Abstract
Familial Hypercholesterolemia (FH) is the most common and serious monogenic disorder of lipoprotein metabolism that leads to premature coronary heart disease. There are over 65,000 people estimated to have FH in Australia, but many remain undiagnosed. Patients with FH are often undertreated, but with early detection, cascade family testing and adequate treatment, patient outcomes can improve. Patient registries are key tools for providing new information on FH and enhancing care worldwide. The development and design of the FH Australasia Network Registry is a crucial component in the comprehensive model of care for FH, which aims to provide a standardized, high-quality and cost-effective system of care that is likely to have the highest impact on patient outcomes. Informed by stakeholder engagement, the FH Australasia Network Registry was collaboratively developed by government, patient and clinical networks and research groups. The open-source, webbased Rare Disease Registry Framework was the architecture chosen for this registry owing to its open-source standards, modular design, interoperability, scalability and security features; all these are key components required to meet the ever changing clinical demands across regions. This paper provides a high level blueprint for other countries and jurisdictions to help inform and map out the critical features of an FH registry to meet their particular health system needs.
Keywords: Disease registry, Familial hypercholesterolaemia, Interoperable, Model of care, Registry framework
Introduction
Familial Hypercholesterolaemia
Familial Hypercholesterolaemia (FH) is a relatively common genetic disorder that is associated with premature coronary heart disease (CHD)1, 2). FH is the most common and serious monogenic disorder of lipoprotein metabolism, and causes severe elevation of total and low-density lipoprotein cholesterol (LDL-C) levels from birth3, 4). Heterozygous FH (HeFH) occurs in as many as 1 in 200 people2), while the prevalence of homozygous FH (HoFH) has recently been estimated to be as high as 1 in 160,000–300,000 people5) for most populations. Higher frequencies of FH occur in first-degree relatives (1 in 2) of index cases, and in populations subject to a “founder gene effect,” such as Afrikaners and Ashkenazi Jews4).
Under-diagnosis of FH is a problem worldwide, with estimates of FH affecting as many as 34.3 million people, with < 1% of cases diagnosed in most countries6, 7). This represents a major gap in care, as 1 in 2 untreated men and 1 in 6 untreated women develop fatal CHD by the age of 60 years. Early diagnosis and treatment can delay the manifestation of atherosclerosis, with the introduction of statins improving the life expectancy of patients with FH which now approaches that of the general population. However, the standardized mortality rate for CHD still remains higher8, 9). HoFH requires therapeutic intervention within the first decade of life due to the development of aggressive CHD during childhood and adolescence, without which they will generally sustain a fatal myocardial infarction before the age of 30 years10).
In Australia, at least 65,000 people are estimated to have FH with the vast majority undiagnosed, and in many diagnosed cases, patients are receiving inadequate treatment1, 11). The Western-Pacific and South-East Asia regions have the highest estimated population density of FH in the world12). Cascade family screening, by which all first-degree relatives of identified index patients are screened for FH, is of critical importance for early diagnosis and treatment of FH to delay or prevent the onset of premature CHD, yet is not often widely performed13). Only three national genetic cascade screening programs are currently operating in the Netherlands, Spain, and Wales, while advanced regional and local programs are operating in a number of countries including Australia, New Zealand, Slovenia, Czech Republic, and Malaysia14).
To bridge this major gap in CHD prevention, the Commonwealth Government funded a flagship program under the Australian Better Health Initiative (ABHI), that resulted in the development of models of care for FH in Western Australia (WA)15). Building on a WA health model of care, the FH Australasia Network published a comprehensive model of care for FH with the aim to provide a standardized, high-quality, and cost-effective system of care that is likely to have the highest impact on patient outcomes11), which was followed by the integrated guidance of care for FH by the International FH Foundation16). One important feature identified as essential to the implementation and effective provision of services, is a patient registry to store clinical and family data13, 15, 17).
The Importance of Registries
Patient registries capture relevant patient information, including clinical and molecular information. Registries are clinical, information rich resources that are essential for the integration of research into clinical practice and translation into therapeutic solutions18). In populations with chronic disease, it has been demonstrated that patient outcomes improve with the use of models of care that integrate patient and clinical information with evidence-based treatments19, 20). As such, the American Heart Association has called for an expansion of clinical registry programmes21). A recent review of current studies of FH patients also concluded that complete national and international FH registries are key instruments for providing new information on FH and enhancing the care of FH patients worldwide22).
Over the last 30 years, several national FH registries (Table 1) and cascade screening programs have been established in various countries, most notably in the United Kingdom (The Simon Broome Register23, 24, 25) and The National Paediatric FH Register26)), the Netherlands (Dutch Lipid Clinic Network27)), Norway (Registry of the Medical Genetics Laboratory28)/Unit of Cardiac and Cardiovascular Genetics Registry29, 30)), the United States (CASCADE FH31, 32) and MED-PED33)), Canada (British Columbia FH Registry34)), and Spain (SAFEHEART Study35, 36)). Recently, the importance of establishing regional FH registries has been noted for the Middle Eastern and North African Region37), and France has also recently created a cohort of FH patients from across the country38). The European Atherosclerosis Society (EAS) recently launched a global “call to arms” initiative to integrate efforts from major FH registries around the world to generate large-scale data on the detection and management of FH (EAS FH Studies Collaboration (FHSC)39)).
