Summary
Reduced activity of nutrient-sensing signaling networks can extend organismal lifespan, yet the underlying biology remains unclear. We show that the anti-aging effects of rapamycin and reduced intestinal insulin/insulin growth factor (IGF) signaling (IIS) require the Drosophila FoxA transcription factor homolog Fork Head (FKH). Intestinal FKH induction extends lifespan, highlighting a role for the gut. FKH binds to and is phosphorylated by AKT and Target of Rapamycin. Gut-specific FKH upregulation improves gut barrier function in aged flies. Additionally, it increases the expression of nutrient transporters, as does lowered IIS. Evolutionary conservation of this effect of lowered IIS is suggested by the upregulation of related nutrient transporters in insulin receptor substrate 1 knockout mouse intestine. Our study highlights a critical role played by FKH in the gut in mediating anti-aging effects of reduced IIS. Malnutrition caused by poor intestinal absorption is a major problem in the elderly, and a better understanding of the mechanisms involved will have important therapeutic implications for human aging.
Keywords: longevity, insulin, enterocytes, lifespan, midgut, Drosophila, absorption, FOXA
Graphical Abstract
Highlights
-
•
Drosophila FKH biochemically interacts with AKT and TOR
-
•
IIS- and rapamycin-induced longevity requires FKH
-
•
Gut tissue, specifically differentiated cells, mediates FKH’s pro-longevity effects
-
•
FKH activity in the gut upregulates intestinal nutrient transporters
Bolukbasi et al. identify the transcription factor FKH as a mediator of nutrient-sensing pathway signaling in Drosophila, and they characterize FKH’s essential involvement in increased longevity via this network. They pinpoint FKH’s pro-longevity effect to the gut, and they show increased expression of nutrient transporters and improvement of barrier function by FKH activity.
Introduction
The signaling network of nutrient-sensing, insulin/insulin growth factor signaling (IIS) and Target of Rapamycin (TOR) influences healthy lifespan in diverse eukaryotic organisms, including mammals (Alic and Partridge, 2011). Specific alleles of IIS genes (Li et al., 2009, Pawlikowska et al., 2009, Suh et al., 2008, Willcox et al., 2008) and transcriptional variation of genes encoding components of the TOR pathway (Passtoors et al., 2013) are associated with survival to advanced ages in humans. Reduced network activity can induce a broad-spectrum resistance to age-related loss of function and disease in animal models (Clancy et al., 2001, Kenyon et al., 1993, Selman et al., 2008, Tatar et al., 2001), making it an attractive target for pharmacological intervention to improve human health during aging (de Cabo et al., 2014). Indeed, attenuation of TOR signaling by rapamycin extends lifespan in diverse species, including mice (Bjedov et al., 2010, Harrison et al., 2009), as does inhibition of the Ras-Erk branch of IIS by the drug trametinib in Drosophila (Slack et al., 2015).
In addition to its effect on aging, the IIS/TOR network regulates growth, metabolism, stress responses, and fecundity, potentially resulting in undesired side effects of reduction of network activity. For example, at some doses, rapamycin is a strong immunosuppressant (de Cabo et al., 2014) and can also impair wound healing (Squarize et al., 2010), while trametinib is a Mek1/2 inhibitor with anti-proliferative properties (Yamaguchi et al., 2011). Therefore, we need to uncover molecular and mechanistic outputs of nutrient-sensing networks in order to triage apart the positive effects of intervention from the negative effects inherent in manipulating upstream network nodes. In particular, we need to determine the tissue-specific effect of signaling activity in lifespan extension and the physiological processes underlying it.
Recent studies identified the intestinal tissue as pivotal in aging (Alic et al., 2014, Biteau et al., 2010, Rera et al., 2012), and they have mainly focused on hyperplastic intestinal pathology resulting from age-dependent intestinal stem cell (ISC) over-proliferation as a major determinant of lifespan (Biteau et al., 2010). However, while stem cell maintenance is no doubt important for intestinal homeostasis, hyperplasia may not occur early enough to influence the early tipping point between young and old metabolic states. Therefore, other aspects of intestinal physiology that determine lifespan still remain to be elucidated.
Outputs of the IIS/TOR signaling network are mediated by several transcription factors (TFs). For instance, in C. elegans and Drosophila, the single Fork Head Box O (FoxO) TF is required for extended lifespan from lowered IIS (Giannakou et al., 2004, Hwangbo et al., 2004, Murphy et al., 2003, Slack et al., 2011, Willcox et al., 2008). In C. elegans the heat shock TF HSF-1 (Hsu et al., 2003) and the Nrf-like xenobiotic response factor SKN-1 (Tullet et al., 2008) are also required. In Drosophila, lowered IIS increases lifespan through both the canonical IIS pathway and its FOXO effector, and through the Ras-Erk-ETS branch and its transcriptional repressor effector anterior open (AOP) (Slack et al., 2015). In Drosophila, at least one other TF is also likely to play a role, because the TF-binding sites upstream of genes regulated by lowered IIS imply the involvement of a FKH box family member other than dFOXO (Alic et al., 2011).
The Fork Head box (FOX) family of TFs shares a conserved DNA-binding domain, whose sequence assigns them to subclasses from FoxA to FoxS (Lam et al., 2013). The Drosophila FoxA homolog FKH is the founding member and namesake, and it plays an essential role in embryonic development (Weigel et al., 1989). FKH also regulates larval cell size in a rapamycin- and TOR-dependent manner (Bülow et al., 2010). Mammalian FoxAs regulate glucose metabolism in the liver, pancreas, and adipose tissue (Friedman and Kaestner, 2006), and liver-specific knockout of FoxA2 results in a premature aging phenotype and increased mTOR activity in mouse (Bochkis et al., 2013).
We have investigated the role of Drosophila FKH in the IIS-and-TOR-signaling network and, in particular, its key role in intestinal aging. We find that FKH interacts with and is phosphorylated by both dAKT and dTOR, placing it as a central transcriptional regulator. Concordantly, we demonstrate an essential requirement for FKH for both IIS- and rapamycin-induced longevity, as well as IIS-induced starvation resistance, a phenotype previously shown to be dFOXO independent (Slack et al., 2011). We locate the longevity effects of FKH to intestinal tissue and specifically to differentiated intestinal cells. We establish that the anti-aging effects of rapamycin and intestinal IIS downregulation both require, and can be recapitulated by, FKH induction in the gut. Gut barrier function loss over aging is improved by intestinal FKH upregulation, while ISC proliferation remains unaffected. Transcriptomic analysis of adult guts revealed FKH-dependent upregulation of nutrient transporters upon reduced IIS. Consistent with this finding, we demonstrate an FKH-dependent increase in nutrient absorption upon reduced IIS and gut-specific FKH overexpression, suggesting improved gut absorption as a possible underlying longevity mechanism. Concordantly, starvation resistance declines over age, but it is rescued by intestinal FKH upregulation in young and old flies. Additionally, we show upregulation of related nutrient transporters in irs1 knockout mouse intestine, suggesting evolutionary conservation of this mechanism. Overall, our results demonstrate FKH-dependent functional consequences of reduced IIS for intestinal absorption, and they imply that FoxA is an evolutionarily conserved regulator of lifespan and gut function, pointing to new directions for therapeutic intervention into aging-related loss of function.
Results
FKH Overexpression Results in Increased Longevity
FOXO overexpression can increase longevity in both worms and flies (Alic and Partridge, 2011), and overexpression of the FoxA homolog pha-4 can also do so in C. elegans (Panowski et al., 2007). To determine whether Drosophila FKH plays a similar role, we assessed the effect of ubiquitous, adult-onset FKH overexpression on lifespan.
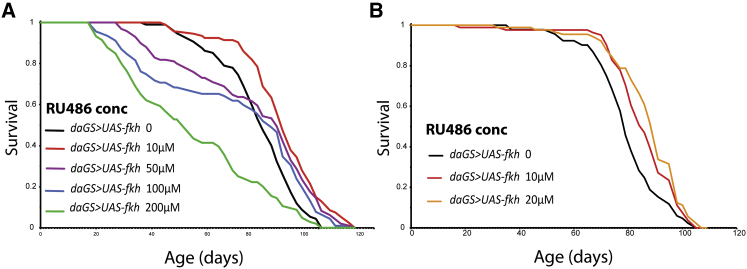
We used the inducible daughterless Gene Switch (daGS) driver to produce a graded increase in FKH expression, by varying the concentration of the activating drug RU486. Strong FKH induction led to short-lived flies (Figure 1A), whereas weaker induction resulted in lifespan extension (Figure 1A; 10 μM median survival + 9%, p = 6.94 × 10−6). Intermediate induction at 50 and 100 μM RU486 resulted in early life mortality, and upregulation at 200 μM RU486 resulted in significantly short-lived flies. To more precisely define the range of induction that maximized lifespan, we used 10 and 20 μM RU486, and we found significant extension at both concentrations (Figure 1B; 10 μM RU486 median survival +9%, p = 0.003 and 20 μM median survival +14%, p = 6.33 × 10−6). Moderate, ubiquitous, adult-specific upregulation of FKH expression is thus sufficient to extend Drosophila lifespan in a dose-dependent manner.
Figure 1.
Ubiquitous Overexpression of FKH Extends Lifespan
(A and B) Survival curves of female flies expressing the UAS-fkh transgene under the control of the daGS driver at different RU486 concentrations. Ubiquitous overexpression of FKH extended lifespan (A) at 10 and 50 μM RU486 (p = 6.94 × 10−6 and p = 0.017, respectively) and (B) at 10 and 20 μM RU486 (p = 0.003 and p = 6.33 × 10−6, respectively). See also Table S1.
Weakening immune response and hence lowered defense against infection contribute to decline of health during aging (Zerofsky et al., 2005). FKH was recently proposed to act as a regulator of innate immune response in Drosophila larvae (Varma et al., 2014). As an indicator of healthspan, we assessed the survival upon systemic infection with Erwinia carotovora carotovora15 (Ecc15), a Gram-negative bacterium that has been used as a model of natural infection in Drosophila larvae and adults (Basset et al., 2000). Systemic Ecc15 infection at 1 week of age resulted in lower mortality than did infection at 7 weeks of age (p = 0.00052), suggesting a decline in immune response over age. Overexpression of FKH led to increased survival at both ages (Figure S1; p = 0.00014 and p = 0.0001 for young and old cohorts, respectively). Hence, FKH activity results in more efficient immunity and attenuates age-related decline in immune function.
