoglobin A-1c level equal to or greater than 6.5% on pre-surgical testing, or if they were on medication for diabetes treatment42. Collected tissue was placed in cold phosphate-buffered saline (PBS) solution containing (in mM): 138 NaCl, 3 KCl, 10 Na2HPO4, 2 NaH2PO4, 5 D-glucose, 0.1 CaCl2, and 0.1 MgSO4, pH 7.4 with NaOH until used.
Vascular Smooth Muscle Cell Isolation
Single vascular smooth muscle cells were dissociated from small diameter adipose arteries from non-diabetic and type 2 diabetic patients using enzymatic digestion as previously described40. Adipose arteries were dissected in ice-cold dissection buffer composed of (in mM): 140 NaCl, 5 KCl, 2 MgCl2, 10 D-glucose, and 10 HEPES, pH 7.4 with NaOH. Following dissection, arteries were cut in small pieces and digested in dissection buffer supplemented with papain (26 U/ml) and dithiothreitol (1 mg/mL) at 37 °C for 15 minutes. After this first incubation period, the solution was exchanged with dissection buffer supplemented with collagenase type H (1.95 U/mL), elastase (0.5 mg/mL) and soybean trypsin Inhibitor (1 mg/ml) at 37 °C for 15 minutes. Cells were then washed in ice-cold dissection buffer three times. Glass pipettes of decreasing diameters were then used to gently triturate arteries and obtain single vascular smooth muscle cells. Isolated cells were maintained in ice-cold dissection buffer until use.
Arterial Diameter Measurements
Freshly dissected small diameter adipose arteries were cannulated on glass micropipettes and mounted in a 5 mL myograph chamber (Living Systems Instrumentation, St. Albans, VT) as described previously9,43,44. The vessels were pressurized to 20 mmHg and allowed to equilibrate while continuously perfused (37 °C, 30 min, 3–5 mL/min) with physiological saline solution (PSS) consisting of (in mM): 119 NaCl, 4.7 KCl, 2 CaCl2, 24 NaHCO3, 1.2 KH2PO4, 1.2 MgSO4, 0.023 ethylenediaminetetraacetic acid (EDTA) and 10 D-glucose aerated with 5% CO2/95% O2, pH 7.35–7.40. Following an equilibration period, arteries were treated with 60 mM KCl (<5 min; isosmotic replacement of NaCl with KCl) to test their viability. Only arteries with ≥50% constriction in response to 60 mM KCl were used for subsequent experiments. Intravascular pressure was increased to 80 mmHg and arteries were allowed to develop myogenic tone. Lumen diameters of the adipose arteries were recorded using IonOptix software (IonOptix LLC, Westwood-MA). To evaluate the impact of BKCa channels on myogenic tone, arteries were treated with 100 nM Iberiotoxin (IBTx) (BKCa channel inhibitor). Percent change in diameter was calculated using the following equation: % myogenic tone = [(DP − DA)/DP] × 100, where DP = passive diameter of the artery in Ca2+-free PSS containing the L-type Ca2+ channel (LTCC) inhibitor nifedipine (10 μM) and DA = active diameter of the artery in Ca2+-containing PSS. Percent constriction in the presence of IBTx or 60 mM K+ was calculated using the following equation: % constriction = [(DP − DT)/DP] × 100, where DT = diameter of the artery in Ca2+-containing PSS with 100 nM IBTx or 60 mM K+ and DP = passive diameter of the artery in Ca2+-free PSS containing 10 μM of the LTCC inhibitor nifedipine.
Electrophysiology
STOCs resulting from the concerted opening of multiple BKCa channels were recorded at different membrane potentials from freshly dissociated vascular smooth muscle from small diameter adipose arteries using the perforated whole-cell configuration of the patch-clamp technique with an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA). Currents were sampled at 10 kHz and low-pass filtered at 2 kHz. The pipette solution consisted of (in mM): 110 K-aspartate, 30 KCl, 10 NaCl, 1 MgCl2, 0.5 EGTA, and 10 HEPES, pH adjusted to 7.3 with KOH. The pipette solution was supplemented with 250 μg/ml of amphotericin B (Sigma, St. Louis, MO). Bath solution consisted of (in mM): 130 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 glucose and 10 HEPES, pH adjusted to 7.4 with NaOH. STOCs were analyzed using the threshold detection algorithm in Clampfit 10 (Axon Instruments, Inc).
