Abstract
Melanopsin (OPN4) is a photo-pigment found in a small subset of intrinsically photosensitive ganglion cells (ipRGCs) of the mammalian retina. These cells play a role in synchronizing the central circadian pacemaker to the astronomical day by conveying information about ambient light to the hypothalamic suprachiasmatic nucleus, the site of the master clock. We evaluated the effect of a heat stimulus (39.5 °C) on clock gene (Per1 and Bmal1) expression in cultured murine Melan-a melanocytes synchronized by medium changes, and in B16-F10 melanoma cells, in the presence of the selective OPN4 antagonist AA92593, or after OPN4 knockdown by small interfering RNA (siRNA). In addition, we evaluated the effects of heat shock on the localization of melanopsin by immunocytochemistry. In both cell lines melanopsin was found in a region capping the nucleus and heat shock did not affect its location. The heat-induced increase of Per1 expression was inhibited when melanopsin was pharmacologically blocked by AA92593 as well as when its protein expression was suppressed by siRNA in both Melan-a and B16-F10 cells. These data strongly suggest that melanopsin is required for thermo-reception, acting as a thermo-opsin that ultimately feeds the local circadian clock in mouse melanocytes and melanoma cells.
Introduction
The canonical role of melanopsin (OPN4) is to act as a photo-pigment in the mammalian intrinsically photosensitive retinal ganglion cells (ipRGCs)1. These cells play a role in synchronizing the central circadian pacemaker2 to the astronomical day by conveying information about ambient light to the hypothalamic suprachiasmatic nucleus (SCN), the site of the master clock3. Another function of melanopsin that was recently described is its participation in early visual system formation4. Upon photo-activation of mammalian ipRGCs, OPN4 triggers a signaling cascade leading to phospholipase C activation and subsequent opening of transient potential receptor channels, TRPC6/7, which ultimately leads to membrane depolarization5.
ipRGC axons release glutamate at the SCN neurons, increasing Per transcripts which reset the clock gene machinery. The biological mechanism of keeping track of time takes place through positive and negative interlaced feedback loops (reviewed in6). In summary, CLOCK and BMAL1 form a heterodimer that activates Per and Cry genes. PER and CRY proteins dimerize and after phosphorylation by casein kinases, are targeted to the nucleus, inhibiting the action of CLOCK/BMAL1. Once PER/CRY heterodimers are degraded, their inhibitory effect is reduced, and then CLOCK/BMAL1 is freed to start a new cycle of transcription. The core of clock gene machinery, described above, is stabilized by Rev-Erbα/β and RORα/β, whose transcripts are induced by CLOCK/BMAL1; Rev-Erbα/β inhibits while RORα/β activates Bmal1 6.
A local temporal controlling machinery has been found in almost every organ tested, comprising a multi-oscillatory system. These peripheral clocks are under the SCN control, which ensures that the whole organism is orchestrated in a single timing zone, allowing a harmonic working relationship among organs and systems6–8.
Interestingly, rhodopsin (OPN2), classically associated with image formation in arthropods and vertebrates9, has been demonstrated to participate in Drosophila temperature sensing. Drosophila larvae lacking rhodopsin lose the ability of thermo-discrimination10–12, which can be rescued by the targeted expression of mouse melanopsin10. In addition, melanopsin has been recently reported in murine blood vessels13, in which it mediates blue-light dependent photo-relaxation. Since the vascular physiology is under circadian control14, one may suggest that melanopsin could act as sensor that ultimately feeds the local temporal controlling system. Following this line, our group has shown that melanopsin and rhodopsin are expressed in murine melanocytes and melanoma cells where they may participate in a photo-sensitive system15. Based on these findings, we questioned whether an opsin could also function as a thermo-sensor in mammalian cells, conveying temperature information to the clock gene machinery of cutaneous melanocytes, cells known to be exposed to cycles of environmental light and temperature16.
Results and Discussion
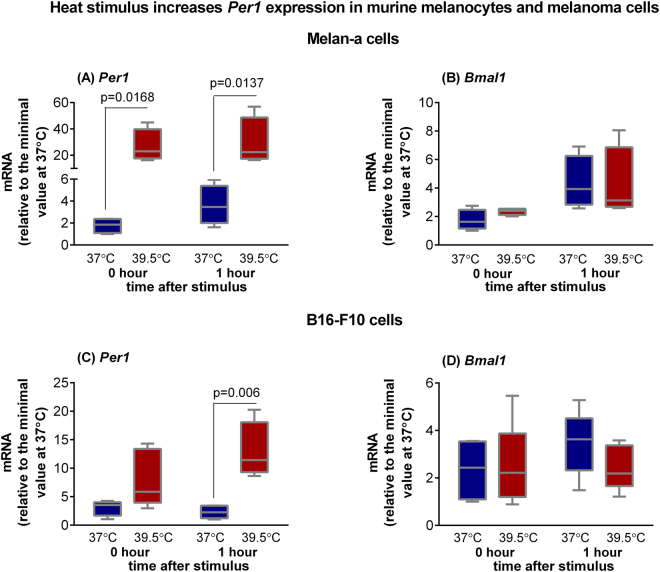
We evaluated the effect of a heat stimulus (39.5 °C) on clock gene expression in cultured Melan-a melanocytes and B16-F10 melanoma cells. Cells were maintained for three days under constant darkness and temperature (37 °C), a situation in which each cell displays its own rhythm of clock gene expression, usually leading to undetectable rhythm of the cell culture17. Melan-a cells exposed to 1 h heat pulse (39.5 °C) showed no difference from control cells in Per1, and Bmal1 expression 0, 1 and 2 h after the stimulus (Supplemental Fig. 1). Because no effect was found in non-synchronized melanocytes, our next step was to repeat the same assay in cells synchronized by two medium changes18,19. In fact, 24 h after cell synchronization, heat shock led to increased Per1 (0 and 1 h after the stimulus, Fig. 1A) but not Bmal1 (Fig. 1B) expression. On the other hand, heat shock induced Per1 increase in B16-F10 cells in constant dark condition 1 h after the end of the stimulus (Fig. 1C). Again, Bmal1 was irresponsive to heat shock (Fig. 1D).