IIS Induces Phosphorylation and a Marked Decrease in Nuclear Localization of FKH
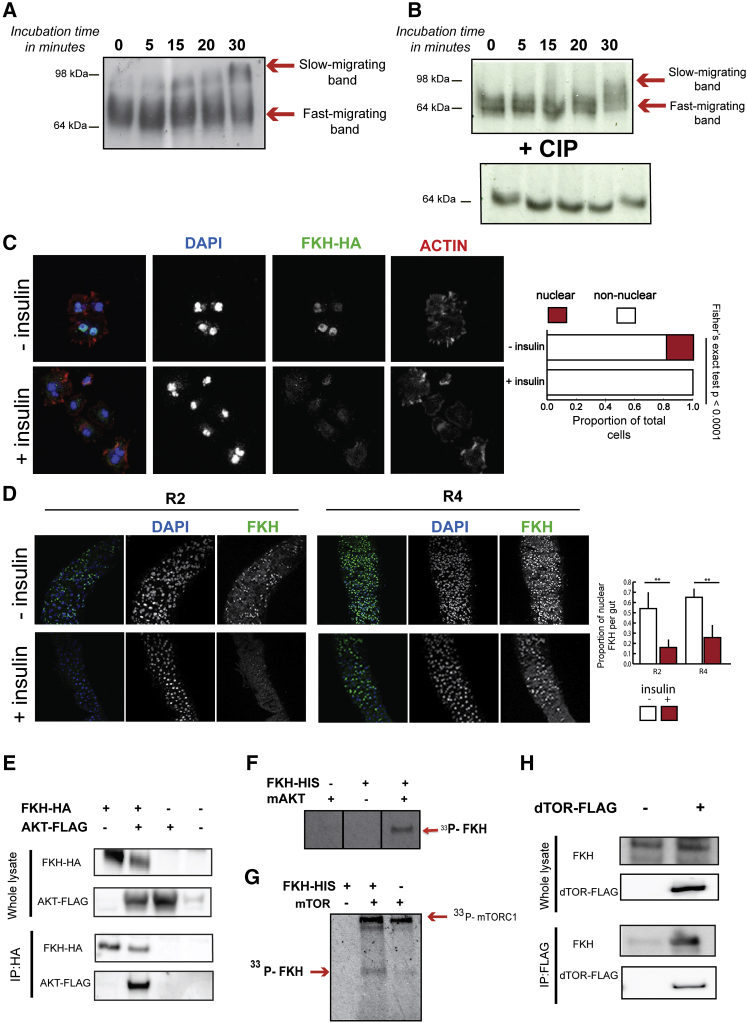
IIS activity results in phosphorylation and nuclear exclusion of dFOXO by Drosophila AKT (dAKT), and, thereby, it controls the expression of dFOXO target genes. We first determined whether IIS activity has similar effects on FKH, by searching for the appearance of a slow-migrating FKH protein band on polyacrylamide gels (Puig et al., 2003). Drosophila S2 cells were transfected with an HA-tagged FKH construct, serum starved, and then stimulated with insulin, and protein extracts were run on polyacrylamide gels containing PhosTag (Kinoshita et al., 2006). Immunoblotting of unstimulated cell extracts revealed a band just above 64 kDa, while extracts from insulin-stimulated cells contained a slower migrating band of around 98 kDa, already apparent at 15 min post-insulin stimulation, with the highest intensity at 30 min (Figure 2A). Phosphatase treatment resulted in a single band at 64 kDa for all time points (Figure 2B), indicating that phosphorylation caused the appearance of the slow-migrating FKH band.
Figure 2.
Activation of IIS Results in FKH Phosphorylation, and FKH Interacts with AKT and TOR In Vitro
(A) S2 cells were transfected with HA-tagged FKH construct and stimulated with insulin for 5, 15, 20, and 30 min. A band above 64 kDa was detected in extracts pre-insulin stimulation. A slower migrating band at ∼98 kDa was present in extracts isolated post-insulin stimulation.
(B) Following phosphatase treatment of the same extracts, a single band corresponding to non-phosphorylated FKH-HA was present (lower panel).
(C) S2 cells were visualized for FKH-HA protein (green), Actin (red), and nuclei stained by DAPI (blue) pre-and post-insulin treatment for 20 min. Quantification of nuclear FKH revealed a significant difference between two conditions (Fisher’s exact test, p < 0.0001).
(D) Ex vivo insulin-stimulated guts were stained for endogenous FKH (green) and DAPI-stained nuclei (blue) and in two different sections of midgut: R2 (left panel) and R4 (right panel). Initially strong nuclear FKH staining was markedly decreased in both R2 (t test, p = 0.045; n = 4) and R4 (t test, p = 0.0014; n = 4) upon insulin stimulation. Error bars represent SD.
(E) S2 cells were transiently transfected with the indicated cDNAs in expression vectors. Anti-HA immunoprecipitates were prepared, and cell lysates were analyzed by immunoblotting with either anti-HA or anti-FLAG antibodies.
(F and G) Recombinant FKH-HIS was incubated in an in vitro kinase reaction with active recombinant (F) Akt1 or (G) mTORC1 radiolabelled [γ-33P]ATP. Reactions lacking either the substrate or the kinase served as negative controls. Proteins were separated by SDS-PAGE and phosphorylated proteins were visualized by autoradiography. In reactions with mTORC1, the incorporation of [γ-33P]ATP was also observed at a higher molecular weight (∼160 kDa), which probably reflected the previously reported phosphorylation of raptor by mTOR (Wang et al., 2009).
(H) S2 cells were transiently transfected with the dTOR-FLAG vector. Anti-FLAG immunoprecipitates were prepared, and cell lysates were analyzed by immunoblotting with either anti-FKH or anti-FLAG antibodies.
We next assessed if the subcellular localization of FKH was changed by IIS, using transiently overexpressed FKH-HA protein and immunofluorescence of S2 cells pre- and post-stimulation with insulin. Around 20% of unstimulated transfected cells showed strong nuclear staining of HA-tagged FKH protein, which was lost upon stimulation with insulin (Figure 2C). To determine whether insulin affected localization of endogenous FKH in adult tissue, guts were isolated from 5-day-old flies and stimulated with insulin ex vivo for 20 min. The Drosophila gut is regionalized in morphology and function (Buchon et al., 2013), and we measured FKH localization in two regions of the midgut, R2 and R4. In both, insulin stimulation led to reduced nuclear FKH staining (Figure 2D).
IIS activity thus results in phosphorylation of FKH in cell culture and a substantial decrease in nuclear localization of FKH in both cell culture and gut ex vivo, demonstrating IIS regulation of FKH function.
FKH Interacts with AKT and TOR In Vitro
We next determined where FKH is integrated into the IIS/TOR network. Since mammalian FoxA2 interacts with mAKT1 in vitro (Wolfrum et al., 2003) and FKH genetically interacts with dTOR in Drosophila larval fat body (Bülow et al., 2010), we investigated AKT and TOR kinases as candidates for a potential interaction with FKH.
Drosophila S2 cells were transiently transfected with HA-tagged FKH and/or a FLAG-tagged dAKT. Following 15 min of insulin stimulation, FKH-HA protein was co-immunoprecipitated as part of a complex with FLAG-tagged dAKT (Figure 2E). Since FKH could physically interact with dAKT, we next assessed whether AKT could phosphorylate FKH. Recombinant, His-tagged FKH was expressed in E.coli and purified. An in vitro kinase assay was carried out using mammalian recombinant active mAKT, and phosphorylation of FKH was assessed by the incorporation of [γ-33P]ATP. FKH-His was phosphorylated upon incubation with active mAKT (Figure 2F).
To assess whether FKH interacted physically with dTOR, S2 cells were transfected with FLAG-tagged dTOR, which was co-immunoprecipitated with endogenous FKH protein (Figure 2H). An in vitro kinase assay with recombinant active mTORC1 and purified FKH-His resulted in the phosphorylation of FKH (Figure 2G).
FKH can interact physically with both AKT and TOR and can be phosphorylated by them, and it could thus integrate signals from these two kinases.
FKH Functions Downstream of IIS to Determine Lifespan
In Drosophila, the reduction of IIS in adult flies results in a complex array of phenotypes, including resistance to starvation, oxidative stress and xenobiotics, reduced fecundity, and increased longevity (Slack et al., 2011).
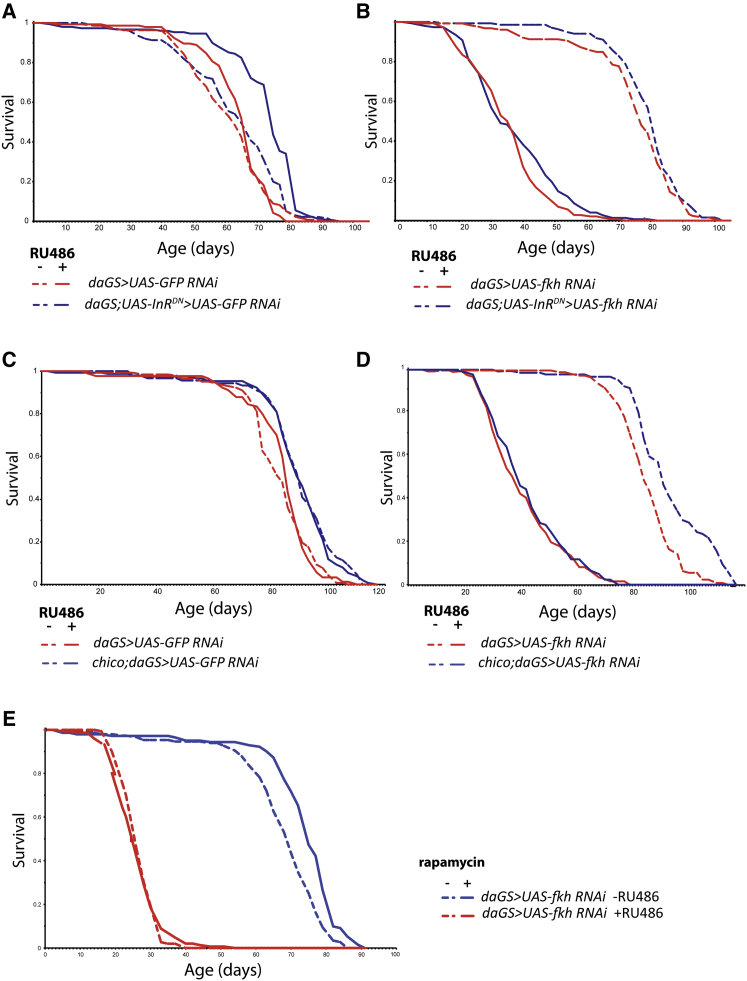
To determine the contribution of FKH function to longevity, we knocked down fkh using RNAi in wild-type flies and flies with a dominant-negative version of the single-fly IIS receptor InRDN. Assessment of transcript levels by qPCR showed that ubiquitous RNAi against fkh resulted in an ∼65% reduction in fkh expression (Figure S2A). Ubiquitous, adult-specific expression of InRDN with the inducible daGS driver resulted in a significant lifespan extension in flies with co-overexpression of GFP RNAi, to control for possible titration of GAL4 (Figure 3A; median survival +14%, p = 4.45 × 10−10). However, RNAi against fkh both substantially reduced the lifespan of wild-type flies and blocked the extension of lifespan by InRDN (Figure 3B; p = 0.08). Cox Proportional Hazard (CPH) analysis established that the response to the presence of the activating drug RU486 in daGS;UAS-InRDN flies was significantly different in the presence and absence of fkh RNAi (Table S3; p = 0.0432). To confirm that IIS was biochemically active upon FKH RNAi, we assessed expression levels of the dFOXO target 4EBP, and we found a significant increase upon reduced IIS on both wild-type and FKH knockdown background (Figure S2E; t test, p < 0.05).
