Abstract
Aim: The effect of nitroglycerin on proper arterial stiffness of the arterial tree has not been fully clarified. The cardio-ankle vascular index (CAVI), which is an application of the stiffness parameter β theory on the arterial tree from the origin of the aorta to the ankle, was developed recently. Furthermore, the stiffness of the aorta (heart-thigh β (htBeta)) and of the femoral-tibial arteries (thigh to ankle β (taBeta)) could be monitored by applying the same theory. The effects of nitroglycerin on CAVI, htBeta, and taBeta were studied comparing the values of healthy people and those of arteriosclerotic patients.
Methods: The subjects were healthy people (CAVI < 7.5, n = 25) and arteriosclerotic patients (CAVI > 9, n = 25). Nitroglycerin (0.3 mg) was administrated sublingually, and various arterial stiffness indices were measured at one-minute intervals for a period of 20 minutes using Vasera VS-1500 (Fukuda Denshi, Tokyo).
Results: After the administration of nitroglycerin in healthy people, CAVI decreased significantly after 5 min. [from 6.76(6.32–7.27) to 5.50(4.70–6.21), P < 0.05], and recovered after 15 min. htBeta [from 5.10(4.76–5.76) to 3.96(3.35–4.79), P < 0.05], and taBeta [from 14.41(10.80–16.33) to 10.72 (9.19–13.01), P < 0.05] also decreased significantly. In arteriosclerotic patients, CAVI decreased after 5 min. [from 10.47(9.67–11.29) to 9.71(8.74–10.57), P < 0.05] and recovered after 15 min. htBeta did not significantly change [from 12.00(11.46–13.21) to 11.81(10.14–13.83), ns], but taBeta decreased significantly [from 18.55(12.93–23.42) to 12.37(9.68–16.99), P < 0.05].
Conclusion: These results indicate that a nitroglycerin-induced decrease of arterial stiffness is more prominent in muscular arteries than in elastic arteries, and this effect was preserved much more prominently in arteriosclerotic patients than in healthy people.
Keywords: Arterial stiffness, Cardio-ankle vascular index, Nitroglycerin, Stiffness parameter
Introduction
Nitroglycerin (NTG) is used to relieve the symptoms of angina pectoris, and this effect is thought to be due to a dilation of the coronary arteries leading to increased coronary blood flow1, 2). Furthermore, veins are also reported to dilate with the use of NTG, and these effects are thought to decrease preload on the heart3). Moreover, it has been suggested that NTG may increase the distensibility of peripheral muscular arteries and reduce systemic resistance; this action would explain the manner by which NTG reduces the myocardial oxygen need and relieves symptoms1, 2, 4, 5). As a method for quantitative assessment of arterial stiffness, pulse wave velocity (PWV) has been used for the last several decades, and it was thought to be a kind of surrogate marker of arteriosclerosis3, 6–9). However, PWV depends inherently on blood pressure (BP) changes at the time of measurement9, 10).
Therefore, there have been difficulties in interpreting the data dealing with various therapies or conditions associated with blood pressure changes9, 10).
Recently, the cardio-ankle vascular index (CAVI) was developed as an arterial stiffness index, which was derived from the stiffness parameter beta theory11) with the application of the Bramwell-Hill equation12). It is essentially independent from BP at the time of measurement, and it reflects the stiffness of the arterial tree from the origin of the aorta to the ankle13). CAVI was almost established to be a surrogate marker of arteriosclerosis14–16) such as coronary arterial disease17, 18), cerebral infarction19), and chronic kidney disease20). CAVI values are also elevated in most patients exhibiting various coronary risk factors and are decreased by treating these risk factors14, 21–24).
The stiffness monitored with CAVI is partly composed of functional stiffness. When the α1 adrenoceptor blocker, doxazosin, was administered to men, blood pressure decreased and CAVI also decreased, indicating that CAVI also reflected the condition of arterial smooth muscle contraction15).
Recently, we reported that CAVI was decreased by NTG administration in healthy people and arteriosclerotic patients25). This is the first report to demonstrate the effect of NTG on proper arterial stiffness of the arterial tree quantitatively in vivo. However, the arterial tree to which CAVI is applied is composed of the aorta, as an elastic artery, and the femoral-tibial arteries as muscular arteries. The specificity of the effect of NTG on a decrease of arterial stiffness in the elastic arteries and muscular arteries has not been determined.
In this paper, we studied whether the arterial stiffness changes caused by NTG are due to its effect on the aorta, or on the femoral and tibial arteries.
In order to measure arterial stiffness of the aorta as an elastic artery, and of the femoral-tibial arteries as muscular arteries separately, we applied the stiffness parameter β theory to those arteries by utilizing Bramwell-Hill's equation in the same way as it is applied when measuring CAVI. In this way, the stiffness of the aortic artery is called heart to thigh Beta (htBeta), and that of the femoral-tibial arteries is called thigh to ankle Beta (taBeta). Furthermore, we compared changes between healthy people (HP) and arteriosclerotic patients (AP).
In addition to this parameter, we analyzed the changes in blood pressure, stroke volume, cardiac output, and systemic vascular resistance during NTG administration in order to understand the role of arterial stiffness of the main arterial tree in systemic circulation.