Single BKCa channel currents were recorded from inside-out membrane patches obtained from freshly dissociated vascular smooth muscle cells from adipose arteries. In some experiments, HEK293 cells expressing either the BKCa α subunit and EGFP or co-transfected with BKCa α + β1 subunit in a 1:1 mix and EGFP using PolyPlus jetPRIME were used as previously described9. Bath and pipette solutions consisted of (in mM) 140 KCl, 1 HEDTA, 10 HEPES, pH adjusted to 7.3 with Tris. Bath solution was supplemented with CaCl2 to achieve the desired free Ca2+ concentration, as determined with the MaxChelator software. Single-channel currents were amplified, low-pass filtered at 1 kHz, and sampled at 20 kHz with the Axopatch 200B amplifier using a DigiData 1440 A acquisition board and pClamp 10 software (Axon Instruments, Inc). Currents were elicited by holding the inside-out patch at the specified voltage. Data were directly stored in a PC hard drive. The half-amplitude algorithm of Clampfit 10 was used to detect single-channel openings and to analyze data of channel activity (e.g. open probability; Po). The number of BKCa channels per patch was estimated by holding the patch at +80 mV in the presence of 10 μM free Ca2+, which maximizes the Po of these channels45. Open time histograms were fit using a log-normal function:
where A is a constant, τ is the time constant, and σ is the standard deviations of τ. This analysis was validated by using an Akaike’s Information Criterion, which determines the probability that a data set could be described by a particular set of competing models46.
Ca2+ Imaging and Analysis
Freshly dissociated vascular smooth muscle cells from small diameter adipose arteries were loaded with the fluorscent Ca2+ indicator fluo-4 AM (5 μM) and imaged using an Andor spinning disk confocal microscope system coupled to an Olympus iX81 inverted microscope equipped with a 60x oil immersion lens (numerical aperture 1.49). Andor IQ software was used for acquisition. Images were acquired at 100–120 Hz. Analysis was performed using custom software (SparkLab) written in LabVIEW that employs a computer algorithm as previously described9,47.
Proximity Ligation Assay (PLA)
The Duolink in situ PLA detection kit was used to determine colocalization between BKCa α and BKCa β1 subunits as previously described40. Freshly dissociated myocytes were plated on glass coverslips and allowed to sit for 30 minutes at room temperature. Cells were then fixed in 4% paraformaldehyde (20 min) and quenched in 100 mM glycine (15 min), followed by 3 min washes (2x) in a phosphate-buffered saline (PBS) solution containing (in mM) 138 NaCl, 3 KCl, 10 Na2HPO4, 2 NaH2PO4, 5 D-glucose, 0.1 CaCl2 and 0.1 MgSO4, pH adjusted to 7.4 with NaOH. Cells were then permeabilized for 20 minutes in 0.1% Triton-100 solution in PBS and blocked in Duolink Blocking Solution for 1 hour at 37 °C in a humidity chamber. Cells were incubated overnight with a specific combination of primary antibodies. Mouse anti-BKCa α (Antibodies Inc; 75–022 1:200) was combined with rabbit anti-BKCa β1 (Abcam; ab3587; 1:200) antibody. As a positive control, cells were stained with mouse anti-BKCa α (Antibodies Inc; 75-022 1:200) and rabbit anti-BKCa α (Alamone; APC-021; 1:200). For negative controls, cells were incubated with only one primary antibody. Antibodies were diluted in Duolink Antibody Diluent Solution per manufacturer instructions. Secondary antibodies containing PLA probes (anti-mouse minus and anti-rabbit plus) were added to the preparation and allowed to incubate for 1 h at 37 °C. A ligation solution consisting of ligase and two distinct oligonucleotides was then added and incubated for 30 minutes at 37 °C. This step was followed by an amplification reaction (100 min, 37 °C), and subsequent washes (2x) for 10 minutes in Duolink Buffer B and 1 × 1 minute in 1% Buffer B per manufacture’s specifications. Coverslips were mounted on a microscope slide with Doulink mounting media. The fluorescence signal was visualized using an Olympus FV1000 confocal system on an Olympus iX81 microscope with a 60X water immersion lens (numerical aperture = 1.4). Images were acquired at different optical planes (z-axis step size = 0.5 μm).
