Abstract
The aim of our research was to examine whether there are differences in the morphology of neuromuscular junctions of different types of muscle fibers in rodents, and after their adaptation to six weeks endurance exercise training. After 5-day acclimation, Wistar rats were subjected to run with the speed 35 m/min during 6 week, 5 days per week and the training volume reached 60 min per day. Muscle samples for ultrastructural studies were fixed, dehydrated and embedded in Epon-812. Ultra-thin sections were cut from longitudinally and transversely oriented blocs, using 4 blocks from each animal. The area of axon terminals on fast- twitch fibers is 1.5 time large (p<0.001) and the perimeter of terminals is 1.7 time large in comparison with slow- twitch oxidative fibers (p<0.001) in control group. There are correlation between cross-sectional area of different muscle fibers and length of axon terminals (r=0.72), between cross-sectional area and with of axon terminal (r=-0.62), and between turnover rate of contractile proteins and length of axon terminal (r=0.75). Fast remodeling of synapse on oxidative and oxidative-glycolytic muscle fibers during endurance training seems to guarantees the intensive renewal of the structures of muscle fibers with higher oxidative capacity.
Key Words: Neuromuscular junction, slow- and fast-twitch fibers, endurance exercise, remodeling of synapses
Competing interest statement
Conflict of Interest
Neuromuscular junction, slow- and fast-twitch fibers, endurance exercise, remodeling of synapses.
List of acronyms
Ach – acetylcholine
AchRs - nicotinic acetylcholine receptors
FT - fast- twitch fibers
NMJ - neuromuscular junction
PSCs - perisynaptic Schwann cells
ST - slow- twitch fibers
The neuromuscular junction (NMJ) is the interface between the motor nervous system and the skeletal muscle fibers. NMJ has shown consists of three cellular components, the nerve terminal, the postsynaptic muscle fiber, and the perisynaptic Schwann cells (PSCs). These cells all together create a chemical synapse that represents the neurological control of muscle contraction.1 Slow- twitch (ST) fibers are innervated by frequently active motoneurons on low frequencies, they have slow conduction velocities, aerobic energy production, and can sustain tension for longer periods. Fast- twitch (FT) fibers fire at high frequency, have fast conduction velocities, but can maintain tension for only short periods and their fiber subtypes differ in energy metablism.2-5 The end-plate region of the muscle fiber is regularly invaginated by postjunctional folds where nicotinic acetylcholine receptors (AChRs) are clustered at the top of these folds and are directly opposed to active zones.6 PSCs have key role in contributions to chemical communication.7,8 The space between the nerve terminal and the postsynaptic membrane is the synaptic cleft. Acetylcholine (ACh) diffuses across the synaptic cleft to active AChRs. Synaptic vesicles contain ACh, each vesicle contain 5000–10000 molecules of ACh.9,10 Acetyl choline esterase in the basal lamina of the postsynaptic membrane and the synaptic cleft accelerates the disappearance of ACh from synaptic cleft, along with the diffusion of ACh out of the cleft.11 Results about size of nerve terminals are conflicting. It has shown that the terminals of ST muscle fibers motor neurons are large and less varicose than those of FT fibers motor neurons,12 yet they release fewer quanta per unit area than those of fast nerve.13 Others had shown that the rat FT and ST muscle fibers terminals are of similar area, but the terminals of FT fibers are shorter.14 The extent of postsynaptic folding is greater in NMJs on FT fibers,15 although this has not been confirmed in all studies.12 In some studies NMJ size and muscle fiber diameter were find positively correlated.16 Exercise training enhanced nerve terminal branching without modifying endplate size. Both increased and decreased physical activity might result in reductions in the ratio between endplate area and length of nerve terminal branches, thus altering the pre- and postsynaptic relationship of NMJs.17 It was shown that the morphology of the NMJ does change significantly in response to exercise training.18 In addition, other authors have shown that the effect of endurance type of exercise training on FT muscle fiber NMJs may reflect some transformation from fast to slow morphological characteristics.3,14 During endurance training NMJs undergo a process of hypertrophy as a compensatory response,17 as well differnt authors have demonstrate that morphology of NMJs does not change significantly.18 Only in one study differences in morphology of NMJs of different fiber types during adaptation to endurance exercise training were described.19
The purpose of this study was to examine whether there are differences in the morphology of neuromuscular junctions in fast fatigue fast-twitch glycolytic, fatigue resistance fast-twitch oxidative-glycolytic and fatigue resistance slow-twitch oxidative muscle fibers, and after their adaptation to six weeks of endurance exercise training. We hypothesized that there exists relation between remodeling of neuromuscular junctions and oxidative capacity of muscle fibers. Our working hypothesis was a strict relation between the turnover of contractile proteins and the neuromuscular junctions morphology, specifically, during adaptation to endurance training.