Methods
Study Subjects:
This study was conducted at Sakura Hospital Medical Center, Toho University. Subjects in the first group were 25 healthy people (HP) aged 25–37 years old. They had no hypertension, diabetes mellitus, or dyslipidemia, and they had no history of arteriosclerotic disease.
The subjects of the second group were 25 people (AP) with a history of cardiovascular disease who undertook percutaneous coronary interventions (PCI) or coronary bypass graft (CABG) operations.
Measuring the Cardio-Ankle Vascular Index (CAVI):
The subjects lay down on a bed in a supine position and took an oral dose of NTG 0.3 mg. From that moment, at intervals of one minute, blood pressure, CAVI, pulse rate, and cardiac output were measured. CAVI was measured using the VaSera VS-1500 machine (Fukuda Denshi, Tokyo) as previously described14). This index was originally derived from stiffness parameter β, proposed by Hayashi11) and was applied to a length of artery with the application of a modified version of Bramwell-Hill's equation12).
CAVI = a{(2 ρ / δP) × ln(Ps/Pd)PWV2} + b ------ CAVI formula
where Ps is systolic blood pressure, Pd is diastolic blood pressure, PWV is pulse wave velocity from the origin of the aorta to the tibial artery at the ankle through the femoral artery, ΔP is Ps-Pd, ρ is blood density, and a and b are constants in order to adjust the values of CAVI to those of Hasegawa's PWV24).
Measuring Heart Thigh Beta (htBeta), and Thigh to Ankle Beta (taBeta) (refer to Fig. 1):
Fig. 1.
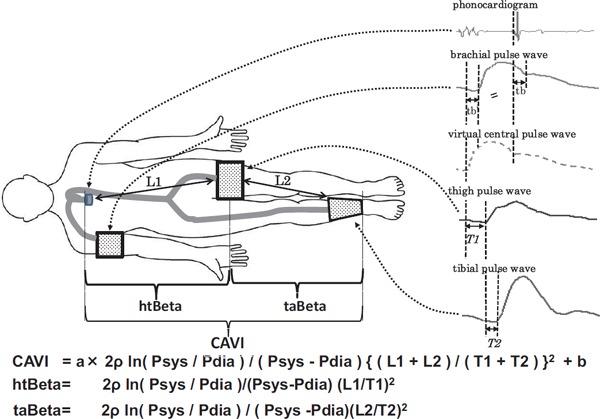
A diagram showing the measurement of arterial stiffness of various portions of the arterial tree with the cardio-ankle vascular index, htBeta and taBeta
htBeta, which indicates the stiffness of the aorta, was calculated by measuring the pulse wave velocity from the origin of the aorta to the upper portion of the femoral artery and blood pressure at the upper brachial artery. These values were introduced into the equation above. taBeta, which indicates the stiffness of the femoral-tibial arteries, was calculated by measuring the pulse wave velocity from the upper portion of the femoral artery to the ankle and blood pressure at the upper brachial artery. These parameter values were introduced into the equation without “a” and “b” constants of the CAVI formula as stated above. htBeta and taBeta values were thus obtained.
htBETA and taBETA were measured essentially using the same method as the CAVI method. Therefore, reproducibility of htBETA and taBETA are supposed to be about the same as CAVI's reproducibility of about a 3.8 percent coefficient of variation13).
In this study, we measured these parameters every minute to clarify the precise dynamics during NTG administration. Furthermore, blood pressure and PWV values were measured at one-minute intervals in each portion.
Blood pressure was measured using an oscillometric method at the right upper brachial portion. The blood pressure value used for taBeta, should ideally be measured in the leg. However, in practice it is difficult to measure every minute in the leg while detecting pulse waves. To confirm the rationale for the usage of blood pressure at the brachial artery in place of the leg, the CAVI values were compared between brachial blood pressure and leg blood pressure. During NTG administration, the CAVI values were not significantly different when utilizing blood pressures at either location (after 5 minutes ΔCAVI: −1.32 ± 0.68 vs. −1.39 ± 0.71, after 10 minutes ΔCAVI: −1.06 ± 0.69 vs. −1.06 ± 0.67, after 15 minutes Δ CAVI: −0.87 ± 0.66 vs. −0.80 ± 0.67, after 20 minutes ΔCAVI: −0.57 ± 0.68 vs. −0.46 ± 0.67, N = 31 in healthy people; after 5 minutes ΔCAVI: −1.66 ± 0.87 vs. −1.65 ± 0.86, after 10 minutes ΔCAVI: −1.79 ± 0.89 vs. −1.79 ± 0.88, after 15 minutes ΔCAVI: −1.59 ± 1.12 vs. −1.60 ± 1.09, after 20 minutes Δ CAVI: −1.37 ± 1.07 vs. −1.37 ± 1.05, N = 25 in arteriosclerotic patients). This was the case among both healthy people and arteriosclerotic patients. Therefore, to measure taBeta, we used blood pressure at the brachial artery in place of the leg artery.
Measurement of Cardiac Stroke Volume and Cardiac Output, and Calculation of the Systemic Vascular Resistance:
The changes of cardiac stroke volume and cardiac output were continuously monitored using the Aesculon mini machine (Osypka medical, California, USA)26). Systemic vascular resistance (SVR) was calculated by dividing the mean brachial blood pressure minus constant central venous pressure with cardiac output27).
SVR = 80(mean arterial pressure-central venous pressure)/cardiac output (dyn sec/cm5).
Central venous pressure was supposed to be 5 mmHg in this case.