Figure 3.
FKH Is Required for Reduced IIS- and Rapamycin-Induced Longevity
(A) daGS;UAS-InRDN> UAS-GFP RNAi females showed increased lifespan in the presence of RU486 (p = 4.45 × 10−10). Survival of daGS > UAS-GFP RNAi females was not different between –RU486 and +RU468 conditions (p = 0.508). See also Table S2A.
(B) daGS > UAS-fkh RNAi and daGS;UAS-InRDN> UAS-fkh RNAi flies showed significantly decreased lifespan in the presence of RU486 (p = 1.97 × 10−54 and p = 2.59 × 10−63, respectively). No significant difference in survival was detected between daGS > UAS-fkh RNAi +RU486 and daGS;UAS-InRDN> UAS-fkh RNAi +RU486 flies (p = 0.08). See also Table S2B.
(C) chico mutation increased lifespan of daGS>UAS-GFP RNAi females in the absence (p = 6.28 × 10−7) and in the presence (p = 2.71 × 10−6) of RU486. See also Table S2C.
(D) chico mutation extended lifespan in daGS > UAS-fkh RNAi female flies in the absence of RU486 (p = 3.86 × 10−8), but not in its presence (p = 0.61). See also Table S2D.
(E) Rapamycin extended the lifespan of daGS > UAS-fkh RNAi female flies in the absence of RU486 (p = 2.37 × 10−8), but not in its presence (p = 0.81). See also Table S2E.
We examined the role of FKH in the extension of lifespan by a second IIS mutant, heterozygous chico1, which has lost a single copy of the insulin receptor substrate and is long-lived (Clancy et al., 2001). chico1 heterozygotes were long-lived with control GFP RNAi (Figure 3C; median survival +8%, p = 2.71 × 10−6), but they lost this lifespan extension with RNAi against fkh (Figure 3D; p = 0.61), confirmed by CPH analysis (Table S3; p = 0.00452). To confirm the specificity of the effect of fkh RNAi, we used a second fkh RNAi line (Bülow et al., 2010) (referred to as UAS-fkh RNAi 2) that also prevented lifespan extension upon overexpression of UAS-InRDN and heterozygous chico1 mutation (Figures S2B–S2D).
These data show that FKH function is essential for mediating the lifespan-extending effects of reduced IIS in Drosophila.
FKH Mediates Lifespan Extension by Rapamycin, but Not by Dietary Restriction
TOR is a conserved serine/threonine kinase that is present as two distinct protein complexes, TORC1 and TORC2. TORC1 activity can be directly inhibited by rapamycin (Cornu et al., 2013, Kapahi et al., 2004), which can also extend lifespan in yeast (Powers et al., 2006), C. elegans (Robida-Stubbs et al., 2012), Drosophila (Bjedov et al., 2010), and mice (Harrison et al., 2009). Our results showed biochemical interaction between FKH and TOR, and we therefore examined whether FKH is required for rapamycin-induced longevity.
Treatment of control flies with 100 μM rapamycin resulted in significant lifespan extension (Figure 3E; median survival +6.5%, p = 2.37 × 10−8), which was abolished by ubiquitous, adult-specific RNAi against fkh (Figure 3E; p = 0.81), confirmed by CPH analysis (Table S3; p = 5.5 × 10−4). The biochemical effect of rapamycin upon FKH RNAi was confirmed by assessing phosphorylation levels of S6K, which were decreased on both wild-type and FKH knockdown backgrounds following rapamycin treatment (Figure S2F). FKH thus functions downstream of TOR to mediate the extension of lifespan by rapamycin.
TOR kinase can play a role in the extension of lifespan by dietary restriction (DR) (Grandison et al., 2009), and the C. elegans homolog of fkh is required for DR-induced longevity (Panowski et al., 2007). We subjected flies overexpressing fkh RNAi and uninduced controls to DR, and we found that DR promoted longevity in both (Figures S3A and S3B; at 0.5× versus 1× yeast concentration, for +RU486 condition median survival +18%, p = 1.02 × 10−10, and for −RU486 condition median survival +3%, p = 0.03, respectively). Importantly, since ubiquitous RNAi again fkh shortened lifespan, the extension of lifespan in these flies by DR indicates that the failure to extend in response to reduced IIS and rapamycin was specific to these interventions.
Together these results indicate that FKH is required for rapamycin-induced, but not DR-induced, longevity.
FKH Is Required for IIS-Induced Starvation Resistance
In addition to its effect on lifespan, lowered IIS is associated with resistance to starvation, oxidative stress, and xenobiotics. We therefore determined whether these types of stress resistance require FKH function.
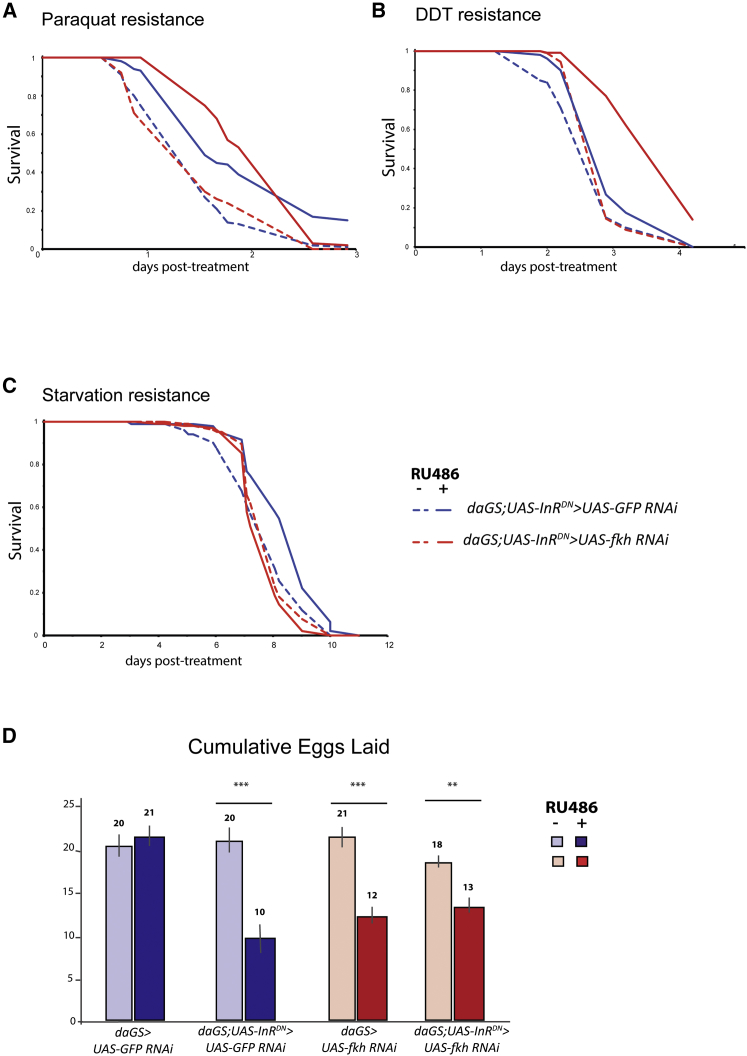
Flies were subjected to oxidative stress by supplementing their food with paraquat, a superoxide generator. Flies expressing InRDN were resistant to paraquat (Figure 4A; p = 0.00058). Ubiquitous RNAi against GFP or fkh did not affect the resistance of either wild-type (Figure S4A) or InRDN-expressing flies (Figure 4A; Table S3). Resistance to xenobiotic treatment was tested by supplementing fly food with dichlorodiphenyltrichloroethane (DDT). Ubiquitous RNAi against GFP or fkh did not affect resistance of wild-type flies to DDT (Figure S4B). Reduction of IIS by InRDN expression resulted in a small, but significant, increase in resistance (Figure 4B; p = 0.0013), but flies lacking fkh function showed even greater resistance to DDT upon reduction of IIS (Figure 4B; p = 0.00028). FKH function is thus not required for reduced IIS to induce resistance to oxidative stress or xenobiotic treatment, and indeed somehow it interferes with the latter.
Figure 4.
Functions of FKH during Lowered IIS-Induced Oxidative Stress, Xenobiotics, Starvation Resistance, and Reduced Fecundity
(A and B) The (A) paraquat and (B) DDT resistance of daGS;UAS-InRDN> UAS-GFP RNAi and daGS;UAS-InRDN> UAS-fkh RNAi females was significantly increased in the presence of RU486 (p = 0.00058 and p = 1.46 × 10−7; p = 0.0013 and p = 3.58 × 10−14, respectively). See also Table S4, A and B.
(C) Starvation resistance of daGS;UAS-InRDN> UAS-GFP RNAi females was significantly increased in the presence of RU486 (p = 3.66 × 10−5), but resistance to starvation was not different for daGS;UAS-InRDN> UAS-fkh RNAi in the presence of RU486 (p = 0.085). See also Table S4C.