Figure 1.
Expression of Per1 (A and C) and Bmal1 (B and D) in murine Melan-a melanocytes and B16-F10 melanoma cells after heat stimulus (39.5 °C). Melan-a or B16-F10 cells were kept for 3 days in constant dark and temperature (37 °C). In the beginning of the 4th day, Melan-a cells were synchronized by two medium changes and after further 24 hours they were heat-stimulated (39.5 °C) during 1 h. B16-F10 cells were heat stimulated in the beginning of the 4th day. Total RNA was extracted immediately and 1 h after the end of the stimulus for Melan-a and B16-F10 cells. Boxplots show the median, quartiles, maximum, and minimum expression values of each gene transcript normalized by 18S ribosomal RNA (for Melan-a cells) and Rpl37a (for B16-F10 cells), and expressed relative to the minimal value at 37 °C (N = 4–6). Statistical analysis was performed by Two-way ANOVA followed by Bonferroni post-test.
In agreement with these findings a 15-min white light pulse (WLP) applied to desynchronized Melan-a cells did not alter clock gene expression in comparison to cells kept in constant dark (DD) condition. Interestingly, in B16-F10 cells the expression of Per1, Per2, and Bmal1 was upregulated in response to WLP15. Although the effects of heat shock or light pulse were not observed in desynchronized Melan-a cells, we cannot rule out that this stimulus may have affected the clock machinery of single cells, which would remain uncoupled to each other, and therefore, the overall oscillation would be unnoticed in the cell population. Another point of view is related to morpho-physiological differences between normal and malignant melanocytes, the latter showing denser dendritic projections among cells, a feature that probably allows more efficient cellular coupling15.
Temperature cycles have been shown to alter rhythmic parameters of clock genes in peripheral tissues20, and are a strong zeitgeber in normal murine keratinocytes21 and fibroblasts22. To our knowledge, this is the first report that a short heat pulse affects clock gene machinery in murine melanocytes and melanoma cells. In fact, in non-mammalian vertebrates, we have already shown that heat shock increases the expression of clock genes in the photosensitive teleost ZEM-2S cell line, but only when cells were synchronized by light/dark (LD) cycles23.
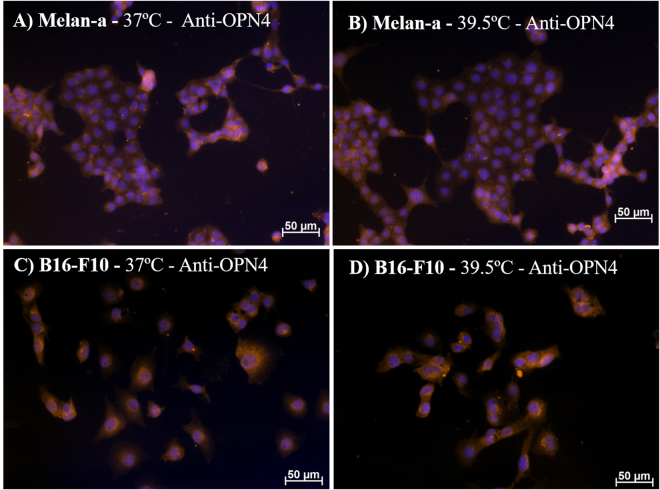
Previous studies using Melan-a and B16-F10 cells15 and human and mouse spermatozoa24 showed immunolabeling of melanopsin in regions capping the nucleus. We have shown that a 15-min WLP promoted OPN4 translocation from nucleus region to the cytoplasm and cell membrane in B16-F10 cells 24 h after the stimulus15. Based on these findings, we investigated whether heat shock was also capable of inducing melanopsin translocation in Melan-a and B16-F10 cells. Our results demonstrate that the cytoplasm and the nucleus capping location of OPN4 in both cell lines was not altered 24 h after the heat shock of 39.5 °C (Fig. 2A–D), suggesting that OPN4 is not required to be inserted into the membrane to detect heat stimulus. In another line of thought, one may consider that the basal level of membrane-bound OPN4 may be enough to detect heat and trigger heat-induced responses in murine melanocytes and melanoma cells.
Figure 2.
Representative fields of melanopsin (OPN4) immunostaining in Melan-a (A,B) and B16-F10 (C,D) cells. Cells were kept in DD for 3 days and at the beginning of the 4th day, cells were divided into 2 groups: (1) Control group kept in constant dark and temperature (37 °C); (2) group in constant dark and exposed to 1 h heat stimulus (39.5 °C). Twenty-four hours later the medium was removed and the cells were fixed with 4% paraformaldehyde. DAPI stained nuclei in blue and OPN4 immunopositivity (1:500 antiserum), revealed with a Cy3-labeled secondary antibody, in orange. Photomicrographies were taken with Axiocam MRm camera (Zeiss) and pseudocolored with Axiovision software (Zeiss). Scale bar 50 μm (200x magnification).
