Abstract
Objective
Formal thought disorder (FTD) is a core symptom in schizophrenia. Here, we focus on resting state cerebral blood flow (rCBF) linked to dimensions of FTD.
Methods
We included 47 schizophrenia spectrum patients and 30 age‐ and gender‐matched healthy controls. We assessed FTD with the assessment of thought, language, and communication (TLC) and imaging on a 3T MRI scanner. Within patients, we tested the association of FTD dimensions and in a subgroup (n = 27) the association of functional outcome after 6 months with whole brain rCBF.
Results
Negative FTD was most prominently associated with perfusion within the superior temporal gyrus, while positive FTD was associated with perfusion within the supplementary motor area, and inferior frontal gyrus. Perfusion within the left supramarginal gyrus was associated with social functioning after 6 months.
Conclusions
Distinguishable associations of rCBF with FTD dimensions point to distinct underlying pathophysiology. The location of aberrant perfusion patterns suggests that negative FTD might reflect defective access to semantic memory while positive FTD likely reflects defective suppression of irrelevant information during increased speech production. Finally, the neural correlates of thought block were also predictive of poor functional outcome. Thus, functional outcome and distinct FTD dimensions may share some pathophysiology.
Keywords: symptom dimensions, formal thought disorder, speech production, semantic processing, social functioning
Significant outcomes.
Resting state perfusion in the language network is associated with FTD.
FTD dimensions are linked to distinguishable rCBF patterns suggesting distinct underlying pathophysiology.
Thought blocking may predict poor functional outcome.
Limitations.
Patients were on antipsychotic medication, which may affect rCBF in schizophrenia.
More patients showed positive than negative and linguistic FTD, which may hamper the search of effects particularly of the linguistic dimension.
We investigated three FTD dimensions. Future studies are required to investigate other dimensions of FTD.
Introduction
Formal thought disorder (FTD) forms a robust and core deficit in individuals with schizophrenia. One key challenge in investigating FTD is the considerable heterogeneity of dysfunctions. In particular, the term formal thought disorder refers to a heterogeneous group of dysfunctions concerning speech production and perception 1. Thus, different FTD dimensions were proposed suspecting a distinct underlying pathobiology. For instance, Andreasen suggested a dichotomous, ‘negative’ (poverty of speech and poverty of content) and ‘positive’ (pressure of speech, tangentiality, derailment, incoherence, and illogicality) structure of FTD 2. In addition, factor analytic studies robustly identified at least two different FTD dimensions 3, 4, 5.
Importantly, FTD dimensions are of clinical relevance 3. In fact, positive FTD and negative FTD are associated with different neuropsychological deficits 6 across multiple neurocognitive domains 7, 8, 9. Moreover, the negative FTD dimension better predicted conversion to schizophrenia in subjects at risk for psychosis, regardless of the genetic risk 10. Likewise, in contrast to overall FTD, distinct dimensions of FTD (positive or negative FTD) predicted poor outcome 3, 11, 12.
Hitherto, the neural mechanisms underlying the proposed FTD dimensions in schizophrenia are unclear. In fact, most neuroimaging studies focused on the overall severity of FTD as a single construct. These studies highlighted alterations in the language network 13, 14, 15, 16, such as increased resting state perfusion in the left superior temporal gyrus (STG), Broca's area (left Brodmann area 44), and the bilateral angular gyrus 17, 18, with some inconsistency in the findings. In contrast, a small number of functional neuroimaging studies directly investigated correlates of FTD dimensions, suggesting distinct pathophysiology of FTD dimensions. While positive FTD was associated with altered task‐based functional activation of the superior temporal (Wernicke's area), the inferior frontal and the parahippocampal gyrus 15, 19, 20, negative FTD is probably less clearly associated with language related areas but in addition with brain areas relevant for higher order control processes (i.e., the parietal lobe, the cuneus, the precuneus, and the posterior frontal lobe) 21, 22. However, no study focused on resting state perfusion (rCBF) as a direct measure of neural metabolism and FTD dimensions. Yet, this pathophysiological knowledge may stimulate the development of targeted treatment approaches such as noninvasive brain stimulation. Likewise, no study tested whether abnormal brain function linked to FTD was associated with subsequent functional outcome in schizophrenia. This is of particular clinical relevance as outcome markers in schizophrenia are missing and modulation of aberrant brain function may ameliorate poor outcome.
Aims of the study
We therefore aimed to (1) confirm an association of the overall formal thought disorder severity and aberrant perfusion in primary language areas; (2) test the association of the severity of formal thought disorder dimensions and resting state cerebral blood flow; and (3) test whether regional resting state cerebral blood flow changes that are linked to formal thought disorder would also be associated with functional outcome after six months. We hypothesized distinguishable regional resting state cerebral blood flow changes in the language network associated with formal thought disorder dimensions. Furthermore, we hypothesized that the severity of negative formal thought disorder is associated with perfusion of brain regions relevant for higher order control processes (i.e., the frontal lobe and the precuneus). In contrast, we hypothesized that the severity of positive formal thought disorder is associated with perfusion of brain areas relevant for speech production, for example, Broca's area. Finally, we suspected that regional resting state cerebral blood flow changes associated with formal thought disorder are also linked to poor functional outcome after 6 months.