Material and Methods
Animals were used in accordance with the European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes and all procedures used in this study were approved by the Animal Experiment Committee of the Ministry of Agriculture, Tallinn, Estonia. All research and animal care was performed according to European guidelines and ethical standards.20
Animals
The animals used were 16-17 weeks old (at the beginning of the experiment) male Wistar rats. All animals were housed in identical conditions in polycarbonated type III cages, at 21 ºC. They received diet [SDS-RM-1(C) 3/8, Witham, Essex, UK] and water ad libitum. The rats were assigned to control group (n=18) and endurance exercise trained group (n=22).
Administration of labeled amino acid
L-[4,53H] leucine (170 Ci/mmol) was infused intraperitoneally, 1.0 ml for 2 hr, 200 μCi per 100 g b.w. before the muscle samples were collected.
Endurance exercise training
After a brief 5-day acclimation that consisted of treadmill running for 5-10 min, rats were subjected to run with the speed 35m/min during 6 week. Rats ran 5 days per week and the training volume reached 60 min per day.
Separation of muscle fiber types
For studies of fast glycolytic (FG) fibers and fast oxidative-glycolytic (FOG) fibers, the quadriceps femoris m. was dissected, liberated from fat and connective tissue and separated into superficial white portion and deep red portion. For further identification of muscle fiber types Cytochromes aa3, Myoglobin and MyHC isoforms were used. Cytochromes aa3 and myoglobin were measured as described previously.21
Separation of myofibrillar protein
Frozen muscle tissue portions were thawed on ice, cut into small pieces, and washed with five volumes 20 mM Na Cl, 5mM sodium phosphate, 1mM EGTA (pH=6.5). Myosin was extracted with three volumes of 100 mM sodium hydrophosphate, 5 mM EGTA, 1mM ditiothreitol (pH=8.5), after 30 min of gentle shaking. Myofibrillar fraction was diluted with one volume glycerol and stored at -20 ºC.
MyHC isoforms separation
MyHC isoforms were separated by 7.2% SDS-PAGE using 0.75 mm thick gels. Myofibrils containing 0.5 μg of protein were loaded on the gel after being incubated for 10 min at 65 ºC in sample buffer containing 62.5 mM Tris. HCl, pH=6.8, 20% (v/v) glycerol, 5% (v/v) 2-mercaptoetanol, 2.0% SDS, 0.05% bromphenol blue. Electrophoresis lasted for 24 h at 120 V.22 Gels were silver-stained by the method of Oakley et al.23 Protein isoform bands were analyzed densitometrically by Image Master 1 D program,Version 3.0 (Amersham Pharmacia Biothech, USA) and the percentage distribution of various isoforms was evaluated.
Turnover rate of MyHC
The relative specific activity, which characterizes the turnover rate of MyHC protein fraction, was calculated as the ratio of the specific activity of the protein fraction to the specific activity of total muscle cell protein and expressed in percentages.24 Specific activity is the ratio between 3H-radioactivity and protein.
Protein assay
Total muscle protein and myofibrillar protein was assayed by using the technique described by Bradford.25
Crossectional area
Crossectional area of muscle fibers was analyzed after labeled ATPase activity (modified method of Brooke and Kaiser.26
Ultrastructural studies
Muscle samples for ultrastructural studies were fixed in 2.5% glutaraldehyde, pos-tfixed in 1% sodium tetroxide, dehydrated in graded alcohol and embedded in Epon-812. Ultra-thin sections were cut from longitudinally and transversely oriented blocs, stained with uranyl acetate and lead hydroxide, using 4 blocks from each animal. In rats of the control group and of the endurance trained groups an electron microscopic examination of 140 axon terminals in each fiber type group, was studied.
Software
The imaging and analysis software (Cell* Soft Imaging System GmbH, Münster, Germany) was used.
Statistics
Means and standard errors were calculated from individual values using standard prcedures of Excel. The data were analyzed by R 2.12.2.27 Pearson correlation coefficients were used for describing relationships between variables. Differences between groups were analyzed by the Wilcoxon rank sum (Mann-Whitney U) test. Probability distribution were compared using the Kolmogorov-Smirnov test. Differences were considered significant at p<0.05.