Statistical Analysis:
The change of CAVI, htBeta, taBeta, blood pressure, stroke volume, heart rate, cardiac output, and systemic vascular resistance during the administration of nitroglycerin in healthy people and arteriosclerotic patients were expressed as the median (interquartile range).
Median values of the parameter before administrated NTG and after NTG administrated were compared using a Wilcoxon signed rank test.
Median values of the change of the parameter after NTG were administered in healthy people and that in arteriosclerotic patients were compared using the Mann-Whitney U-test.
Clinical backgrounds of the studied subjects were expressed as the mean ± standard deviation. The mean values were compared using Unpaired Student t-test.
As for the analogy compared between brachial blood pressure and ankle blood pressure for the calculation of CAVI, data were expressed as the mean ± standard deviation. The mean values were compared using Paired t-test.
Statistically significant differences were considered at P < 0.05.
All statistical analyzes were performed using the statistical package SPSS Version 22.0 (IBM, Chicago, IL, USA).
Ethics
All participants gave written informed consent after a detailed description of the procedures in accordance with the Declaration of Helsinki, and the study protocol was approved by the ethics committee of the Faculty of Medicine, Toho University (Approved No. 26001).
Measurement of Intimal Wall Thickness of Carotid Artery:
Cardiac echocardiography were taken by skilled technician using Philips iE33 (Amsterdam, The Netherlands) and carotid ultrasonography were taken by skilled technician using Toshiba AplioXG (Tokyo, Japan).
Plaque score was calculated as reported previously28). IMT was measured as reported previously29).
Results
Patient characteristics are shown in Table 1.
Table 1. The clinical backgrounds of the studied subjects.
| Variable | Healthy people | Arteriosclerotic patients | Significance |
|---|---|---|---|
| (n = 25) | (n = 25) | ||
| Age (years) | 30.9 ± 3.9 | 72 ± 6.4 | P < 0.001 |
| Male | 25 (100%) | 21 (84%) | |
| Height (cm) | 173.2 ± 4.9 | 163.8 ± 7.7 | P < 0.001 |
| Weight (kg) | 66.8 ± 7.1 | 65.6 ± 10.6 | NS |
| BMI (kg/m2) | 22.3 ± 2.1 | 24.3 ± 2.5 | P < 0.01 |
| Current smoker | 0 (0%) | 2 (8%) | |
| Total cholesterol (mg/dl) | 187.1 ± 29.7 | 173.7 ± 34.3 | NS |
| Triglyceride (mg/dl) | 87.0 ± 57.2 | 137.7 ± 72.7 | P < 0.001 |
| HDL cholesterol (mg/dl) | 64.7 ± 15.3 | 52.3 ± 13.9 | P < 0.01 |
| LDL cholesterol (mg/dl) | 106.6 ± 30.6 | 98.2 ± 27.2 | NS |
| HbA1c (%) | 4.9 ± 0.2 | 6.8 ± 1.0 | P < 0.001 |
| Serum creatinine (mg/dl) | 0.77 ± 0.14 | 0.95 ± 0.28 | P < 0.001 |
| BNP (pg/ml) | 52.1 ± 42.5 | ||
| EF (%) | 65.1 ± 11.2 | ||
| E/e' | 11.3 ± 3.5 | ||
| E/A | 1.1 ± 1.5 | ||
| LVH (mm) | 21.0 ± 2.0 | ||
| Max IMT (mm) | 2.57 ± 0.77 | ||
| Mean IMT (mm) | 0.88 ± 0.15 | ||
| Plaque score | 10.93 ± 3.44 | ||
| Coronary Artery Bypass Grafting (CABG) | 15 (60%) | ||
| Percutaneous Coronary Intervention (PCI) | 10 (40%) | ||
| Prior medications | |||
| Anti-hypertension drug use, n (%) | 0 (0%) | 24 (96%) | |
| Anti-dyslipidemia drug use, n (%) | 0 (0%) | 20 (80%) | |
| Anti-diabetes mellitus drug use, n (%) | 0 (0%) | 14 (56%) | |
| Cardio-Ankle Vascular Index (CAVI) | 6.73 ± 0.66 | 10.72 ± 1.73 | P < 0.001 |
| heart-thigh Beta (htBeta) |  |
P < 0.001 | |
| thigh-ankle Beta (taBeta) | P < 0.05 | ||
Data is Mean ± SD
*: P < 0.05
***: P < 0.001
1). Vascular parameters of healthy people during NTG administration:
When healthy people took an oral dose of NTG (0.3 mg), their arterial stiffness from the origin of the aorta to the ankle was measured using CAVI at one-minute intervals for 20 minutes. Blood pressure, pulse rate, stroke volume, and cardiac output were also measured. After administration of NTG in healthy people, the CAVI value decreased significantly after 5 min. [from 6.76(6.32–7.27) to 5.50(4.70–6.21), P < 0.05 at 5 min.], and recovered after 15 min., as shown in Fig. 2.
Fig. 2.
