Abstract
White adipose tissue (WAT) stores energy as triacylglycerol in preparation for fasting state. In contrast, brown adipose tissue (BAT) consumes energy and produces heat in a cold environment. One of the major differences between these two adipose tissues is the morphology of the intracellular lipid droplet (LD), which is large and unilocular in WAT and small and multilocular in BAT. Although the fat-specific protein 27 alpha (FSP27α), belonging to the cell death-inducing DNA fragmentation factor A (DFFA)-like effector (Cide) family, was known to be indispensable for large unilocular LD formation in WAT, the mechanism that regulated small multilocular LD formation in BAT remained unknown. We recently uncovered that FSP27β, a novel isoform of FSP27 abundantly expressed in BAT, plays a crucial role in small multilocular LD formation by inhibiting the homodimerization of CideA in BAT. We speculate that unilocular LD formation is ideal for efficient lipid storage in WAT because lipolysis from the LD surface is restricted due to the minimum LD surface area. In addition, hydrolyzed free fatty acid (FFA) and glycerol can efficiently flow out into the circulation from the cell surface. In contrast, small multilocular LD formation is ideal for efficient intracellular lipolysis from the LD surface and the subsequent facilitation of FFA transport to mitochondria that are adjacent to LDs for β-oxidation in BAT. Thus, intracellular LD morphology is closely related to the functions and characteristics of adipose tissues. Given that the browning of adipose tissue leads to enhanced energy expenditure and the prevention of obesity, clarification of the mechanism with respect to intracellular LD formation is very meaningful.
Keywords: Lipid droplet, Adipocyte, Cell death-inducing DNA fragmentation factor A (DFFA)-like effector A (CideA), Fat-specific protein 27 (FSP27), Brown adipose tissue (BAT), White adipose tissue (WAT)
Introduction
Obesity and obesity-related disorders, including type 2 diabetes, dyslipidemia, hypertension, fatty liver, and atherosclerosis, are increasing worldwide1). Obesity is the result of energy imbalance caused by sufficient nutrient intake and poor energy expenditure and is characterized by excessive lipid accumulation in adipose tissues2). In particular, visceral adiposity is believed to cause insulin resistance and is associated with a variety of diseases including diabetes and cardiovascular diseases3, 4). Furthermore, the adipose tissue plays crucial roles in the regulation of whole-body energy homeostasis. Adipose tissue is not only highly adapted to store surplus energy as triacylglycerol (TAG) but also secretes various mediators and cytokines (adipokines) that affect whole-body metabolism5) and heart diseases6). Excessive TAG storage in adipose tissues causes adipose tissue inflammation and the deregulation of adipokines, leading to insulin resistance in remote insulin-sensitive tissues, for example, the liver, skeletal muscle and heart. In addition, surplus lipid that exceeds the storage capacity of adipocytes induces ectopic TAG accumulation in other tissues, such as the liver, skeletal muscle, heart, or pancreas. It induces not only insulin resistance in insulin-sensitive tissues but also impairment of insulin secretion in β cells7). These imply that TAG storage in adipocyte is associated with obesity, diabetes, and cardiovascular diseases. Adipocytes can be classified into energy-storing white adipocytes and energy-consuming brown ones according to their distinct function and morphology8). It is important to unveil the mechanism for efficient TAG storage in white adipocytes and for efficient energy expenditure in brown adipocytes. Although the transcriptional factors controlling the differentiation between white and brown adipocytes have been extensively investigated9, 10), it is not enough to examine the difference of lipid droplet (LD) formation between the two adipocytes. We assume that the functions of these adipocytes are associated with the intracellular LD morphology. Thus, it is crucial to disclose the mechanism for lipid storage and consumption in white and brown adipocytes. We describe the recent progress regarding the relationship between LD morphology and energy metabolism in white and brown adipocytes.
White Adipose Tissue and Brown Adipose Tissue
Energy storage as droplets containing neutral lipids (mainly TAG and steryl esters) in the cytoplasm in preparation for starvation is a common and widespread feature among eukaryotic cells. LDs of all types of cells share a general structure. A hydrophobic core of neutral lipids in LD is surrounded by a membrane monolayer of phospholipids (phosphatidylcholone and phosphatidylethanolamine) 11, 12). LDs are believed to derive from the endoplasmic reticulum (ER). Neutral lipids are synthesized in the interior of the bilayer of the ER and enlarge into spheres between the bilayer; they eventually bud from the ER into the cytoplasm surrounded by a phospholipid monolayer derived from the ER13). In addition, the phospholipid monolayer of LDs contains a variety of proteins involved in the appropriate regulation of LD formation and degradation14). Among them, perilipin 1 was identified as the first LD protein expressed abundantly in adipocytes15). It is a major phosphorylated protein by protein kinase A in adipocytes, and its phosphorylation is essential for catecholamine-stimulated lipolysis16). Furthermore, perilipin 1 was also found to regulate cellular lipid metabolism17). LDs exist ubiquitously in various organs. In non-adipocyte cells, the LD is very small, and its size is usually smaller than 1 µm in diameter, except for extreme pathological states such as hepatocytes in steatosis18). However, adipocytes have large LDs because adipocytes are cells in which the lipid storage function has specifically developed. There are two types of adipocytes that play different roles in energy metabolism8). One is the white adipocyte that stores lipid as a large unilocular lipid droplet that occupies almost all the cytoplasmic space and can be in the 100 µm range18). The other is the brown adipocyte that stores lipid as small multilocular lipid droplets. Both adipocytes have the common characteristic of storing lipid efficiently for each tissue-specific purpose. White adipocyte uptakes glucose and free fatty acids (FFA), synthesizes TAG, and stores it as lipid in the postprandial period. In the fasting, it hydrolyzes TAG to FFA and glycerol. The former is utilized in skeletal muscles and the heart as an energy source instead of glucose. The latter is used in the liver as a substrate for the production of glucose. Conversely, brown adipocytes dissipate energy for heat production by using FFA generated by hydrolyzing intracellular LD through the proton leak via the activation of the BAT-specific protein, uncoupling protein 1 (UCP1)19, 20).