Material and Methods
Subjects
We included 47 clinically stable patients (38 in‐patients and nine out‐patients) with schizophrenia spectrum disorder according to the diagnostic and statistical manual of mental disorders (DSM 5) and 30 healthy control subjects, matched for age and gender. General exclusion criteria for all subjects were substance abuse or dependence other than nicotine, history of head trauma, and specific exclusion criteria for MRI scans (e.g., metallic implants, claustrophobia and pregnancy). Additional exclusion criteria for controls were a history of any psychiatric disorder as well as first‐degree relatives with schizophrenia spectrum disorders. All participants provided written informed consent. The study protocol adhered to the Declaration of Helsinki and was approved by the local Ethics Committee, Bern (KEK). Of the subjects, 20 subjects participated in a previous study 23.
All, but four patients received treatment with antipsychotic medication. Patients were on a stable dosage of antipsychotics at least 4 weeks before scanning. Of the medicated patients, 39 received second generation while two received first generation, and two‐first‐ and second‐generation antipsychotics. We calculated chlorpromazine equivalent dosages (CPZ) 24.
Procedure
We interviewed all participants with the mini international neuropsychiatric interview version 6.0 (MINI). We diagnosed patients following the MINI, clinical interviews, and review of all available records 25. Moreover, we assessed nonverbal intelligence with the test of nonverbal intelligence TONI version 4 26. In patients, we assessed psychopathology and symptom severity with the positive and negative syndrome scale (PANSS) 27, and severity of FTD with the score for the assessment of thought, language, and communication (TLC) 28. Briefly, the TLC contains 18 items and an overall rating (global TLC). Severity ratings of the items 1–9 range from 0 (absent) to 4 (extreme), while severity ratings from the items 10–18 range from 0 (absent) to 3 (severe). To assess the severity of FTD dimensions, we calculated severity ratings of three dimensions derived from the TLC according to Nagels et al. 5. In particular, factor analysis identified a three‐factor solution 5. The identified three factors comprise a dimension mostly including symptoms related to positive FTD (derailment, loss of goal, circumstantiality, pressure of speech, tangentiality, distractible speech, self‐reference, preservation, incoherence, and stilted speech), termed disorganization subtype (TLC‐Dis). A second dimension included symptoms related to negative FTD or alogia (poverty of speech, blocking, and poverty of content), termed emptiness subtype (TLC‐Emp). Finally, a third dimension contained the symptoms paraphasia, neologism, illogicality, and word approximations and was termed linguistic subtype (TLC‐Lin) 5. In addition, within patients we assessed global functioning (Global Assessment of Functioning: GAF) and social functioning (social and occupational functioning: SOFAS 29.
All patients were approached for follow‐up assessments after 6 months. In total, 27 patients had follow‐up data including the PANSS, as well as measures of functional outcome: the SOFAS 29, the GAF, and the brief version of the University of California San Diego Performance‐Based Assessment (UPSA brief: UPSA‐B) 30 (Table S2).
Structural and functional MRI acquisition
We acquired structural and functional imaging data on a 3T MRI scanner (Siemens Magnetom Trio; Siemens Medical Solutions, Erlangen, Germany) with a 12‐channel radio frequency head coil for signal reception. Structural 3D‐T1‐weighted (modified driven equilibrium Fourier transform pulse sequence; MDEFT) 31 images for each subject were obtained, providing 176 sagittal slices with 256 × 256 matrix points with a non‐cubic field of view (FOV) of 256 mm, yielding a nominal isotopic resolution of 1 mm³ (i.e., 1 × 1 × 1 mm). Additional scan parameters for the anatomical data were 7.92 ms repetition time (TR), 2.48 ms echo time (TE), and a flip angle of 16° (FA). Furthermore, we obtained 110 functional images [pseudo continuous arterial spin labeling (pCASL) sequence] 32, 33. Scanning parameters for the functional images were as follows: 20 slices (in ascending order) with 64 × 64 matrix points with a non‐cubic FOV of 230 mm, yielding a nominal isotopic resolution of 4.27 mm³ (i.e., 3.6 × 3.6 × 6 mm), TR of 4000 ms, TE of 18 ms, and a FA of 25°.
Data processing
For structural and perfusion image processing, we used SPM version 8 (Wellcome Trust Center for Neuroimaging, London; http://www.fil.ion.ucl.ac.uk/spm). We preprocessed structural and perfusion images with an in‐house written MATLAB program toolbox 34, 35, 36. In detail, calibration of rCBF was performed with the following parameters: bolus duration of 1.6 s, postlabel delay of 1.5 s, relaxation time of blood at 3T of 1.65 s, blood/tissue water partition coefficient λ = 0.9 [g/ml], and tagging efficiency assumed to be α = 0.95. ASL images were realigned. From the time series of these realigned ASL signal, the mean regional rCBF flow was calculated voxel‐wise and stored as a rCBF map. We used individual mean gray matter rCBF values as a covariate. In addition, we coregistered all these rCBF maps to the T1 weighted images, normalized, and smoothed with 8 mm full width at half maximum (FWHM) kernel.