(D) Cumulative number of eggs per female over 3 weeks following RU486 induction. Data are presented as the mean number of eggs laid per female over a 24-hr period each week ± SEM; t test revealed a significant difference between +RU486 and −RU486 conditions for daGS;UAS-InRDN> UAS-GFP RNAi (p = 2.04 × 10−7), daGS;UAS-InRDN> UAS-fkh RNAi (p = 0.0037), and daGS > UAS-fkh RNAi (p = 1.63 × 10−5).
Resistance to starvation was assessed by exposing flies only to agar. Ubiquitous RNAi against GFP or fkh did not affect the response to starvation (Figure S4C). Flies with reduced IIS were resistant to starvation (Figure 4C; p = 3.66 × 10−5). Knocking down fkh resulted in a loss of starvation resistance in flies expressing InRDN (Figure 4C; p = 0.085), with CPH revealing a significant difference between these conditions (Table S3; p = 4 × 10−5). Resistance to starvation upon the downregulation of IIS thus requires FKH function.
IIS mutant females often have a fecundity deficit (Slack et al., 2011), and we assessed the role of FKH in this trait. RNAi against GFP did not affect egg-laying (Figure 4D; p = 0.47). Reduction of IIS resulted in a substantial decrease in fecundity (Figure 4D; p = 2.04 × 10−7), as did fkh RNAi alone (Figure 4D; p = 1.63 × 10−5). Knocking down fkh in flies with lowered IIS neither restored nor exacerbated their fecundity deficit. These results suggest that FKH is not required for the reduced fecundity observed upon the downregulation of IIS. The decrease in fecundity observed upon reduced IIS and fkh RNAi alone was not additive, and, therefore, it was likely to be occurring through independent mechanisms.
Upregulation of FKH in the Gut Extends Lifespan and Improves Gut Barrier Function
Many targets of TFs are cell type specific (Heintzman et al., 2009). Indeed, both dFOXO (Alic et al., 2014, Teleman et al., 2008) and AOP (Alic et al., 2014) have distinct targets in different tissues. Interestingly, dfoxo or aop activity in the abdominal fat body and adult midgut results in longevity, whereas their upregulation in the gut alone does not (Alic et al., 2014). A possible reason for the requirement of several distinct TFs for IIS-mediated lifespan is, thus, the tissue specificity of their action. We therefore investigated possible tissue specificity of FKH action to affect lifespan. A plethora of studies in both C. elegans (Libina et al., 2003, Tullet et al., 2008) and Drosophila (Alic et al., 2014, Biteau et al., 2010, Regan et al., 2016, Slack et al., 2015) has pointed to adipose and/or intestinal tissue as key mediators of the effects of reduced IIS on lifespan, and we therefore investigated the role of FKH in these two tissues.
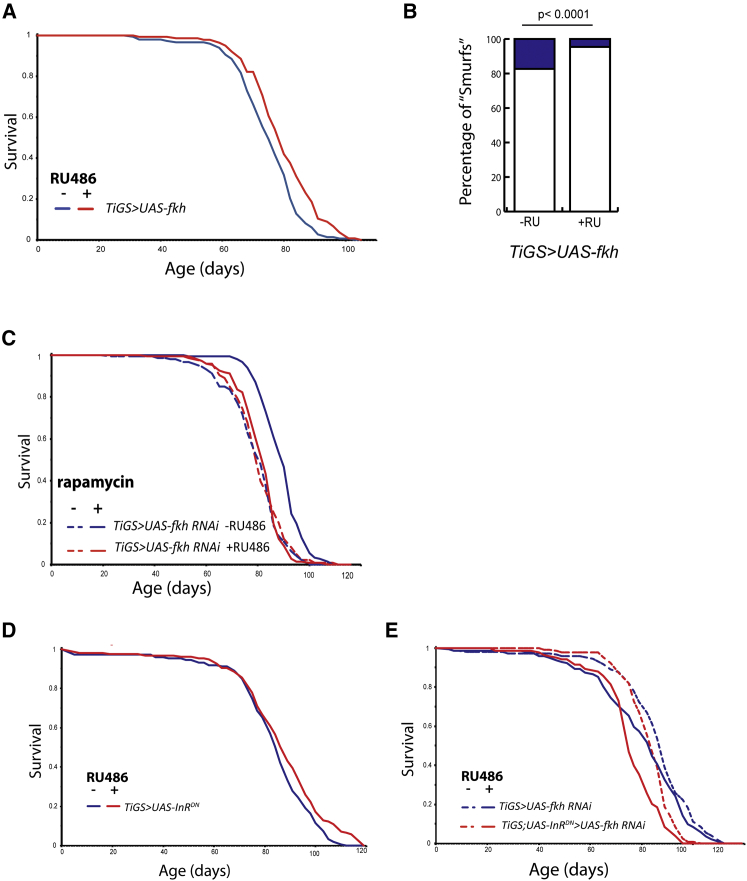
Overexpression of FKH in the adult gut using the inducible TiGS driver, which expresses throughout the gut including differentiated cells (Alic et al., 2014), significantly increased lifespan (Figure 5A; median survival +6%, p = 0.00015), but its overexpression simultaneously in abdominal fat body and gut with S1106 driver did not (Figure S5A; p = 0.093). Furthermore, FKH overexpression restricted solely to ISCs and enteroblasts (EBs) with GS5961 driver (Biteau et al., 2010) did not affect longevity (Figure S5B; p = 0.21). RU486 feeding had no effect on the lifespan of control flies with the UAS transgene or driver alone (Figures S5D–S5H).
Figure 5.
FKH Upregulation in the Gut Extends Lifespan, and Intestinal FKH Function Mediates Longevity Effects of Rapamycin and Reduced IIS
(A) Adult gut-specific overexpression of UAS-fkh under the control of TiGS driver extended lifespan (p = 0.00015). See also Table S5A.
(B) Quantification of the proportion of “Smurf” flies with leaky guts at 10 weeks of age in TiGS>UAS-fkh flies revealed a significant reduction in Smurf numbers in the +RU486 condition (two-tailed Fisher’s exact test, p < 0.0001; n > 200 flies per condition).
(C) Rapamycin extended the lifespan of TiGS > UAS-fkh RNAi females in the absence of RU486 (p = 1.79 × 10−12), but not in its presence (p = 0.57). See also Table S5B.
(D) TiGS < UAS-InRDN females were long-lived in the presence of RU486 (p = 0.014). See also Table S5C.
(E) Survival of TiGS > UAS-fkh RNAi females was not affected by RU468 (p = 0.077). The reduction of IIS upon overexpression of UAS-fkh RNAi led to significantly short-lived flies (p = 8.97 × 10−7). See also Table S5, D and E.
These results show that at least part of the lifespan-extending effect of FKH activity occurs in the gut, in contrast to dFOXO. However, FKH upregulation solely in the progenitor cells (ISCs and EBs) is not sufficient to extend lifespan, suggesting involvement of other gut cell types.
To determine how FKH acts in the gut to extend lifespan, we examined the gut barrier function by feeding aged flies with a blue dye that does not normally leak into the body cavity (Rera et al., 2012), and we scored the proportion of “Smurf” flies, in which leakage occurred. FKH overexpression resulted in a significant decrease in the number of Smurfs at 10 week of age (Figure 5B; Fisher’s exact test, p < 0.0001), indicating stronger gut barrier function.
ISC proliferation increases with age, eventually leading to intestinal dysplasia (Biteau et al., 2008, Choi et al., 2008). To determine whether FKH function affects age-related proliferation of ISCs, we assessed the number of PH3-positive cells in the gut, but we did not find any difference at either young or old age between FKH-overexpressing and control flies (Figure S5C; t test, p = 0.42 and p = 0.51).
These results suggest that FKH overexpression in the gut maintains barrier function during aging, but it does not affect ISC proliferation.
FKH Function in the Gut Mediates Rapamycin-Induced Longevity
Rapamycin reduces age-related intestinal pathologies in flies (Fan et al., 2015). Given the increased lifespan upon gut-targeted FKH overexpression, we assessed the requirement for FKH for rapamycin-induced longevity. We knocked down fkh in the gut by RNAi with the TiGS driver. Treatment of uninduced flies with rapamycin significantly extended lifespan (Figure 5C; median survival + 11%, p = 1.79 × 10−12), and this extension was lost upon gut-specific RNAi against FKH (Figure 5C; p = 0.57), confirmed by CPH analysis (Table S3; p = 6.66 × 10−15). Hence, FKH function in the gut is necessary for rapamycin-induced longevity.
Reduced IIS in the Gut Induced an FKH-Dependent Extension of Lifespan
Downregulating IIS activity in progenitor cells of the gut extends Drosophila lifespan (Biteau et al., 2010). We examined the effect of a broader IIS reduction, and the contribution of FKH, by expressing InRDN and RNAi against fkh under the control of the TiGS driver. The gut-targeted reduction of IIS resulted in a small, but significant, increase in lifespan (Figure 5D; median survival + 3%, p = 0.014), while RNAi against fkh resulted in slightly elevated early life mortality (Figure 5E; p = 0.077). Importantly, a reduction of IIS in the gut in combination with fkh knockdown failed to extend lifespan, and indeed it led to significantly short-lived flies (Figure 5E; p = 8.92 × 10−7). Hence, the decrease of IIS activity in the gut alone results in long-lived flies, and this not only requires FKH function but also is deleterious without it.
Reduced IIS Increases the Expression of Intestinal Transmembrane Transporters through FKH, and It Induces Enterocyte-Specific Nuclear Localization of FKH
Our results indicate that FKH function in the gut is necessary for both rapamycin- and reduced IIS-induced longevity. Given the detrimental effect of FKH knockdown in guts with reduced IIS, in our subsequent analysis we focused on the identification of genes regulated by FKH in the gut upon downregulation of IIS. We isolated guts from flies with ubiquitous reduction of IIS with or without knockdown of FKH, and controls, and we carried out genome-wide transcript profiling using next-generation RNA sequencing.