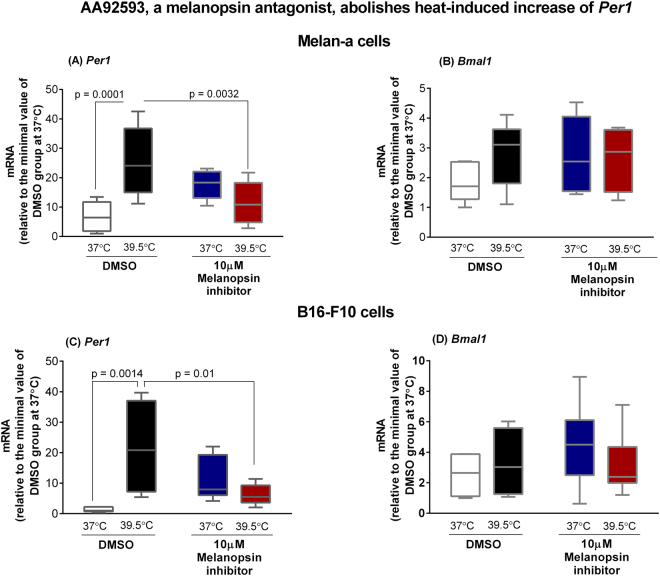
We then pharmacologically inhibited melanopsin with the antagonist AA92593, shown to be specific because it competes with retinaldehyde for the melanopsin retinal binding site which is very distinct from other opsins. Its administration to mice in vivo specifically and reversibly modified melanopsin-dependent light responses including the pupillary light reflex and light aversion25. Our data show that the increase of Per1 level induced by heat in Melan-a and B16-F10 cells was significantly reduced in the presence of the melanopsin antagonist (Fig. 3A,C) whereas Bmal1 expression was not affected (Fig. 3B,D). Surprisingly, in Melan-a cells the group incubated with the antagonist and kept at 37 °C showed a statistically significant increase of Per1 transcript when compared to DMSO-treated control group (p = 0.0478, Fig. 3A); a phenomenon that showed no statistical significance (p = 0.08) in B16-F10 cells. The apparent intrinsic effect of AA92593 on control cells kept at 37 °C could be due to the following reasons: I) melanopsin acts as a thermosensor, and when inhibited, the cell would lose its ability to sense temperature, and the response would resemble the heat-evoked behavior; II) another possibility lies on a partial agonistic activity of AA92593 which, although not reported by Jones and co-workers25, could explain the response found in the control group treated with AA92593; III) since AA92593 is a competitive melanopsin antagonist, its presence in the retinal-binding pocket of melanopsin leads to the displacement of retinal, which could trigger a downstream signaling that would ultimately result in Per1 increased expression.
Figure 3.
Expression of Per1 (A and C) and Bmal1 (B and D) in murine Melan-a melanocytes and B16-F10 melanoma cells after heat stimulus (39.5 °C) in the presence of AA92593. Melan-a or B16-F10 cells were kept for 3 days in constant dark and temperature (37 °C). In the beginning of the 4th day, Melan-a cells were synchronized by two medium changes, and after further 24 hours cells were heat-stimulated. For B16-F10 cells, the heat shock (39.5 °C) was applied at the beginning of the 4th day. In both scenarios, cells were divided into four groups: (1) control group at 37 °C in the presence of DMSO (0.1%); (2) heat-stimulated (39.5 °C) group in the presence of DMSO (0.1%); (3) group kept at 37 °C in the presence of AA92593 (10 µM), a selective OPN4 antagonist; (4) heat-stimulated (39.5 °C) group in the presence of AA92593 (10 µM). Total RNA was extracted immediately and 1 h after the end of the stimulus for Melan-a and B16-F10 cells, respectively. Boxplots show the median, quartiles, maximum, and minimum expression values of each gene transcript normalized by Rpl 37a and expressed relative to the minimal value of DMSO group at 37 °C (N = 5–9). Statistical analysis was performed by One-way ANOVA followed by Tukey’s test.