Statistical analyses
To analyze demographic variables, for clinical characterization and to test effects of categorical and continuous variables on rCBF, we used SPSS version 22.0. (SPSS Inc., Chicago, IL, USA) as well as SPM routines. We applied two sample t‐tests, chi‐square tests (χ2), and multiple regression analysis respectively. For all imaging analysis, we included age and individual head motion parameters as covariates and excluded all voxels with less than 10 [ml/100 g/min] blood flow.
First, to assess the effect of group on whole brain rCBF we compared rCBF between patients and controls (t‐tests).
Within patients, we then focused on the association of severity of FTD and rCBF. Therefore, we calculated correlations of the severity of overall FTD (global TLC) and rCBF using multiple regression analyses. Likewise, we tested the association of severity of FTD of each dimension on rCBF (TLC‐Dis, TLC‐Emp, TLC‐Lin) using multiple regression analyses for each dimension separately. Moreover, we tested the association of severity of FTD of each dimension on rCBF independent of severity of FTD of the other two dimensions and dosage of antipsychotic medication. Therefore, we included in one multiple regression analysis, severity ratings of all three dimensions. We then calculated effects of each dimension on rCBF separately covarying for the other two dimensions (e.g., negative FTD dimension independent of positive and linguistic FTD) and average CPZ of the past 5 years. Thus, in these latter analyses we included motion parameters, age, average CPZ of the past 5 years, and the severity ratings of the other two FTD dimensions as covariates.
We generated permutation‐based output images applying threshold‐free cluster enhancement (TFCE) as implemented in the TFCE toolbox for SPM 37 and applied a statistical threshold of P < 0.05 family‐wise error corrected for multiple testing (FWE‐corr). Briefly, in TFCE, the value of the statistics at each voxel is replaced by a composition of the statistics observed in that voxel and neighbors' voxels. They follow certain spatial properties considering both intensity and signal extent family‐wise error controlled for multiple comparisons. Figures were produced using SPM8 and MRIcron (12/2012) 38.
For illustration purposes, we extracted the data post hoc from significant clusters of the whole brain analyses for each subject with the SPM toolbox MarsBaR (0.44) 39 and calculated simple correlations of extracted mean perfusion values (rCBF) in patients and FTD severity (global TLC, TLC‐Dis, TLC‐Emp, TLC‐Lin). In addition, we calculated simple correlations of extracted mean perfusion values (rCBF) in patients and scores of functional outcome measures at baseline and follow‐up (GAF, SOFAS, UPSA brief, and PANSS).
Finally, we tested the effect of a nonlinear regression model for our main analyses applying the ‘fit nonlinear regression model’ for MATLAB.
Results
Demographic and clinical characteristics are given in Table 1. None of our patients had mental retardation or markedly reduced nonverbal intelligence as measured with the TONI. In addition, we were able to conduct follow‐up assessments in 27 patients, while 20 patients were lost to follow‐up. At baseline, patients with follow‐up data did not differ from those without regarding demographic and clinical characteristics, particularly the severity of FTD (see Table S2).
Table 1.
Demographic and clinical characteristics of the sample
| Patients (n = 47) | Controls (n = 30) | df | T/X2 | P | |
|---|---|---|---|---|---|
| Age (years) ± SD | 38.2 ± 11.4 | 36.7 ± 12.9 | 75 | 0.5 | 0.609 |
| Gender (n; % men) | 29; 62% | 17; 43% | 1 | 0.2 | 0.660 |
| Education (years) ± SD | 13.4 ± 3.1 | 12.9 ± 2.9 | 75 | −1.6 | 0.105 |
| TONI index | 11.3 ± 1.7 | 10.5 ± 2.0 | 75 | −5.4 | <0.001 |
| Duration of illness (years) ± SD | 12.2 ± 12.3 | – | – | – | – |
| Number of episodes ± SD | 6.7 ± 7.1 | – | – | – | – |
| PANSS pos ± SD | 18.2 ± 6.4 | – | – | – | – |
| PANSS neg ± SD | 18.4 ± 5.1 | – | – | – | – |
| PANSS tot ± SD | 72.6 ± 17.1 | – | – | – | – |
| TLC global ± SD | 1.4 ± 1.3 | – | – | – | – |
| TLC‐Emp ± SD | 0.8 ± 1.7 | – | – | – | – |
| TLC‐Lin ± SD | 1.1 ± 2.8 | – | – | – | – |
| TLC‐Dis ± SD | 4.1 ± 6.2 | – | – | – | – |
| CPZ ± SD | 400.2 ± 344.2 | – | – | – | – |
TONI, Test of nonverbal intelligence; PANSS, Positive And Negative Syndrome Scale; pos, positive symptom scores; neg, negative symptom scores; tot, total scores; CPZ, chlorpromazine equivalent doses; TLC, scale for the assessment of thought, language and communication; TLC‐Dis, TLC disorganization subtype; TLC‐Emp, TLC emptiness subtype; TLC‐Lin, TLC linguistic subtype.