WAT is mainly located in the visceral or subcutaneous space, whereas BAT is mainly located in the interscapular and perirenal spaces in rodents. Visceral fat accumulation has been shown to increase with age21). BAT was traditionally thought to exist only in the neonatal and early childhood periods in the interscapular region and to disappear with age in humans22). However, it was revealed that adult humans also have BAT depots, for example, in the cervical, supraclavicular, and paravertebral areas23–28).
WAT and BAT originate by a distinct developmental program. White adipocytes originate from myogenic lineage marker Myf5-negative mesenchymal precursor cells. On the contrary, brown adipocytes arise from precursors that express Myf5, and the transcriptional profiles of brown adipocytes are similar to those of the skeletal muscle29, 30). Several factors have been identified as brown fat-specific gene induction factors, such as peroxisome proliferator-activated receptor (PPAR) γ-coactivator-1α (PGC-1α)31), PR domain-containing protein-16 (PRDM16)32), bone morphogenic protein family (BMP)33), CCAAT/enhancer binding protein β (C/EBPβ)34), and lysine-specific demethylase 1 (Lsd1)35).
Recently, another type of thermogenic adipocyte, designated as “beige” or “brite” cells, was demonstrated to be induced in the WAT of rodents and humans36, 37). Beige adipocytes derive from Myf5-negative cells in WAT30), and their development is induced in response to environmental stimulation, such as cold exposure, PPARγ agonists, and exercise38). They are highly energy expending and characterized by plenty of mitochondria, small multilocular LDs, and increased expression of UCP1 similar to brown adipocytes39). In recent years, brown and beige adipocytes are suggested as therapeutic targets for weight loss40).
Importance of LD Morphology in the Characteristics of Adipocytes with Respect to Energy Metabolism
In mammals, white adipocytes play an important role as the primary reservoir of excess energy. Large unilocular LDs in white adipocytes are thought to be the ideal structure to store TAG. In this form, the surface area of the LD becomes minimum. Thus, the area of the contact site of the LD with lipase becomes small, resulting in the restriction of lipolysis. In addition, white adipocytes need to supply FFA and glycerol to other tissues in case of energy demand, such as the fasting state. In such cases, FFA and glycerol generated on the LD surface that is close to the plasma membrane can efficiently flow out of the cells into the circulation. On the other hand, brown adipocytes possess small multilocular LDs in their cytoplasm and effectively conduct thermogenesis by uncoupling substrate oxidation and adenosine triphosphate production through the proton leak. Small multilocular LD formation increases the contact area of the LD with lipase, which efficiently promotes lipolysis and facilitates FFA transport to the mitochondria adjacent to LDs for β-oxidation and heat production in mitochondria (Fig. 1). As just described, these differences in the lipid accumulation pattern between the two adipocytes are thought to reflect their functional characteristics. However, the mechanism responsible for the formation of LDs in white and brown adipocytes has remained unknown.
Fig. 1.
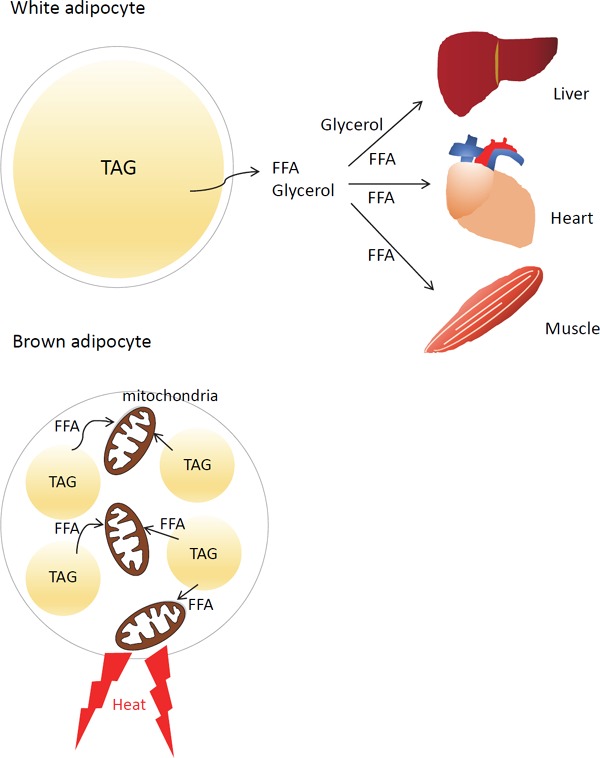
Lipid droplet formation and function in white and brown adipocytes
White adipocyte stores TAG as a unilocular large LD. In a fasting state, stored TAG is hydrolyzed to FFA and glycerol, which are delivered to the skeletal muscle, heart, and liver. They are utilized as substrates for ATP production in the muscles and heart or for glucose production in the liver. Brown adipocyte forms small multilocular LDs and effectively induces lipolysis from the enlarged total surface area of multilocular LDs. Generated FFAs flow into adjacent mitochondria and are utilized as a source for β-oxidation and the production of heat by uncoupling.
Cide Family Proteins Regulate Whole-Body Energy Homeostasis
Cell death-inducing DNA fragmentation factor A (DFFA)-like effector (Cide) family proteins, containing CideA, CideB, and the fat-specific protein 27 (FSP27) (CideC in humans), are among the LD-associated proteins and have been shown to play a crucial role in lipid and energy metabolism, including lipolysis, lipid oxidation, and LD formation41). They share homology with the N-terminal region of the DNA fragmentation factor DFF45 (DFFA), which regulates apoptosis42).