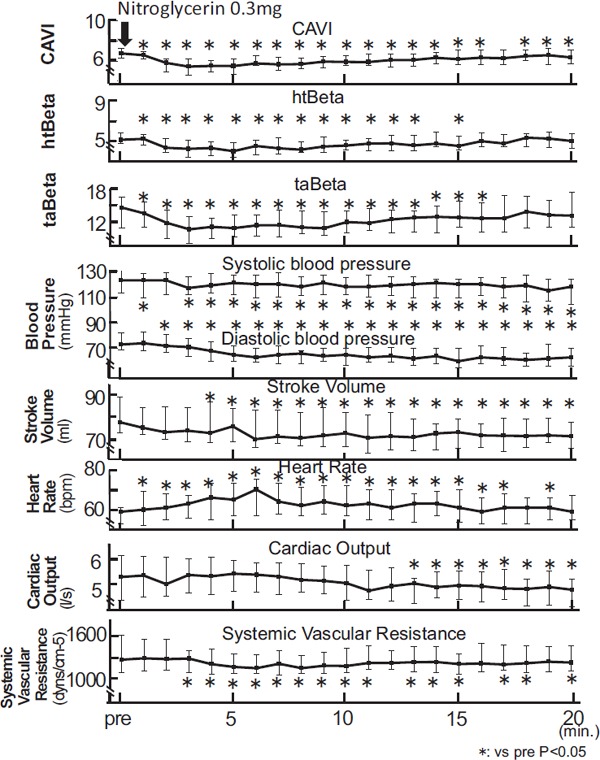
The change of CAVI, htBeta, taBeta, blood pressure, stroke volume, heat rate, cardiac output, and systemic vascular resistance during the administration of nitroglycerin in healthy people
Notes: Data were presented as median (interquartile range error bars). Wilcoxon signed rank test was used to compare pre administration and after administration.
In this study, we measured htBeta and taBeta, simultaneously. htBeta decreased a little [from 5.10 (4.76–5.76) to 3.96(3.35–4.79), P < 0.05 at 5 min. after 5 min., then returned to the previous value. taBeta decreased [from 14.41(10.80–16.33) to 10. 72 (9.19–13.01), P < 0.05 at 5 min.] after 5 min., and returned to its previous value after 20 min.
Systolic blood pressure showed a tendency to decrease [from 123(113–132) to 121(112–127), P < 0.05 at 5 min.]. Diastolic blood pressure showed a significant decrease during the 20 min. [from 73(69–82) to 65(60–72), P < 0.05 at 5 min.]. Stroke volume decreased after 5 min. (from 77.5(72.8–89.2) to 75.6 (68.8–86.6), P < 0.05 at 5 min.). Heart rate increased significantly after 5 min. [from 59(53–61) to 65(57–73), P < 0.05 at 5 min.], then it decreased. Cardiac output was not changed during the first 10 min. [from 5.30(4.39–6.15) to 5.43(4.74–5.95), ns at 5 min.], but decreased after 12 min. Systemic vascular resistance decreased significantly after 3 min. [from 1273(1084–1625) to 1169(1078–1354), P < 0.05 at 5 min.].
2). Vascular parameters of arteriosclerotic patients during NTG administration:
When arteriosclerotic patients took NTG (0.3 mg) sublingually, the CAVI value decreased after 5 min. [from 10.47(9.67–11.29) to 9.71(8.74–10.57), P < 0.05 at 5 min.], and recovered after 15 min. as shown in Fig. 3. htBeta did not change except after 7 min. [from 12.00(11.46–13.21) to 11.81(10.14–13.83), ns at 5 min.]. taBeta decreased significantly [from 18.55(12.93–23.42) to 12.37(9.68–16.99), P < 0.05 at 10 min.] after 5 min.
Fig. 3.
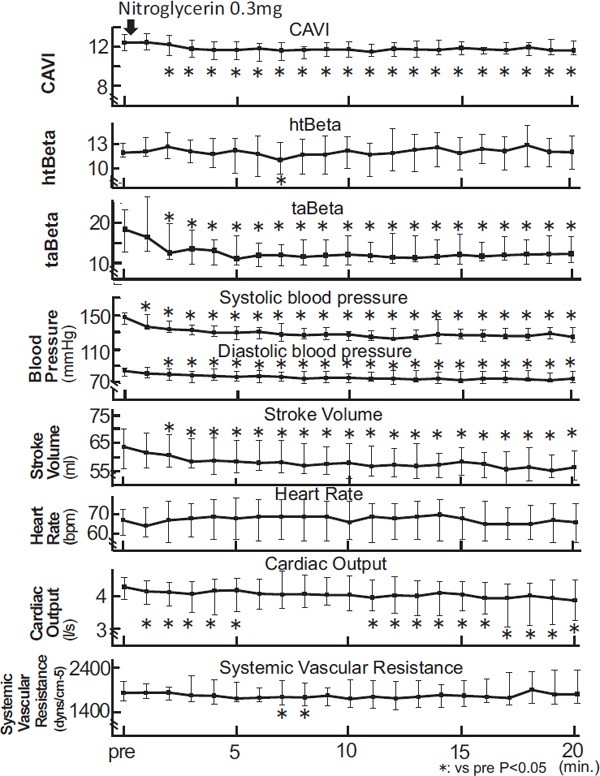
The change of CAVI, htBeta, taBeta, blood pressure, stroke volume, heat rate, cardiac output, and systemic vascular resistance during the administration of nitroglycerin in arteriosclerotic patients
Notes: Data were presented as median (interquartile range error bars). Wilcoxon signed rank test was used to compare pre administration and after administration.