Immunofluorescence
Immunofluorescence labeling of freshly dissociated vascular smooth muscle from small diameter adipose arteries from non-diabetic and diabetic patients was performed as described previously10,48 using a rabbit anti-BKCa α (Alamone; APC-021; 1:200) and a rabbit anti-BKCa β1 (Abcam; ab3587; 1:200). The secondary antibody was an Alexa Fluor 568-conjugated donkey anti-rabbit (5 mg/mL) from Molecular Probes. Cells were visualized (512 × 512 pixel images) using an Olympus FV1000 confocal microscope coupled with an Olympus X60 water immersion lens (NA = 1.4) and a zoom of 3.5 (pixel size = 0.1 μm). Images were collected at multiple optical planes (z axis step size = 0.25 μm). The specificity of the primary antibody was tested in negative control experiments in which the primary antibody was substituted with PBS. Cells for each group were imaged using the same laser power, gain settings and pinhole for all treatments.
Western Blot Analysis
Whole tissue homogenates were prepared from dissected adipose arteries. In some experiments, protein lysates were obtained from HEK293 cells expressing either the BKCa α or BKCa β1 subunit or cells that have not been transfected. Samples were flash frozen in liquid nitrogen and homogenized on ice with lysis buffer containing (in mM) 150 NaCl, 10 Na2HPO4, 1 EDTA with 1% deoxycholic acid, 0.1% sodium dodecyl sulfate, 40 β-glycerophosphate, 20 Na pyrophosphate, 30 NaF, 1 dithiothreitol and protease inhibitors (Complete Mini protease inhibitor cocktail, Roche, San Francisco, CA) in a glass dounce homogenizer. Whole lysates were sonicated for 1 min, rested on ice for 20 min, and centrifuged (5000 rpm, 4 °C) to yield clarified supernatant. Equal amounts of protein (approximately 10 µg) were separated under reducing conditions on a 4–20% gradient polyacrylamide gel according to the manufacturer’s specifications (Bio-Rad, Hercules, CA). Proteins were transferred to polyvinylidene difluoride (PVDF) or nitrocellulose membranes for Western immunoblotting using enhanced chemiluminescence (ECL) and X-ray film or using an Odyssey scanner (LI-COR; Lincoln, NE, USA) with far red or near-infrared (NIR) labeled secondary antibodies. For the Odyssey scanner, nitrocellulose membranes were blocked with 50% Odyssey blocking buffer (LI-COR; Lincoln, NE, USA) (40 min) in Tris-buffered saline (TBS), then incubated with BKCa subunit specific primary antibodies on a rotating platform for 18–24 h (4 °C). Primary antibodies were rabbit anti-BKCa α (Alamone; APC-021; 1:500), rabbit anti-BKCa β1 (Genetex; GTX105666; 1:5000; or Abcam; ab3587; 1:500), anti-BKCa γ (Alamone; APC-021; 1:500), and mouse anti-β actin (Sigma; AC-40; 1:4000; as a loading control). These antibodies were diluted in TBS with 0.05% tween-20 (t) and 5% Odyssey blocking solution. Membranes were washed 3 times in TBS-t, then incubated (2 h, room temperature) with appropriate secondary antibodies: goat anti-rabbit 680LT (LI-COR; 925-68021; 1:15,000; with 0.1% SDS), 800CW (LI-COR; 925-32211; 1:15,000; for dual staining) or goat anti-mouse 680LT (LI-COR; 925-68020 1:10,000) in TBS-t. For the far red or NIR dyes, band images were acquired using an Odyssey scanner. In some experiments, detection of BKCa α was acquired by X-ray film. PVDF membranes were blocked with 10% nonfat milk for 1 hour, incubated in rabbit rabbit anti-BKCa α (Alomone, 1:500) for 2 hours, washed 3 times in TBS-t, incubated in HRP labeled goat anti-mouse (sc-2005; 1:5000; Santa Cruz), then washed 3 times in TBS-t. Bands were identified by ECL and exposure to X-ray film, followed by conventional scanning. Densitometry for immunoreactive bands was performed with ImageJ software (National Institutes of Health). Band quantification was normalized to β actin, and expressed as a percentage of control.