CideA is strongly and almost exclusively expressed in BAT in mice43). CideA-deficient mice show lean phenotype and are resistant to diet-induced obesity and diabetes. CideA appears to be a mitochondrial protein that suppresses UCP1 activity and regulates thermogenesis and lipolysis43). Lipid accumulation and LD size in brown adipocytes decreased in CideA-deficient mice stimulated by cold exposure43). Increased expression level of CideA was also observed in the livers of high-fat diet (HFD)-fed mice and leptin-deficient (ob/ob) mice44, 45). In recent years, CideA was demonstrated as mediating LD fusion at the LD–LD contact site between two LDs in white and brown adipose tissues46, 47). CideB is highly expressed in the liver and kidney42, 48). Energy expenditure was increased and insulin sensitivity was improved in CideB-null mice48). CideB is also localized on the LD–LD contact sites and promotes LD fusion and growth in hepatocytes49). Moreover, CideB plays an important role in mature very-low-density lipoprotein secretion50–52).
FSP27 was initially reported to be one of the LD proteins in 3T3-L1 adipocytes53). Thereafter, FSP27 was found to be predominantly expressed in adipose tissues, and it contributes to the efficient storage of TAG and the large unilocular LD formation in white adipocytes of mice54). FSP27 knockout (KO) mice showed reduction of lipid storage in WAT and were protected from HFD-induced obesity and insulin resistance54, 55). Interestingly, WAT in FSP27 KO mice displayed multilocular LD formation that is similar to BAT54). In addition, insulin sensitivity was also improved in CideA and FSP27 double-KO mice, and their white and brown adipose tissues showed smaller LDs than those of CideA or FSP27 single deficient mice46). These results suggest that in the Cide family, CideA, CideB, and FSP27 are all involved in the enlargement of LD. In particular, FSP27 is essential for unilocular LD formation that is the characteristic LD morphology of WAT.
In humans, the results reported on CideA and CideC are inconsistent with those in mice. CideA and CideC are both expressed in WAT in humans; however, CideA is abundantly expressed in BAT, but not in WAT, in mice. The expression levels of CideA and CideC correlate positively with the insulin sensitivity in obese people56). In addition, a patient with homozygous nonsense mutation in CideC was reported to be characterized by lipodystrophic phenotype, i.e., white adipocytes partially with multilocular LDs and insulin resistance supposedly caused by fatty liver57). Increased hepatic expressions of CideA and FSP27 were also observed under the condition of liver steatosis in humans58). In the mice model, FSP27-decifient mice appeared to be protected against weight gain and insulin resistance54, 55). However, small LDs in WAT were observed, and hepatosteatosis and insulin resistance developed on HFD feeding in adipocyte-specific FSP27 KO mice59). Furthermore, insulin resistance and hepatic steatosis developed in FSP27 KO mice that were overloaded with excess lipid in the context of leptin deficiency or long-term exposure to HFD60). Anyway, the effects of FSP27 on whole-body energy metabolism may be different between mice in which CideA is expressed exclusively in BAT and humans in whom CideA is also expressed in WAT. However, at least in humans, adipose FSP27 is supposed to improve insulin sensitivity by increasing lipid accumulation in adipose tissues and, resultantly, minimizing ectopic fat accumulation in other insulin-sensitive tissues, for example, the liver or skeletal muscle. Therefore, it is unclear whether the silencing of FSP27 that resulted in the reduction of WAT mass and the enhanced insulin sensitivity without leading to hepatosteatosis in obese mice61) can also be of therapeutic application to obese people.
FSP27 Regulates the Formation of Large Unilocular LD in White Adipocytes
Ectopic expression of FSP27 led to the enlargement of LDs and TAG accumulation in non-adipose cells54, 62–65), whereas depletion of FSP27 in cultured adipocytes resulted in small LD formation and increased lipolysis54, 62, 63). Moreover, depletion of FSP27 by siRNA in HW adipocytes that show the morphological characteristics of white adipocytes resulted in the formation of many small LDs similar to brown adipocytes54). In fact, WAT mass was reduced, and the LDs in WAT were also of a small multilocular pattern in FSP27 KO mice (Fig. 2)54, 55). Energy expenditure was significantly increased in FSP27 KO mice due to enhanced mitochondrial biogenesis and FFA oxidation in WAT54). These results suggest that FSP27 plays essential roles in large unilocular LD formation in WAT. In addition, the mechanism by which FSP27 promotes LD enlargement is also clarified. FSP27 is highly enriched at an LD–LD contact site and forms homodimers that are involved in directional lipid transfer from small to large LDs between adjacent LDs due to the higher internal pressure in small LDs66, 67). Furthermore, structure-function analysis reveals that the carboxy-terminal domain of FSP27 (amino acids 131–239) plays a crucial role in LD expansion, possibly by homodimerization, whereas the amino-terminal domain (amino acids 1–130) has a supportive role65). On the other hand, there are studies showing the importance of the amino-terminal region of FSP27 in promoting LD growth67). However, considering the report that a patient carrying a homozygous nonsense mutation in CIDEC, the human homologue of FSP27, which is predicted to truncate the protein at amino acid 186, shows small and multilocular LDs in white adipocytes57), the carboxy-terminal domain of FSP27 is essential for large unilocular LD formation in human WAT. Besides, FSP27 was found to store TAG efficiently in cooperation with several proteins: perilipin 167, 68), adipose triacylglycerol lipase69), and Egr170).
Fig. 2.