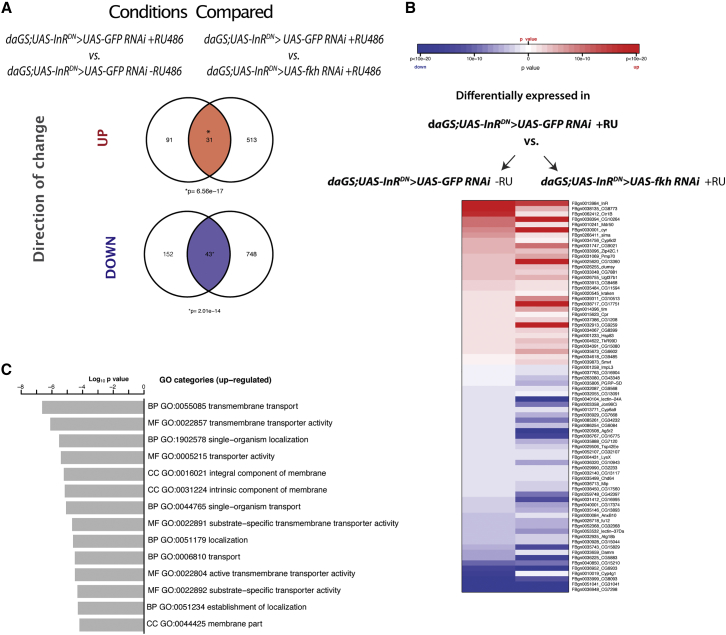
We first identified the set of genes differentially expressed in wild-type and reduced-IIS guts (daGS;UAS-InRDN > UAS-GFP RNAi + RU486 versus daGS;UAS-InRDN > UAS-GFP RNAi − RU486) and the set of genes differentially expressed in reduced-IIS guts with or without RNAi against FKH (daGS;UAS-InRDN > UAS-GFP RNAi + RU486 versus daGS;UAS-InRDN > UAS-fkh RNAi + RU486). We reasoned that the transcriptional response regulated by FKH in response to reduced IIS should be indicated by genes whose expression levels are reverted back to wild-type levels upon FKH knockdown in reduced-IIS flies (Figures 6A and 6B). We carried out gene ontology (GO) category analysis on genes in the overlap between these two sets.
Figure 6.
Reduced IIS in the Gut Results in a Transcriptional Response that Upregulates Transmembrane Transporters via FKH Function
(A) In the adult gut, the overlap between the transcriptional response induced by reduced IIS compared to controls (daGS;UAS-InRDN> UAS-GFP RNAi +RU486 versus daGS;UAS-InRDN> UAS- GFP RNAi –RU486) and compared to flies with reduced IIS and fkh knockdown (daGS;UAS-InRDN> UAS-GFP RNAi +RU486 versus daGS;UAS-InRDN> UAS-fkh RNAi +RU486) revealed 31 upregulated and 43 downregulated genes.
(B) Heatmap showing genes significantly upregulated or downregulated in both comparisons.
(C) Significantly upregulated GO categories induced by reduced IIS in an FKH-dependent manner in the adult gut.
We found GO enrichment only among genes upregulated in reduced-IIS flies, particularly for genes encoding proteins involved in transmembrane transport (Figure 6C). Within this GO category were a number of genes responsible for the transport and uptake of nutrients, such as copper and zinc (ctr1b and zip1), glucose (CG1208), phospholipids (mdr50), and fatty acids (pmp70) (Figure 6B), suggesting that FKH could be increasing nutrient uptake and absorption in reduced-IIS flies.
Drosophila enterocytes (ECs) regulate nutrient transport and absorption in the midgut (Lemaitre and Miguel-Aliaga, 2013). We next investigated whether reduced IIS affected subcellular FKH localization, and we found that it increased nuclear localization of FKH specifically in ECs, which are characterized by polyploidy and large nuclei (Figure S6; t test, p < 0.0001). In cells with smaller nuclei, possibly consisting of ISCs, EBs, and enteroendocrine cells (EEs), nuclear localization of FKH did not change (Figure S6; t test, p = 0.78). Additionally, and in agreement with previously published work (Biteau et al., 2010), in the IIS mutant there were fewer cells with smaller nuclei, consistent with reduced ISC proliferation. These observations suggest a role for FKH in regulating transcription in ECs, including of genes related to nutrient transport and absorption, upon reduced IIS activity.
FKH Function Promotes Nutrient Absorption, and FKH Activity in Differentiated Gut Cells Results in Increased Longevity
To confirm our transcriptomic data, we assessed by qRT-PCR the expression levels of transmembrane transporters, including ctr1B, zip1, mdr50, pmp70, and CG1208 (Figure S7A), and we found that they were increased in the IIS mutant (t test, p = 0. 0029, p = 0.0035, p = 0.031, p = 0.043, and p = 0.03, respectively; n = 3–4). RNAi against fkh abolished the differential expression of ctr1B, mdr50, pmp70, or CG1208 (t test, p > 0.05; n = 3–4), but zip1 expression levels remained significantly increased (t test, p = 0.0085; n = 4). Linear model analysis showed a significant interaction between RU and genotype (p = 0.0004), showing that the expression of genes involved in transport of nutrients was increased in the IIS mutant in an FKH-dependent manner.
We next assessed the effect of gut-specific upregulation of FKH on the expression of nutrient transporters, and we found a significant increase in ctr1B, zip1, and mdr50 expression in guts with the induction of fkh (Figure S7B; t test, p = 0.02, p = 0.006, and p = 0.007, respectively). Therefore, FKH overexpression in the gut alone can upregulate expression of nutrient transporters.
To determine whether the upregulation of nutrient transporters also increased nutrient uptake, we assessed intestinal metal and lipid absorption. Drosophila metallothioneins are cysteine-rich proteins that bind heavy metals, such as zinc and copper, and their expression levels are used as a proxy measure of metal content of the Drosophila gut (Binks et al., 2010, Qin et al., 2013). We assessed by qRT-PCR the expression levels of the metallothioneinB (mtnB) and metallothioneinC (mtnC) in guts isolated from flies with fkh knockdown alone or with reduced IIS with or without fkh knockdown. Reduced IIS increased expression of both metallothionein genes, and this increase was dependent on FKH (Figure S7C), as confirmed by linear model analysis (interaction between RU and genotype, p = 0.0015). Next, we quantified mtnB and mtnC expression in guts with intestinal FKH overexpression and controls at 1 and 7 weeks of age. mtnC levels were significantly upregulated in fkh-overexpressing guts at both ages (Figure S7D; t test, p = 0.02 and p = 0.005), whereas mtnB levels were significantly increased only at 7 weeks of age (Figure S7D; t test, p = 0.01). These results indicate that FKH promotes intestinal metal uptake in the intestine upon reduced IIS activity.
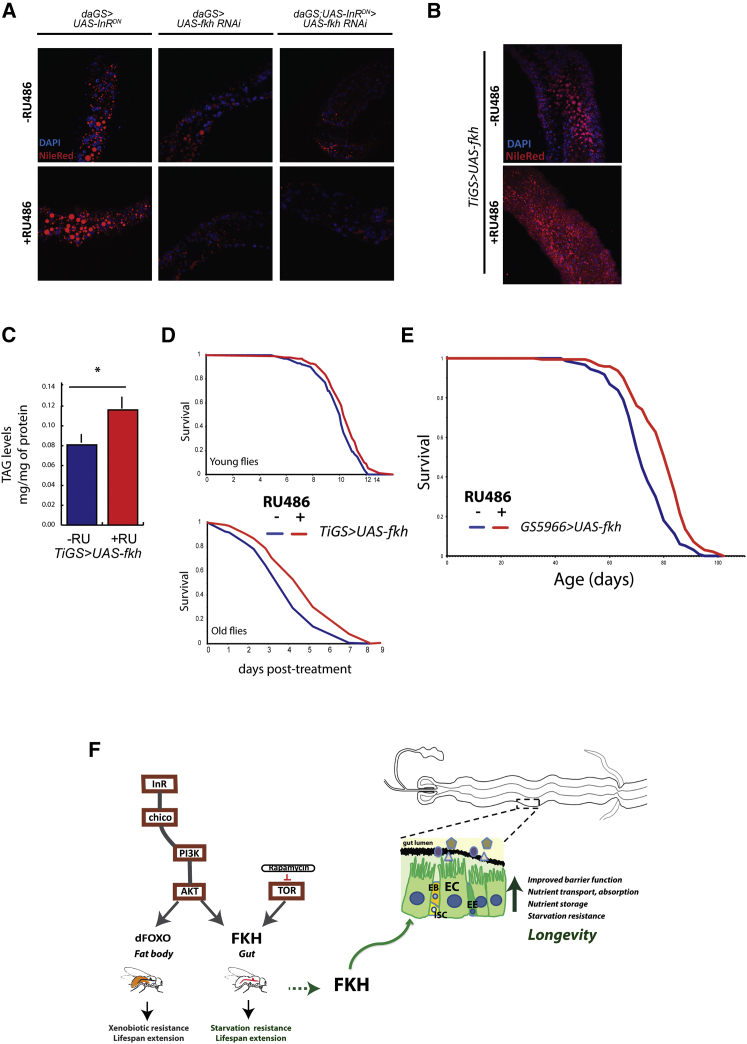
Previous studies highlighted a key role for the fly gut in absorption and metabolism of dietary lipids (Karpac et al., 2013, Luis et al., 2016). The midgut displays an age-related decline in lipid storage, probably due to an overall reduction in intestinal lipid absorption, which results in increased starvation sensitivity (Karpac et al., 2013). We assessed intestinal lipid uptake with Nile Red staining to visualize lipid droplets in midguts with reduced IIS and with or without fkh knockdown. In agreement with previously published work, we detected lipid droplets in the anterior region of the midgut (Karpac et al., 2013, Luis et al., 2016), with a significant increase upon reduced IIS (Figures 7A and S7E; t test, p = 0.0019). This was lost upon fkh knockdown specifically in the guts of reduced-IIS flies (Figures 7A and S7E; t test, p = 0.094). These results suggest that FKH promotes intestinal lipid storage in response to reduced IIS. Gut-specific FKH overexpression also increased Nile Red staining in the midgut (Figures 7B and S7F; t test, p = 2.29 × 10−8), TAG levels in whole flies (Figure 7C; t test, p = 0.033), and resistance to starvation in both young and old flies (Figure 7D; p = 0.0047 and p = 1.74 × 10−5).
Figure 7.
FKH Promotes Intestinal Nutrient Uptake upon Overexpression and in Response to Reduced IIS, and FKH Activity in Differentiated Cells of the Gut Induces Longevity
(A) Nile Red (red) and DAPI (blue) staining of the anterior region of midgut isolates from flies of genotypes daGS > UAS-InRDN, daGS;UAS-InRDN> UAS-fkh RNAi, and daGS > UAS-fkh RNAi induced or not with 200 μM RU486. See also Figure S7E.
(B) Nile Red (red) and DAPI (blue) staining of the anterior region of midgut isolates from flies of genotype TiGS > UAS-fkh induced or not with 200 μM RU486. See also Figure S7F.
