Abstract
Introduction
Extra-anastomotic intraluminal recurrence of the colon cancer after curative surgery was rarely reported but intraluminal ileal relapse has not been described to date.
We report a case of intraluminal ileal tumor arising after curative right hemicolectomy that could be ascribed to an implantation of exfoliated cancer cells.
Case report
A 71-years old man was admitted with no metastatic stenotic adenocarcinoma of the hepatic flexure and submitted, without preoperative bowel preparation, to right hemicolectomy using a “no-touch” technique. Histology showed moderately differentiated adenocarcinoma without lymph nodes involvement (pT3N0). No adjuvant therapy was prescribed. First colonoscopy three months after surgery was negative but a second endoscopic examination nine months later revealed an ileal neoplasia, presenting like an ulcer 10 cm proximally to ileocolic anastomosis. A new ileo-colic resection including past anastomosis was performed with curative intent. Pathological examination showed moderately differentiated adenocarcinoma extended to peri-visceral fat tissue with 10 tumor-free lymph nodes. (pT3N0). Six courses of Capecitabine adjuvant chemotherapy was prescribed and 32 months after second surgery, the patient is alive without disease.
Discussion
In the present case, the relatively short time from the primary surgery and the fact that recurrence occurred outside the anastomosis suggest that implantation of exfoliated malignant cells seems to be the main pathogenetic mechanism. We suppose that the high grade of primary cancer and the occlusive condition could have promoted the cancer cells reflux through the ileocecal orifice and in the transverse colon.
Conclusion
This case seems to confirm the intraluminal implanting capacity of exfoliated carcinoma cells.
Keywords: Colon cancer, Locoregional relapse, Intraluminal recurrence, Exfoliated cancer cells
Highlights
-
•
Extra-anastomotic intraluminal recurrence of the colon cancer after surgery was rarely reported.
-
•
We report a unique case of intraluminal ileal tumor recurrence after curative right hemicolectomy.
-
•
This case confirms the capacity of implanting of the exfoliated carcinoma cells in the bowel in the extra-anastomotic site.
1. Introduction
In patients submitted to curative surgery for colon cancer, distant relapse remains the most common cause of cancer-related mortality [1]. In a large series, locoregional recurrence (LR) is estimated to occur in 11.5% of cases, with severe impact on survival and morbidity [2]. Anastomotic recurrence has been described with higher incidence after anterior resection of the rectum in comparison to proximal colo-colic or ileo-colic anastomosis [3]. Extra-anastomotic intraluminal recurrence in the proximal lumen of the colon has been reported in only two single case reports after surgery for obstructive sigmoid or rectal cancer [4], [5] but proximal intraluminal ileal recurrence of colon cancer has not been described to date.
Here we report a case of intraluminal ileal tumor arising one year after curative right hemicolectomy for hepatic flexure obstructive adenocarcinoma that could be ascribed to an implantation of exfoliated cancer cells. The work has been reported in line with SCARE criteria [6].
2. Case report
A 71-years old caucasian man was admitted to the Surgical Department for bowel occlusion. His medical history revealed mild hypertension and diabetes under medication. He had no family history of gastrointestinal neoplasia. A CT scan revealed bowel obstruction in hepatic flexure of the colon but no metastases in other organs were detected. Colonoscopy identified a stenotic adenocarcinoma. Circulating CEA level was normal. Because of intestinal occlusion no bowel preparation was administered and, five days after admission, the patient was submitted to surgery. The procedure was performed by M.C. a surgeon with great experience in colonic cancer surgery.
At laparotomy, the presence of a stenotic neoplasia of the hepatic flexure was confirmed with distension of cecum and distal ileum due to ileo-cecal valve incontinence. A right hemicolectomy was performed using a “no-touch” technique followed by hand-sewn latero-lateral iso-peristaltic ileocolic anastomosis. Histology showed adenocarcinoma moderately differentiated (G2) with widespread necrosis, extended from visceral wall to peri-colic fat tissue. Resection's margins (38 cm proximally and 17 cm distally) were free from disease. No metastasis in 29 loco-regional lymph nodes were found (pT3N0 according to TNM classification) (Fig. 1). Post-operative course was uneventful and patient was discharged in the eighth post-operative day. No adjuvant therapy was prescribed. Endoscopic follow-up at three months was negative for luminal recurrence. One year after surgery further follow-up colonoscopy showed an ulcerative lesion (diameter about 7 mm) on the ileum. Biopsy of lesion revealed moderately differentiated adenocarcinoma. No distant metastases were detected by CT-scan and CEA circulating level was normal. Based on these findings, we performed an ileo-colic resection including past anastomosis (Fig. 2). A new hand-sewn latero-lateral iso-peristaltic ileocolic anastomosis was made between resected viscera. This second procedure was performed with curative intent by the same surgical team that carried out previous left hemicolectomy.