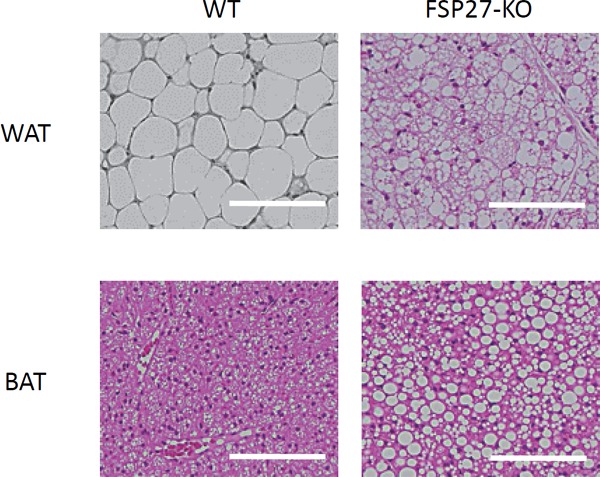
WAT and BAT of FSP27 KO mouse
Hematoxylin and eosin staining of epididymal WAT and BAT (Scale bar: 100 µm). LDs are smaller and multilocular in the epididymal WAT of an FSP27 knockout (KO) mouse. In contrast, LDs in the BAT of an FSP27 KO mouse are larger than those of a wild-type (WT) mouse.
CideA and FSP27 β Coordinately Regulate LD Formation in Brown Adipocytes
Recently, a new isoform of FSP27, FSP27β, was identified in the liver71). It contains 10 additional amino acids at the amino-terminal domain of the conventional isoform of FSP27, designated as FSP27α. Both isoforms of FSP27 are alternatively transcripted from the same gene and driven by distinct promoters. Although FSP27α is mainly expressed in WAT, FSP27β is expressed at a high level in the liver and BAT in mice71, 72). FSP27α is transcriptionally regulated by PPARγ, and FSP27β is regulated by the liver-enriched transcription factor cyclic-AMP-responsive-element-binding protein H (CREBH) in the liver71). CREBH is activated by ER stress and proinflammatory stimuli and induces the expression of acute phase response genes73). CREBH expression increases during fasting through FFA and PPARα74). It regulates glucose and lipid metabolism in the liver75–77). CREBH-deficient mice have been reported as developing hepatic steatosis due to increased lipolysis in adipose tissues when fasted or fed a ketogenic diet78). A recent study revealed that the loss of CREBH decreases the hepatic expression level of FSP27β in fasted mice, and the overexpression of CREBH induces LD growth and TAG accumulation through FSP27β by suppressing lipolysis in the liver of mice71). In the liver, FSP27β is thought to be associated with LD growth in the same manner as FSP27α71). FSP27β is also expressed in BAT along with the simultaneous abundant expression of CideA72). However, the roles of FSP27β in BAT were not clarified.
Although CideA is known to be exclusively expressed in BAT and known to promote LD enlargement, the molecular mechanism of CideA that forms the small multilocular LD in BAT has remained unknown. We recently demonstrated that CideA and FSP27β coordinately regulate LD formation in brown adipocytes72). We found that the overexpression of FSP27α or CideA promoted the formation of large LDs in COS cells, as was reported previously; however, the sole expression of FSP27β did not induce the enlargement of LDs72). Interestingly, the simultaneous overexpression of FSP27β and CideA in COS cells that imitated the expression pattern of the Cide family proteins in BAT resulted in smaller LD formation compared with the cells that overexpressed CideA only. RNAi-mediated FSP27β knockdown in HB2 adipocytes, which possess the characteristics of brown adipocytes, resulted in enlarged LDs. Furthermore, FSP27 KO mice in which FSP27β was not expressed in BAT had larger LDs in BAT compared with wild-type mice (Fig. 2). FSP27β and CideA formed a complex in the BAT of mice72). FSP27β inhibited the homodimerization of CideA by binding to CideA in COS cells72). Co-expressed Cide proteins, including CideA, CideB, and FSP27, are shown to localize on the LD surface and form a complex at the LD–LD contact site in non-adipose cells49). These results indicate that FSP27β negatively regulates CideA-promoted enlargement of LD size by inhibiting the homodimerization of CideA on the LD surface of brown adipocytes (Fig. 3). β3-adrenergic agonist-stimulated oxygen consumption was increased in isolated white adipocytes from FSP27 KO mice that show multilocular LD. In contrast, oxygen consumption was reduced in isolated brown adipocytes of FSP27 KO mice that showed large LDs compared with those of wild-type mice54). Given that the multiloculization of LDs resulted in increased oxygen consumption in white adipocytes and the enlargement of LDs resulted in decreased oxygen consumption in brown adipocytes, cellular LD morphology can affect cellular energy metabolism. Thus, FSP27β, which is essential for small multilocular LD formation, may be a potential target for application in therapies aiming to switch adipocytes into energy-dissipating adipocytes.
Fig. 3.
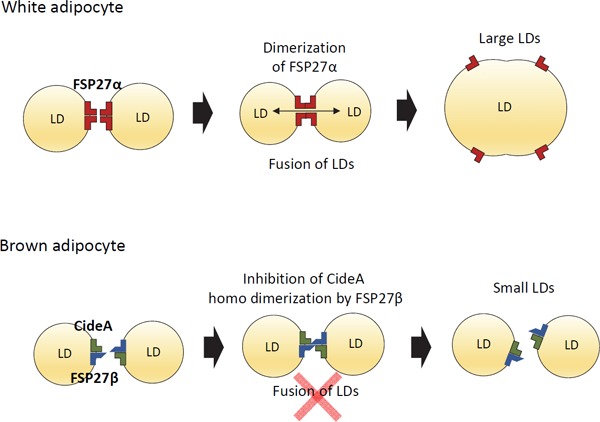
Proposed mechanisms by which the Cide protein family regulates LD size in WAT and BAT
In WAT, FSP27α on neighboring LDs forms homodimerization, resulting in the fusion of LD, subsequent lipid exchange, and formation of larger LDs. CideA also promotes large LD formation by forming homodimerization. However, in BAT, FSP27β inhibits the homodimerization of CideA and suppresses the formation of large LDs, resulting in the formation of multilocular LDs.