Results
MyHC isoforms relative content in differnt muscle fibers
In FG fibers myosin heavy chain (MyHC) IIb isoforms relative content was 97±6%, MyHC IId isoform relative content 3±0.3%; cytochromes aa3 concentration was 9.4±0.9 ng/g muscle wet weight and myoglobin concentration 0.9±0.09 mg/g wet weight. In FOG fibers MyHC IIb isoform relative content was 23±2%, MyHC IId isoform relative content was 25±2%, MyHC IIa 44±4% and MyHC I isoform relative content was 8±0.8%. Cytochrome aa3 concentration was 32±3 nm/g and myoglobin concentration 4±0.4 mg/g wet weight. Slow oxidative (SO) fibers were separated from the m. soleus.
Turnover rate of MyHC in different muscle fibers
In ST O fibers protein turnover rate is 0.93±0.009; in FT O-G 0.90±0.01, and in FT G fibers 0.60±0.008.
Axon terminal morphology
Structures of neuromuscular synapses on different skeletal muscle fiber types are different. The axon terminals of rat ST O fibers are relatively short, round or oval shaped and are morphologically similar (Fig 1A). The sarcoplasm near the terminals contains a great number of mitochondria, which are full of cristae. The axon terminals of rat FT O-G fibers (Fig 1B, 2A) and FT G fibers (Fig 1C) are elliptical and 2.5 times longer than the terminals of ST O fibers (p<0.001). The postsynaptic folds of neighboring synapses on FT O-G and G fibers have linked with each other. In comparison with OG fibers the postsynaptic folds of G fibers are longer, more regular and they cover between them a much larger area of sarcoplasm. The area of axon terminals in this group is 1.7 time larger than the same area in ST O fibers (p < 0.001) in control groups (Table 1). Enduranc training increased the axon terminal area of about 10% in each fiber types groups but this change was not statistically significant. There are correlation between CSA of different muscle fibers and length of axon terminals (r=0.72), between CSA and width of axon terminal (r=-0.62), and change in turover rate of contractile proteins and width of axon terminal (r=0.75).
Fig. 1.
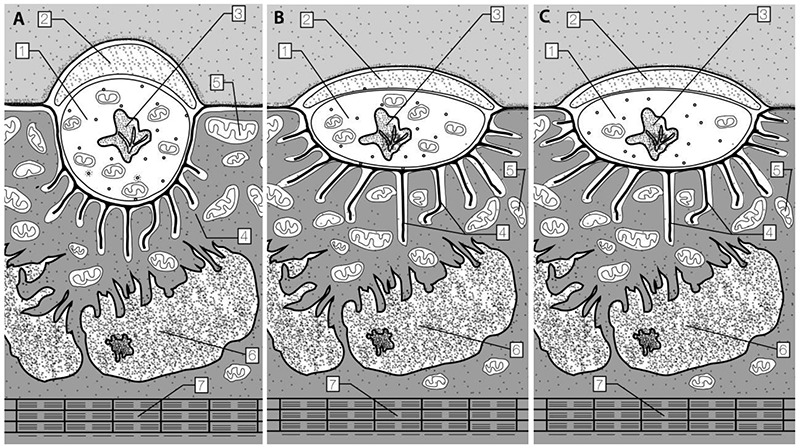
Schematic representation of the synapse of oxidative (A), oxidative-glycolytic (B) and glycolytic (C) muscle fibers. 1 - axon terminal; 2 - Schwann’s cell; 3 - autofagosome; 4 - postsynaptic folds; 5 - mitochondria; 6 - nucleus of the muscle fiber; 7 – myofibrils
Fig. 2.
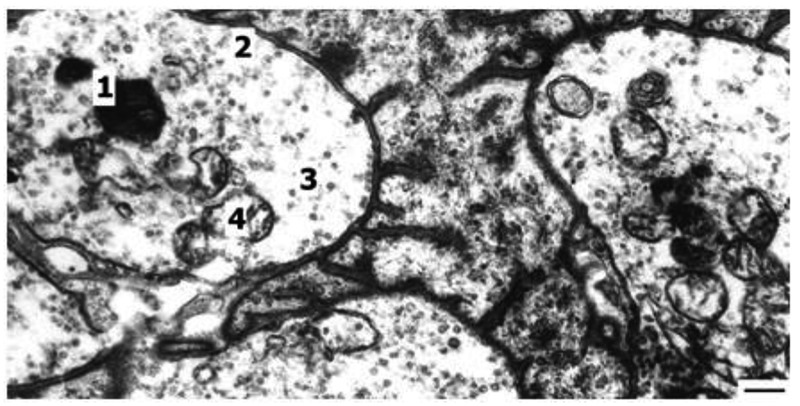
Myelin figure in synapse of oxidative muscle fiber after six weeks of endurance training. 1. myelin figure; 2. synapse; 3. synaptic vesicles; 4. mitochondria; Bar 0.5 μm
Table 1.