(C) Quantification of whole-animal TAG levels normalized to total protein in flies of genotype TiGS < UAS-fkh induced or not with RU486 revealed significant increases upon induction (t test, p = 0.033; n = 10; error bars represent SEM).
(D) Starvation resistance of TiGS > UAS-fkh females was significantly increased in the presence of RU486 at 1 week (p = 0.0047) and 10 weeks of age (p = 1.74 × 10−5).
(E) Intestinal differentiated cell-specific overexpression of UAS-fkh under the control of GS5966 driver extended lifespan (p = 2.03614 × 10−7). See also Table S5.
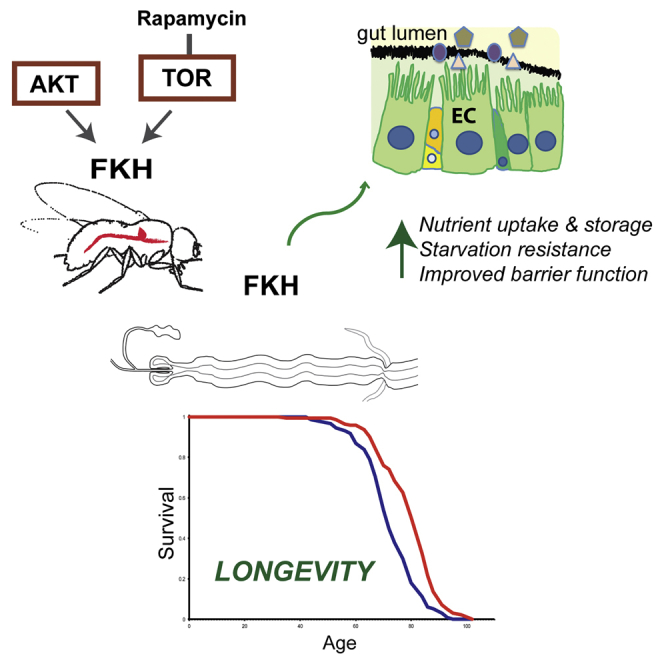
(F) Model. FKH integrates signals from AKT and TOR. dFOXO and FKH exert their pro-longevity effects in different tissues. In the gut, FKH improves barrier function and upregulates the expression of nutrient transporters, resulting in increased nutrient storage and resistance to starvation and overall contributing to increased longevity.
Our findings suggest a role for FKH in nutrient absorption. We next assessed whether increasing FKH activity specifically in differentiated gut cells, namely EEs and absorptive ECs, affected longevity. Upregulation of FKH in differentiated cells resulted in a significant increase in lifespan (Figure 7E; median survival +15%, p = 2.03 × 10−7). This finding further supports the transcriptomic and molecular analysis, and it pinpoints the pro-longevity effect of FKH to EEs and ECs.
irs1 Knockout Upregulates CG1208, pmp70, and zip1 Orthologs in Mouse Small Intestine
Mouse small intestine is functionally and morphologically similar to Drosophila midgut, and it plays an essential role in the uptake of nutrients (Radtke and Clevers, 2005). We therefore measured expression levels of orthologous nutrient transporters in duodenum from female mice with intestinal Irs1 knockout (VillCre::Irs1lox/lox) and controls (Irs1lox/lox) at 3 months of age. We quantified mRNA levels of Glut8, Abcd3, Slc39a3, and Slc31a1 (mouse orthologs of CG1208, pmp70, zip1, and ctr1B, respectively), and we found a significant increase in Abcd3, Glut8, and Slc39a3 expression (Figure S7G; t test, p = 0.006562, p = 0.029, and p = 0.019, respectively) upon intestinal Irs1 knockout. Thus, consistent with our observations in the Drosophila gut, reduced IIS in mouse small intestine upregulates the expression of genes involved in nutrient transport.
Discussion
The major role played by the IIS-and-TOR-signaling network during the control of metabolism, cell growth, and organismal aging has been under investigation for over two decades (Alic and Partridge, 2011). In Drosophila, dFOXO integrates signals from the insulin-PI3K-AKT branch of the IIS pathway (Jünger et al., 2003), and it has been considered to be the key TF mediating many of its transcriptional outputs and its effect on organismal lifespan (Slack et al., 2011). Here we have shown that a second FOX family member, FKH, interacts with both the AKT and TOR branches to regulate organismal lifespan.
While the functionality of the phosphorylation events remains to be determined, based on our biochemical data, we propose a model in which FKH can be phosphorylated by AKT and TOR and integrate signals from them (Figure 7F). IIS activity leads to FKH phosphorylation, potentially contributing to a decrease in nuclear FKH. Insulin-dependent subcellular localization of FoxA2 in mammalian cells has been subject to conflicting reports. While Wolfrum et al., 2003, Wolfrum et al., 2004, reported complete nuclear exclusion of FoxA2, in vitro and in vivo in response to insulin, a study by Zhang et al. (2005) showed that FoxA2 remained nuclear under all metabolic states in mouse hepatocytes. We did not detect complete nuclear FKH exclusion in the fly but a decrease in strong nuclear FKH staining upon in vitro and ex vivo insulin stimulation. Rapamycin feeding was previously shown to increase nuclear FKH in larvae (Bülow et al., 2010, Varma et al., 2014), lending further support to a regulating role of nutrient signaling in FKH subcellular localization in the fly.
We have established an important role for FKH in Drosophila longevity. Ubiquitous upregulation of FKH extends lifespan, and FKH is required for reduced IIS- and rapamycin-induced longevity. We found that FKH mediates starvation resistance of IIS mutants. Interestingly, dfoxo plays no role in either extension of lifespan by rapamycin or in the starvation resistance of Drosophila IIS mutants (Slack et al., 2011). Furthermore, dFOXO is required for xenobiotic resistance of IIS mutants while FKH is not. These findings highlight different functional roles of dFOXO and FKH in determination of the phenotypes of IIS mutants and the response of lifespan to TORC1 inhibition. However, both TFs are necessary for reduced IIS-induced longevity, suggesting that they are functionally complementary. The identification of FKH as a TF downstream of both IIS and TOR branches enables a more insightful picture of the transcriptional regulation within the nutrient-signaling network, and it defies the notion that dFOXO is the key TF regulating Drosophila lifespan downstream of AKT kinase.
We found that the response of lifespan to DR in Drosophila is independent of FKH function, as has previously been shown for dFOXO (Slack et al., 2015). In contrast, the C. elegans FoxA homolog PHA-4 is required for DR-induced longevity, but not reduced IIS-induced lifespan extension (Panowski et al., 2007). Hence, our results show a major evolutionarily functional divergence between PHA-4 and FKH, suggesting distinct mechanisms of organismal lifespan regulation by the worm and fly FoxA orthologs.
Here, we show that intestinal FKH upregulation is sufficient to extend lifespan, whereas gut-specific dFOXO overexpression does not (Alic et al., 2014). The two TFs may, therefore, be modulating lifespan through different target genes in different tissues. We propose that tissue specificity might be the reason for the requirement of multiple TFs with lifespan-extending effects within the nutrient-signaling network, as is the case in worms (Bishop and Guarente, 2007, Libina et al., 2003).
The Drosophila midgut is the functional equivalent of the mammalian stomach and small intestine, and similarly it contains distinct cell types with diverse functions (Lemaitre and Miguel-Aliaga, 2013). ISCs and EBs are progenitor cells, whereas EEs and ECs are the sole two differentiated intestinal cell types. EEs primarily play a secretory role, while ECs are responsible for immune response, absorption of nutrients, and secretion of digestive enzymes. The interaction between IIS activity in Drosophila gut and aging has been intensively studied (Alic et al., 2014, Biteau et al., 2010, Rera et al., 2012). Major interest has focused on ISCs, and previous work showed that reduced IIS in progenitor cells decreases age-induced proliferation of ISCs and extends lifespan (Biteau et al., 2010). However, an exclusively deterministic role for ISCs during aging is contentious (Petkau et al., 2014, Resnik-Docampo et al., 2017). In addition to intestinal homeostasis and stem cell maintenance, other aspects of gut physiology, such as immunity, secretion, and absorption, are also likely to influence organismal aging.
We found that a broader intestinal reduction of IIS activity with TiGS driver also results in lifespan extension but becomes detrimental upon gut-specific FKH knockdown. Despite increased longevity upon broad intestinal upregulation of FKH, restricting its overexpression to intestinal progenitor cells does not affect lifespan. Increasing FKH activity specifically in differentiated cells significantly increases longevity, suggesting this pro-longevity effect is ISC independent. Concordantly, FKH-overexpressing guts display improved barrier function without any changes in age-related ISC proliferation.
Induction of FKH activity by either lowered IIS or direct overexpression induced the expression of nutrient transporters. Aging results in decreased intestinal lipid storage and whole-body nutrient stores (Karpac et al., 2013, Rera et al., 2012), which correlates with age-related repression of lipases essential for lipid absorption (Karpac et al., 2013). Reduced intestinal storage may be due to an overall decline in absorptive capacity, resulting in decreased whole-body metabolic stores. We propose a model whereby FKH-dependent expression of nutrient transporters overcomes this decline and contributes to starvation resistance and longevity (Figure 7F). Concordantly, we show that starvation resistance declines over aging, likely due to increasingly poor nutrient uptake, but it can be enhanced by intestinal FKH induction at both young and old age. Similarly, upon reduced IIS, FKH activity may promote nutrient uptake, leading to better nutrient stores, contributing to increased starvation resistance. Recent work (Luis et al., 2016) revealed increased midgut lipid storage and expression of nutrient transporters in Drosophila ECs in response to DR. It is possible that upregulation of nutrient transporters is promoted by a number of lifespan-extending interventions. Intestinal barrier function is another important aspect of gut physiology improved by FKH, also likely to be contributing to its pro-longevity effects. Absorptive and barrier capacity of gut epithelial cells may well be functionally linked and also reflective of an overall improvement in the maintenance of EC physiology over age. Altogether, our data suggest that the pro-longevity effect of FKH in the gut involves ISC-independent mechanisms.
Finally, irs1 knockout led to the upregulation of three orthologous nutrient transporters in the mouse small intestine. This suggests that an evolutionarily conserved mechanism, by which the reduction of IIS upregulates intestinal nutrient absorption, is also present in mammals. Intriguingly, mammalian FoxAs provide protection against hypoglycemia (Friedman and Kaestner, 2006). We propose that fly and mammalian FoxAs might show functional convergence in promoting nutrient uptake. Further studies are required to dissect out detailed consequences of the upregulation of nutrient transporters on digestive and absorptive capacity of the fly and mammalian gut over aging. In humans, aging causes a decrease in nutrient absorption, and malnourishment is a major problem for many elderly people (Woudstra and Thomson, 2002). Our findings may, therefore, represent new directions for therapeutic interventions to improve human health during aging.