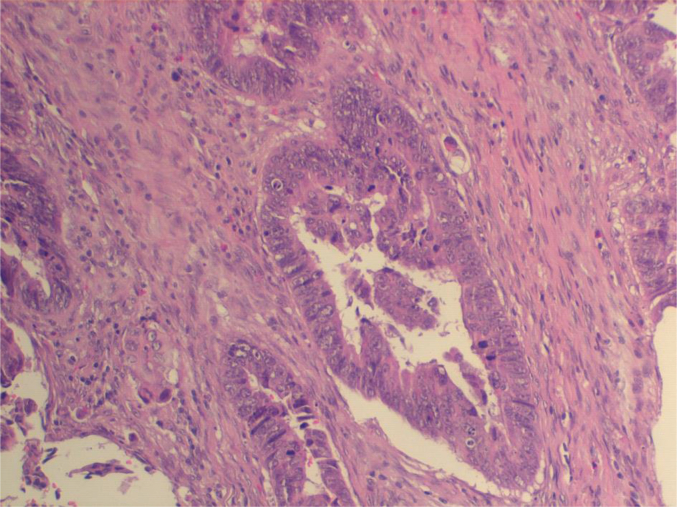
Fig. 1.
Adenocarcinoma of right colon, moderately differentiated (G2), extended from visceral wall to peri-colic fat tissue with widespread necrosis. he; 20 ×.
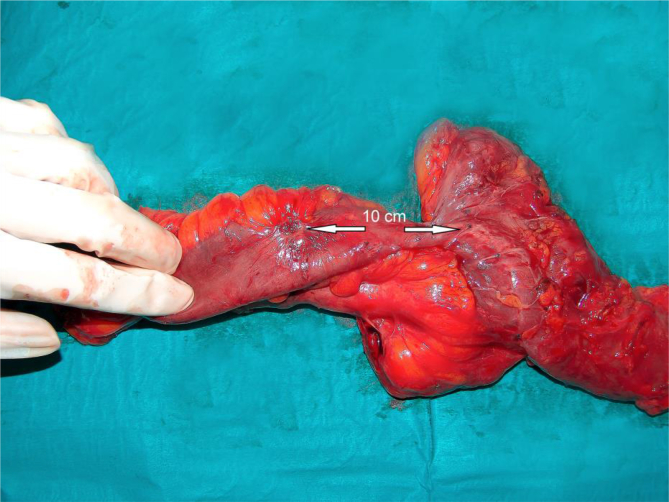
Fig. 2.
Ileal lesion is situated about 10 cm from previous anastomosis.
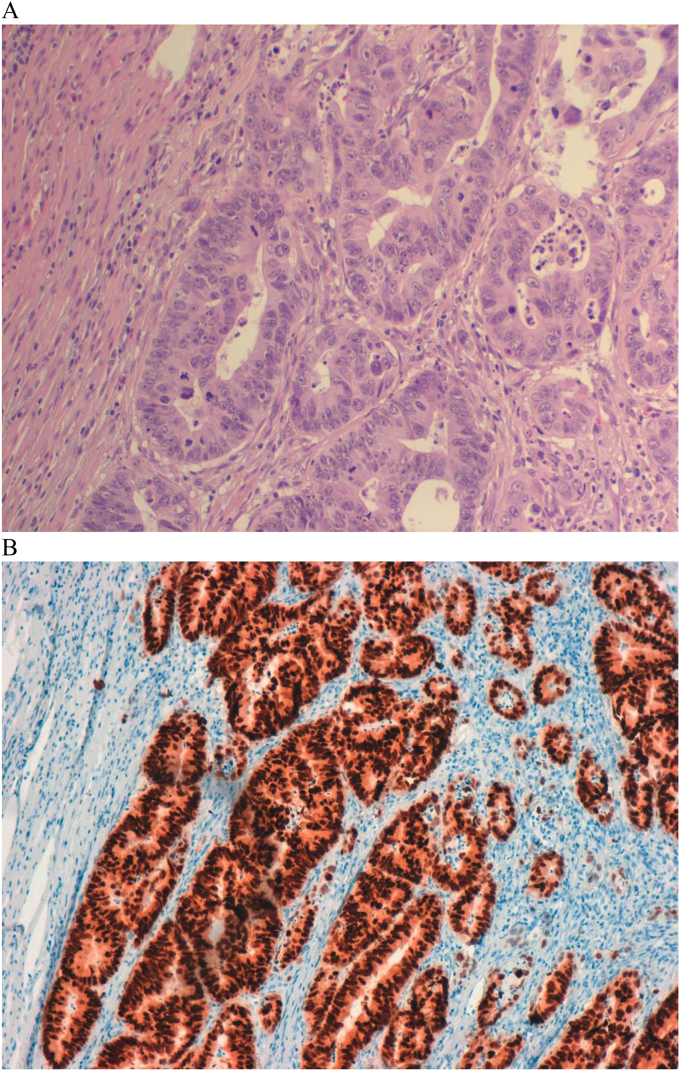
Pathological examination confirmed the pre-operative diagnosis of moderately differentiated (G2) adenocarcinoma (Fig. 3a), located 10 cm proximally to anastomosis, extended to peri-visceral fat tissue, with 10 tumor-free lymph nodes. CDX2 Immunostaining was coherent with colonic type adenocarcinoma. (Fig. 3b). Resection's margins were free from disease (pT3N0 according to TNM classification). Post-operative course was uneventful and the patient was discharged in ninth post-operative day. The patient received Capecitabine adjuvant chemotherapy for six courses one month after surgery and was followed-up in outpatient department. He was undervent every three months to clinical evaluation, abdominal ultrasound and circulating CEA determination. Colonscopy was performed three months after surgery and thereafter early as well as CT scan. Thirty-two months after second surgery, the patient is alive without disease.
Fig. 3.
A: Colonic adenocarcinoma (ileal lesion), moderately differentiated (G2), extended to peri-visceral fat tissue. he; 20 ×. B: CDX2 Immunostaining is coherent with colonic type adenocarcinoma.
3. Discussion
LR after curative colectomy for adenocarcinoma of the intraperitoneal colon has been described with a wide variability (6%–28%) [6], [7]. This seems to reflect a not homogeneous definition of “local” recurrence, with some authors reporting only intraluminal anastomotic recurrence as local failure after resection with curative intents [7], [8].
The pathogenesis has been related to several factors including tumor and host characteristics and technical aspects such as the wide resection of the mesocolon, the extent of lymphadenectomy and the resection margins [7], [8], [9]. As concerning the tumor characteristics, LR occurred rarely in the absence of primary T4 or N2 disease [10].