Systolic blood pressure showed a significant decrease during the 20 minutes [from 146(140–153) to 130(122–137), P < 0.05 at 5 min.]. Diastolic blood pressure also showed a significant decrease during the 20 minutes [from 85(79–88) to 79(74–87), P < 0.05 at 5 min.], and stroke volume decreased [from 63.7 (56.13–70.23) to 58.58(54.62–66.20), P < 0.05 at 5 min.]. Heart rate did not significantly increase [from 67(59–73) to 68(57–79) ns at 5 min.]. Cardiac output also decreased significantly [from 4.28(3.89–4.57) to 4.18(3.51–4.56), P < 0.05 at 5 min.]. Systemic vascular resistance decreased significantly after 7 min. [from 1842(1661–2103) to 1750(1567–2123), P < 0.05 at 7 min.].
3). Comparison of Δ vascular parameter changes between healthy people and arteriosclerotic patients during NTG administration
We compared the changes of arterial stiffness and other circulation factors between healthy people (HP) and arteriosclerotic patients (AP) as shown in Fig. 4. The differences between those factors are shown by Δ, which is the difference compared to previous values. The maximum depression of the Δ CAVI value was similar in HP and in AP [HP − 1.10(−1.83 − −0.72) at 5 min. vs. AP − 1.08(−1.55 − −0.34) at 8 min., ns].
Fig. 4.
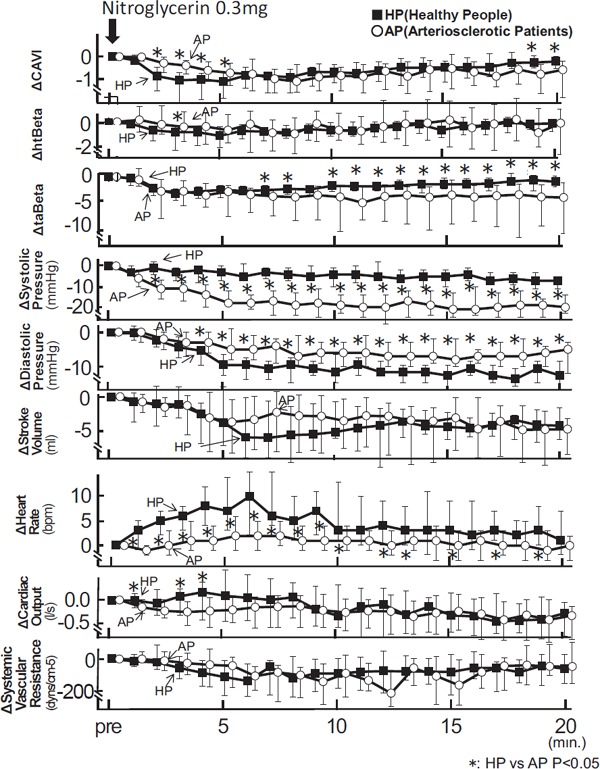
The Δ changes of CAVI, htBeta, taBeta blood pressure, stroke volume, heart rate, cardiac output, and systemic vascular resistance during the administration of nitroglycerin in healthy people and arteriosclerotic patients
Notes: Data were presented as median (interquartile range error bars). Mann-Whitney U-test was used to compare the two groups.
However, ΔCAVI of HP was significantly greater than that of AP until 5 min. [ HP − 0.86(−1.49 − −0.47) vs. AP − 0.28(−0.70 − −0.03), P < 0.05 at 2 min.; HP − 1.04(−2.06 − −0.71) vs. AP − 0.38(−0.74 − −0.16), P < 0.05 at 3 min.].
And then, ΔCAVI in HP recovered faster than that of AP after about 17 min. [HP − 0.26(−0.50 − 0.03) vs. AP − 0.76(−1.62 − −0.35), P < 0.05 at 19 min.; HP − 0.22(−0.53 − −0.01) vs. −0.57(−1.79 − −0.20), P < 0.05, at 20 min.]. As for Δ stiffness of the aorta, a decreased Δ htBeta of HP was more prominent than that of AP at 3 min. [HP − 0.76(−2.22 − −0.35) vs. AP − 0.41(−1.60–0.80), P < 0.05, at 3 min.]. However, decreased Δ taBeta of AP was much greater than that of HP after 10 min. [HP − 1.98 (−3.15 − −1.57) vs. AP −4.35(−11.72 − −2.17), P < 0.05, at 10 min.]. Decreased Δ systolic blood pressure of AP was larger than the value in HP (HP −3(−7 − 0) vs. AP −18(−19 − −12), P < 0.05, at 5 min.]. Decreased Δ diastolic blood pressure was more pronounced in HP, more than that of AP (HP −9(−12 − −6) vs. AP −5 (−8 − 1), P < 0.05, at 5 min.]. Decreased Δ stroke volume was not different between the two groups. [HP −3.95(−7.49–1.56) vs. AP −3.92(−8.73 − −0.49), ns, at 5 min.], and an increased Δ heart rate was much higher in HP than in AP [HP 7(3–14) vs. AP 2(−1–6), P < 0.05, at 5 min.]. Decreased Δ cardiac output was much more pronounced in AP than in HP [HP 0.09(−0.41–0.59) vs. AP −0.19(−0.43–0.05), P < 0.05, at 5 min.]. Decreased Δ systemic vascular resistance was not different between both groups. [HP −135(−270− −14) vs. AP −28(−186− 36), ns at 5 min.].