Differences between axon terminal areas of different types of muscle fibers.
| Group | Number of animals per group | Total number of synapsees per fiber type | Axon terminal area (μm2) | ||
|---|---|---|---|---|---|
| O | O-G | G | |||
| Control | 18 | 216 | 259 ± 34 | 381 ± 48 | 326 ± 33 |
| * | |||||
| Endurance training | 22 | 264 | 291 ± 26 | 420 ± 44 | 355 ± 34 |
| * | |||||
O – oxidative type of muscle fibers; O-G – oxidative-glycolytic type of muscle fibers; G – glycolytic type of muscle fibers. * - p<0.05 in comparison with oxidative fiber;
Remodelling of synapses
In terminal profiles of O (1.67±0.27) and O-G (1.70±0.26) fibers coated vesicles are present from 10-15% more than coated vesicles in terminal profiles of G fibers control group (Table 2).
Table 2.
Remodelling of synapses according to changes of coated vesicles in terminals during endurance exercise training.
| Group | Number of synapsees per fiber type per animal | Number of coated vesicles per cut of terminal | ||
|---|---|---|---|---|
| O | O-G | G | ||
| Control | 12 | 1.67 ± 0.2 | 1.70 ± 0.26* | 1.35 ± 0.20 |
| Endurance | 12 | 5.06 ± 0.42 | 6.45 ± 0.44 | 2.05 ± 0.22 |
| *** | *** | |||
| xxx | xxx | x | ||
O – oxidative muscle fibers; O-G – oxidative-glycolytic muscle fibers; G – glycolytic muscle fibers. *** - p<0.001 in comparison with glycolytiv fibers; xxx - p<0.001 in comparison with control group; x - p<0.05 in comparison with control goup
Effect of endurance training on the morphology of synapses
Endurance training causes the heterogeneity of the structures of neuromuscular synapses which is clearly expressed in muscle fibers with higher oxidative capacity (O and O-G fibers). These fibers have faster turnover rate (subsequently 0.93±0.04 and 0.90±0.04) of muscle proteins as only a well developed synaptic apparatus guarantees intensive renewal of the structures of muscle fibers. The surface of the neighbouring neuromuscular contacts is smooth, the sarcoplasm near the terminals of the muscle fiber contains a great number of mitochondria which are full of cristae. After one week of endurance training as a result of branching terminals number increase (Fig 3B). One axon terminal may branch into 2-4 branches. The number of axon terminals of motor nerve endings on one cut may exeed ten. In some terminals lysosome like formations and myelin corpuscles number increase (Fig 2, 4B). It is typical of synapses of O-G fibers to have large postsynaptic folds area and as a result of endurance training the surface of nuclei on the side of terminals is covered with many extensions (Fig 4A). As a result of endurance training large complexes of mitochondria are located between the nucleas of muscle fibers and myofibrils, surrounding from each side the connection between the nerve and muscle (Fig 4A).
Fig 3, B.