Table 1. Details of existing FH Registries: Type: DNA positive indicates a molecular genetic diagnosis of FH. Clinical indicates a clinical diagnosis of FH by DLCNS or other criteria. Hybrid indicates a mixture of molecular genetic and clinical diagnosis for inclusion in the registry.
| Country/Region | Registry | Type | Time-frame | Number (year confirmed) | System | Further details |
|---|---|---|---|---|---|---|
| UK | The Simon Broome Register of FH23–25) | Hybrid | 1980- | 3,382 heFH patients from 21 lipid clinics | SPSS | Developed the Simon Broome Criteria to determine definite or probable FH status. Reported significant decreases in excess coronary heart disease mortality for patients who received early diagnosis and treatment. |
| USA | Make Early Diagnosis, Prevent Early Deaths (MEDPED)33) | Hybrid | 1989–2004 | ∼8,000 definite or probable FH patients31) | MEDPED criteria created and validated to estimate the probability of FH. | |
| Norway | Unit of Cardiac and Cardiovascular Genetics Registry28–30) | DNA positive | 1992- | 7,091 (2016) | Filemaker from Apple | Registry data can be linked to several other National health registries to assess mortality, cardiovascular disease and pregnancy outcomes in FH patients. |
| The Netherlands | Dutch Lipid Clinic Network27) | Hybrid | 1994- | > 30,00031) | PASS Clinical ® Vascular | Developed and validated a set of diagnostic criteria for FH. |
| Czech Republic | Czech MED-PED Registry | Hybrid | 1998- | 6919 (2016) | Online database (PAGEWISER) | MedPed project initiative. |
| Spain | Spanish FH Longitudinal Cohort Study (SAFEHEART)35, 36) | Hybrid | 2004- | 4,615 (2016) | Dinahosting (network server) | Approximately 3,000 individuals have a positive genetic test. The registry is run by the Fundacion HF. Website: https://www.colesterolfamiliar.org/ |
| Wales and England | Pass Database54, 55) | Hybrid | 2010- | 2587 (2016) | PASS Clinical ® Vascular | |
| UK | The National Paediatric FH Register26) | Clinical | 2012- | 380 (2016) | Electronic data capture | Established to collect baseline and long-term follow-up data on all children (0–18 years) with FH in the UK. Approximately 60% have a DNA family mutation recorded. |
| USA | CASCADE FH31, 32) | Hybrid | 2013- | 3,030 (2016) | Bespoke built in partnership with the Duke Clinical Research Institute. | A national, multicentre initiative that longitudinally tracks FH therapy, family screening, clinical outcomes and patient-reported outcomes. |
| Canada | FH Canada Registry34) | Hybrid | 2014- | 738 (2016); 2900 expected by the end of 2016 | iCAPTURE (bespoke built) from the James Hogg Research Centre, UBC | Started in 2014 from the existing British Columbia FH Registry6). Established to diagnose, educate and treat individuals with heFHheFH. Website: www.fhcanada.net |
| France | French Cohort of patients with FH38) | Clinical | 2015- | ∼3,263 (2016) | Integralis | Primary objective is to create a cohort of French patients with FH to evaluate screening and clinical management. |
| Taiwan | Taiwan Familial Hypercholesterolemia Registry Study | Hybrid | 2015- | 500 (2016) | Clinical Study Information System (CIMS) | Organised by the Taiwan Society of Lipid & Atherosclerosis. |
| International | European Atherosclerosis Society (EAS) FH Studies Collaboration (FHSC)39, 56) | Hybrid | 2015- | Data to be received from 10/2016. | Bespoke system | A global initiative from the EAS that, through a consortium of major FH registries worldwide, aims to generate large-scale robust data. Investigators from over 5 countries have formally committed to contribute their data to date. |
| International | Homozygous autosomal dominant hypercholsterolemia (HoADH) International Clinical Collaboration (HICC) | Hybrid | 2015- | REDCap | The HICC will evaluate the true prevalence and phenotypic and genetic characterisation of HoADH. |
Bamimore et al.37) noted that there is no “standard rule” for building a FH registry; however, they suggest following the consensus-based guidance for the management of FH16) as the starting point. A number of public and commercially available systems have been utilized in the creation of FH registries to date (see Table 1 for an overview). For example, PASS Clinical Vascular is a commercially available software package for clinical management and cascade screening, used in Latvia, Germany, Ireland, the United Kingdom, and the Netherlands40). REDCap is a webbased application for building and managing online surveys and databases that is free to non-profit organizations that are members of the REDCap consortium41), used in some Japanese Atherosclerosis Society registry projects.
Registry Design
There are several fundamental factors that need to be considered when designing a registry including purpose, ethical and legal requirements, documentation, governance, design, and sustainability42). With a wide range of bespoke, commercially available, and open source tools available for the establishment of registries, choosing which system to use can be difficult due to the diverse range of functionality and technology choices offered18).
To this point, several groups have recently developed key criteria for robust and sustainable registry implementation including, the Joint Declaration of 10 Key Principles for Rare Disease Patient Registries issued by the European Organisation for Rare Diseases (EURORDIS), the National Organisation for Rare Diseases (NORD), and the Canadian Organization for Rare Disease (CORD)43), the recommendations published by the European Union Committee of Experts on Rare Diseases44), and Rare Disease and Patient Registry Checklists to guide future registry development18, 45, 46).