Discussion
The median CAVI value in arteriosclerotic patients was higher than that in healthy people. As for the median htBeta and taBeta, taBeta was significantly higher than htBeta in both groups, indicating that stiffness of muscular arteries (femoral and tibial arteries) was higher than that of elastic arteries (the aortic artery). Furthermore, both htBeta and taBeta were significantly higher in arteriosclerotic patients than in healthy people, indicating that both muscular and elastic arteries increased their stiffness with arteriosclerosis.
Furthermore, we evaluated the effect of NTG administration on CAVI, and htBeta and taBeta to clarify the specificity of the responsiveness to NTG in each artery.
Administration of NTG decreased CAVI in both healthy people (HP) and arteriosclerotic patients (AP) (Fig. 2, 3).
The maximum depression of ΔCAVI was similar in both HP and AP (Fig. 4). These results were consistent with those reported by Shimizu et al.25).
It was already reported that NTG decreases the stiffness of the peripheral arteries by monitoring the pulse wave velocity (PWV)4, 6). However, PWV is essentially changed by blood pressure at the time of measurement9, 10), therefore, accurate arterial stiffness changes could not been shown using PWV. Our results shown in Fig. 2, 3 indicate that nitroglycerin-induced dilatation of the arteries is accompanied by increased proper elasticity of the arteries. Furthermore, the responsiveness to NTG of the whole arterial tree from the origin of the aorta to the ankle was almost the same in both HP and AP. However, the time of maximum depression of CAVI in HP was faster than that in AP. This indicates that the response of vascular smooth muscle to administrated NTG was faster in HP than in AP. Furthermore, ΔCAVI in HP was less than that in AP after about 17 min. It may indicate that the recovery of the smooth muscle vasodilatation was much faster in HP than in AP.
htBeta in HP decreased, but htBeta in AP scarcely decreased. This result might indicate that the elasticity of the aorta decreased with the progression of arteriosclerosis (Fig. 2, 3).
On the other hand, taBeta decreased in both HP and AP groups almost at the same rate in the first 5 min (Fig. 2, 3, 4).
Therefore, the significant decrease in CAVI in HP compared with that in AP might be due to the decreased htBeta in HP.
On the contrary, ΔtaBeta in AP was greater than that in HP after 10 min. (Fig. 4).
ΔhtBeta in HP and, ΔhtBeta in AP were not different after 10 min. But ΔtaBeta in AP was significantly greater than that in HP. Therefore, significantly decreased ΔCAVI in AP after 10 min. might be due to the decreased taBeta in AP.
These results indicated that nitroglycerin-induced vasodilatation was maintained in femoral and tibial arteries, as muscular arteries, in AP. Namely, it suggested that the responsiveness of smooth muscle cells to NTG in the muscular arteries was maintained even in AP.
These results are consistent with the clinical observation that administration of nitroglycerin to arteriosclerotic patients is effective in the treatment of angina pectoris30).
Systolic blood pressure decreased in both groups, and decreased Δsystolic blood pressure was much greater in AP than in HP. Whereas, diastolic blood pressure decreased in both groups, and decreased Δ diastolic blood pressure was much greater in HP than in AP.
Considering the maintained responsiveness of taBeta to NTG in AP and larger decreased Δsystolic blood pressure in AP, systolic blood pressure might be much more dependent on muscular artery elasticity.
Considering that htBeta of the aorta was scarcely decreased by NTG and diastolic blood pressure was less decreased in AP than in HP, it might be suggested that diastolic blood pressure was much more affected by the aorta as an elastic artery than by the arterioles as muscular arteries. To confirm this hypothesis, further studies will be required.
Cardiac output in HP was maintained during the first 10 min. and decreased after 12 min. during NTG administration. However, in the AP group, cardiac output decreased from the first few mins. Stroke volume decreased in both HP and AP at almost the same rate whereas heart rate increased in HP, but not in AP. The different heart rate changes might be due to the different response of autonomic nerve reflection, but this needs to be clarified. Systemic vascular resistance decreased in both HP and AP at almost the same rate during NTG administration, and the difference was not significant. This effect of NTG on peripheral vascular resistance has already been reported by Taira et al.31). Interestingly, a correlation between changes of CAVI and systemic vascular resistance was observed. The correlation rate was r = 0.727, P < 0.001 in HP and r = 0.636, P = 0.002 in AP (data was shown only here). CAVI reflects stiffness or elasticity of the arterial tree from the origin of the aorta to the ankle, including elastic arteries and muscular arteries, but not the arterioles. It has been suggested that blood pressure was mainly regulated with the resistance of peripheral arteries32). Our results show that CAVI is well correlated with systemic vascular resistance, and this might indicate that CAVI reflects vascular resistance in the main arterial tree. Therefore, CAVI may be a useful index to evaluate the involvement of the main arterial tree in the blood control system in vivo.
Study Limitation:
Blood pressure for the calculation of CAVI, htBeta, and taBeta should ideally be measured in each arterial segment, but this was not possible in this clinical study. We used blood pressure at the upper brachial artery. CAVI values obtained using the blood pressures both at the ankle and upper brachial artery were almost the same during NTG administration.
The CAD patients were examined under the controlled state of their various coronary risk factors. The point that we cannot remove the effects of their medications is a limitation of our study.