Profile of FTD in schizophrenia patients
PANSS total scores indicated moderate severity of psychotic symptoms in patients. In addition, FTD (TLC global ratings > 0) was currently present in 33 of 47 patients. According to FTD factor categories 5, 25 patients had positive FTD (TLC‐Dis > 0), 16 patients had negative FTD (TLC‐Emp > 0), while only 10 patients had linguistic FTD (TLC‐Lin > 0) (Figure S1). This distribution of FTD symptoms and severity is comparable to previous reports 5.
rCBF alterations in patients compared to controls irrespective of FTD
In line with the literature, we detected reduced rCBF within the temporal, frontal, and parietal lobe in all patients compared to healthy controls independent of FTD (TableS3 and Figure S2) 36, 40, 41, 42, 43.
rCBF alterations linked to overall severity of FTD
Overall severity of FTD was associated with regional perfusion within brain areas relevant for language processing. In fact, perfusion within the bilateral superior temporal gyrus (STG, Heschl's gyrus) and in the left middle temporal gyrus demonstrated a positive linear association with overall FTD severity (TLC global) (see Table 2 and Figure 1). Thus, higher perfusion in these regions was associated with increased FTD severity. In contrast, we detected no brain region with lower perfusion associated with overall severity of FTD and rCBF. Applying a nonlinear regression model yielded substantially the same results (see Table S7).
Table 2.
Linear association of severity of overall FTD within schizophrenia patients
| T‐test within patients (n = 47): Positive linear association | ||||||
|---|---|---|---|---|---|---|
| Brain region | Cluster | Peak | ||||
| Size | P (FWE‐cor) | TFCE | MNI coordinates (x, y, z) | |||
| R superior temporal gyrus (Heschl's gyrus) | 272 | 0.006 | 445.5 | 58 | −10 | 6 |
| 0.012 | 373.5 | 48 | −12 | 6 | ||
| L middle temporal gyrus extending to superior temporal gyrus and angular gyrus | 194 | 0.028 | 272.0 | −56 | −56 | 2 |
| 0.030 | 262.4 | −54 | −48 | 6 | ||
| 0.033 | 249.8 | −58 | −60 | 26 | ||
| 9 | 0.040 | 227.0 | −60 | −54 | 26 | |
| 1 | 0.046 | 215.7 | −62 | −50 | 30 | |
Covariates: movement parameters and age.
Figure 1.
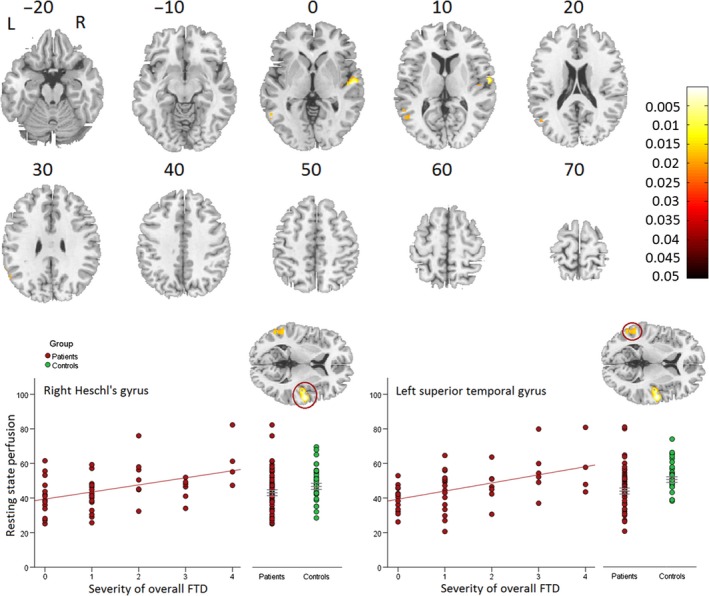
Positive linear association of severity of overall FTD and resting state perfusion (rCBF) within schizophrenia patients. Increased resting state perfusion (rCBF) bilateral in the temporal lobe was associated with increased severity of overall FTD. Covariates: movement parameters and age. We displayed images on the rendered surface and the sections of the standard MNI‐template. Color bars reflect P‐values of the whole brain analysis.
rCBF alterations linked to the severity of distinct FTD dimensions
To assess associations of rCBF and distinguishable aspects of FTD, we calculated the linear association between rCBF and FTD severity in three dimensions according to Nagels et al. 5. Neither for positive FTD (TLC‐Dis) nor linguistic FTD (TLC‐Lin) did we detect linear associations with rCBF applying FWE correction.
In contrast, we found a linear association of rCBF and negative FTD (TLC‐Emp) in several brain regions: bilaterally in the temporal lobe, the cuneus, the precuneus, the cerebellum as well as the right inferior parietal lobe. In fact, higher perfusion in these regions indicated increased negative FTD (Table 3 and Figure 2). These findings held true when controlling for the severity of positive and linguistic FTD as well as chlorpromazine equivalent dosage (see Table S4). Results of the nonlinear regression model are presented in the Table S7.
Table 3.