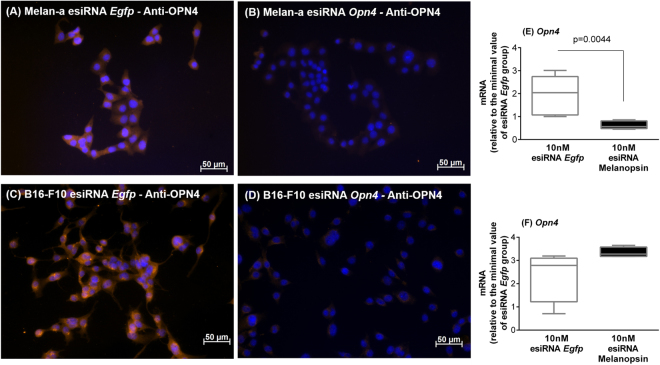
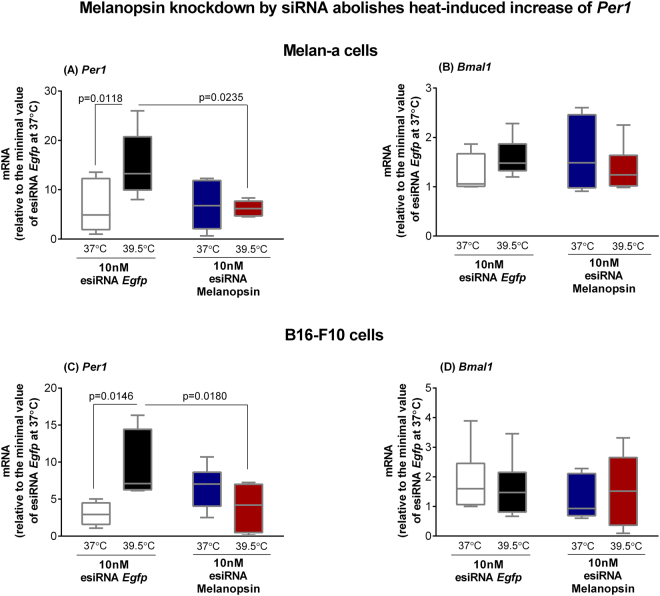
Next, we performed gene knockdown assays using the endoribonuclease-prepared siRNAs (esiRNA), which consist of a heterogeneous mixture of siRNAs against murine melanopsin mRNA. These siRNAs selectively suppress gene expression with low off-target effects26. In fact, we show a significant reduction of Opn4 transcription as well as melanopsin protein level 48 h after transfection in Melan-a cells (Fig. 4A,B,E). In B16-F10 cells, although the mRNA levels of melanopsin did not decrease 48 h after transfection (Fig. 4F), a fact that could be due to a faster mRNA turnover, OPN4 protein level was drastically reduced (Fig. 4C,D). Then, our next step was to heat shock Melan-a and B16-F10 cells with reduced melanopsin expression, and the results demonstrated that the heat-induced increase of Per1 was significantly reduced in both cell lines (Fig. 5A,C) while no effect on Bmal1 expression was found (Fig. 5B,D). We have demonstrated that an opsin in a skin cell type can be activated not only by photons15 but also by thermal energy. In fact, thermo-isomerization of rhodopsin and cone opsins has been shown to occur and it requires about half the energy necessary to photo-activate the photo-pigment27. Therefore, taken together our results clearly demonstrate– for the first time – the involvement of melanopsin in thermo-responses of mammalian cells.
Figure 4.
Representative fields of melanopsin (OPN4) immunostaining in Melan-a (A,B) and B16-F10 (C,D) cells. Cells were kept in DD for 24 hours, on the 2nd day, were transfected with esiRNA against melanopsin or EGFP (both at 10 nM), and 48 h after transfection the cells were immunostained for melanopsin (OPN4). (A and C) esiRNA against mRNA of EGFP transfected cells (control group) and (B and D) esiRNA against mRNA of OPN4 transfected cells. Photo-micrographies were obtained with 200 x magnification in an inverted fluorescence microscope Axiovert 40CFL (Zeiss, Oberkochen, Germany) with a mercury lamp of 50 W, and DAPI (excitation 358 and emission 463 nm) and Cy3 (excitation 549 and emission 562 nm) filters. Melanopsin gene and protein knockdown by endoribonuclease-prepared siRNAs (esiRNA). Gene expression of Opn4 (melanopsin encoding gene) in esiRNA against mRNA of EGFP (control group) or of melanopsin transfected cells. Melan-a (E) or B16-F10 (F) cells were kept during three days in constant dark and temperature (37 °C). At the beginning of the 4th day, Melan-a cells were synchronized by two medium changes, and after further 24 hours they were transfected with esiRNA. B16-F10 cells were transfected at the beginning of the 4th day. In both cases, gene expression was evaluated 48 h after transfection with esiRNA. Boxplots show the median, quartiles, maximum, and minimum expression values of each gene transcript normalized by Rpl 37a and expressed relative to the minimal value of the esiRNA EGFP group kept at 37 °C (N = 5–6). Statistical analysis was performed by Student’s t test.
Figure 5.
Expression of Per1 (A and C) and Bmal1 (B and D) in esiRNA transfected murine Melan-a melanocytes and B16-F10 melanoma cells after heat stimulus (39.5 °C). Melan-a or B16-F10 cells were kept during three days in constant dark and temperature (37 °C). At the beginning of the 4th day, Melan-a cells were synchronized by two medium changes, and after further 24 h cells were transfected with esiRNA against melanopsin or EGFP (both at 10 nM) using Lipofectamine 3000 transfection kit. B16-F10 cells were transfected with esiRNA, as described above, at the beginning of the 4th day. In both experimental scenarios, 48 hours after transfection, cells were divided into four groups: (1) control group at 37 °C in the presence of esiRNA EGFP (10 nM); (2) heat-stimulated (39.5 °C) group in the presence of esiRNA EGFP (10 nM); (3) group at 37 °C in the presence of esiRNA melanopsin (10 nM); (4) heat-stimulated (39.5 °C) group in the presence of esiRNA melanopsin (10 nM). Boxplots show the median, quartiles, maximum, and minimum expression values of each gene transcript normalized by Rpl 37a and expressed relative to the minimal value of the esiRNA EGFP group at 37 °C (N = 5–11). Total RNA was extracted immediately and 1 h after the end of the stimulus for Melan-a and B16-F10 cells, respectively. Statistical analysis was performed by One-way ANOVA followed by Tukey’s test.