Conclusion
NTG administration decreased CAVI values, the beta theory-applied index of the aorta (htBeta), and the femoral and tibial arteries (taBeta) with varying rates in each index. It is noteworthy that muscular arteries in arteriosclerotic patients maintained their responsiveness to NTG much more than those in healthy people.
The response of CAVI was well correlated with systemic vascular resistance, indicating that CAVI might reflect the vascular resistance of the arterial tree.
These results indicate that measuring arterial stiffness with β theory-derived vascular indices might contribute to studies on the role of segmental arterial stiffness in systemic circulation.
Acknowledgment
We would like to express our sincere gratitude to Dr. Hisayuki Tsukuma who is a statistical associated professor in Toho University and gave us useful comments and inspiring discussions about statistics.
Disclosures
Tomoyuki Yamamoto belongs to Fukuda Denshi Co. Ltd, and was involved in the development of the VaSera machine for measuring CAVI.
Kazuhiro Shimizu had no conflict of interest concerning this paper.
Mao Takahashi had no conflict of interest concerning this paper.
Ichiro Tatsuno had no conflict of interest concerning this paper.
Kohji Shirai was a visiting professor of the Department of Vascular Function in Toho University, which received donations from Fukuda Denshi Co. Ltd., but has no patent and has received no financial profit.
References
- 1). Smulyan H, Mookherjee S, Warner RA: The effect of nitroglycerin on forearm arterial distensibility. Circulation, 1986; 73: 1264-1269 [DOI] [PubMed] [Google Scholar]
- 2). McGregor M: The nitrates and myocardial ischemia. Circulation, 1982; 66: 689-692 [DOI] [PubMed] [Google Scholar]
- 3). Fam WM, McGregor M: Effect of nitroglycerin and dipyridamole on regional coronary resistance. Circ Res, 1968; 22: 649-659 [DOI] [PubMed] [Google Scholar]
- 4). Wilkinson IB, Qasem A, McEniery CM, Webb DJ, Avolio AP, Cockcroft JR: Nitric oxide regulates local arterial distensibility in vivo. Circulation, 2002; 105: 213-217 [DOI] [PubMed] [Google Scholar]
- 5). Feldman RL, Pepine CJ, Conti CR: Magnitude of dilatation of large and small coronary arteries of nitroglycerin. Circulation, 1981; 64: 324-333 [DOI] [PubMed] [Google Scholar]
- 6). Pauca AL, Kon ND, O'Rourke MF: Benefit of glyceryl trinitrate on arterial stiffness is directly due to effects on peripheral arteries. See comment in PubMed Commons belowHeart, 2005; 91: 1428-1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7). Asmar R, Benetos A, Topouchian J, Laurent P, Pannier B, Brisac AM, Target R, Levy BI: Assessment of arterial distensibility by automatic pulse wave velocity measurement. Validation and clinical application studies. Hypertension, 1995; 26: 485-490 [DOI] [PubMed] [Google Scholar]
- 8). Sawabe M, Takahashi R, Matsushita S, Ozawa T, Arai T, Hamamatsu A, Nakahara K, Chida K, Yamanouchi H, Murayama S, Tanaka N: Aortic pulse wave velocity and the degree of atherosclerosis in the elderly: a pathological study based on 304 autopsy cases. Atherosclerosis, 2005; 179: 345-351. Epub 2004 Dec 13 [DOI] [PubMed] [Google Scholar]
- 9). Nye ER: The effect of blood pressure alteration on the pulse wave velocity. Br Heart J, 1964; 266: 261-265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10). Van Bortel LM, Duprez D, Starmans-Kool MJ, Safar ME, Giannattasio C, Cockcroft J, Kaiser DR, Thuillez C: Clinical applications of arterial stiffness, Task Force III: recommendations for user procedures. Am J Hypertens, 2002; 15: 445-452 [DOI] [PubMed] [Google Scholar]
- 11). Hayashi K, Handa H, Nagasawa S, Okumura A, Moritake K: Stiffness and elastic behavior of human intracranial and extracranial arteries. J Biomech, 1980; 13: 175-184 [DOI] [PubMed] [Google Scholar]
- 12). Bramwell JC, Hill AV: Velocity of transmission of the pulse and elasticity of arteries. Lancet, 1922; 1: 891 [Google Scholar]
- 13). Shirai K, Utino J, Otsuka K, Takata M: A novel blood pressure-independent arterial wall stiffness parameter; cardio-ankle vascular index (CAVI). J Atheroscler Thromb, 2006; 13: 101-107 [DOI] [PubMed] [Google Scholar]
- 14). Shirai K, Hiruta N, Song M, Kurosu T, Suzuki J, Tomaru T, Miyashita Y, Saiki A, Takahashi M, Suzuki K, Takata M: Cardio-ankle vascular index (CAVI) as a novel indicator of arterial stiffness: theory, evidence and perspectives. J Atheroscler Thromb, 2011; 18: 924-938 [DOI] [PubMed] [Google Scholar]