Linear association of severity of negative FTD within schizophrenia patients
| T‐test within patients (n = 47): positive linear association: negative FTD | ||||||
|---|---|---|---|---|---|---|
| Brain region | Cluster | Peak | ||||
| Size | P (FWE‐cor) | TFCE | MNI coordinates (x, y, z) | |||
| R, L posterior/middle cingulate cortex, precuneus | 993 | 0.006 | 709.5 | 6 | −40 | 30 |
| 0.006 | 681.0 | 0 | −34 | 30 | ||
| 0.007 | 647.0 | −6 | −44 | 32 | ||
| R superior temporal gyrus, middle temporal gyrus, supramarginal gyrus, rolandic operculum | 683 | 0.012 | 514.3 | 58 | −26 | 20 |
| 0.014 | 477.2 | 54 | −32 | 6 | ||
| 0.015 | 464.5 | 46 | −18 | 18 | ||
| R inferior parietal lobe | 542 | 0.016 | 458.1 | 60 | −44 | 34 |
| 0.020 | 413.5 | 54 | −60 | 36 | ||
| 0.020 | 403.5 | 54 | −54 | 44 | ||
| L middle temporal gyrus, angular gyrus | 92 | 0.016 | 448.4 | −44 | −68 | 34 |
| R, L cuneus | 757 | 0.024 | 367.0 | 2 | −66 | 8 |
| 0.025 | 365.9 | 4 | −62 | 18 | ||
| 0.031 | 333.3 | −12 | −56 | 2 | ||
| R cerebellum | 39 | 0.031 | 329.1 | 8 | −70 | −12 |
| L cerebellum | 5 | 0.038 | 295.2 | −22 | −68 | −12 |
| L superior temporal gyrus, supramarginal gyrus, rolandic operculum | 66 | 0.039 | 292.8 | −58 | −50 | 28 |
| 0.043 | 276.8 | −58 | −54 | 36 | ||
| 0.050 | 255.9 | −54 | −46 | 40 | ||
| L supramarginal gyrus | 40 | 0.046 | 268.3 | −54 | −52 | 6 |
| 0.048 | 261.5 | −60 | −48 | 2 | ||
| R supramarginal gyrus | 3 | 0.049 | 258.7 | 50 | −42 | 24 |
Covariates: movement parameters and age.
Figure 2.
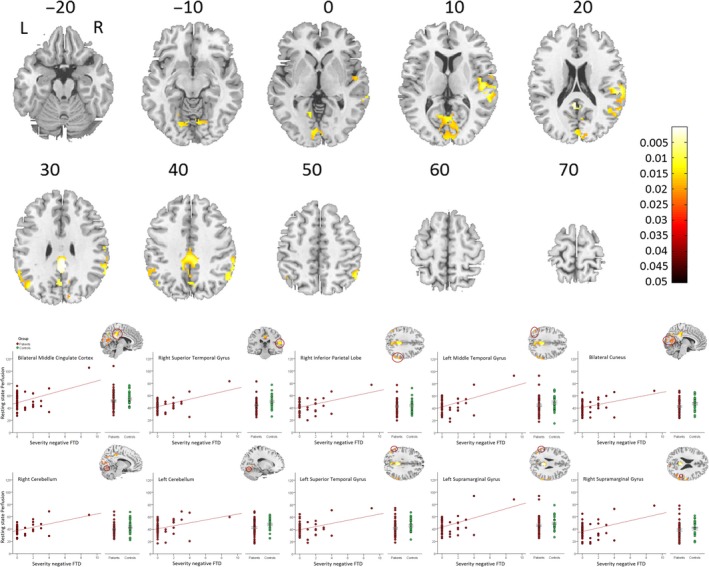
Positive linear association of severity of negative FTD and resting state perfusion (rCBF) within schizophrenia patients. Increased resting state perfusion (rCBF) bilateral in the temporal lobe was associated with increased severity of negative FTD. Upper panel: whole brain effects; Lower panel: extracted values within significant whole brain clusters. Covariates: movement parameters and age. We displayed images on the sections of the standard MNI‐template. Color bars reflect P‐values of the whole brain analysis.
Furthermore, we tested whether a linear association of rCBF and positive FTD or linguistic FTD dimensions would appear at a more liberal threshold (P (FWE‐corrected) < 0.1). Again, we detected no significant cluster with the positive FTD and linguistic FTD dimensions. However, the exploratory analysis exclusively in patients with positive FTD (TLC‐dis > 0; n = 25) detected a positive association with rCBF within a large cluster including the supplementary motor area (SMA), mid cingulum, and superior frontal gyrus as well as within the left middle temporal gyrus and the inferior frontal gyrus (see Figure S3, Table S5). This association of rCBF and positive FTD within the SMA remained significant (P (FWE‐corrected) < 0.05) when controlling for the severity of chlorpromazine equivalent dosage, negative FTD, and linguistic FTD (see Table S7).
Regional rCBF alterations in the left supramarginal gyrus linked to social functioning after 6 month
We tested whether regional rCBF was associated with FTD symptom severity and measures of functional outcome. Baseline symptom severity (PANSS scores) and functioning (GAF and SOFAS) were not associated with rCBF. Likewise, we detected no association of functional capacity (UPSA brief) after 6 months and rCBF. However, social functioning (SOFAS) at a 6 months follow‐up had a negative linear association with baseline perfusion of the left supramarginal gyrus (r = −0.386; P = 0.046) (Figure S4). In fact, patients with poor social functioning (low SOFAS scores) at follow‐up had higher perfusion in the left supramarginal gyrus at baseline. Likewise, baseline perfusion of the supramarginal gyrus was linked to the severity of negative FTD at baseline. In contrast, negative FTD at baseline was not associated with social functioning at follow‐up (r = −0.144; P = 0.474).