Experimental Procedures
Fly Husbandry
Stocks were maintained and experiments conducted at 25°C on a 12-hr light/dark cycle at 60% humidity, on food containing 10% (w/v) brewer’s yeast, 5% (w/v) sucrose, and 1.5% (w/v) agar, unless otherwise noted. Please see the Supplemental Experimental Procedures for further details.
Statistical Analysis
Statistical analyses were performed in JMP (version 9) software (SAS Institute), R, or Excel (Microsoft). Survival data were analyzed with either log rank test or CPH using Excel (Microsoft) or R using the survival package (Terry Therneau, https://cran.r-project.org/web/packages/survival/index.html). qPCR analysis was performed by using either Student’s t test or a linear model.
Mouse Models and Husbandry
All mice were maintained at 22°C under a 12-hr light/dark cycle (lights on from 6:00 a.m. to 6:00 p.m.). Mice were housed in groups of three to five same-sex littermates under specific pathogen-free conditions within individually ventilated cages (Techniplast UK, Kettering, Northamptonshire, UK). Mice had ad libitum access to normal chow (ssniff R/M-H phytoestrogen-poor [9% fat, 34% protein, and 57% carbohydrate], ssniff Spezialdiäten, Soest, Germany) and water. Female mice were sacrificed at 3 months. Duodenums were dissected and tissues were snap-frozen in liquid nitrogen. Details of the generation of tissue-specific Irs1 knockout (KO) mouse are described in the Supplemental Experimental Procedures.
Ethics Statement
This study was performed in strict accordance with the recommendations and guidelines of the Federation of European Laboratory Animal Science Associations (FELASA). The protocol was approved by the Landesamt für Natur, Umwelt und Verbraucherschutz Nordrhein-Westfalen.
Author Contributions
E.B. and L.P. designed the experiments. E.B., M.K., J.C.R., J.A., M.C.D., and T.N. performed the experiments. E.B., N.A., and D.K.I. analyzed data. J.M.T. and L.P. supervised the project. E.B. and L.P. wrote the manuscript. All authors approved the final submission.
Acknowledgments
We thank Dr. M. Junger for providing valuable reagents and insightful information at the initial steps of the project. This work was funded by a Wellcome Trust Strategic Award (098565), Alzheimer’s Research UK (ART-PG2009-4), and the Max Planck Society.
Published: October 17, 2017
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, seven figures, and eight tables and can be found with this article online at https://doi.org/10.1016/j.celrep.2017.09.042.
Data and Software Availability
The accession number for the microarray data reported in this paper is ArrayExpress: E-MTAB-6056.
Supplemental Information
References
- Alic N., Partridge L. Death and dessert: nutrient signalling pathways and ageing. Curr. Opin. Cell Biol. 2011;23:738–743. doi: 10.1016/j.ceb.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alic N., Andrews T.D., Giannakou M.E., Papatheodorou I., Slack C., Hoddinott M.P., Cochemé H.M., Schuster E.F., Thornton J.M., Partridge L. Genome-wide dFOXO targets and topology of the transcriptomic response to stress and insulin signalling. Mol. Syst. Biol. 2011;7:502. doi: 10.1038/msb.2011.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alic N., Giannakou M.E., Papatheodorou I., Hoddinott M.P., Andrews T.D., Bolukbasi E., Partridge L. Interplay of dFOXO and two ETS-family transcription factors determines lifespan in Drosophila melanogaster. PLoS Genet. 2014;10:e1004619. doi: 10.1371/journal.pgen.1004619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basset A., Khush R.S., Braun A., Gardan L., Boccard F., Hoffmann J.A., Lemaitre B. The phytopathogenic bacteria Erwinia carotovora infects Drosophila and activates an immune response. Proc. Natl. Acad. Sci. USA. 2000;97:3376–3381. doi: 10.1073/pnas.070357597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binks T., Lye J.C., Camakaris J., Burke R. Tissue-specific interplay between copper uptake and efflux in Drosophila. J. Biol. Inorg. Chem. 2010;15:621–628. doi: 10.1007/s00775-010-0629-y. [DOI] [PubMed] [Google Scholar]
- Bishop N.A., Guarente L. Two neurons mediate diet-restriction-induced longevity in C. elegans. Nature. 2007;447:545–549. doi: 10.1038/nature05904. [DOI] [PubMed] [Google Scholar]
- Biteau B., Hochmuth C.E., Jasper H. JNK activity in somatic stem cells causes loss of tissue homeostasis in the aging Drosophila gut. Cell Stem Cell. 2008;3:442–455. doi: 10.1016/j.stem.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biteau B., Karpac J., Supoyo S., Degennaro M., Lehmann R., Jasper H. Lifespan extension by preserving proliferative homeostasis in Drosophila. PLoS Genet. 2010;6:e1001159. doi: 10.1371/journal.pgen.1001159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjedov I., Toivonen J.M., Kerr F., Slack C., Jacobson J., Foley A., Partridge L. Mechanisms of life span extension by rapamycin in the fruit fly Drosophila melanogaster. Cell Metab. 2010;11:35–46. doi: 10.1016/j.cmet.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochkis I.M., Shin S., Kaestner K.H. Bile acid-induced inflammatory signaling in mice lacking Foxa2 in the liver leads to activation of mTOR and age-onset obesity. Mol. Metab. 2013;2:447–456. doi: 10.1016/j.molmet.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchon N., Osman D., David F.P., Fang H.Y., Boquete J.P., Deplancke B., Lemaitre B. Morphological and molecular characterization of adult midgut compartmentalization in Drosophila. Cell Rep. 2013;3:1725–1738. doi: 10.1016/j.celrep.2013.04.001. [DOI] [PubMed] [Google Scholar]
- Bülow M.H., Aebersold R., Pankratz M.J., Jünger M.A. The Drosophila FoxA ortholog Fork head regulates growth and gene expression downstream of Target of rapamycin. PLoS ONE. 2010;5:e15171. doi: 10.1371/journal.pone.0015171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi N.H., Kim J.G., Yang D.J., Kim Y.S., Yoo M.A. Age-related changes in Drosophila midgut are associated with PVF2, a PDGF/VEGF-like growth factor. Aging Cell. 2008;7:318–334. doi: 10.1111/j.1474-9726.2008.00380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy D.J., Gems D., Harshman L.G., Oldham S., Stocker H., Hafen E., Leevers S.J., Partridge L. Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science. 2001;292:104–106. doi: 10.1126/science.1057991. [DOI] [PubMed] [Google Scholar]
- Cornu M., Albert V., Hall M.N. mTOR in aging, metabolism, and cancer. Curr. Opin. Genet. Dev. 2013;23:53–62. doi: 10.1016/j.gde.2012.12.005. [DOI] [PubMed] [Google Scholar]
- de Cabo R., Carmona-Gutierrez D., Bernier M., Hall M.N., Madeo F. The search for antiaging interventions: from elixirs to fasting regimens. Cell. 2014;157:1515–1526. doi: 10.1016/j.cell.2014.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X., Liang Q., Lian T., Wu Q., Gaur U., Li D., Yang D., Mao X., Jin Z., Li Y., Yang M. Rapamycin preserves gut homeostasis during Drosophila aging. Oncotarget. 2015;6:35274–35283. doi: 10.18632/oncotarget.5895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman J.R., Kaestner K.H. The Foxa family of transcription factors in development and metabolism. Cell. Mol. Life Sci. 2006;63:2317–2328. doi: 10.1007/s00018-006-6095-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannakou M.E., Goss M., Jünger M.A., Hafen E., Leevers S.J., Partridge L. Long-lived Drosophila with overexpressed dFOXO in adult fat body. Science. 2004;305:361. doi: 10.1126/science.1098219. [DOI] [PubMed] [Google Scholar]
- Grandison R.C., Piper M.D., Partridge L. Amino-acid imbalance explains extension of lifespan by dietary restriction in Drosophila. Nature. 2009;462:1061–1064. doi: 10.1038/nature08619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison D.E., Strong R., Sharp Z.D., Nelson J.F., Astle C.M., Flurkey K., Nadon N.L., Wilkinson J.E., Frenkel K., Carter C.S. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heintzman N.D., Hon G.C., Hawkins R.D., Kheradpour P., Stark A., Harp L.F., Ye Z., Lee L.K., Stuart R.K., Ching C.W. Histone modifications at human enhancers reflect global cell-type-specific gene expression. Nature. 2009;459:108–112. doi: 10.1038/nature07829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu A.L., Murphy C.T., Kenyon C. Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science. 2003;300:1142–1145. doi: 10.1126/science.1083701. [DOI] [PubMed] [Google Scholar]
- Hwangbo D.S., Gershman B., Tu M.P., Palmer M., Tatar M. Drosophila dFOXO controls lifespan and regulates insulin signalling in brain and fat body. Nature. 2004;429:562–566. doi: 10.1038/nature02549. [DOI] [PubMed] [Google Scholar]
- Jünger M.A., Rintelen F., Stocker H., Wasserman J.D., Végh M., Radimerski T., Greenberg M.E., Hafen E. The Drosophila forkhead transcription factor FOXO mediates the reduction in cell number associated with reduced insulin signaling. J. Biol. 2003;2:20. doi: 10.1186/1475-4924-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapahi P., Zid B.M., Harper T., Koslover D., Sapin V., Benzer S. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr. Biol. 2004;14:885–890. doi: 10.1016/j.cub.2004.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpac J., Biteau B., Jasper H. Misregulation of an adaptive metabolic response contributes to the age-related disruption of lipid homeostasis in Drosophila. Cell Rep. 2013;4:1250–1261. doi: 10.1016/j.celrep.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon C., Chang J., Gensch E., Rudner A., Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- Kinoshita E., Kinoshita-Kikuta E., Takiyama K., Koike T. Phosphate-binding tag, a new tool to visualize phosphorylated proteins. Mol. Cell. Proteomics. 2006;5:749–757. doi: 10.1074/mcp.T500024-MCP200. [DOI] [PubMed] [Google Scholar]