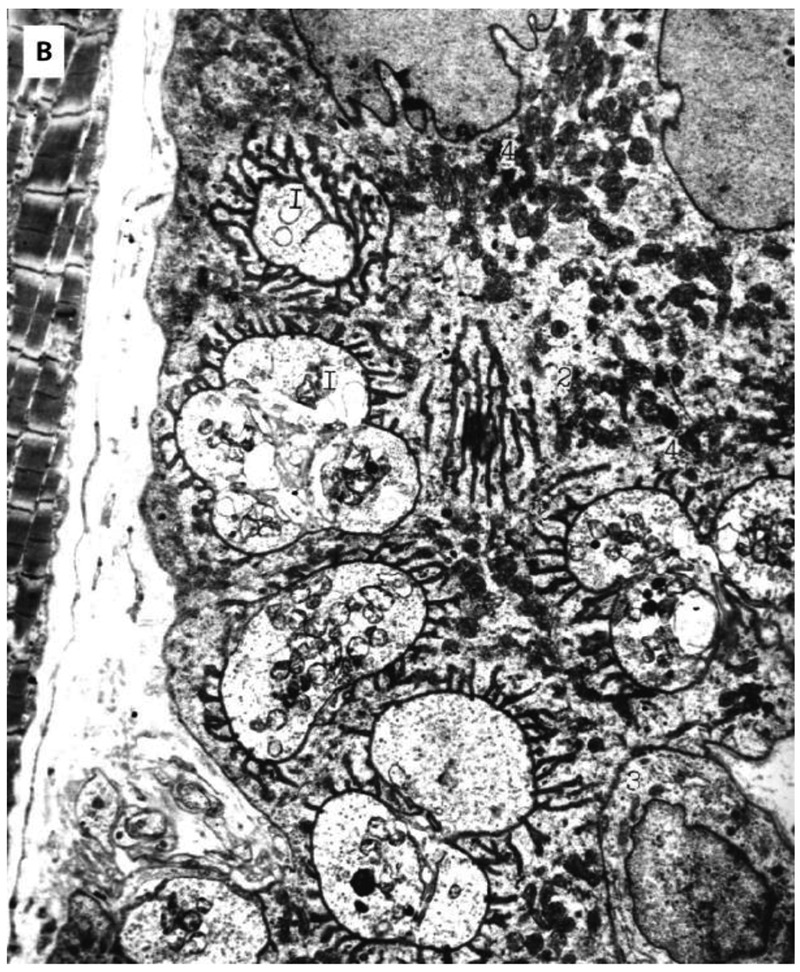
Effect of endurance training on structure of nerve synapse of oxidative-glycolytic muscle fiber after one week of endurance training. 1. neuromuscular synapse; 2. mitochondria in postsynaptic area; 3. satellite cell in synaptic area; Bar 1μm.
Fig, 4 B.
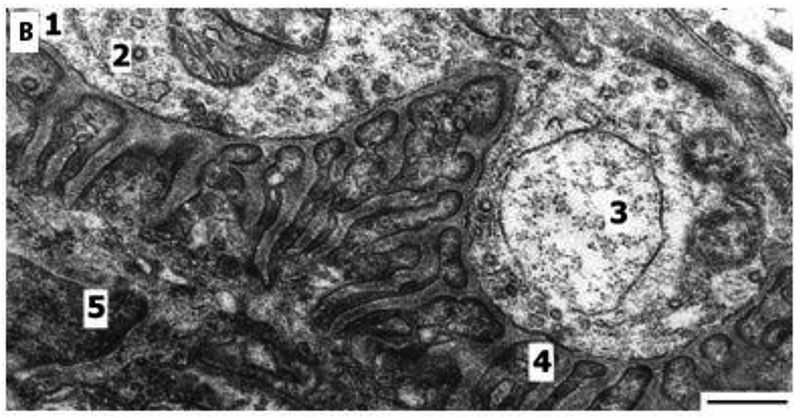
Structure of synapse of oxidative-glycolytic muscle fiber after recovery from endurance training. 1. synapse; 2. coated vesicles in terminal; 3. large vacuole in axon terminal; 4. postsynaptic folds; 5. nucleus of muscle fiber. Bar 1μm
Fig. 4, A.
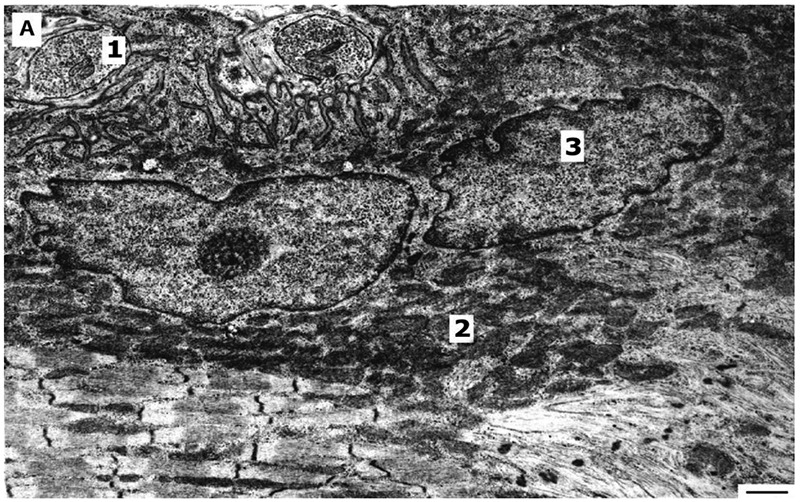
Effect of endurance training on the structure of synapse of oxidative-glycolytic muscle fiber. 1. synapse, terminal is filled with synaptic vesicles; 2. mitochondria in postsynaptic zone; 3. nuclei of muscle fiber. Bar 1μm
Effect of endurance training on the remodeling of synapses
The increase of the number of coated vesicles in terminals (Fig 4B) is connected with the resynthesis of Ach after the active functioning of the synapse. Coated vesicles appear mainly in the sarcoplasm of the presynaptic area of O and O-G fibers after endurance training and they are not only related to the resynthesis of Ach in nerve endings, but these vesicles also carry the proteins of choline receptors in the postsynaptic membrane. Remodeling of NMJs during 6 w of endurance training, according to change of coated vesicles in terminal (number of vesicles per profile of terminal) in comparison with control group increased about 300% (from 1.67±0.27 to 5.06±0.42; p< 0.001) in O fibers, 380 % (from 1.70±0.26 to 6.45±0.44; p< 0.001) in O-G fibers, and in G fibers 150% (p<0.05).