These recommendations and checklists highlight the effective sharing of data, and the ability to link patients, samples, and analyses are essential to the success of disease research and improving outcomes for patients. This has led to international initiatives to harmonize legacy systems such as the European Union Framework Program 7, RD-Connect (http://rd-connect.eu/), established to develop an integrated platform that connects key infrastructure tools such as databases, registries, biobanks, and clinical bioinformatics47).
The open-source Rare Disease Registry Framework (RDRF) was therefore developed as a viable solution to registry development18, 48–50). Rather than focusing on the deployment of single, stand-alone registries, the RDRF enables the dynamic creation of web-based patient registries without the need for software development18, 48–50). By incorporating key criteria required for a registry such as modular design, interoperability, and security features, the RDRF allows the deployment of national and international registries that are able to meet ever-changing clinical demands.
Aim
This paper details the development and design of the FH Australasia Network Registry utilizing the RDRF. It provides a high level blueprint for other countries and jurisdictions to help inform and map out the critical features of a FH registry to meet their particular health system needs. The primary purpose of the FH Australasia Network Registry is to collate data to facilitate clinical service planning and to inform clinical best practices17). The registry will also enable research on aggregated data and the identification of eligible volunteers for clinical trials. The specific aims include:
Facilitate data collection to inform best practices, models of care, and health service planning;
Data analyses and reporting by the registry on estimated prevalence; geographical distribution, genetic variants associated with disease, clinical features, clinical management and patient outcomes;
Enable research by providing aggregate, deidentified data to research entities;
Facilitate identification and recruitment of eligible volunteers for clinical trials; and
Coordinate and administer education to registrants and participating centers.
Methods
Development of the FH Australasia Network Registry
Extensive stakeholder engagements led to the recognition of the need to improve outcomes for Australian and New Zealand FH patients under the ABHI. This resulted in the establishment of clinical models of care11, 15) and the development of a simple relational database. Through an iterative process, an international registry previously titled the “Australia and New Zealand FH Registry (ANZFHR)”17) was developed. The registry was collaboratively developed by the Office of Population and Health Genomics (Department of Health, Government of Western Australia), the FH Australasia Network (Australian Atherosclerosis Society), clinical networks, and the Centre for Comparative Genomics (Murdoch University)13, 17).
The Registry Framework
The RDRF allows the efficient deployment of web-based registries that can be modified dynamically as registry requirements evolve18, 48–50). The RDRF not only facilitates the effective capture, storage, management, and access of patient information, it is designed to be interoperable to capture, import, and store data from other systems such as the RD-Connect platform49) (see Fig. 1). The ability to re-use data elements (DEs) across multiple registries greatly assists with the standardization of data capture, allowing for effective data sharing for research purposes.
Fig. 1.
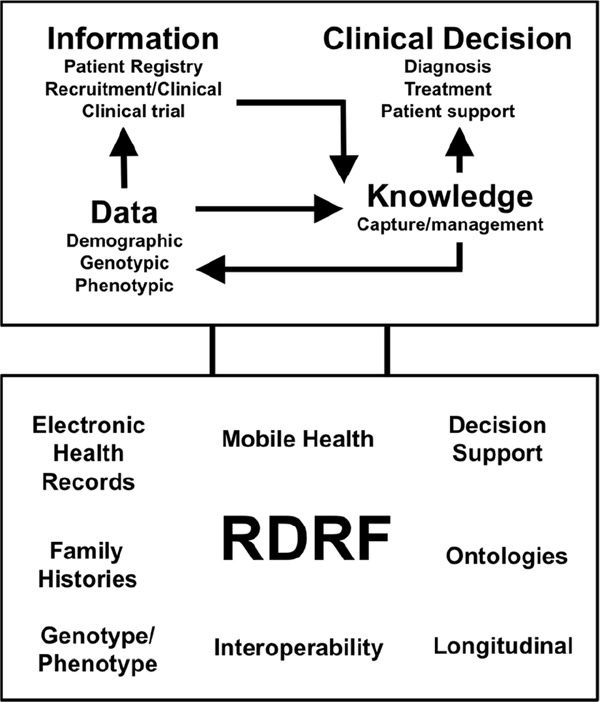
The Rare Disease Registry Framework (RDRF) is a cloud-technology based platform that is focused on analytics
The RDRF facilitates the capture of information in the form of patient registries or clinical trials through the capture and storage of data (demographics, genotypic and phenotypic), which then in turn informs knowledge such as clinical decisions (diagnosis, treatment, and patient support). The RDRF is designed to “talk” and connect to other systems such as biobanks, registries and Electronic Health Records to capture data and ultimately provide decision support.
The RDRF fulfills the key criteria required for sustainable registry development18, 43, 44, 46), has continued to evolve since first described by Bellgard et al.18, 48–50). Additional features and enhancements of the RDRF were implemented specific to the FH Australasia Network Registry, described in Napier et al.51).
Modular Design
The modular design of the RDRF enables simple configuration and creation of DEs, Sections, and Forms, such that FH Australasia Network Registry can continue to evolve over time. Currently, the patient data captured by the registry includes demographics and clinical information for each patient captured by the “Demographics” and “Consents” modules and six additional Forms titled Clinical Data, Medications, Genetic Data, Imaging, Apheresis and Follow Up (Table 2, also see Napier et al.51)).