- 15). Shirai K, Song M, Suzuki J, Kurosu T, Oyama T, Nagayama D, Miyashita Y, Yamamura S, Takahashi M: Contradictory effects of β1 - and α1-aderenergic receptor blockers on cardio-ankle vascular stiffness index (CAVI)-the independency of CAVI from blood pressure. J Atheroscler Thromb, 2011; 18: 49-55 [DOI] [PubMed] [Google Scholar]
- 16). Namekata T, Suzuki K, Ishizuka N, Shirai K: Establishing baseline criteria of cardio-ankle vascular index as a new indicator of arteriosclerosis: a cross-sectional study. BMC Cardiovasc Disord, 2011; 11:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17). Nakamura K, Tomaru T, Yamamura S, Miyashita Y, Shirai K, Noike H: Cardio-ankle vascular index is a candidate predictor of coronary atherosclerosis. Circ J, 2008; 72: 598-604 [DOI] [PubMed] [Google Scholar]
- 18). Horinaka S, Yabe A, Yagi H, Ishimura K, Hara H, Iemua T, Matsuoka H: Comparison of atherosclerotic indicators between cardio-ankle vascular index and brachial ankle pulse wave velocity. Angiology, 2009; 60: 468-476 [DOI] [PubMed] [Google Scholar]
- 19). Suzuki J, Sakakibara R, Tomaru T, Tateno F, Kishi M, Ogawa E, Kurosu T, Shirak K: Stroke and cardio-ankle vascular stiffness index. J Stroke Cerebrovasc Dis, 2011; 22: 171-175. 61 [DOI] [PubMed] [Google Scholar]
- 20). Kubozono T, Miyata H, Uegama K, Nagaki A, Hamasaki S, Kusano K, Kubozono O, Tei C: Association between arterial stiffness and estimated glomerular filtration rate in the Japanese general population. J Atheroscler Thromb, 2009; 16: 840-845 [DOI] [PubMed] [Google Scholar]
- 21). Noike H, Nakamura K, Sugiyama Y, Iizuka T, Shimizu K, Takahashi M, Hirano K, Suzuki M, Mikamo H, Nakagami T, Shirai K: Changes in cardio-ankle vascular index in smoking cessation. J Atheroscler Thromb, 2010; 17: 517-525 [DOI] [PubMed] [Google Scholar]
- 22). Kasai T, Inoue K, Kumagai T, Kato M, Kawana F, Sagara M, Ishiwata S, Ohno M, Yamaguchi T, Momomura S, Narui K: Plasma pentraxin3 and arterial stiffness in men with obstructive sleep apnea. Am J Hypertens, 2011; 24: 401-407 [DOI] [PubMed] [Google Scholar]
- 23). Satoh-Asahara N, Suganami T, Majima T, Kotani K, Kato Y, Araki R, Koyama K, Okajima T, Tanabe M, Oishi M, Himeno A, Kono S, Sugawara A, Hattori M, Ogawa Y, Shimatsu A, Japan Obesity and Metabolic Syndrome Study (JOMS) Group : Urinary Cystatin C as a Potential Risk Marker for Cardiovascular Disease and Chronic Kidney Disease in Patients with Obesity and Metabolic Syndrome. Clin J Am Soc Nephrol, 2011; 6: 265-273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24). Hayashi K, Yamamoto T, Takahara A, Shirai K: Clinical assessment of arterial stiffness with cardio-ankle vascular index: theory and applications. J Hypertens, 2015; 33: 1742-1757 [DOI] [PubMed] [Google Scholar]
- 25). Shimizu K, Yamamoto T, Takahashi M, Sato S, Noike H, Shirai K: Effect of nitroglycerin administration on cardioankle vascular index. Vasc Health Risk Manag, 2016; 12: 313-319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26). Zoremba N, Bickenbach J, Krauss B, Rossaint R, Kuhlen R, Schälte G: Comparison of electrical velocimetry and thermodilution techniques for the measurement of cardiac output. Acta Anaesthesiol Scand, 2007; 51: 1314-1319 [DOI] [PubMed] [Google Scholar]
- 27). Paul L Marino, Kenneth M Sutin: The ICU Book, 3rd Edition Philadelphia: Lippincott Williams & Wilkins, 2007; 20-21 [Google Scholar]
- 28). Handa N, Matsumoto M, Maeda H, Hougaku H, Ogawa S, Fukunaga R, Yoneda S, Kimura K, Kawada T: Ultrasonic evaluation of early carotid atherosclerosis. Stroke, 1990; 21: 1567-1572 [DOI] [PubMed] [Google Scholar]
- 29). Nagai Y, Kitagawa K, Yamagami H, Kondo K, Hougaku H, Hori M, Matsumoto M: Carotid artery intima-media thickness and plaque score for the risk assessment of stroke subtypes. Ultrasound Med Biol, 2002; 28: 1239-1243 [DOI] [PubMed] [Google Scholar]
- 30). Egashira K, Inou T, Hirooka Y, Yamada A, Urabe Y, Takeshita A: Evidence of impaired endothelium-dependent coronary vasodilatation in patients with angina pectoris and normal coronary angiograms. N Engl J Med, 1993; 328: 1659-1664 [DOI] [PubMed] [Google Scholar]
- 31). Taira N, Imai Y, Hiwatari M: Differential effects of nitroglycerin, trimetazidine, verapamil and SK&F 24260 on venous return as revealed by the open-loop method in the dog. Jpn J Pharmacol, 1980; 30: 449-461 [DOI] [PubMed] [Google Scholar]
- 32). Jamil Mayet, Alun Hughes: Cardiac and vascular pathophysiology in hypertension. Heart, 2003; 89: 1104-1109. See comment in PubMed Commons below [DOI] [PMC free article] [PubMed] [Google Scholar]