Discussion
We investigated the association between local rCBF as a measure of neural activity and the severity of FTD, focusing on overall FTD as well as distinct FTD dimensions in patients with schizophrenia. In line with previous results, patients had reduced rCBF in fronto‐temporal brain areas. Furthermore, we detected that rCBF in primary language areas was associated with overall FTD severity in patients. Moreover, we confirmed our hypothesis of distinct associations between rCBF and FTD dimensions. In fact, negative FTD was most prominently linked to perfusion of brain areas relevant for semantic processing, while positive FTD was linked to rCBF in brain areas relevant for speech production. We failed to detect an association of functional capacity and rCBF associated with FTD. However, perfusion of the left supramarginal gyrus was linked to negative FTD and associated with social functioning after 6 months.
FTD is linked to dysfunction of primary language areas
We noted an association of overall FTD severity and rCBF exclusively in brain areas of the language network (the right superior and the left middle temporal gyrus). In fact, the superior and the medial temporal gyrus are key regions of semantic processing 44. Thus, we were able to replicate the link between FTD severity or disorganized speech and rCBF alterations in the language network in schizophrenia 16, 17, 45, 46, 47. In contrast, temporal hypoactivity during speech production was associated with positive FTD 15, 19. Previously, Liu et al. 42 demonstrated that baseline rCBF may influence the BOLD signal. First evidence suggests an inverse relationship between task‐related BOLD signal and resting state CBF in schizophrenia: Local BOLD signal increases were linked to rCBF reduction, while increased resting state rCBF was associated with decreased task‐related BOLD signal 48. Therefore, the BOLD signal decrements during speech production in previous reports may reflect a relative task‐based hypoactivation on top of a baseline hyperperfusion of these primary language areas in subjects with severe FTD. In addition, increased severity of FTD was associated with deviant semantic network activity in patients 49. Our findings support the notion that FTD in schizophrenia involve a disturbance of basic language functions 50. This argues against FTD as a simple byproduct of general cognitive impairments related to psychosis 3 but rather an independent phenomenon. This is aligned with previous findings that negative FTD was linked to objective quality of life independent of the level of depression and neurocognitive impairments 51.
FTD dimensions have distinct pathobiology
This is the first study applying rCBF as a measure of baseline neural metabolism in FTD dimensions. FTD is a heterogeneous construct in terms of prevalence, longitudinal course, associated clinical variables, and influence on outcome 3. Given that a behavioural dissociation of FTD dimensions exists, one would expect differential perfusion patterns of the FTD dimensions. In fact, we detected distinct rCBF patterns associated with FTD dimensions in line with modern models of FTD 1.
In particular, negative FTD (e.g., decreased speech initiation and thought blocking) was associated with increased rCBF particularly in key regions of semantic processing within the temporal lobe. Thus, patients with negative FTD may be engaged in an ineffective struggle accessing the lexical‐semantic memory store. In addition, negative FTD was associated with perfusion in the precuneus. Suspected functions of the precuneus include episodic, working, and semantic memory, as well as retrieval of verbal information 52. It seems reasonable to assume that these processes are insufficiently engaged during thought blocking. In fact, the relative hyperperfusion of the precuneus in subjects with negative FTD may reflect increased, albeit insufficient, effort to retrieve verbal information. Furthermore, this relative hyperperfusion may compensate for hippocampal dysfunction previously detected during word association in schizophrenia 14. Thus, it may also reflect a compensation for poor retrieval of the lexical‐semantic memory store 53, 54. In addition, we detected inferior parietal lobe (IPL) perfusion to be linked to negative FTD. The IPL was previously suggested in the categorical representation of verbal information, obtained in the auditory system (e.g., the STG) 55, 56, 57. However, the specific role of the IPL for FTD dimensions has to be further evaluated. Finally, the association between cerebellar perfusion and negative FTD may indicate increased and insufficient processes during mental search, preparation of responses and verbal self‐monitoring; functions which are linked to the cerebellum 58, 59, 60. Yet, cerebellar activation has also been associated with positive FTD in schizophrenia 15. Therefore, cerebellar involvement may not be specific to one FTD dimension. Taken together, our findings are partly consistent with reports on task‐based functional imaging 21, 22. In particular, negative FTD was also linked to aberrant neural activity in the IPL, precuneus, and cuneus 22.
Regarding positive FTD, we found rCBF to be linked to FTD but exclusively within patients with positive FTD. In fact, we identified a distinguishable pattern of rCBF linked to positive in comparison with negative FTD, overlapping in the left middle temporal gyrus. Current symptoms of increased speech production and loosening of associations were associated with rCBF in two brain areas relevant for speech production: the SMA and the inferior frontal gyrus. The interpretation of this finding may be that patients with positive FTD ineffectively struggle to suppress inappropriate mental activity along with increased speech production. In line with previous reports, increased severity of positive FTD was associated with higher perfusion of a large cluster in the frontal lobe, interpreted as a faulty suppression of irrelevant material 18, 19, 61, 62. In addition, the association within the SMA seems to be specific for positive FTD, as it remained significant when controlling for the severity of negative and linguistic FTD. Finally, we failed to detect an association of linguistic FTD and rCBF. The low number of patients with symptoms of linguistic FTD (21%) may account for the missing correlation.