Table 2. Capture of patient data in the FH Australasia Network Registry.
| FORM NAME | SUMMARY |
|---|---|
| Consents | Customisable consent sections easily allow new consent questions to be added or existing questions to be amended. Consent is captured via boolean Data Elements (DEs) presented as check boxes. A file DE allows the upload of a hard copy of a consent document if required. |
| Demographics | Patient's personal and contact details are captured, along with details of their doctors. For Index Patients, a Patient Relatives section is also present, which stores the details of relatives and allows a Patient Relative to be created in the registry. A Pedigree section allows the upload of family pedigree files and the capture of details on founder effect origin and the number of first, second and third degree relatives. The family linkage module, which details the relationships of Patient Relatives to Index Patients, is also accessed from this form. |
| Clinical Data | Clinical data is captured through various DEs, including family history, clinical history, physical examination, plasma LDL-cholesterol, biochemistry profile, risk factors and clinical trials. Derived DEs are utilised which allow automatic calculations of the ‘Dutch Lipid Clinic Network Score’, ‘FH Diagnostic category’, ‘LDL-cholesterol adjusted for treatment’, and ‘BMI’. |
| Medications | Details on patient's medications and drug intolerances are captured. Multi-sections, which allow the addition of the same section, are utilised to add additional drug intolerances if required. |
| Genetic Data | This form captures a patient's genetic data, such as the genotype and gene variants. Laboratory Reports can also be uploaded to the registry through the use of a file DE. |
| Imaging | This form captures details of various imaging tests, such as carotid ultrasonography, echocardiograms, coronary artery calcium scores, angiograms, and nuclear perfusion scans. Multi-sections are utilised so additional tests may be easily added as required. |
| Apheresis | This form captures the type, frequency and complications related to apheresis through a multi-section. |
| Follow-Up | This form captures additional clinical data at follow up assessments, such as events, hypertension, diabetes, antithrombotic, biochemistry profiles, and death. |
Interoperability
The ability for patient registries to communicate and share data with systems such as other registries or biobanks is critical. Interoperability in the RDRF is achieved through an application programming interface (API), which can interrogate other systems via their API's. In addition, the RDRF is able to share and re-use DEs, sections, and forms across multiple registries, assisting in the standardization of data capture.
Security
The RDRF provides distinct levels of inbuilt security, including Secure Socket Layer security encrypting all web traffic to and from the application, Cross-Site Request Forgery checking (a method of ensuring falsifying form submissions are near impossible), and the storage of identifying patient demographic data in a distinct database to any clinical data (refer to Napier et al.51))18, 48, 50). In addition, all user log-ins (successful and failed) are also recorded within the RDRF. The RDRF also has multiple levels of access with configurable permission levels (role based security model), which restricts the visibility of forms and fields to specified user groups.
Governance and Access
Standard international ethical principles52), supplemented by local legislation and requirements, were followed when initiating and deploying the FH Australasia Network Registry. The FH Australasia Network Registry is hosted and maintained at Murdoch University, WA, with strategic direction and oversight provided by an advisory board of key stakeholders (FH Australasia Network Registry Advisory Board). A charter, protocol, and guidelines for the registry were developed by the WA Department of Health in conjunction with the FH Australasia Network and Australian Atherosclerosis Society, and are available from https://fhregistry-international.com.
The FH Australasia Network Registry is a multicenter collaboration, coordinated through the FH Australasia Network, with a national coordinator responsible for ensuring cross-site coordination. A principal investigator from each State or jurisdictional clinical service is responsible for the data collation of their patients. Applications for access to data are considered for approval by the FH Australasia Network Registry Advisory Board.
Discussion
In this paper, we describe the development and design of an international clinical registry for FH in Australia and New Zealand, the FH Australasia Network Registry. The open-source nature and modular design of the RDRF, upon which the registry is deployed, contributes to its long-term sustainability. A key merit of the RDRF is that is has an open-source license (GNU GPL v3), which allows it to be freely shared. The RDRF is therefore more flexible as it is not bound by licensing agreements like other registry systems such as PASS Clinical Vascular and REDCap. The RDRF is also much more cost effective in comparison to commercial software such as PASS Clinical Vascular. New features can also be easily incorporated into an existing registry as they are made available, which allows for the continual evolution over time as required. For example, a dynamic application to draw patient pedigrees based on the diagnosis status and relationships of patients can be designed and implemented in a future release of the RDRF.
The FH Australasia Network Registry also provides a great opportunity for recruiting participants and planning clinical trials. Information can be easily gathered for future clinical trials, simply by defining a new “Clinical Trials” form and DEs customized to capture the appropriate data in the RDRF. Existing features, as well as DEs and sections defined for the FH Australasia Network Registry, can also be utilized and shared by other registries. The RDRF has been endorsed by RD-Connect (see: http://rd-connect.eu/tools-resources/rare-disease-registry-framework-rdrf/), which emphasizes the value and long-term sustainability of the RDRF, and its ability to be interoperable with other registries and systems.
Through its multi-level user access, the FH Australasia Network Registry also has the ability to easily convert to a multi-national FH registry. As additional countries from across the Australasia-Pacific region join this registry, access will be easily configured through the use of customizable working groups and user permissions.