Discussion
Motoneuron type and not muscle fiber type determines the fast or slow character of NMJ. Not only size of the muscle fibers, but also the type and firing pattern of the motoneurons and the spatial constraints at preformed endplates influence the relation between junction size and muscle fiber diameter.16 FT fast fatigued muscle terminals are active in short bursts at a high frequency,28 while ST fatigue- resistant muscle with active terminals have longer periods of burst at low frequency.13 FT muscle terminals loos a higher proportion of their vesicles per impulse and would, therefore, require that the vesicles return to the pool more rapidly in order to maintain transmitter release.13 There are significant differences in synaptic vesicles trafficking in motor nerve terminals. ST muscle terminals support sustained quantal transmitter release much better than the terminals of FT muscle.13 NMJs undergoes a continual process of remodeling and expansion during normal sedentary activity and exercise training.17 Exercise training induces hypertrophy of the NMJs and nerve terminal branching independently of muscle hypertrophy and intensity of exercise.29 Treadmill running increases the area of the nerve terminals, the corresponding muscle fiber diameter reducing, but significant differences between the trained group and the control group were obtained only in FT muscle.14 If there is greater area nerve terminals in endurance trained group, it may be the result of intensive branching of terminals.30 In some terminals there are lysosome-like structures, myelin corpuscles, synaptic vesicles and numerous coates vesicles. Appearance of coated vesicles is connected with the resynthesis of ACh after the active function of the synapse. Endurance training causes heterogeneity of neuro muscular synapses on O and O-G muscle fibers. Some terminals of endurance trained rats are filled with a great amount of synaptic vesicles, the others have few of them, but both have lots of mitochondria. There are also completely ’’clean’’ terminals with reduced postsynaptic folds. It is typical of the synapses of O-G fibers to have a large postsynaptic area. At NMJs the most striking structural features of the postsynaptic region are the deep infolding of the sarcolemma. The chrests of the postsynaptic membrane infolding contain a very high density of AchRs,6 which may explain the characteristic curvature of the membrane in this region. Between the nerve and muscle fibers there is a synaptic cleft about 50–100 nm wide. Especially large complexes of mitochondria are located between the nuclei as of muscle fibers and myofibrils, surrounding from each side the connection between the nerve and muscle after endurance training. A mechanism seems to link mitochondria and myofibrils in specific structures - intracellular energetic units, as was previously shown .31,32 The reliability of neuromuscular transmission normally results from the release of more quanta of ACh than quanta required to initiate an action potential. The safety factor for neuromuscular transmission is used to describe this excess, i.e., a safety factor in terms of the number of ACh quanta actually released compared to the numbers needed to generate an action potential.12 Due to the above mentioned morphological changes in NMJs, adaptive process in FT muscles shows high potential of recruitment of FT muscle fibers during endurance exercise.33
Axon terminals of ST O fibers are relatively short, round or oval shaped. The sarcoplasm near the terminals contains a great number of mitochondria. The axon terminals of FT O-G fibers and FT G fibers are elliptical and 2.5 times longer than the terminals of ST O fibers. In comparison with OG fibers the postsynaptic folds of G fibers are longer, more regular and they cover between them a much larger area of sarcoplasm. The area of axon terminals on FT OG and G fibers and the perimeter of terminals is are respectively 1.5 time and 1.7 time larger than the same in ST O fibers in control group. Increase of axon terminals area exceeds about 10% during 6 week endurance exercise training period when compared with G fibers in control groups. Endurance training causes the heterogeneity of the structures of neuromuscular synapses which is clearly expressed in muscle fibers with higher oxidative capacity. The surface of the neighbor neuromuscular contacts is smoother than the sarcoplasm near the terminals of the muscle fiber containing a great number of mitochondria full of cristae. Remodeling of NMJs during 6 w of endurance training, in comparison with control groups is in exceed of about 300% and 380% in O fibers and in O-G fibers respectively. Fast remodeling of synapse in O and O-G fibers during endurance training guarantees the intensive renewal of the structures of the muscle fibers.