When LR occurred inside the lumen, it seems arise quite exclusively at the anastomotic site, with an incidence all the more relevant given the primary tumor proximity and for left-sided in comparison to right-sided or ileo-colic anastomosis [11]. The two most credited pathogenetic theories are: 1) the implantation of exfoliated cancerous cells in the suture line [12]; 2) the metachronous carcinogenesis of another colonic tumor [13].
Several studies have indicated that cancer cells are shed into the bowel lumen during colorectal cancer resection and that these cells are viable and capable of implanting at the site of tissue injury [14], [15]. Using irrigation-fluid specimens from patients with colon cancer, Umpleby et al. [14] demonstrated that exfoliated malignant cells existed at the oral and anal stumps in 57% and 84% of their patients, respectively. Hasegawa et al. [11] examined the lavage fluid obtained from both bowel stumps after right hemicolectomy, detecting exfoliated malignant cells in the terminal ileum and distal colon samples from 11.1% to 55.6% of the patients, respectively. The authors speculate that the lower frequency of exfoliated malignant cells in the distal ileum should be explained by the reflux prevention of the ileocecal orifice and bowel peristalsis.
More recently other Authors reported in patients submitted to colon resection with functional end-to-end colo-colic anastomosis that the detection rate of exfoliated colon cancer cells on the linear stapler cartridges was 20% and it was significantly associated with depth of tumor invasion and lack of preoperative bowel preparation [16]. About cancer characteristics, aggressive histological type, poor differentiation (G3) and advanced tumor stage are the main biological risk factors [17].
Intraluminal extra-anastomotic recurrence of colonic cancer is an extremely rare phenomenon and has been reported only in two single case reports. The first case was an intraluminal metastasis at the colostomy site after Hartmann's procedure for obstructive rectal cancer [4]. The second case was a recurrence of sigmoid cancer in the proximal colonic lumen affected by obstructive colitis [5]. It should be noted that in both cases the luminal recurrences arise from an injured colonic mucosa confirming the experimental hypothesis that a damaged mucosa in the presence of viable colon cancer cells may favorite implantation and intraluminal tumor growth [18].