The FH Australasia Network Registry, deployed utilizing the RDRF, is a secure and comprehensive clinical registry that allows harmonization of data collection across different states, clinical jurisdictions, and countries. The RDRF is highly interactive and flexible, and has the ability to connect to other registries, systems, and data repositories, thus distinguishing the FH Australasia Network Registry from other FH registries developed to date. The full potential of this registry will be realized through its ability to connect with other data linkage systems.
The FH Australasia Network Registry has many functions. It is not only a repository of data, it also serves to support clinicians, researchers and patient groups, enables improvements to models of care, education, and communication, and helps focus and drive research and the development of new treatments.
Conclusion
The features described and the processes articulated in the development of the FH Australasia Network Registry encompasses the key features that should be considered when choosing a system for building patient registries. The FH Australasia Network Registry can be readily adapted to other conditions related to FH, such as familial combined hyperlipidemia and elevated lipoprotein(a)53). This framework is also applicable to other inherited cardiovascular conditions, such as familial hypertrophic cardiomyopathy.
Acknowledgments
The FH Australasia Network Registry was established with the support from the development grants from the Office and Population Health Genomics, Government of Western Australia, and the FH Australasia Network of the Australian Atherosclerosis Society. The authors gratefully acknowledge the combined support-in-part funding for this work. This includes the RD-Connect-European Union Seventh Framework Programme (FP7/2007–2013 program HEALTH. 2012.2. 1.1-1-C) under grant agreement number 305444: RD Connect: An integrated platform connecting databases, registries, biobanks, and clinical bioinformatics for rare disease research, the financial support of Australian National Health and Medical Research Council (APP1055319) under the NHMRC–European Union Collaborative Research Grants scheme, and the Wellcome Trust [REF 104746]. The authors wish to acknowledge the FH Australasia Network and the Australian Atherosclerosis Society for their partnership in the FH Australasia Network Registry. The Australian Atherosclerosis Society and the FH Australasia Network are supported by Sanofi, Amgen, and MSD Australia.
The Office of Population Health Genomics, in collaboration with the Department of Internal Medicine at Royal Perth Hospital and the UWA School of Medicine and Pharmacology and Primary Care, were supported by the Australian Better Health Initiative: A joint Australian, State and Territory government initiative to run the Familial Hypercholesterolaemia Cascade Screening Project and the development of the Familial Hypercholesterolaemia Model of Care. The key outcome of this Flagship project was a national framework for the early detection and treatment of familial hypercholesterolemia in affected Australian families. The authors wish to thank Professors Peter O'Leary and John Burnett for their dedication and commitment to leading the pilot programs and developing the model of care framework and the context for the clinical enabling registry described in this paper.
Conflicts of Interest
Warrick Bishop has been supported by Amgen for educational and advisory board work. David Sullivan has been supported by Amgen, Abbott, AstraZenica, MS&D, Sanofi, and NHMRC for clinical research, and by Abbott, MS&D, Pfizer, and NPS for education and consultancy work. Gerald F Watts has received honoraria for lectures, research studies or scientific advisory boards from Merck Sharp and Dohme, Novartis, Kowa, Amgen, Sanofi, and Regeneron.
References
- 1). Watts GF, Shaw JE, Pang J, Magliano DJ, Jennings G. Carrington MJ: Prevalence and treatment of familial hypercholesterolaemia in Australian communities. Int J Cardiol, 2015; 185: 69-71 [DOI] [PubMed] [Google Scholar]
- 2). Benn M, Watts GF, Tybjaerg-Hansen A, Nordestgaard BG: Familial hypercholesterolemia in the Danish general population: prevalence, coronary artery disease, and cholesterol-lowering medication. J Clin Endocrinol Metab, 2012; 97: 3956-3964 [DOI] [PubMed] [Google Scholar]
- 3). Goldstein JL, Brown MS: The LDL receptor. Arterioscler Thromb Vasc Biol, 2009; 29: 431-438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4). Austin MA, Hutter CM, Zimmern RL, Humphries SE: Genetic causes of monogenic heterozygous familial hypercholesterolemia: a HuGE prevalence review. Am J Epidemiol, 2004; 160: 407-420 [DOI] [PubMed] [Google Scholar]