According to Colin Pittendrigh Escape from Light theory28, higher temperatures are found during the photo-phase of the day and, therefore, temperature and light are environmental entities exerting simultaneous selective pressures on the organisms. It is not surprising, therefore, that light and temperature may be perceived by the same conserved proteins, having in mind that photo- and thermo-sensitive systems probably co-evolved during evolution. Considering that the skin is constantly exposed to both physical stimuli16, it is relevant to better understand how the skin perceives heat and light. In fact, our data add another layer of complexity for this system: an opsin, which is classically a light sensor, also acts as a thermo-sensor that ultimately feeds the local circadian clock. Interestingly, the opsin-mediated clock gene activation is conserved in malignant melanocytes, which warrants further investigation whether this event was pro- or anti-tumorigenic. Within this line, it has been recently shown that clock gene machinery of melanoma cells is suppressed15,29, but clock gene activation by dexamethasone, forskolin, or heat shock results in reduced melanoma proliferation in vitro and in vivo without leading to cell death29. Taken altogether, our data bring convincing evidence of a new role for a canonical mammalian light sensor, which challenges the current paradigm that mammalian opsins exclusively function as photo-pigments.
Material and Methods
Cell Culture
Immortalized murine Melan-a melanocytes and B16-F10 malignant melanocytes were cultured in RPMI 1640 medium without phenol red (Atená, Campinas, SP, Brazil), supplemented with 14.3 mM NaHCO3, 15 mM HEPES, 10% fetal bovine serum (FBS) (Atená, Campinas, SP, Brazil), 1% antibiotic/antimycotic solution (10,000 U/mL penicillin, 10,000 μg/mL streptomycin, and 25 μg/mL amphotericin B, ThermoFisher, Waltham, MA, USA), and 100 nM of all-trans retinal (Sigma-Aldrich, St. Louis, MO, USA). Phorbol 12-myristate 13-acetate (TPA, Sigma-Aldrich, St. Louis, MO, USA) at 200 nM was added to Melan-a medium, since it is required to maintain cell viability in culture30. The pH was adjusted to 7.2, and the cells were kept at constant temperature (37 °C) with 5% CO2. Previous cell maintenance and experiment set up were carried out under ambient lighting.
Experimental Design
In all experiments, Melan-a and B16-F10 cells were maintained in the medium described above but FBS was reduced to 2% (experimental medium). Cell manipulation during the experiments was carried out under red dim light (7 W Konex bulb and Safe-Light filter GBX-2, Kodak, Rochester, NY, USA). For all protocols described below, Melan-a and B16-F10 cells were kept in constant dark and temperature (37 °C) during 3 days. At the beginning of the 4th day the medium of Melan-a cells was changed twice with a 2-hour interval, after which the cells were kept in DD during further 24 h. This procedure has been shown to synchronize clock genes in this cell line31.
Effect of Heat Stimulus on Clock Gene Machinery of Melan-a and B16-F10 Cells
Melan-a or B16-F10 cells were seeded at the density of 106 and 105 cells respectively in 25 cm2 flasks. In the beginning of the 4th or 5th day (24 h after medium changes), B16-F10 and Melan-a cells were, respectively, heat-stimulated (39.5 °C) during 1 h while the control group remained at 37 °C. Total RNA was initially extracted immediately, 1 and 2 h after the end of the heat stimulus, and the time points showing maximal response of clock gene expression were adopted for subsequent assays.
Effect of Heat Stimulus on OPN4 Localization in Melan-a and B16-F10 Cells
Immunocytochemistry assays were performed as previously described15. A peptide comprised by the 15 N-terminal amino acid sequence of mouse melanopsin (Genbank accession NP_038915) with an appended C-terminal cysteine (MDSPSGPRVLSSLTQC) (Uniformed Services University of the Health Biomedical Instrumentation Center, Bethesda, MD, USA) was conjugated to keyhole limpet hemocyanin and used to immunize rabbits (Covance Labs, Denver, PA, USA). The antisera were used with no further purification1. Previous studies showed that increasing the concentration of the antigenic peptide led to the loss of immunoreactivity by pre-absorption in a dose-dependent manner, and that retinas of OPN4 knockout mice showed lack of immunoreactivity32.
Melan-a or B16-F10 cells were seeded (104/well) into 8-chamber slides in the experimental medium as described above. The cells were kept in DD at 37 °C for 3 days and at the beginning of the 4th day they were divided into 2 groups: the control remained in DD at 37 °C while the experimental group was exposed to 1 h of heat stimulus (39.5 °C). Twenty-four hours later the medium was removed and the cells were fixed in 4% paraformaldehyde as described below.
To verify the effectiveness of the Opn4 silencing Melan-a or B16-F10 cells were seeded (104/well) into 8-chamber slides and kept in DD for 24 hours. On the 2nd day, the cells were transfected with esiRNA, and immunostaining of OPN4 was performed 48 h after transfection. The cells were incubated in the primary antibody anti-melanopsin (1:500, Covance Laboratories, Denver, PA, USA) overnight at 4 °C. A Cy3-labeled anti-rabbit secondary antibody (1:500, Jackson Immunolab, West Grove, PA, USA) was applied for 1 h at room temperature. Photo-micrographies were obtained with 200 x magnification in an inverted fluorescence microscope Axiovert 40CFL (Zeiss, Oberkochen, Germany) with a mercury lamp of 50 W, and DAPI (excitation 358 and emission 463 nm) and Cy3 (excitation 549 and emission 562 nm) filters.