Association of rCBF linked to negative FTD and social functioning
Research on the prognostic value of FTD dimensions on functional outcome is limited and inconclusive 12. However, negative FTD has been reported as predictive for occupation and rehospitalization rates in first episode schizophrenia 63. On a behavioral level, negative FTD was linked to global functioning (GAF) 64. In contrast, Roche and colleges detected positive FTD (disorganized FTD) as the only FTD dimension associated with functional outcome after one year in first episode psychosis 12. Thus, there is growing evidence that dimensions of FTD have a selective impact on functional outcome 12.
We failed to detect an association of functional capacity and rCBF associated with FTD dimensions. However, we detected that social functioning after 6 months correlated with rCBF in the left supramarginal gyrus, which is also linked to negative FTD. Precisely, higher perfusion in the left supramarginal gyrus was associated with poorer social functioning. Still, the common ground for these observations is the rCBF in the supramarginal gyrus, because negative FTD at baseline and social functioning at follow‐up was not related. In line with this finding, a recent meta‐analysis reported a cluster including the supramarginal gyrus to be associated with both social cognition and language processing 65. Language facilitates the exchange of social information 66. Thus, our results suggest that altered metabolism in a brain area implicated in speech processing and social cognition (the supramaginal gyrus) was correlated with poorer social functioning in the future in patients with schizophrenia. On the other hand, we cannot exclude that hyperperfusion in this region reflects an, albeit insufficient, compensatory mechanism. If these findings can be replicated in larger patient groups, our results may help to stimulate research on new treatment strategies such as targeted noninvasive brain stimulation in patients with negative FTD. We may hope that these interventions will prevent functional decline in patients with FTD.
Limitations
Some limitations require discussion. First, all but four subjects were on antipsychotic medication at the time of study. In general, antipsychotic medication may affect rCBF in schizophrenia 67. However, all medicated patients were on a stable medication with first‐ or second‐generation antipsychotics. Furthermore, more patients presented positive FTD than linguistic or negative FTD on the behavioral level. This may hamper the search for effects particularly in the linguistic FTD dimension. In addition, results of positive FTD have to be interpreted with caution as we report results exclusively in patients with positive FTD (TLC‐dis > 0; n = 25). While the course of FTD is still a matter of debate, negative FTD (TLC emptiness or alogia) has been consistently suggested to be more persistent than positive FTD 68 and perfusion alterations linked to negative FTD may be easier to detect than to the other FTD dimensions. In addition, we investigated three FTD dimensions derived from the thought and language communication scale (TLC). Future studies are required to investigate other dimensions of FTD. For instance, the dimensions proposed in the thought and language disorder scale (TALD) account for subjective and objective symptoms, which was not the case in the FTD dimensions applied in our study 69. Finally, we performed no categorical comparison between patients exclusively showing positive, negative, or linguistic FTD due to overlap of symptoms of FTD dimensions within single patients.
We confirmed that resting state perfusion in the language network was associated with global FTD. Moreover, we detected distinct associations of rCBF and three FTD dimensions. These findings argue for a distinct underlying pathophysiology of FTD dimensions. In particular, negative FTD might be associated with ineffective access to the lexical‐semantic memory store, while positive FTD is likely to be associated with defective suppression of irrelevant material along with increased speech production. Finally, perfusion associated with thought blocking (negative FTD) in one cluster relevant for speech production and social cognition was associated with social functioning in 6 months. Our results stress the relevance of adequate verbal communication for social interaction. In the future, larger studies in distinct FTD dimensions may further explore the neural correlates and clinical relevance of FTD.
Declaration of interest
The authors (Katharina Stegmayer, Marius Stettler, Werner Strik, Andrea Federspiel, Roland Wiest, Stephan Bohlhalter, and Sebastian Walther) declare no conflict of interest.
Supporting information
Figure S1. Frequency of overall FTD and FTD dimensions within patients.
Figure S2. Group comparison of schizophrenia patients and healthy controls.
Figure S3. Positive linear association of severity of positive FTD and resting state perfusion (rCBF) within a subgroup of schizophrenia patients with positive FTD (n = 25).
Figure S4. Linear association of social functioning after 6 month (n = 27) and resting state perfusion (rCBF) within the left supramarginal gyrus (left panel).
Table S1. Demographic and clinical characteristics of the sample grouped according to symptoms of FTD dimensions.
Table S2. Demographic and clinical characteristics of the sample baseline and follow‐up.
Table S3. Group comparison of resting state cerebral blood flow (rCBF) in schizophrenia patients compared to healthy controls.
Table S5. Linear association of severity of positive FTD within a subgroup of schizophrenia patients with positive FTD.
Table S6. Linear association of severity of positive FTD within a subgroup of schizophrenia patients with positive FTD independent of positive and linguistic FTD.
Table S7. Nonlinear association of severity of A: overall and B: negative FTD within schizophrenia patients.