Chemicals and Statistics
All chemical reagents were from Sigma-Aldrich (St. Louis, MO) unless otherwise stated. Iberiotoxin was from Peptides International (Louisville, KY). Data were analyzed using GraphPad Prism software and expressed as mean ± SEM. Data were assessed for potential outliers using the GraphPad Prism Outlier Test and for normality of distribution using the Shapiro-Wilk or KS normality tests. Statistical significance was then determined using appropriate paired or unpaired Student’s t-test, nonparametric tests or One-way analysis of variance (ANOVA) for multiple comparisons with appropriate post hoc test. P < 0.05 was considered statistically significant (denoted by * in figures).
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author in a reasonable request.
Electronic supplementary material
Acknowledgements
This work was supported by NIH grants R01HL098200 and R01HL121059 and AHA grant 14GRNT18730054 (to MFN), NIH grant T32HL086350 (to AUS and MAN), AHA 16SDG27260070 (to MAN), NIH grant R01DK057236 (to SMW), NIH grant R01HL085870 (to LFS), NIH grants R01MH097887, R01AG055357 and R01NS078792 (to JWH) and a UC Davis Academic Federation Innovative Development Award (to MN-C).
Author Contributions
M.N.-C. and M.F.N. conceived, designed, and executed experiments; collected, analyzed and interpreted data; and wrote and revised the manuscript. A.U.S., O.R.B., R.R.R., M.A.N. and D.G. executed experiments, collected, and analyzed data, and revised the manuscript. K.C.S. and S.M.W. contributed human samples critical to this work, information about patients, and revised the manuscript. L.F.S. and J.W.H. designed experiments, interpreted results and revised the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-14565-9.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Madeline Nieves-Cintrón, Email: mcnieves@ucdavis.edu.
Manuel F. Navedo, Email: mfnavedo@ucdavis.edu
References
- 1.WHO. http://www.who.int/mediacentre/factsheets/fs312/en/index.html (2011).
- 2.Cooper ME, Bonnet F, Oldfield M, Jandeleit-Dahm K. Mechanisms of diabetic vasculopathy: an overview. Am J Hypertens. 2001;14:475–486. doi: 10.1016/S0895-7061(00)01323-6. [DOI] [PubMed] [Google Scholar]
- 3.Sonoyama K, Greenstein A, Price A, Khavandi K, Heagerty T. Vascular remodeling: implications for small artery function and target organ damage. Therapeutic advances in cardiovascular disease. 2007;1:129–137. doi: 10.1177/1753944707086358. [DOI] [PubMed] [Google Scholar]
- 4.Aronson D. Hyperglycemia and the pathobiology of diabetic complications. Adv Cardiol. 2008;45:1–16. doi: 10.1159/000115118. [DOI] [PubMed] [Google Scholar]
- 5.Brown A, Reynolds LR, Bruemmer D. Intensive glycemic control and cardiovascular disease: an update. Nature reviews. Cardiology. 2010;7:369–375. doi: 10.1038/nrcardio.2010.35. [DOI] [PubMed] [Google Scholar]
- 6.Schofield I, Malik R, Izzard A, Austin C, Heagerty A. Vascular structural and functional changes in type 2 diabetes mellitus: evidence for the roles of abnormal myogenic responsiveness and dyslipidemia. Circulation. 2002;106:3037–3043. doi: 10.1161/01.CIR.0000041432.80615.A5. [DOI] [PubMed] [Google Scholar]
- 7.Williams SB, Cusco JA, Roddy MA, Johnstone MT, Creager MA. Impaired nitric oxide-mediated vasodilation in patients with non-insulin-dependent diabetes mellitus. Journal of the American College of Cardiology. 1996;27:567–574. doi: 10.1016/0735-1097(95)00522-6. [DOI] [PubMed] [Google Scholar]