Pharmacological Inhibition of Melanopsin
We used the selective competitive antagonist of melanopsin, AA92593 (Sigma-Aldrich, St. Louis, MO, USA), at 10 µM, based on a previous study25. Melan-a and B16-F10 cells were seeded at the density of 106 and 105 cells respectively in 25 cm2 flasks, and after the procedure described in the Experimental Design section, they were divided into four groups: (1) control group at 37 °C in the presence of DMSO (0.1%); (2) heat-stimulated group (39.5 °C) in the presence of DMSO (0.1%); (3) group kept at 37 °C in the presence of the OPN4 antagonist AA92593 (10 µM); (4) heat-stimulated group (39.5 °C) in the presence of AA92593 (10 µM). Total RNA of Melan-a cells was extracted immediately and of B16-F10 cells 1 h after the end of the heat stimulus.
Melanopsin Knockdown by Endoribonuclease Small Interfering RNA (esiRNA)
We used esiRNA as gene silencing tool (Sigma-Aldrich, St. Louis, MO, USA) that targets mouse Opn4 variants 1 and 2 (access numbers NM_001128599.1 and NM_013887.2). The esiRNA results from the cleavage of long double-stranded RNA (dsRNA). This process generates a heterogeneous mixture of siRNAs, all of which target the same mRNA of interest. This methodology provides highly selective gene suppression with lower off-target effects than single or pooled siRNAs26. As a control for our experiments, we used an esiRNA that targets the mRNA of Enhanced Green Fluorescent Protein (EGFP), which can be used as a negative control in systems that lack this protein.
Melan-a or B16-F10 cells were seeded (105 and 5 × 104/well respectively) in a 12-well plate, and after the procedure described in the Experimental Design section they were transfected with esiRNA against melanopsin or EGFP (both at 10 nM) using Lipofectamine 3000 transfection kit (ThermoFisher, Waltham, MA, USA) according to the manufacturer’s instructions.
To verify the functional role of melanopsin in perceiving heat, Melan-a or B16-F10 cells were divided into four groups 48 hours after transfection: (1) control group transfected with esiRNA EGFP (10 nM) at 37 °C; (2) heat-stimulated (39.5 °C) group transfected with esiRNA EGFP (10 nM); (3) group transfected with esiRNA melanopsin (10 nM) at 37 °C; (4) heat-stimulated (39.5 °C) group transfected with esiRNA melanopsin (10 nM). Total RNA was extracted immediately after the end of heat stimulus and gene expression of Opn4 (melanopsin encoding gene) was assessed by qPCR as previously described15 in Melan-a and B16-F10 cells transfected with esiRNA against EGFP (control group) or against melanopsin mRNA. Lipofectamine 3000 displayed no effect per se on clock gene expression (data not shown).
RNA Extraction, Purification, and cDNA Synthesis
RNA was extracted with trizol (Ambion, Carlsbad, CA, USA), and purified (Direct-zol™ Zymo Research, Irvine, CA, USA) according to the manufacturers’ instructions. RNA concentration and quality (OD260/OD280) were determined in a NanoDrop spectrophotometer (NanoDrop, Wilmington, DE, USA), and 1 µg of total RNA was reverse transcribed to cDNA using random hexamer primers and Superscript III, following the manufacturer’s instruction (ThermoFisher, Waltham, MA, USA).
Quantitative PCR
Quantitative PCR reactions were performed in an iQ5 thermocycler (Bio-Rad Laboratories, Hercules, CA, USA) with the products of reverse transcription using primers spanning introns, designed, and synthesized by IDT (Coralville, IA, USA), and based on sequences obtained from GenBank (http://www.ncbi.nlm.nih.gov/genbank). The access number of each gene, the respective primer sequences, and concentrations are shown in Table 1. The qPCR reactions were performed using two different protocols: multiplex for simultaneous analysis of multiple genes (TaqMan®) and SYBR® GreenER™. The TaqMan® solutions contained Per1 and Bmal1 respective primers and fluorescent probes (Table 1), and iQ Multiplex Powermix (Bio-Rad Laboratories, Hercules, CA, USA) or Kapa Probe Fast qPCR Mix 2X (Kapa Biosystems, Wilmington, MA, USA). Each experimental cDNA was run in triplicates (1 µl of cDNA per reaction) in 96 well plates. The assays were performed under the following conditions: 7 min at 95 °C, followed by 45 cycles of 30 s at 95 °C and 30 s at 55 °C.
Table 1.