- 5). Cuchel M, Bruckert E, Ginsberg HN, Raal FJ, Santos RD, Hegele RA, Kuivenhoven JA, Nordestgaard BG, Descamps OS, Steinhagen-Thiessen E: Homozygous familial hypercholesterolaemia: new insights and guidance for clinicians to improve detection and clinical management. A position paper from the Consensus Panel on Familial Hypercholesterolaemia of the European Atherosclerosis Society. Eur Heart J, 2014; 35: 2146-2157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6). Nordestgaard BG, Chapman MJ, Humphries SE, Ginsberg HN, Masana L, Descamps OS, Wiklund O, Hegele RA, Raal FJ, Defesche JC: Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease. Eur Heart J, 2013; 34: 3478-3490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7). Pang J, Lansberg PJ, Watts GF: International Developments in the Care of Familial Hypercholesterolemia: Where Now and Where to Next? J Atheroscler Thromb, 2016; 23:505-519 [DOI] [PubMed] [Google Scholar]
- 8). Versmissen J, Oosterveer DM, Yazdanpanah M, Defesche JC, Basart DC, Liem AH, Heeringa J, Witteman JC, Lansberg PJ, Kastelein JJ: Efficacy of statins in familial hypercholesterolaemia: a long term cohort study. BMJ, 2008; 337: a2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9). Harada-Shiba M, Sugisawa T, Makino H, Abe M, Tsushima M, Yoshimasa Y, Yamashita T, Miyamoto Y, Yamamoto A, Tomoike H: Impact of statin treatment on the clinical fate of heterozygous familial hypercholesterolemia. J Atheroscler Thromb, 2010; 17: 667-674 [DOI] [PubMed] [Google Scholar]
- 10). Raal FJ, Santos RD: Homozygous familial hypercholesterolemia: current perspectives on diagnosis and treatment. Atherosclerosis, 2012; 223: 262-268 [DOI] [PubMed] [Google Scholar]
- 11). Watts GF, Sullivan DR, Poplawski N, van Bockxmeer F, Hamilton-Craig I, Clifton PM, O'Brien R, Bishop W, George P, Barter PJ: Familial hypercholesterolaemia: a model of care for Australasia. Atheroscler Suppl, 2011; 12: 221-263 [DOI] [PubMed] [Google Scholar]
- 12). Watts GF, George P, Hagger MS, Hu M, Lin J, Lin KK, Marais AD, Miida T, Nawawi HM, Pang J, Park E, Gonzalez-Santos LB, Su TC, Troung TH, Santos RD, Soran H, Yamashita Y, Tomlinson B, Translational Research for Improving the Care of Familial Hypercholesterolaemia : The “Ten Countries Study” and Beyond. J Atheroscler Thromb, 2016; 23: 891-900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13). Hamilton-Craig I, Watts G, Hammond E, Sullivan D, George P, Clifton P, Nicholls S, van Bockxmeer F, Bishop W, O'Brien R, Bell D: Establishing an Australian and New Zealand Registry for Patients with Familial Hypercholesterolaemia. Heart Lung Circ, 2013; 22: S229 [Google Scholar]
- 14). Defesche JC: Defining the challenges of FH screening for familial hypercholesterolemia. J Clin Lipidol, 2010; 4: 338-341 [DOI] [PubMed] [Google Scholar]
- 15). Watts G, Dimmitt S, Redgrave T, Bates T, Emery J, Burnett J, van Bockxmeer F, Poke S, Maxwell S, O'Leary P: Model of Care: Familial Hypercholesteroleamia, Perth, Western Australia, 2008 [Google Scholar]
- 16). Watts GF, Gidding S, Wierzbicki AS, Toth PP, Alonso R, Brown WV, Bruckert E, Defesche J, Lin KK, Livingston M: Integrated guidance on the care of familial hypercholesterolaemia from the International FH Foundation. Int J Cardiol, 2014; 171: 309-325 [DOI] [PubMed] [Google Scholar]
- 17). Hammond E, Watts GF, Rubinstein Y, Farid W, Livingston M, Knowles JW, Lochmüller H, Bellgard M, Dawkins HJ: Role of international registries in enhancing the care of familial hypercholesterolaemia. Int J Evid Based, 2013; 11: 134-139 [DOI] [PubMed] [Google Scholar]
- 18). Bellgard MI, Beroud C, Parkinson K, Harris T, Ayme S, Baynam G, Weeramanthri T, Dawkins H, Hunter A: Dispelling myths about rare disease registry system development. Source Code Biol Med, 2013; 8: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19). Young AS, Chaney E, Shoai R, Bonner L, Cohen AN, Doebbeling B, Dorr D, Goldstein MK, Kerr E, Nichol P: Information technology to support improved care for chronic illness. J Gen Intern Med, 2007; 22: 425-430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20). Bodenheimer T: Interventions to improve chronic illness care: evaluating their effectiveness. Dis Manag, 2003; 6: 63-71 [DOI] [PubMed] [Google Scholar]
- 21). Bufalino VJ, Masoudi FA, Stranne SK, Horton K, Albert NM, Beam C, Bonow RO, Davenport RLV, Girgus M, Fonarow GC: The American Heart Association's recommendations for expanding the applications of existing and future clinical registries a policy statement from the American Heart Association. Circulation, 2011; 123: 2167-2179 [DOI] [PubMed] [Google Scholar]
- 22). Mundal L, Retterstøl K: A systematic review of current studies in patients with familial hypercholesterolemia by use of national familial hypercholesterolemia registries. Curr Opin Lipidol, 2016; 27: 388-397 [DOI] [PubMed] [Google Scholar]