Fig. 3, A.
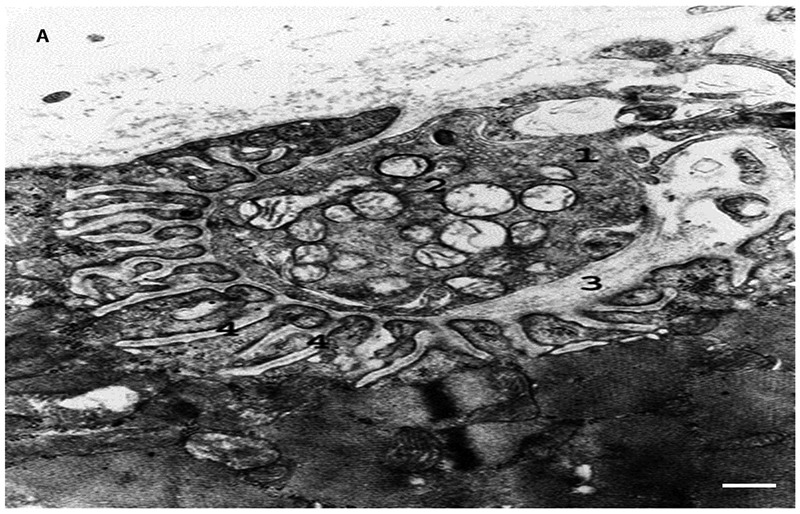
Electron micrograph of synapse of oxidative-glycolytic muscle fiber. 1. axon terminal; 2. synaptic vesicles; 3. synaptic cleft; 4. postsynaptic folds. Bar 0.5 μm
Acknowledgments
This study was supported by the Estoninan Research Council, research project number TKKSB1787.
References
- 1.Auld S, Colomar A, Belair E-L, et al. Modulation of neurotransmission by reciprocal synapse-glial interactions at the neuromuscular junction. J Nerocytol 2003;32:1003-15. [DOI] [PubMed] [Google Scholar]
- 2.Pette D, Staron RS. Transitions of muscle fibre phenotypic profiles. Histochem Cell Biol 2001;115:359-72. [DOI] [PubMed] [Google Scholar]
- 3.Seene T, Kaasik P, Umnova M. Structural rearrangements in contractile apparatus and resulting skeletal muscle remodelling: effect of exercise training. J Sports Med Phys Fitness 2009;49:410-23. [PubMed] [Google Scholar]
- 4.van Wessel T, de Haan A, van der Laarse WJ, Jaspers RT. The muscle fiber type-fiber size paradox: hypertrophy or oxidative metabolism? Eur J Appl Physiol 2010;110:665 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seene T, Kaasik P. Biological characteristics of structural and functional remodelling in sekeletal muscle: effect of exercise. Advanced Studies in Biology 2013;6(5):251-78. [Google Scholar]
- 6.Salpeter MM. Vertebrate neuromuscular junctions: general morphology, molecular organization, and functional consequence. In: Salpeter MM. ed. The vertebrate neuromuscular junction. Alan Liss: New York; 1987 pp 1-54. [Google Scholar]
- 7.Auld DS, Robitaille R. Perisynaptic Sshwann cell at the neuromuscular junction: Nerve-and activity-dependent contributions to synaptic efficacy, plasticity and reinnervation. Neuroscientist 2001;9:144-57. [DOI] [PubMed] [Google Scholar]
- 8.Castonguay A, Levesque S, Robitaille R. Clial cells as activate partners in synaptic functions. Progress in Brain Research 2001;132:227-40. [DOI] [PubMed] [Google Scholar]
- 9.Martin AR. Quantal nature of synaptic transmission. Physiol Rev 1965;46:51-66. [Google Scholar]
- 10.Kuffler SW, Yoshikami D. The number of transmitter molecules in a quantum: an estimate from iontophoretic application of acetycholine at the neuromuscular synapse. J Physiol (Lond) 1975;251:465-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McMahan UJ, Sanes JR, Marshall LM. Cholinesterase is associated with the basal lamina at the neuromuscular junction. Nature 1978;271:172-4. [DOI] [PubMed] [Google Scholar]
- 12.Wood SJ, Slater CR. The contribution of postsynaptic folds to the safety factor for neuromuscular transmission in rat fast- and slow-twitch muscles. J Physiol (Lond) 1997;500:165-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reid B, Salter CR, Bewick GS. Synaptic vesicle dynamics in rat fast and slow motor nerve terminals. J Neurosci 1999;19:2511-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Werhaug O, Dahl HA, Kardel K. Different effects of physical trainig on morphology of motor nerve terminals in rat extensor digitorum longus and soleus muscles. Anat Embryol 1992;186:125-8. [DOI] [PubMed] [Google Scholar]
- 15.Ogata T. Structure of motor endoplates in the different fiber types of vertebrate skeletal muscles. Arch Histol Cytol 1988;51:385-424. [DOI] [PubMed] [Google Scholar]