We reported a case of intraluminal cancer localized 10 cm before the ileo-colic anastomosis, diagnosed one year after right hemicolectomy for obstructive adenocarcinoma. In our case, the pathological examination of all specimens showed tumor-free margins, as well as the absence of lymph-vascular invasion and no lymph node involvement. The relatively short time from the primary surgery and the fact that recurrence occurred outside the anastomosis suggest that metachronous carcinogenesis is highly doubtful and the implantation of exfoliated malignant cells seems to be the most likely mechanism of recurrence.
Studies aimed at preventing anastomotic recurrence attributable to exfoliated cancer cells have been conducted mainly in patients undergoing anterior resection but at the present, efficacy of these procedures are controversial. Some authors reported that irrigating the intestinal lumen by normal saline may be an effective measure to clear exfoliated carcinoma cells [14]. Other researchers have demonstrated that irrigation of the lumen with 5% povidone-iodine is useful to prevent anastomotic recurrence [19]. Regarding preoperative bowel preparation with powder polyethylene glycol (PEG), Ikehara et al. [16] found that the rate of exfoliated cancer cells was 11.8% in patients with PEG, while it was 37.5% in patients without PEG.
In our case, no preoperative bowel preparation with PEG was administered as consequence of the intestinal occlusion and we suppose that the high grade of primary cancer and the occlusive condition could have promoted the cancer cells reflux through the ileocecal orifice and in the transverse colon.
As regarding the surgical therapy of LR, the ability to achieve complete resection was associated with single site recurrence, disease limited to the peri-anastomotic region, absence of distant metastases and normal CEA value [20]. The patients with extraluminal tumor recurrence have a lower rate of curative resections and, even when surgery with curative intents was carried out, patients with extraluminal tumor recurrence survived lower compared to patients with an intraluminal tumor recurrence [20]. Our patient develops localized intraluminal recurrence without distant metastases and normal CEA value. The patient received a curative resection respecting the preoperative perspective and he is alive without disease 32 months after second surgery.
In conclusion, intraluminal extra-anastomotic recurrence of colon cancer is a very rare form of cancer relapse and reported in only two single case report. We report the first case described in literature of colon cancer recurrence in the ileal stump after curative right hemicolectomy. We retain that this case confirms the possibility of exfoliated carcinoma cells implantation in the extra-anastomotic site. The hypothesis of metachronous carcinogenesis of another colonic tumor is, in our opinion, less convincing.
Sources of funding
All the authors declare that they have no source of funding.
Author contribution
Clementi M and Colozzi S contributed the original idea and steasure of the manuscript.
Clementi M and Schietroma M contributed by conceptualization and performing the surgical procedures.
Chiominto A, Colozzi S, Sista F and Della Penna A contributed by collecting all the data.
Guadagni S. contributed by conceptualization and revision of the manuscript.
The final manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us.
Conflicts of interest
All the authors declare that they have no conflict of interest.
Research registry
researchregistry2438.
Guarantor
Marco Clementi.
Consent
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
References
- 1.Elferink M.A., Visser O., Wiggers T. Prognostic factors for locoregional recurrences in colon cancer. Ann. Surg. Oncol. 2012;19(7):2203–2211. doi: 10.1245/s10434-011-2183-4. [DOI] [PubMed] [Google Scholar]
- 2.Sjovall A., Granath F., Cedermark B., Glimelius B., Holm T. Loco-regional recurrence from colon cancer: a population-based study. Ann. Surg. Oncol. 2007;14(2):432–440. doi: 10.1245/s10434-006-9243-1. [DOI] [PubMed] [Google Scholar]
- 3.Kyzer S., Gordon P.H. The stapled functional end-to-end anastomosis following colonic resection. Int. J. Colorectal Dis. 1992;7:125–131. doi: 10.1007/BF00360351. [DOI] [PubMed] [Google Scholar]