- 8.Montero D, et al. Vascular smooth muscle function in type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetologia. 2013;56:2122–2133. doi: 10.1007/s00125-013-2974-1. [DOI] [PubMed] [Google Scholar]
- 9.Nystoriak MA, et al. AKAP150 Contributes to Enhanced Vascular Tone by Facilitating Large-Conductance Ca2+-Activated K+ Channel Remodeling in Hyperglycemia and Diabetes Mellitus. Circ Res. 2014;114:607–615. doi: 10.1161/CIRCRESAHA.114.302168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nieves-Cintron M, et al. Selective downregulation of Kv2.1 function contributes to enhanced arterial tone during diabetes. Journal of Biological Chemistry. 2015;290:7918–7929. doi: 10.1074/jbc.M114.622811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Navedo MF, Takeda Y, Nieves-Cintron M, Molkentin JD, Santana LF. Elevated Ca2+ sparklet activity during acute hyperglycemia and diabetes in cerebral arterial smooth muscle cells. American journal of physiology. Cell physiology. 2010;298:C211–220. doi: 10.1152/ajpcell.00267.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jackson WF. Ion channels and vascular tone. Hypertension. 2000;35:173–178. doi: 10.1161/01.HYP.35.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jaggar JH, et al. Ca2+ channels, ryanodine receptors and Ca2+-activated K+ channels: a functional unit for regulating arterial tone. Acta Physiol Scand. 1998;164:577–587. doi: 10.1046/j.1365-201X.1998.00462.x. [DOI] [PubMed] [Google Scholar]
- 14.Amberg GC, Navedo MF. Calcium dynamics in vascular smooth muscle. Microcirculation. 2013;20:281–289. doi: 10.1111/micc.12046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nelson MT, et al. Relaxation of arterial smooth muscle by calcium sparks. Science. 1995;270:633–637. doi: 10.1126/science.270.5236.633. [DOI] [PubMed] [Google Scholar]
- 16.Patterson AJ, Henrie-Olson J, Brenner R. Vasoregulation at the molecular level: a role for the beta1 subunit of the calcium-activated potassium (BK) channel. Trends Cardiovasc Med. 2002;12:78–82. doi: 10.1016/S1050-1738(01)00146-3. [DOI] [PubMed] [Google Scholar]
- 17.Brenner R, et al. Vasoregulation by the beta1 subunit of the calcium-activated potassium channel. Nature. 2000;407:870–876. doi: 10.1038/35038011. [DOI] [PubMed] [Google Scholar]
- 18.Nieves-Cintrón M, Amberg GC, Nichols CB, Molkentin JD, Santana LF. Activation of NFATc3 Down-regulates the β1 Subunit of Large Conductance, Calcium-activated K+ Channels in Arterial Smooth Muscle and Contributes to Hypertension. J Biol Chem. 2007;282:3231–3240. doi: 10.1074/jbc.M608822200. [DOI] [PubMed] [Google Scholar]
- 19.Amberg GC, Santana LF. Downregulation of the BK channel b1 subunit in genetic hypertension. Circ Res. 2003;93:965–971. doi: 10.1161/01.RES.0000100068.43006.36. [DOI] [PubMed] [Google Scholar]
- 20.Amberg GC, Bonev AD, Rossow CF, Nelson MT, Santana LF. Modulation of the molecular composition of large conductance, Ca2+ activated K+ channels in vascular smooth muscle during hypertension. J Clin Invest. 2003;112:717–724. doi: 10.1172/JCI200318684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dong L, et al. Functional and molecular evidence for impairment of calcium-activated potassium channels in type-1 diabetic cerebral artery smooth muscle cells. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2008;28:377–386. doi: 10.1038/sj.jcbfm.9600536. [DOI] [PubMed] [Google Scholar]