Summary
FSP27α and FSP27β are indispensable when regulating the morphology of LDs in adipocytes. FSP27α promotes unilocular LD growth in white adipocytes, and FSP27β inhibits the enlargement of LDs induced by CideA and contributes to the multilocularization of LDs in brown adipocytes (Table 1). Consequently, Cide family proteins regulate energy metabolism efficiently through the modulation of intracellular LD morphology in WAT and BAT.
Table 1. Characteristics and functions of two FSP27 isoforms.
| FSP27α | FSP27β | |
|---|---|---|
| Structure | 1–239 amino acids | 10 amino acids longer at the N-terminus of FSP27α |
| Major expression tissues | WAT | Liver, BAT |
| Transcription factor | PPARγ | CREBH (liver), Unknown (BAT) |
| Function in adipose tissue | Promote the formation of large lipid droplet in white adipocytes Effective energy storage in WAT |
Maintain the formation of small lipid droplets in brown adipocytes Increase energy expenditure in BAT |
Conflict of Interest Statement
The authors have no conflicts of interest.
Footnotes
The following abbreviations are used: WAT, white adipose tissue; BAT, brown adipose tissue; LD, lipid droplet; FSP27, fat-specific protein 27; Cide, cell death-inducing DFF45-like effector; TAG, triacylglycerol; ER, endoplasmic reticulum; FFA, free fatty acids; KO, knockout; HFD, high-fat diet; CREBH, cyclic-AMP-responsive-element-binding protein H.
References
- 1). GBD 2015 Obesity Collaborators : Health effects of over-weight and obesity in 195 countries over 25 years. N Engl J Med, 2017; 377: 13-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2). Spiegelman BM, Flier JS: Obesity and the regulation of energy balance. Cell, 2001; 104: 531-543 [DOI] [PubMed] [Google Scholar]
- 3). Fujimoto WY, Bergstrom RW, Boyko EJ, Chen KW, Leonetti DL, Newell-Morris L, Shofer JB, Wahl PW: Visceral adiposity and incident coronary heart disease in Japanese-American men. The 10-year follow-up results of the Seattle Japanese-American Community Diabetes Study. Diabetes Care, 1999; 22: 1808-1812 [DOI] [PubMed] [Google Scholar]
- 4). Morigami H, Morioka T, Yamazaki Y, Imamura S, Numaguchi R, Asada M, Motoyama K, Mori K, Fukumoto S, Shoji T, Emoto M, Inaba M: Visceral adiposity is preferentially associated with vascular stiffness rather than thickness in men with type 2 diabetes. J Atheroscler Thromb, 2016; 23: 1067-1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5). Rosen ED, Spiegelman BM: Adipocytes as regulators of energy balance and glucose homeostasis. Nature, 2006; 444: 847-853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6). Ouchi N: Adipocytokines in cardiovascular and metabolic diseases. J Atheroscler Thromb, 2016; 23: 645-654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7). Simha V, Garg A: Lipodystrophy: lessons in lipid and energy metabolism. Curr Opin Lipidol, 2006; 17: 162-169 [DOI] [PubMed] [Google Scholar]
- 8). Cohen P, Spiegelman BM: Cell biology of fat storage. Mol. Biol. Cell, 2016: 27: 2523-2527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9). Giralt M, Villarroya F: White, brown, beige/brite: different adipose cells for different functions? Endocrinology, 2013; 154: 2992-3000 [DOI] [PubMed] [Google Scholar]
- 10). Sidossis L, Kajimura S: Brown and beige fat in humans: thermogenic adipocytes that control energy and glucose homeostasis. J Clin Invest, 2015; 125: 478-486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11). Zweytick D, Athenstaedt K, Daum G: Intracellular lipid particles of eukaryotic cells. Biochim. Biophys. Acta, 2000; 1469: 101-120 [DOI] [PubMed] [Google Scholar]
- 12). Tauchi-Sato K, Ozeki S, Houjou T, Taguchi R, Fujimoto T: The surface of lipid droplets is a phospholipid monolayer with a unique fatty acid composition. J Biol Chem, 2002; 277: 44507-44512 [DOI] [PubMed] [Google Scholar]
- 13). Wilfling F, Haas JT, Walther TC, Farese RV, Jr: Lipid droplet biogenesis. Curr Opin Cell Biol, 2014; 29: 39-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14). Brasaemle DL: Thematic review series: adipocyte biology. The perilipin family of structural lipid droplet proteins: stabilization of lipid droplets and control of lipolysis. J Lipid Res, 2007; 48: 2547-2559 [DOI] [PubMed] [Google Scholar]
- 15). Greenberg AS, Egan JJ, Wek SA, Garty NB, Blanchette-Mackie EJ, Londos C: Perilipin, a major hormonally regulated adipocyte-specific phosphoprotein associated with the periphery of lipid storage droplets. J Biol Chem, 1991; 266: 11341-11346 [PubMed] [Google Scholar]
- 16). Miyoshi H, Perfield JW, 2nd, Souza SC, Shen WJ, Zhang HH, Stancheva ZS, Kraemer FB, Obin MS, Greenberg AS: Control of adipose triglyceride lipase action by serine 517 of perilipin A globally regulates protein kinase A-stimulated lipolysis in adipocytes. J Biol Chem, 2007; 282: 996-1002 [DOI] [PubMed] [Google Scholar]
- 17). Bickel PE, Tansey JT, Welte MA: PAT proteins, an ancient family of lipid droplet proteins that regulate cellular lipid stores. Biochim Biophys Acta, 2009; 1791: 419-440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18). Suzuki M, Shinohara Y, Ohsaki Y, Fujimoto T: Lipid droplets: size matters. J Electron Microsc (Tokyo), 2011; 60: S101-116 [DOI] [PubMed] [Google Scholar]
- 19). Enerbäck S, Jacobsson A, Simpson EM, Guerra C, Yamashita H, Harper ME, Kozak LP: Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature, 1997; 387: 90-94 [DOI] [PubMed] [Google Scholar]