- 23). Humphries SE, Whittall RA, Hubbart CS, Maplebeck S, Cooper JA, Soutar A, Naoumova R, Thompson GR, Seed M, Durrington PN: Genetic causes of Familial Hypercholesterolaemia in UK patients: relation to plasma lipid levels and coronary heart disease risk. J Med Genet, 2006; 43: 943-949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24). Neil A, Cooper J, Betteridge J, Capps N, McDowell I, Durrington P, Seed M, Humphries SE: Reductions in all-cause, cancer, and coronary mortality in statin-treated patients with heterozygous familial hypercholesterolaemia: a prospective registry study. Eur Heart J, 2008; 29: 2625-2633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25). Neil H, Hammond T, Huxley R, Matthews D, Humphries S: Extent of underdiagnosis of familial hypercholesterolaemia in routine practice: prospective registry study. BMJ, 2000; 321: 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26). Ramaswami U, Cooper J, Humphries SE: The UK Paediatric Familial Hypercholesterolaemia Register: preliminary data. Arch Dis Child, 2017; 102: 255-260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27). Umans-Eckenhausen MA, Defesche JC, Sijbrands EJ, Scheerder RL, Kastelein JJ: Review of first 5 years of screening for familial hypercholesterolaemia in the Netherlands. Lancet, 2001; 357: 165-168 [DOI] [PubMed] [Google Scholar]
- 28). Toleikyte I, Retterstøl K, Leren TP, Iversen PO: Pregnancy Outcomes in Familial Hypercholesterolemia A Registry-Based Study. Circulation, 2011; 124: 1606-1614 [DOI] [PubMed] [Google Scholar]
- 29). Mundal L, Sarancic M, Ose L, Iversen PO, Borgan JK, Veierød MB, Leren TP, Retterstøl K: Mortality Among Patients With Familial Hypercholesterolemia: A Registry? Based Study in Norway, 1992–2010. J Am Heart Assoc, 2014; 3: e001236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30). Mundal L, Veierød MB, Halvorsen T, Holven KB, Ose L, Iversen PO, Tell GS, Leren TP, Retterstøl K: Cardiovascular disease in patients with genotyped familial hypercholesterolemia in Norway during 1994–2009, a registry study. Eur J Prev Cardiol, 2016;. 2047487316666371 [DOI] [PubMed] [Google Scholar]
- 31). O'Brien EC, Roe MT, Fraulo ES, Peterson ED, Ballantyne CM, Genest J, Gidding SS, Hammond E, Hemphill LC, Hudgins LC: Rationale and design of the familial hypercholesterolemia foundation CAscade SCreening for Awareness and DEtection of Familial Hypercholesterolemia registry. Am Heart J, 2014; 167: 342-349 [DOI] [PubMed] [Google Scholar]
- 32). deGoma EM, Ahmad ZS, O'Brien EC, Kindt I, Shrader P, Newman CB, Pokharel Y, Baum SJ, Hemphill LC, Hudgins LC, Ahmed CD: Treatment Gaps in Adults with Heterozygous Familial Hypercholesterolemia in the United States: Data from the CASCADE-FH Registry. Circ Cardiovasc Genet, 2016; 10.1161/CIRCGENETICS.116.001381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33). Williams RR, Hamilton-Craig I, Kostner GM, Hegele RA, Hayden MR, Pimstone SN, Faergeman O, Schuster H, Steinhagen-Thiessen E, Beisiegel U, Keller C, Czeizel AE, Leitersdore E, Kastelein JC, Defesche JJP, Ose L, Leren TP, Seftel HC, Raal FJ, Marais AD, Eriksson M, Keller U, Miserez AR, Jeck T, Betterridge DJ, Humphries SE, Day INM, Kwiterovich PO, Lees RS, Stein E, Illingworth R, Kane J, Boulyjenkov V: MED-PED: An Integrated Genetic Strategy for Preventing Early Deaths. In: Genetic Approaches to Noncommunicable Diseases, ed by Berg K, Boulyjenkov V, Christen Y. pp35-45, Springer Berlin Heidelberg, Berlin, Heidelberg, 1996 [Google Scholar]
- 34). Wong S, Taraboanta C, Francis GA, Ignaszewski A, Frohlich J: The British Columbia Familial Hypercholesterolemia Registry. BCMJ, 2013; 55: 326-330 [Google Scholar]
- 35). Mata P, Alonso R, Pérez-Jiménez F: Screening for Familial Hypercholesterolemia: a Model for Preventive Medicine. Rev Esp Cardiol, 2014; 67: 685-688 [DOI] [PubMed] [Google Scholar]
- 36). Mata N, Alonso R, Badimón L, Padró T, Fuentes F, Muñiz O, Perez-Jiménez F, López-Miranda J, Díaz JL, Vidal JI: Clinical characteristics and evaluation of LDLcholesterol treatment of the Spanish Familial Hypercholesterolemia Longitudinal Cohort Study (SAFEHEART). Lipids Health Dis, 2011; 10: 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37). Bamimore MA, Zaid A, Banerjee Y, Al-Sarraf A, Abifadel M, Seidah NG, Al-Waili K, Al-Rasadi K, Awan Z: Familial hypercholesterolemia mutations in the Middle Eastern and North African region: A need for a national registry. J Clin Lipidol, 2015; 9: 187-194 [DOI] [PubMed] [Google Scholar]
- 38). Béliard S, Millier A, Bruckert É: A French cohort of patients with familial hypercholesterolemia. 11ème congrès NSFA, 2015; http://www.creativ-ceutical.com/sites/default/files/NSFA_2015_17-4_French_cohort_familial_hypercholesterolemia.pdf [Google Scholar]