- 22.Rueda A, Fernandez-Velasco M, Benitah JP, Gomez AM. Abnormal Ca2+ spark/STOC coupling in cerebral artery smooth muscle cells of obese type 2 diabetic mice. PLoS One. 2013;8:e53321. doi: 10.1371/journal.pone.0053321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu T, et al. Impaired Ca2+ -dependent activation of large-conductance Ca2+ -activated K+ channels in the coronary artery smooth muscle cells of Zucker Diabetic Fatty rats. Biophys J. 2008;95:5165–5177. doi: 10.1529/biophysj.108.138339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang Y, et al. Function of BKCa channels is reduced in human vascular smooth muscle cells from Han Chinese patients with hypertension. Hypertension. 2013;61:519–525. doi: 10.1161/HYPERTENSIONAHA.111.00211. [DOI] [PubMed] [Google Scholar]
- 25.McGahon MK, et al. Diabetes downregulates large-conductance Ca2+ -activated potassium beta 1 channel subunit in retinal arteriolar smooth muscle. Circ Res. 2007;100:703–711. doi: 10.1161/01.RES.0000260182.36481.c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bolton TB, Imaizumi Y. Spontaneous transient outward currents in smooth muscle cells. Cell Calcium. 1996;20:141–152. doi: 10.1016/S0143-4160(96)90103-7. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka Y, Meera P, Song M, Knaus HG, Toro L. Molecular constituents of maxi KCa channels in human coronary smooth muscle: predominant alpha + beta subunit complexes. J Physiol. 1997;502(Pt 3):545–557. doi: 10.1111/j.1469-7793.1997.545bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dick GM, Sanders KM. (Xeno)estrogen sensitivity of smooth muscle BK channels conferred by the regulatory beta1 subunit: a study of beta1 knockout mice. J Biol Chem. 2001;276:44835–44840. doi: 10.1074/jbc.M106851200. [DOI] [PubMed] [Google Scholar]
- 29.Detweiler ND, et al. BK channels in rat and human pulmonary smooth muscle cells are BKalpha-beta1 functional complexes lacking the oxygen-sensitive stress axis regulated exon insert. Pulmonary circulation. 2016;6:563–575. doi: 10.1086/688838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fredriksson S, et al. Protein detection using proximity-dependent DNA ligation assays. Nature biotechnology. 2002;20:473–477. doi: 10.1038/nbt0502-473. [DOI] [PubMed] [Google Scholar]
- 31.Wellman GC, et al. Ca2+ sparks and their function in human cerebral arteries. Stroke. 2002;33:802–808. doi: 10.1161/hs0302.104089. [DOI] [PubMed] [Google Scholar]
- 32.Hempelmann RG, Seebeck J, Ziegler A, Mehdorn HM. Effects of potassium channel inhibitors on the relaxation induced by the nitric oxide donor diethylamine nitric oxide in isolated human cerebral arteries. Journal of neurosurgery. 2000;93:1048–1054. doi: 10.3171/jns.2000.93.6.1048. [DOI] [PubMed] [Google Scholar]
- 33.Gokina NI, et al. Role of Ca(2+)-activated K+ channels in the regulation of membrane potential and tone of smooth muscle in human pial arteries. Circ Res. 1996;79:881–886. doi: 10.1161/01.RES.79.4.881. [DOI] [PubMed] [Google Scholar]
- 34.Howitt L, et al. Differential effects of diet-induced obesity on BKCa {beta}1-subunit expression and function in rat skeletal muscle arterioles and small cerebral arteries. Am J Physiol Heart Circ Physiol. 2011;301:H29–40. doi: 10.1152/ajpheart.00134.2011. [DOI] [PubMed] [Google Scholar]