- 4.Papaziogas B., Koutelidakis G., Chatzimavroudis G., Tsiaousis P., Atmatzidis K. Intraluminal metastasis at the colostomy site after Hartmann's procedure for obstructive rectal cancer. Tech. Coloproctol. 2006;10(4):365–366. [PubMed] [Google Scholar]
- 5.Mekata E., Shimizu T., Endo Y., Tani T. The rapid growth of intraluminal tumor metastases at the intestinal wall sites damaged by obstructive colitis due to sigmoid colon cancer: report of a case. Surg. Today. 2008;38:862–865. doi: 10.1007/s00595-007-3708-0. [DOI] [PubMed] [Google Scholar]
- 6.Agha R.A., Fowler A.J., Saetta A., Barai I., Rajmohan S., Orgill D.P., the SCARE Group The SCARE statement: consensus-based surgical case report guidelines. Int. J. Surg. 2016;34:180–186. doi: 10.1016/j.ijsu.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 7.Enker W.E., Laffer U.T., Block G.E. Enhanced survival of patients with colon and rectal cancer is based upon wide anatomic resection. Ann. Surg. 1979;190:350–360. doi: 10.1097/00000658-197909000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Slanetz C.A., Jr., Grimson R. Effect of high and intermediate ligation on survival and recurrence rates following curative resection of colorectal cancer. Dis. Colon Rectum. 1997;40:1205–1218. doi: 10.1007/BF02055167. [DOI] [PubMed] [Google Scholar]
- 9.Guadagni S., Aigner K.R., Fiorentini G., Cantore M., Clementi M., Chiominto A. Pelvic perfusion for rectal cancer. In: Aigner K.R., Stephens F.O., editors. Induction Chemotherapy. Springer-Verlag Berlin Heidelberg; 2016. pp. 293–307. [Google Scholar]
- 10.Read T.E., Mutch M.G., Chang B.W., McNevin M.S., Fleshman J.W., Birnbaum E.H. Locoregional recurrence and survival after curative resection of adenocarcinoma of the colon. J. Am. Coll. Surg. 2002 Jul;195(1):33–40. doi: 10.1016/s1072-7515(02)01224-3. [DOI] [PubMed] [Google Scholar]
- 11.Hasegawa J., Nishimura J., Yamamoto S., Yoshida Y., Iwase K., Kawano K. Exfoliated malignant cells at the anastomosis site in colon cancer surgery: the impact of surgical bowel occlusion and intraluminal cleaning. Int. J. Colorectal Dis. 2011 Jul;26(7):875–880. doi: 10.1007/s00384-011-1148-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Umpleby H.C., Williamson R.C. Anastomotic recurrence in large bowel cancer. Br. J. Surg. 1987;74:873–878. doi: 10.1002/bjs.1800741003. [DOI] [PubMed] [Google Scholar]
- 13.Roe R., Fermor B., Williamson R.C. Proliferative instability and experimental carcinogenesis at colonic anastomoses. Gut. 1987;28:312–315. doi: 10.1136/gut.28.7.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Umpleby H.C., Fennor B., Symes M.O., Williamson R.C.N. Viability of exfoliated colorectal carcinoma cells. Br. J. Surg. 1984;71:659–663. doi: 10.1002/bjs.1800710902. [DOI] [PubMed] [Google Scholar]
- 15.Skipper D., Jeffrey M., Cooper A.J., Taylor I., Alexander P. Enhanced growth of tumor cells in healing colonic anastomoses and laparotomy wounds. lnt. J. Color. Dis. 1989;4:172–177. doi: 10.1007/BF01649697. [DOI] [PubMed] [Google Scholar]
- 16.Ikehara K., Endo S., Kumamoto K., Hidaka E., Ishida F., Tanaka J., Kudo S.E. Positive detection of exfoliated colon cancer cells on linear stapler cartridges was associated with depth of tumor invasion and preoperative bowel preparation in colon cancer. World J. Surg. Oncol. 2016 Aug 31;14(1):233–237. doi: 10.1186/s12957-016-0991-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Law W.L., Chu K.W. Local recurrence following total mesorectal excision with double-stapling anastomosis for rectal cancers: analysis of risk factors. World J. Surg. 2002;26:1272–1276. doi: 10.1007/s00268-002-6560-9. [DOI] [PubMed] [Google Scholar]
- 18.Broyn T. Tumour implantation in ischemic, thermic and mechanical lesions of the colonic mucosa. An experimental study in rats. Acta Chir. Scand. 1972;138(2):202–206. [PubMed] [Google Scholar]
- 19.Agaba E.A. Does rectal washout during anterior resection prevent local tumor recurrence? Dis. Colon Rectum. 2004;47:291–296. doi: 10.1007/s10350-003-0046-1. [DOI] [PubMed] [Google Scholar]
- 20.Bowne W.B., Lee B., Wong W.D., Ben-Porat L., Shia J., Cohen A.M. Operative salvage for locoregional recurrent colon cancer after curative resection: an analysis of 100 cases. Dis. Colon Rectum. 2005 May;48(5):897–909. doi: 10.1007/s10350-004-0881-8. [DOI] [PubMed] [Google Scholar]