- Lam E.W., Brosens J.J., Gomes A.R., Koo C.Y. Forkhead box proteins: tuning forks for transcriptional harmony. Nat. Rev. Cancer. 2013;13:482–495. doi: 10.1038/nrc3539. [DOI] [PubMed] [Google Scholar]
- Lemaitre B., Miguel-Aliaga I. The digestive tract of Drosophila melanogaster. Annu. Rev. Genet. 2013;47:377–404. doi: 10.1146/annurev-genet-111212-133343. [DOI] [PubMed] [Google Scholar]
- Li Y., Wang W.J., Cao H., Lu J., Wu C., Hu F.Y., Guo J., Zhao L., Yang F., Zhang Y.X. Genetic association of FOXO1A and FOXO3A with longevity trait in Han Chinese populations. Hum. Mol. Genet. 2009;18:4897–4904. doi: 10.1093/hmg/ddp459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libina N., Berman J.R., Kenyon C. Tissue-specific activities of C. elegans DAF-16 in the regulation of lifespan. Cell. 2003;115:489–502. doi: 10.1016/s0092-8674(03)00889-4. [DOI] [PubMed] [Google Scholar]
- Luis N.M., Wang L., Ortega M., Deng H., Katewa S.D., Li P.W., Karpac J., Jasper H., Kapahi P. Intestinal IRE1 Is Required for Increased Triglyceride Metabolism and Longer Lifespan under Dietary Restriction. Cell Rep. 2016;17:1207–1216. doi: 10.1016/j.celrep.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy C.T., McCarroll S.A., Bargmann C.I., Fraser A., Kamath R.S., Ahringer J., Li H., Kenyon C. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature. 2003;424:277–283. doi: 10.1038/nature01789. [DOI] [PubMed] [Google Scholar]
- Panowski S.H., Wolff S., Aguilaniu H., Durieux J., Dillin A. PHA-4/Foxa mediates diet-restriction-induced longevity of C. elegans. Nature. 2007;447:550–555. doi: 10.1038/nature05837. [DOI] [PubMed] [Google Scholar]
- Passtoors W.M., Beekman M., Deelen J., van der Breggen R., Maier A.B., Guigas B., Derhovanessian E., van Heemst D., de Craen A.J., Gunn D.A. Gene expression analysis of mTOR pathway: association with human longevity. Aging Cell. 2013;12:24–31. doi: 10.1111/acel.12015. [DOI] [PubMed] [Google Scholar]
- Pawlikowska L., Hu D., Huntsman S., Sung A., Chu C., Chen J., Joyner A.H., Schork N.J., Hsueh W.C., Reiner A.P., Study of Osteoporotic Fractures Association of common genetic variation in the insulin/IGF1 signaling pathway with human longevity. Aging Cell. 2009;8:460–472. doi: 10.1111/j.1474-9726.2009.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkau K., Parsons B.D., Duggal A., Foley E. A deregulated intestinal cell cycle program disrupts tissue homeostasis without affecting longevity in Drosophila. J. Biol. Chem. 2014;289:28719–28729. doi: 10.1074/jbc.M114.578708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers R.W., 3rd, Kaeberlein M., Caldwell S.D., Kennedy B.K., Fields S. Extension of chronological life span in yeast by decreased TOR pathway signaling. Genes Dev. 2006;20:174–184. doi: 10.1101/gad.1381406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig O., Marr M.T., Ruhf M.L., Tjian R. Control of cell number by Drosophila FOXO: downstream and feedback regulation of the insulin receptor pathway. Genes Dev. 2003;17:2006–2020. doi: 10.1101/gad.1098703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Q., Wang X., Zhou B. Functional studies of Drosophila zinc transporters reveal the mechanism for dietary zinc absorption and regulation. BMC Biol. 2013;11:101. doi: 10.1186/1741-7007-11-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radtke F., Clevers H. Self-renewal and cancer of the gut: two sides of a coin. Science. 2005;307:1904–1909. doi: 10.1126/science.1104815. [DOI] [PubMed] [Google Scholar]
- Regan J.C., Khericha M., Dobson A.J., Bolukbasi E., Rattanavirotkul N., Partridge L. Sex difference in pathology of the ageing gut mediates the greater response of female lifespan to dietary restriction. eLife. 2016;5:e10956. doi: 10.7554/eLife.10956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rera M., Clark R.I., Walker D.W. Intestinal barrier dysfunction links metabolic and inflammatory markers of aging to death in Drosophila. Proc. Natl. Acad. Sci. USA. 2012;109:21528–21533. doi: 10.1073/pnas.1215849110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnik-Docampo M., Koehler C.L., Clark R.I., Schinaman J.M., Sauer V., Wong D.M., Lewis S., D’Alterio C., Walker D.W., Jones D.L. Tricellular junctions regulate intestinal stem cell behaviour to maintain homeostasis. Nat. Cell Biol. 2017;19:52–59. doi: 10.1038/ncb3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robida-Stubbs S., Glover-Cutter K., Lamming D.W., Mizunuma M., Narasimhan S.D., Neumann-Haefelin E., Sabatini D.M., Blackwell T.K. TOR signaling and rapamycin influence longevity by regulating SKN-1/Nrf and DAF-16/FoxO. Cell Metab. 2012;15:713–724. doi: 10.1016/j.cmet.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selman C., Lingard S., Choudhury A.I., Batterham R.L., Claret M., Clements M., Ramadani F., Okkenhaug K., Schuster E., Blanc E. Evidence for lifespan extension and delayed age-related biomarkers in insulin receptor substrate 1 null mice. FASEB J. 2008;22:807–818. doi: 10.1096/fj.07-9261com. [DOI] [PubMed] [Google Scholar]
- Slack C., Giannakou M.E., Foley A., Goss M., Partridge L. dFOXO-independent effects of reduced insulin-like signaling in Drosophila. Aging Cell. 2011;10:735–748. doi: 10.1111/j.1474-9726.2011.00707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack C., Alic N., Foley A., Cabecinha M., Hoddinott M.P., Partridge L. The Ras-Erk-ETS-Signaling Pathway Is a Drug Target for Longevity. Cell. 2015;162:72–83. doi: 10.1016/j.cell.2015.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squarize C.H., Castilho R.M., Bugge T.H., Gutkind J.S. Accelerated wound healing by mTOR activation in genetically defined mouse models. PLoS ONE. 2010;5:e10643. doi: 10.1371/journal.pone.0010643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh Y., Atzmon G., Cho M.O., Hwang D., Liu B., Leahy D.J., Barzilai N., Cohen P. Functionally significant insulin-like growth factor I receptor mutations in centenarians. Proc. Natl. Acad. Sci. USA. 2008;105:3438–3442. doi: 10.1073/pnas.0705467105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatar M., Kopelman A., Epstein D., Tu M.P., Yin C.M., Garofalo R.S. A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science. 2001;292:107–110. doi: 10.1126/science.1057987. [DOI] [PubMed] [Google Scholar]
- Teleman A.A., Hietakangas V., Sayadian A.C., Cohen S.M. Nutritional control of protein biosynthetic capacity by insulin via Myc in Drosophila. Cell Metab. 2008;7:21–32. doi: 10.1016/j.cmet.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Tullet J.M., Hertweck M., An J.H., Baker J., Hwang J.Y., Liu S., Oliveira R.P., Baumeister R., Blackwell T.K. Direct inhibition of the longevity-promoting factor SKN-1 by insulin-like signaling in C. elegans. Cell. 2008;132:1025–1038. doi: 10.1016/j.cell.2008.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma D., Bülow M.H., Pesch Y.Y., Loch G., Hoch M. Forkhead, a new cross regulator of metabolism and innate immunity downstream of TOR in Drosophila. J. Insect Physiol. 2014;69:80–88. doi: 10.1016/j.jinsphys.2014.04.006. [DOI] [PubMed] [Google Scholar]
- Wang L., Lawrence J.C., Jr., Sturgill T.W., Harris T.E. Mammalian target of rapamycin complex 1 (mTORC1) activity is associated with phosphorylation of raptor by mTOR. J. Biol. Chem. 2009;284:14693–14697. doi: 10.1074/jbc.C109.002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel D., Jürgens G., Küttner F., Seifert E., Jäckle H. The homeotic gene fork head encodes a nuclear protein and is expressed in the terminal regions of the Drosophila embryo. Cell. 1989;57:645–658. doi: 10.1016/0092-8674(89)90133-5. [DOI] [PubMed] [Google Scholar]
- Willcox B.J., Donlon T.A., He Q., Chen R., Grove J.S., Yano K., Masaki K.H., Willcox D.C., Rodriguez B., Curb J.D. FOXO3A genotype is strongly associated with human longevity. Proc. Natl. Acad. Sci. USA. 2008;105:13987–13992. doi: 10.1073/pnas.0801030105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfrum C., Besser D., Luca E., Stoffel M. Insulin regulates the activity of forkhead transcription factor Hnf-3beta/Foxa-2 by Akt-mediated phosphorylation and nuclear/cytosolic localization. Proc. Natl. Acad. Sci. USA. 2003;100:11624–11629. doi: 10.1073/pnas.1931483100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfrum C., Asilmaz E., Luca E., Friedman J.M., Stoffel M. Foxa2 regulates lipid metabolism and ketogenesis in the liver during fasting and in diabetes. Nature. 2004;432:1027–1032. doi: 10.1038/nature03047. [DOI] [PubMed] [Google Scholar]
- Woudstra T., Thomson A.B. Nutrient absorption and intestinal adaptation with ageing. Best Pract. Res. Clin. Gastroenterol. 2002;16:1–15. doi: 10.1053/bega.2001.0262. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T., Kakefuda R., Tajima N., Sowa Y., Sakai T. Antitumor activities of JTP-74057 (GSK1120212), a novel MEK1/2 inhibitor, on colorectal cancer cell lines in vitro and in vivo. Int. J. Oncol. 2011;39:23–31. doi: 10.3892/ijo.2011.1015. [DOI] [PubMed] [Google Scholar]
- Zerofsky M., Harel E., Silverman N., Tatar M. Aging of the innate immune response in Drosophila melanogaster. Aging Cell. 2005;4:103–108. doi: 10.1111/j.1474-9728.2005.00147.x. [DOI] [PubMed] [Google Scholar]
- Zhang L., Rubins N.E., Ahima R.S., Greenbaum L.E., Kaestner K.H. Foxa2 integrates the transcriptional response of the hepatocyte to fasting. Cell Metab. 2005;2:141–148. doi: 10.1016/j.cmet.2005.07.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.