- 35.Xu H, et al. BK channel beta1-subunit deficiency exacerbates vascular fibrosis and remodelling but does not promote hypertension in high-fat fed obesity in mice. Journal of hypertension. 2015;33:1611–1623. doi: 10.1097/HJH.0000000000000590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.ZhuGe R, et al. Dynamics of signaling between Ca(2+) sparks and Ca(2+)- activated K(+) channels studied with a novel image-based method for direct intracellular measurement of ryanodine receptor Ca(2+) current. J Gen Physiol. 2000;116:845–864. doi: 10.1085/jgp.116.6.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leo MD, et al. Dynamic regulation of beta1 subunit trafficking controls vascular contractility. Proc Natl Acad Sci USA. 2014;111:2361–2366. doi: 10.1073/pnas.1317527111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leo MD, et al. Angiotensin II stimulates internalization and degradation of arterial myocyte plasma membrane BK channels to induce vasoconstriction. Am J Physiol Cell Physiol. 2015;309:C392–402. doi: 10.1152/ajpcell.00127.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nishijima Y, et al. Contribution of KV1.5 Channel to Hydrogen Peroxide-Induced Human Arteriolar Dilation and Its Modulation by Coronary Artery Disease. Circ Res. 2017;120:658–669. doi: 10.1161/CIRCRESAHA.116.309491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nystoriak, M. A. et al. Ser1928 phosphorylation by PKA stimulates L-type Ca2+ channel Cav1.2 and vasoconstriction during acute hyperglycemia and diabetes. Science signaling10 (2017). [DOI] [PMC free article] [PubMed]
- 41.Morotti, S., Nieves-Cintrón, M., Nystoriak, M. A., Navedo, M. F. & Grandi, E. Predominant contribution of L-type Cav1.2 channel stimulation to impaired intracellular calcium and cerebral artery vasoconstriction in diabetic hyperglycemia. Channels, 1–7 (2017). [DOI] [PMC free article] [PubMed]
- 42.Association AD. Standards of Medical Care in Diabetes - 2017. Diabetes Care. 2017;40:S1–S135. doi: 10.2337/dc17-S001. [DOI] [PubMed] [Google Scholar]
- 43.Amberg GC, Navedo MF, Nieves-Cintrón M, Molkentin JD, Santana LF. Calcium Sparklets Regulate Local and Global Calcium in Murine Arterial Smooth Muscle. J Physiol. 2007;579:187–201. doi: 10.1113/jphysiol.2006.124420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nieves-Cintron M, Amberg GC, Navedo MF, Molkentin JD, Santana LF. The control of Ca2+ influx and NFATc3 signaling in arterial smooth muscle during hypertension. Proc Natl Acad Sci USA. 2008;105:15623–15628. doi: 10.1073/pnas.0808759105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meera P, Wallner M, Jiang Z, Toro L. A calcium switch for the functional coupling between alpha (hslo) and beta subunits (Kv,Caβ) of maxi K channels. FEBS Lett. 1996;385:127–128. doi: 10.1016/0014-5793(96)83884-1. [DOI] [PubMed] [Google Scholar]
- 46.Akaike H. A new look at the statistical model identification. IEEE Trans. Autom. Control. 1974;AC19:716–723. doi: 10.1109/TAC.1974.1100705. [DOI] [Google Scholar]
- 47.Banyasz T, Chen-Izu Y, Balke CW, Izu LT. A new approach to the detection and statistical classification of Ca2+ sparks. Biophys J. 2007;92:4458–4465. doi: 10.1529/biophysj.106.103069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Navedo MF, et al. Cav1.3 channels produce persistent calcium sparklets, but Cav1.2 channels are responsible for sparklets in mouse arterial smooth muscle. Am J Physiol Heart Circ Physiol. 2007;293:H1359–1370. doi: 10.1152/ajpheart.00450.2007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author in a reasonable request.