- 16.Werhaug O, Lømo T. Factors causing different propertied at neuromuscular junctions in fast and slow rat skeletal muscles. Anat Embryol (Berl) 1994;190:113-25. [DOI] [PubMed] [Google Scholar]
- 17.Fahim MA. Endurance exercise modulates neuromuscular junction of C57BL\6Nnia aging mice. J Appl Physiol 1997;83:59-66. [DOI] [PubMed] [Google Scholar]
- 18.Andonian MH, Fahim MA. Effects of endurance exercise on the morphology of mouse neuromuscular junctions during ageing. J Nerocytol 1987;16:589-99. [DOI] [PubMed] [Google Scholar]
- 19.Deschenes MR, Roby MA, Glass EK. Aging influences adaptations of the neuromuscular junction to endurance training. Neuroscience 2011;190:56-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harris DJ, Atkinson G. Update-ethical standards in sport and exercise science research. Int J Sports Med 2011;32:819-21. [DOI] [PubMed] [Google Scholar]
- 21.Seene T, Alev K. Effect of glucocorticoids on the turnover rate of actin and myosin heavy and light chains on different types of skeletal muscle fibers. J Steroid Biochem 1985;22:767-71. [DOI] [PubMed] [Google Scholar]
- 22.Hämäläinen N, Pette D. Slow-to-fast transitions in myosin expression of rat soleus muscle by phasic high-frequency stimulation. FEBS Lett 1996;399:220-2. [DOI] [PubMed] [Google Scholar]
- 23.Oakley B, Kirsh D, Morris N. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem 1980;105:361-3. [DOI] [PubMed] [Google Scholar]
- 24.Pehme A, Alev K, Kaasik P, Julkunen A, Seene T. The effect of mechanical loading on the MyHC synthesis rate and composition in rat plantari muscle. Int Sports Med 2004;25:332-8. [DOI] [PubMed] [Google Scholar]
- 25.Bradford A. A rapid sensitive method for the quantitation of microgram quantities of protein utilizig the principe of protein-dye binding. Anal Biochem 1976;72:248-54. [DOI] [PubMed] [Google Scholar]
- 26.Brooke MH, Kaiser KK. Some comments on the histochemical characterization of muscle adenosine triphospahte. J Histochem Cytochem 1969;17:431-2. [DOI] [PubMed] [Google Scholar]
- 27.R Development Core Team R. A language and environment for statical computing. R Foundation for statical computing. Vienna, Austria. 2011; ISBN 3-900051-07-0. URL http://www.R-projectorg/ [Google Scholar]
- 28.Henning R, Lömo T. Firing patterns of motor units in normal rats. Nature 1985;314:164-6. [DOI] [PubMed] [Google Scholar]
- 29.Deschenes MR, Maresh CM, Crivello JF, et al. The effects of exercise training of different intensities on neuromuscular junction morphology. J Neurocytol 1993;22(8):603-15. [DOI] [PubMed] [Google Scholar]
- 30.Andonian MH, Fahim MA. Endurance exercise alters commorphology of fast- and slow-twitch rat neuromuscular junctions. Int J Sports Med 1988;9(3):218-23. [DOI] [PubMed] [Google Scholar]
- 31.Saks VA, Kaambre T, Sikk P, et al. Intracellular energetics units in red muscle cells. Biochem J 2001;35:643 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seppet EK, Eimre M, Anmann T, et al. Intracellular energetic units in healthy and diseased hearts. Exp Clin Cardiol 2005;10:173 83. [PMC free article] [PubMed] [Google Scholar]
- 33.Seene T, Alev K, Kaasik P, Pehme A. Changes in fast-twitch muscle oxidative capacity and myosin isoforms modulation during endurance training. J Sports Med Phys Fitness 2007;47:124-32. [PubMed] [Google Scholar]