- 20). Ricquier D, Bouillaud F: The uncoupling protein homologues: UCP1, UCP2, UCP3, StUCP and AtUCP. Biochem J, 2000; 345: 161-179 [PMC free article] [PubMed] [Google Scholar]
- 21). Hirose H, Takayama M, Iwao Y, Kawabe H: Effects of aging on visceral and subcutaneous fat areas and on homeostasis model assessment of insulin resistance and insulin secretion capacity in a comprehensive health checkup. J Atheroscler Thromb, 2016; 23: 207-215 [DOI] [PubMed] [Google Scholar]
- 22). Enerbäck S: Human brown adipose tissue. Cell Metab, 2010; 11: 248-252 [DOI] [PubMed] [Google Scholar]
- 23). Garcia CA, Van Nostrand D, Atkins F, Acio E, Butler C, Esposito G, Kulkarni K, Majd M: Reduction of brown fat 2-Deoxy-2-[F-18]fluoro-d-glucose uptake by controlling environmental temperature prior to positron emission tomography scan. Mol Imagin Biol, 2006; 8: 24-29 [DOI] [PubMed] [Google Scholar]
- 24). Nedergaard J, Bengtsson T, Cannon B: Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab, 2007; 293: E444-452 [DOI] [PubMed] [Google Scholar]
- 25). van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ: Cold-activated brown adipose tissue in healthy men. N Engl J Med, 2009; 360: 1500-1508 [DOI] [PubMed] [Google Scholar]
- 26). Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, Kolodny GM, Kahn CR: Identification and importance of brown adipose tissue in adult humans. N Engl J Med, 2009; 360: 1509-1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27). Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerbäck S, Nuutila P: Functional brown adipose tissue in healthy adults. N Engl J Med, 2009; 360: 1518-1525 [DOI] [PubMed] [Google Scholar]
- 28). Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio-Kobayashi J, Iwanaga T, Miyagawa M, Kameya T, Nakada K, Kawai Y, Tsujisaki M: High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes, 2009; 58: 1526-1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29). Timmons JA, Wennmalm K, Larsson O, Walden TB, Lassmann T, Petrovic N, Hamilton DL, Gimeno RE, Wahlestedt C, Baar K, Nedergaard J, Cannon B: Myogenic gene expression signature establishes that brown and white adipocytes originate from distinct cell lineages. Proc Natl Acad Sci USA, 2007; 104: 4401-4406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30). Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, Scimè A, Devarakonda S, Conroe HM, Erdjument-Bromage H, Tempst P, Rudnicki MA, Beier DR, Spiegelman BM: PRDM16 controls a brown fat/skeletal muscle switch. Nature, 2008; 454: 961-967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31). Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM: A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell, 1998; 92: 829-839 [DOI] [PubMed] [Google Scholar]
- 32). Seale P, Kajimura S, Yang W, Chin S, Rohas LM, Uldry M, Tavernier G, Langin D, Spiegelman BM: Transcriptional control of brown fat determination by PRDM16. Cell Metab, 2007; 6: 38-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33). Tseng YH, Kokkotou E, Schulz TJ, Huang TL, Winnay JN, Taniguchi CM, Tran TT, Suzuki R, Espinoza DO, Yamamoto Y, Ahrens MJ, Dudley AT, Norris AW, Kulkarni RN, Kahn CR: New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure, Nature, 2008; 454: 1000-1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34). Kajimura S, Seale P, Kubota K, Lunsford E, Frangioni JV, Gygi SP, Spiegelman BM: Initiation of myoblast to brown fat switch by a PRDM16-C/EBP-beta transcriptional complex. Nature, 2009; 460: 1154-1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35). Duteil D, Tosic M, Lausecker F, Nenseth HZ, Müller JM, Urban S, Willmann D, Petroll K, Messaddeq N, Arrigoni L, Manke T, Kornfeld JW, Brüning JC, Zagoriy V, Meret M, Dengjel J, Kanouni T, Schüle R: Lsd1 Ablation triggers metabolic reprogramming of brown adipose tissue. Cell Reports, 2016; 17: 1008-1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36). Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J: Chronic peroxisome proliferatoractivated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem, 2010; 285: 7153-7164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37). Wu J, Boström P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, Huang K, Tu H, van Marken Lichtenbelt WD, Hoeks J, Enerbäck S, Schrauwen P, Spiegelman BM: Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell, 2012; 150: 366-376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38). Kajimura S, Saito M: A new era in brown adipose tissue biology: molecular control of brown fat development and energy homeostasis. Annu Rev Physiol, 2014; 76: 225-249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39). Lee YH, Mottillo EP, Granneman JG: Adipose tissue plasticity from WAT to BAT and in between. Biochim Biophys Acta, 2014; 1842: 358-369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40). Warner A, Mittag J: Breaking BAT: can browning create a better white? J Endocrinol, 2016; 228: R19-29 [DOI] [PubMed] [Google Scholar]
- 41). Xu L, Zhou L, Li P: CIDE proteins and lipid metabolism. Arterioscler Thromb Vasc Biol, 2012; 32: 1094-1098 [DOI] [PubMed] [Google Scholar]