Sequences and Final Concentrations of Primers and Probes. Access Numbers in Between Parentheses.
| Templates | Primers and probes | Final Concentration |
|---|---|---|
| Per1 (NM_0011065.3) | Forward: 5′-AGCAGGTTCAGGCTAACCAGGAAT-3′ | 300 nM |
| Reverse: 5′-AGGTGTCCTGGTTTCGAAGTGTGT-3′ | 300 nM | |
| Probe:5′-/6FAM/AGCCTTGTGCCATGGACATGTCTACT/BHQ_1/-3′ | 200 nM | |
| Bmal1 (NM_001243048) | Forward: 5′-AGCTTCTGCACAATCCACAGCAC-3′ | 300 nM |
| Reverse: 5′-TGTCTGGCTCATTGTCTTCGTCCA-3′ | 300 nM | |
| Probe:5′-/5HEX/-AAAGCTGGCCACCCACGAAGATGGG/BHQ_1–3′ | 200 nM | |
| Opn4 (NM_001128599.1) | Forward: 5′-ACATCTTCATCTTCAGGGCCA-3′ | 300 nM |
| Reverse: 5′-ACTCACCGCAGCCCTCAC-3′ | 300 nM | |
| Rpl37a (NM_009084.4) | Forward: GCATGAAAACAGTGGCCGGT | 300 nM |
| Reverse: CAGGGTCACACAGTATGTCTCAAAA | 300 nM | |
| 18S RNA | Forward: 5′-CGGCTACCACATCCAAGGAA-3′ | 50 nM |
| Reverse: 5′-GCTGGAATTACCGCGGCT-3′ | 50 nM |
The solutions for Opn4, Rpl 37a or 18S RNA contained the respective primers (Table 1) and Kapa SYBR® Fast qPCR Master Mix 2X (Kapa Biosystems, Wilmington, MA, USA). Each experimental cDNA was run in duplicates (1 µl of cDNA per well) in 96 well plates. These assays were performed under the following conditions: 10 min at 95 °C, followed by 45 cycles of 15 s at 95 °C, 1 min at 60 °C, and 80 cycles of 10s at 55 °C, with a gradual increase of 0.5 °C. Ribosomal 18S RNA and Rpl 37a were used as reference genes in both TaqMan® and SYBR® GreenER™ methodologies since they did not vary with time under our experimental conditions.
Statistical Analyses
To analyze qPCR data, we used the 2−ΔΔCT method as previously described33. The temporal effect of heat stimulus on clock gene expression of Melan-a and B16-F10 cells was analyzed by Two-way ANOVA followed by Bonferroni post-test. For the pharmacological and gene knockdown assays, Student’s t test or One-way ANOVA followed by Tukey post-test were used according to the number of groups. Significance was set for p < 0.05. All analyses were carried out in GraphPad Prism Version 6.0 (La Jolla, CA, USA).
Electronic supplementary material
Acknowledgements
This work was partially supported by the Sao Paulo Research Foundation (FAPESP, grant 2012/50214-4) and by the National Council of Technological and Scientific Development (CNPq, grants 301293/2011-2 and 303070/2015-3). MN Moraes, LVM de Assis, and LHRG Lima are fellows of FAPESP (2014/16412-9, 2013/24337-4 and 2009/53533-0 respectively). We are thankful to Prof. Ignacio Provencio from the University of Virginia for providing theoretical and methodological insights and thoughtful discussions.
Author Contributions
All authors designed the study. M.N.M., L.V.M.D.A. and K.K.M.M. acquired and analyzed the data. M.N.M. and L.V.M.D.A. drafted the manuscript. A.M.L.C. discussed the data and critically revised the draft. All authors have approved the final version of the manuscript and agreed to be accountable for all aspects of the study in ensuring that questions related to the accuracy or integrity of any part of the study are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Maria Nathália Moraes and Leonardo Vinícius Monteiro de Assis contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-13939-3.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Provencio I, Rollag MD, Castrucci AML. Photoreceptive net in the mammalian retina. This mesh of cells may explain how some blind mice can still tell day from night. Nature. 2002;415(6871):493. doi: 10.1038/415493a. [DOI] [PubMed] [Google Scholar]
- 2.Panda S, et al. Melanopsin is required for non-image-forming photic responses in blind mice. Science. 2003;301(5632):525–527. doi: 10.1126/science.1086179. [DOI] [PubMed] [Google Scholar]
- 3.Ralph MR, Foster RG, Davis FC, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990;247(4945):975–978. doi: 10.1126/science.2305266. [DOI] [PubMed] [Google Scholar]
- 4.Allen AE, Storchi R, Martial FP, Bedford RA, Lucas RJ. Melanopsin contributions to the representation of images in the early visual system. Curr. Biol. 2017;27(11):1623–1632. doi: 10.1016/j.cub.2017.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hughes S, et al. Signalling by melanopsin (OPN4) expressing photosensitive retinal ganglion cells. Eye (Lond) 2016;30(2):247–254. doi: 10.1038/eye.2015.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takahashi JS. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 2017;18(3):164–179. doi: 10.1038/nrg.2016.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Husse J, Eichele G, Oster H. Synchronization of the mammalian circadian timing system: Light can control peripheral clocks independently of the SCN clock: alternate routes of entrainment optimize the alignment of the body’s circadian clock network with external time. Bioessays. 2015;37(10):1119–1128. doi: 10.1002/bies.201500026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bass J, Lazar MA. Circadian time signatures of fitness and disease. Science. 2016;354(6315):994–999. doi: 10.1126/science.aah4965. [DOI] [PubMed] [Google Scholar]
- 9.Terakita A, Nagata T. Functional properties of opsins and their contribution to light-sensing physiology. Zoolog. Sci. 2014;31(10):653–659. doi: 10.2108/zs140094. [DOI] [PubMed] [Google Scholar]