- 39). Vallejo-Vaz AJ, Kondapally SS, Cole D, Hovingh GK, Kastelein J, Mata P, Raal FJ, Santos RD, Soran H, Watts GF: Familial hypercholesterolaemia: A global call to arms. Atherosclerosis, 2015; 243: 257. [DOI] [PubMed] [Google Scholar]
- 40). PASS Software : PASS Clinical Vascular. 2010; 2015: http://www.pass-software.com/Producten/PASSClinical-Vascular/tabid/116/language/en-US/Default.aspx [Google Scholar]
- 41). Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG: Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform, 2009; 42: 377-381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42).TREAT-NMD: Registries tool kit. 2015; http://www.treat-nmd.eu/resources/patient-registries/tookit/
- 43). EURORDIS-NORD-CORD : Joint Declaration 10 Key Principles of Rare Disease Patient Registries. 2012; http://download.eurordis.org/documents/pdf/EURORDIS_NORD_CORD_JointDec_Registries_FINAL.pdf
- 44). EUCERD : Core Recommendations on Rare Disease Patient Registration and Data Collection. 2013; http://www.eucerd.eu/wp-content/uploads/2013/06/EUCERD_Recommendations_RDRegistryDataCollection_adopted.pdf
- 45). Bellgard MI, Sleeman MW, Guerrero FD, Fletcher S, Baynam G, Goldblatt J, Rubinstein Y, Bell C, Groft S, Barrero R: Rare Disease Research Roadmap: Navigating the bioinformatics and translational challenges for improved patient health outcomes. Health Pol Tech, 2014; 3: 325-335 [Google Scholar]
- 46). Lindoerfer D, Mansmann U: A Comprehensive Assessment Tool for Patient Registry Software Systems: The CIPROS Checklist. Methods Inf Med, 2015; 54: 447-454 [DOI] [PubMed] [Google Scholar]
- 47). Thompson R, Johnston L, Taruscio D, Monaco L, Béroud C, Gut IG, Hansson MG, Peter-Bram A, Patrinos GP, Dawkins H: RD-Connect: an integrated platform connecting databases, registries, biobanks and clinical bioinformatics for rare disease research. J Gen Intern Med, 2014; 29: 780-787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48). Bellgard MI, Macgregor A, Janon F, Harvey A, O'Leary P, Hunter A, Dawkins H: A modular approach to disease registry design: Successful adoption of an internet - based rare disease registry. Hum Mutat, 2012; 33: E2356-E2366 [DOI] [PubMed] [Google Scholar]
- 49). Bellgard MI, Render L, Radochonski M, Hunter A: Second generation registry framework. Source Code Biol Med, 2014; 9: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50). Bellgard MI, Napier K, Render L, Radochonski M, Lamont L, Graham C, Wilton SD, Fletcher S, Goldblatt J, Hunter AA, Weeramanthri T: A Registry Framework Enabling Patient-Centred Care. Stud Health Technol Inform, 2015; 214: 8-14 [PubMed] [Google Scholar]
- 51). Napier KR, Pang J, Lamont L, Walker CE, Dawkins HJS, Hunter AA, van Bockxmeer FM, Watts GF, Bellgard MI: A web-based registry for familial hypercholesterolaemia. Heart Lung Circ, 2017; 10.1016/j.hlc.2016.10.019 [DOI] [PubMed] [Google Scholar]
- 52). World Medical Association : World Medical Association Declaration of Helsinki - ethical principles for medical research involving human subjects. J Am Heart Assoc, 2013; 310: 2191-2194 [DOI] [PubMed] [Google Scholar]
- 53). Ellis KL, Hooper AJ, Burnett JR, Watts GF: Progress in the care of common inherited atherogenic disorders of apolipoprotein B metabolism. Nat Rev Endocrinol, 2016; 12: 467-484 [DOI] [PubMed] [Google Scholar]
- 54). Haralambos K, Whatley S, Butler R, Datta D, Townsend D, Gingell R, Edwards R, Howard K, McDowell I: Familial Hypercholesterolaemia (FH) in Wales - Integrating R&D with Service Delivery (Conference Poster). 2013; http://www.fhservice.wales.nhs.uk/sitesplus/docu-ments/1072/Conference [Google Scholar]
- 55). Haralambos K, Whitmore J: Using Pass Database and Geographic Information Systems (GIS) to map Familial Hypercholesterolaemia (FH) diagnoses and England and Wales. 2016; Unpublished Poster [Google Scholar]
- 56). Vallejo-Vaz A, Akram A, Kondapally Seshasaic S, Cole D, Watts G, Hovingh G, Kastelein J, Mata P, Raal F, Santos R, Soran H, Freiberger T, Abifadel M, Aguilar-Salinas C, Alnouri F, Alonso R, Al-Rasadi K, Banach M, Bogsrud M, Bourbon M, Bruckert E, Car J, Ceska R, Corral P, Descamps O, Dieplinger H, Do C, Durst R, Ezhov M, Fras Z, Gaita D, Gaspar I, Genest J, Harada-Shiba M, Jiang L, Kayikcioglu M, Lam C, Latkovskis G, Laufs U, Liberopoulos E, Lin J, Lin N, V M, Majano N, Marais A, März W, Mirrakhimov E, Miserez A, Mitchenko O, Nawawi H, Nilsson L, Nordestgaard B, Paragh G, Petrulioniene Z, Pojskic B, Reiner Z, Sahebkar A, Santos L, Schunkert H, Shehab A, Slimane M, Stoll M, Su T, Susekov A, Tilney M, Tomlinson B, Tselepis A, Vohnoutbo B, Widén E, Yamashita S, Catapano A, Ray K: Pooling and expanding registries of familial hypercholesterolaemia to assess gaps in care and improve disease management and outcomes: Rationale and design of the global EAS Familial Hypercholesterolaemia Studies Collaboration. Atheroscler Suppl, 2016; 22: 1-32 [DOI] [PubMed] [Google Scholar]