- 42). Inohara N, Koseki T, Chen S, Wu X, Núñez G: CIDE, a novel family of cell death activators with homology to the 45 kDa subunit of the DNA fragmentation factor. EMBO J, 1998; 17: 2526-2533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43). Zhou Z, Yon Toh S, Chen Z, Guo K, Ng CP, Ponniah S, Lin SC, Hong W, Li P: Cidea-deficient mice have lean phenotype and are resistant to obesity. Nat Genet, 2003; 35: 49-56 [DOI] [PubMed] [Google Scholar]
- 44). Matsusue K, Kusakabe T, Noguchi T, Takiguchi S, Suzuki T, Yamano S, Gonzalez FJ: Hepatic steatosis in leptindeficient mice is promoted by the PPARgamma target gene Fsp27. Cell Metab, 2008; 7: 302-311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45). Zhou L, Xu L, Ye J, Li D, Wang W, Li X, Wu L, Wang H, Guan F, Li P: Cidea promotes hepatic steatosis by sensing dietary fatty acids. Hepatology, 2012; 56: 95-107 [DOI] [PubMed] [Google Scholar]
- 46). Wu L, Zhou L, Chen C, Gong J, Xu L, Ye J, Li D, Li P: Cidea controls lipid droplet fusion and lipid storage in brown and white adipose tissue. Sci China Life Sci, 2014; 57: 107-116 [DOI] [PubMed] [Google Scholar]
- 47). Barneda D, Planas-Iglesias J, Gaspar ML, Mohammadyani D, Prasannan S, Dormann D, Han GS, Jesch SA, Carman GM, Kagan V, Parker MG, Ktistakis NT, Klein-Seetharaman J, Dixon AM, Henry SA, Christian M: The brown adipocyte protein CIDEA promotes lipid droplet fusion via a phosphatidic acid-binding amphipathic helix. ELife, 2015; 4: e07485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48). Li JZ, Ye J, Xue B, Qi J, Zhang J, Zhou Z, Li Q, Wen Z, Li P: Cideb regulates diet-induced obesity, liver steatosis, and insulin sensitivity by controlling lipogenesis and fatty acid oxidation. Diabetes, 2007; 56: 2523-2532 [DOI] [PubMed] [Google Scholar]
- 49). Xu W, Wu L, Yu M, Chen FJ, Arshad M, Xia X, Ren H, Yu J, Xu L, Xu D, Li JZ, Li P, Zhou L: Differential roles of cell death-inducing DNA fragmentation factor-α-like effector (CIDE) proteins in promoting lipid droplet fusion and growth in subpopulations of hepatocytes. J Biol Chem, 2016; 291: 4282-4293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50). Chen Z, Norris JY, Finck BN: Peroxisome proliferatoractivated receptor-gamma coactivator-1alpha (PGC-1alpha) stimulates VLDL assembly through activation of cell death-inducing DFFA-like effector B (CideB). J Biol Chem, 2010; 285: 25996-26004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51). Tiwari S, Siddiqi S, Siddiqi SA: CideB protein is required for the biogenesis of very low density lipoprotein (VLDL) transport vesicle. J Biol Chem, 2013; 288: 5157-5165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52). Ye J, Li JZ, Liu Y, Li X, Yang T, Ma X, Li Q, Yao Z, Li P: Cideb, an ER- and lipid droplet-associated protein, mediates VLDL lipidation and maturation by interacting with apolipoprotein B. Cell Metab, 2009; 9: 177-190 [DOI] [PubMed] [Google Scholar]
- 53). Brasaemle DL, Dolios G, Shapiro L, Wang R: Proteomic analysis of proteins associated with lipid droplets of basal and lipolytically stimulated 3T3-L1 adipocytes. J Biol Chem, 2004; 279: 46835-46842 [DOI] [PubMed] [Google Scholar]
- 54). Nishino N, Tamori Y, Tateya S, Kawaguchi T, Shibakusa T, Mizunoya W, Inoue K, Kitazawa R, Kitazawa S, Matsuki Y, Hiramatsu R, Masubuchi S, Omachi A, Kimura K, Saito M, Amo T, Ohta S, Yamaguchi T, Osumi T, Cheng J, Fujimoto T, Nakao H, Nakao K, Aiba A, Okamura H, Fushiki T, Kasuga M: FSP27 contributes to efficient energy storage in murine white adipocytes by promoting the formation of unilocular lipid droplets. J Clin Invest, 2008; 118: 2808-2821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55). Toh SY, Gong J, Du G, Li JZ, Yang S, Ye J, Yao H, Zhang Y, Xue B, Li Q, Yang H, Wen Z, Li P: Up-regulation of mitochondrial activity and acquirement of brown adipose tissue-like property in the white adipose tissue of fsp27 deficient mice. PLoS One, 2008; 3: e2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56). Puri V, Ranjit S, Konda S, Nicoloro SM, Straubhaar J, Chawla A, Chouinard M, Lin C, Burkart A, Corvera S, Perugini RA, Czech MP: Cidea is associated with lipid droplets and insulin sensitivity in humans. Proc Natl Acad Sci U. S. A., 2008; 105: 7833-7838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57). Rubio-Cabezas O, Puri V, Murano I, Saudek V, Semple RK, Dash S, Hyden CS, Bottomley W, Vigouroux C, Magré J, Raymond-Barker P, Murgatroyd PR, Chawla A, Skepper JN, Chatterjee VK, Suliman S, Patch AM, Agarwal AK, Garg A, Barroso I, Cinti S, Czech MP, Argente J, O'Rahilly S, Savage DB: Partial lipodystrophy and insulin resistant diabetes in a patient with a homozygous nonsense mutation in CIDEC. EMBO Mol Med, 2009; 1: 280-287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58). Hall AM, Brunt EM, Klein S, Finck BN: Hepatic expression of cell death-inducing DFFA-like effector C in obese subjects is reduced by marked weight loss. Obesity, 2010; 18: 417-419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59). Tanaka N, Takahashi S, Matsubara T, Jiang C, Sakamoto W, Chanturiya T, Teng R, Gavrilova O, Gonzalez FJ: Adipocyte-specific disruption of fat-specific protein 27 causes hepatosteatosis and insulin resistance in high-fat diet-fed mice. J Biol Chem, 2015; 290: 3092-3105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60). Zhou L, Park SY, Xu L, Xia X, Ye J, Su L, Jeong KH, Hur JH, Oh H, Tamori Y, Zingaretti CM, Cinti S, Argente J, Yu M, Wu L, Ju S, Guan F, Yang H, Choi CS, Savage DB, Li P: Insulin resistance and white adipose tissue inflammation are uncoupled in energetically challenged Fsp27-deficient mice. Nat Commun, 2015; 6: 5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61). Langhi C, Arias N, Rajamoorthi A, Basta J, Lee RG, Baldán Á: Therapeutic silencing of fat-specific protein 27 improves glycemic control in mouse models of obesity and insulin resistance. J Lipid Res, 2017; 58: 81-91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62). Puri V, Konda S, Ranjit S, Aouadi M, Chawla A, Chouinard M, Chakladar A, Czech MP: Fat-specific protein 27, a novel lipid droplet protein that enhances triglyceride storage. J Biol Chem, 2007; 282: 34213-34218 [DOI] [PubMed] [Google Scholar]