- 10.Shen WL, et al. Function of rhodopsin in temperature discrimination in Drosophila. Science. 2011;331:1333–1336. doi: 10.1126/science.1198904. [DOI] [PubMed] [Google Scholar]
- 11.Sokabe T, Chen HC, Luo J, Montell CA. A switch in thermal preference in Drosophila larvae depends on multiple rhodopsins. Cell Rep. 2016;17:336–344. doi: 10.1016/j.celrep.2016.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leung, N. Y. & Montell, C. Unconventional roles of opsins. Annu. Rev. Cell Dev. Biol. 10.1146/annurev-cellbio-100616-060432 (2017). [DOI] [PMC free article] [PubMed]
- 13.Sikka G, et al. Melanopsin mediates light-dependent relaxation in blood vessels. Proc. Natl. Acad. Sci. USA. 2014;111(50):17977–17982. doi: 10.1073/pnas.1420258111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richards J, Diaz AN, Gumz ML. Clock genes in hypertension: novel insights from rodent models. Blood Press. Monit. 2014;19(5):249–254. doi: 10.1097/MBP.0000000000000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Assis LV, Moraes MN, da Silveira Cruz-Machado S, Castrucci AML. The effect of white light on normal and malignant murine melanocytes: A link between opsins, clock genes, and melanogenesis. Biochim. Biophys. Acta. 2016;1863:1119–1133. doi: 10.1016/j.bbamcr.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 16.Slominski AT, et al. Sensing the environment: Regulation of local and global homeostasis by the skin neuroendocrine system. Adv. Anat. Embryol. Cell Biol. 2012;212:v–115. doi: 10.1007/978-3-642-19683-6_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Assis LV, Moraes MN, Castrucci AML. Heat shock antagonizes UVA-induced responses in murine melanocytes and melanoma cells: an unexpected interaction. Photochem. Photobiol. Sci. 2017;16:633–648. doi: 10.1039/C6PP00330C. [DOI] [PubMed] [Google Scholar]
- 18.Liu AC, et al. Intercellular coupling confers robustness against mutations in the SCN circadian clock network. Cell. 2007;129(3):605–616. doi: 10.1016/j.cell.2007.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown, S. A. & Azzi, A. Peripheral circadian oscillators in mammals. Handb. Exp. Pharmacol. (217), 45–66 (2013). [DOI] [PubMed]
- 20.Buhr ED, Yoo SH, Takahashi JS. Temperature as a universal resetting cue for mammalian circadian oscillators. Science. 2010;330:379–385. doi: 10.1126/science.1195262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sporl F, et al. A circadian clock in HaCaT keratinocytes. J. Invest. Dermatol. 2011;131(2):338–348. doi: 10.1038/jid.2010.315. [DOI] [PubMed] [Google Scholar]
- 22.Saini C, Morf J, Stratmann M, Gos P, Schibler U. Simulated body temperature rhythms reveal the phase-shifting behavior and plasticity of mammalian circadian oscillators. Genes Dev. 2012;26(6):567–580. doi: 10.1101/gad.183251.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeronimo R, et al. Thermal stress in Danio rerio: A link between temperature, light, thermo-TRP channels, and clock genes. J. Thermal Biol. 2017;68(A):128–138. doi: 10.1016/j.jtherbio.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 24.Pérez-Cerezales S, et al. Involvement of opsins in mammalian sperm thermotaxis. Sci. Rep. 2015;5:16146. doi: 10.1038/srep16146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones KA, et al. Small-molecule antagonists of melanopsin-mediated phototransduction. Nature Chem. Biol. 2013;9(10):630–635. doi: 10.1038/nchembio.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Theis, M. & Buchholz, F. MISSION esiRNA for RNAi screening in mammalian cells. J. Visual Exp. 39, pii 2008. Advance online publication. 10.3791/2008 (2010). [DOI] [PMC free article] [PubMed]
- 27.Wang T, Facciotti MT, Duan Y. Schiff base switch II precedes the retinal thermal isomerization in the photocycle of bacteriorhodopsin. PLoS. 2013;8(7):e69882. doi: 10.1371/journal.pone.0069882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pittendrigh CS. Temporal organization: reflections of a Darwinian clock-watcher. Annu. Rev. Physiol. 1993;55:17–54. doi: 10.1146/annurev.ph.55.030193.000313. [DOI] [PubMed] [Google Scholar]
- 29.Kiessling S, et al. Enhancing circadian clock function in cancer cells inhibits tumor growth. BMC Biol. 2017;15(1):13. doi: 10.1186/s12915-017-0349-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bennett DC, Cooper DC, Hart IR. A line of non-tumorigenic mouse melanocytes, syngeneic with the B16 melanoma and requiring a tumour promoter for growth. Int. J. Cancer. 1987;39(3):414–418. doi: 10.1002/ijc.2910390324. [DOI] [PubMed] [Google Scholar]
- 31.Poletini MO, de Assis LV, Moraes MN, Castrucci AML. Estradiol differently affects melanin synthesis of malignant and normal melanocytes: a relationship with clock and clock-controlled genes. Mol. Cell Biochem. 2016;421(1–2):29–39. doi: 10.1007/s11010-016-2781-3. [DOI] [PubMed] [Google Scholar]
- 32.Panda S, et al. Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting. Science. 2002;298(5601):2213–2216. doi: 10.1126/science.1076848. [DOI] [PubMed] [Google Scholar]
- 33.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–428. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.