- 63). Keller P, Petrie JT, De Rose P, Gerin I, Wright WS, Chiang SH, Nielsen AR, Fischer CP, Pedersen BK, MacDougald OA: Fat-specific protein 27 regulates storage of triacylglycerol. J Biol Chem, 2008; 283: 14355-14365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64). Jambunathan S, Yin J, Khan W, Tamori Y, Puri V: FSP27 promotes lipid droplet clustering and then fusion to regulate triglyceride accumulation. PLoS One, 2011; 6: e28614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65). Tamori Y, Tateya S, Ijuin T, Nishimoto Y, Nakajima S, Ogawa W: Negatively-charged residues in the polar carboxy-terminal region in FSP27 are indispensable for expanding lipid droplets. FEBS Lett, 2016; 590: 750-759 [DOI] [PubMed] [Google Scholar]
- 66). Gong J, Sun Z, Wu L, Xu W, Schieber N, Xu D, Shui G, Yang H, Parton RG, Li P: Fsp27 promotes lipid droplet growth by lipid exchange and transfer at lipid droplet contact sites. J Cell Biol, 2011; 195: 953-963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67). Sun Z, Gong J, Wu H, Xu W, Wu L, Xu D, Gao J, Wu J.W, Yang H, Yang M, Li P: Perilipin1 promotes unilocular lipid droplet formation through the activation of Fsp27 in adipocytes. Nat Commun, 2013; 4: 1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68). Grahn TH, Zhang Y, Lee MJ, Sommer AG, Mostoslavsky G, Fried SK, Greenberg AS, Puri V: FSP27 and PLIN1 interaction promotes the formation of large lipid droplets in human adipocytes. Biochem Biophys Res Commun, 2013; 432: 296-301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69). Grahn TH, Kaur R, Yin J, Schweiger M, Sharma VM, Lee MJ, Ido Y, Smas CM, Zechner R, Lass A, Puri V: Fatspecific protein 27 (FSP27) interacts with adipose triglyceride lipase (ATGL) to regulate lipolysis and insulin sensitivity in human adipocytes. J Biol Chem, 2014; 289: 12029-12039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70). Singh M, Kaur R, Lee MJ, Pickering RT, Sharma VM, Puri V, Kandror KV: Fat-specific protein 27 inhibits lipolysis by facilitating the inhibitory effect of transcription factor Egr1 on transcription of adipose triglyceride lipase. J Biol Chem, 2014; 289: 14481-14487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71). Xu X, Park JG, So JS, Lee AH: Transcriptional activation of Fsp27 by the liver-enriched transcription factor CREBH promotes lipid droplet growth and hepatic steatosis. Hepatology, 2015; 61: 857-869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72). Nishimoto Y, Nakajima S, Tateya S, Saito M, Ogawa W, Tamori Y: Cell death-inducing DNA fragmentation factor A-like effector A and fat-specific protein 27β coordinately control lipid droplet size in brown adipocytes. J Biol Chem, 2017; 292: 10824-10834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73). Zhang K, Shen X, Wu J, Sakaki K, Saunders T, Rutkowski DT, Back SH, Kaufman RJ: Endoplasmic reticulum stress activates cleavage of CREBH to induce a systemic inflammatory response. Cell, 2006; 124: 587-599 [DOI] [PubMed] [Google Scholar]
- 74). Danno H, Ishii KA, Nakagawa Y, Mikami M, Yamamoto T, Yabe S, Furusawa M, Kumadaki S, Watanabe K, Shimizu H, Matsuzaka T, Kobayashi K, Takahashi A, Yatoh S, Suzuki H, Yamada N, Shimano H: The liver-enriched transcription factor CREBH is nutritionally regulated and activated by fatty acids and PPARalpha. Biochem Biophys Res Commun, 2010; 391: 1222-1227 [DOI] [PubMed] [Google Scholar]
- 75). Lee MW, Chanda D, Yang J, Oh H, Kim SS, Yoon YS, Hong S, Park KG, Lee IK, Choi CS, Hanson RW, Choi HS, Koo SH: Regulation of hepatic gluconeogenesis by an ER-bound transcription factor, CREBH. Cell Metab, 2010; 11: 331-339 [DOI] [PubMed] [Google Scholar]
- 76). Lee JH, Giannikopoulos P, Duncan SA, Wang J, Johansen CT, Brown JD, Plutzky J, Hegele RA, Glimcher LH, Lee AH: The transcription factor cyclic AMP-responsive element-binding protein H regulates triglyceride metabolism. Nat Med, 2011; 17: 812-815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77). Zhang C, Wang G, Zheng Z, Maddipati KR, Zhang X, Dyson G, Williams P, Duncan SA, Kaufman RJ, Zhang K: Endoplasmic reticulum-tethered transcription factor cAMP responsive element-binding protein, hepatocyte specific, regulates hepatic lipogenesis, fatty acid oxidation, and lipolysis upon metabolic stress in mice. Hepatology, 2012; 55: 1070-1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78). Park JG, Xu X, Cho S, Hur KY, Lee MS, Kersten S, Lee AH: CREBH-FGF21 axis improves hepatic steatosis by suppressing adipose tissue lipolysis. Sci Rep, 2016; 6: 27938. [DOI] [PMC free article] [PubMed] [Google Scholar]