Acknowledgements
This work was supported by the Swiss National Science Foundation (SNSF: 152619/1 to S.W., A.F. and S.B.) and the Bangerter‐Rhyner Foundation (to S.W.).
Stegmayer K, Stettler M, Strik W, Federspiel A, Wiest R, Bohlhalter S, Walther S. Resting state perfusion in the language network is linked to formal thought disorder and poor functional outcome in schizophrenia.
References
- 1. DeLisi LE. Speech disorder in schizophrenia: review of the literature and exploration of its relation to the uniquely human capacity for language. Schizophr Bull 2001;27:481–496. [DOI] [PubMed] [Google Scholar]
- 2. Andreasen NC. Thought, language, and communication disorders. II. Diagnostic significance. Arch Gen Psychiatry 1979;36:1325–1330. [DOI] [PubMed] [Google Scholar]
- 3. Roche E, Creed L, MacMahon D, Brennan D, Clarke M. The epidemiology and associated phenomenology of formal thought disorder: a systematic review. Schizophr Bull 2015;41:951–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Weintraub S. Risk factors in schizophrenia: the Stony Brook High‐Risk Project. Schizophr Bull 1987;13:439–450. [DOI] [PubMed] [Google Scholar]
- 5. Nagels A, Stratmann M, Ghazi S et al. The German translation and validation of the scale for the assessment of thought, language and communication: a factor analytic study. Psychopathology 2013;46:390–395. [DOI] [PubMed] [Google Scholar]
- 6. Nagels A, Fahrmann P, Stratmann M et al. Distinct neuropsychological correlates in positive and negative formal thought disorder syndromes: the thought and language disorder scale in endogenous psychoses. Neuropsychobiology 2016;73:139–147. [DOI] [PubMed] [Google Scholar]
- 7. Tan EJ, Yelland GW, Rossell SL. Characterising receptive language processing in schizophrenia using word and sentence tasks. Cogn Neuropsychiatry 2016;21:14–31. [DOI] [PubMed] [Google Scholar]
- 8. Tan EJ, Neill E, Rossell SL. Assessing the relationship between semantic processing and thought disorder symptoms in schizophrenia. J Int Neuropsych Soc 2015;21:629–638. [DOI] [PubMed] [Google Scholar]
- 9. Tan EJ, Rossell SL. Formal thought disorder and neurocognition in schizophrenia: the question of individual mechanisms. Schizophr Res 2017. https://doi.org/10.1016/j.schres.2017.03.039. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 10. Ott SL, Roberts S, Rock D, Allen J, Erlenmeyer‐Kimling L. Positive and negative thought disorder and psychopathology in childhood among subjects with adulthood schizophrenia. Schizophr Res 2002;58:231–239. [DOI] [PubMed] [Google Scholar]
- 11. Roche E, Segurado R, Renwick L et al. Language disturbance and functioning in first episode psychosis. Psychiatry Res 2016;235:29–37. [DOI] [PubMed] [Google Scholar]
- 12. Roche E, Lyne J, O'Donoghue B et al. The prognostic value of formal thought disorder following first episode psychosis. Schizophr Res 2016;178:29–34. [DOI] [PubMed] [Google Scholar]
- 13. Stegmayer K, Strik W, Federspiel A, Wiest R, Bohlhalter S, Walther S. Specific cerebral perfusion patterns in three schizophrenia symptom dimensions. Schizophr Res 2017. https://doi.org/10.1016/j.schres.2017.03.018. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 14. Kircher T, Whitney C, Krings T, Huber W, Weis S. Hippocampal dysfunction during free word association in male patients with schizophrenia. Schizophr Res 2008;101:242–255. [DOI] [PubMed] [Google Scholar]
- 15. Kircher TTJ, Liddle PF, Brammer MJ, Williams SCR, Murray RM, McGuire PK. Neural correlates of formal thought disorder in schizophrenia – preliminary findings from a functional magnetic resonance imaging study. Arch Gen Psychiatry 2001;58:769–774. [DOI] [PubMed] [Google Scholar]
- 16. Kircher TT, Liddle PF, Brammer MJ, Williams SC, Murray RM, McGuire PK. Severity of ‘positive’ formal thought disorder in schizophrenia is inversely correlated with activation in left superior temporal cortex. Schizophr Res 2001;49:179–180.11343876 [Google Scholar]
- 17. Horn H, Federspiel A, Wirth M et al. Structural and metabolic changes in language areas linked to formal thought disorder. Br J Psychiatry 2009;194:130–138. [DOI] [PubMed] [Google Scholar]
- 18. Liddle PF, Friston KJ, Frith CD, Hirsch SR, Jones T, Frackowiak RS. Patterns of cerebral blood flow in schizophrenia. Br J Psychiatry 1992;160:179–186. [DOI] [PubMed] [Google Scholar]
- 19. McGuire PK, Quested DJ, Spence SA, Murray RM, Frith CD, Liddle PF. Pathophysiology of ‘positive’ thought disorder in schizophrenia. Brit J Psychiatry 1998;173:231–235. [DOI] [PubMed] [Google Scholar]
- 20. Kircher TTJ, Liddle PF, Brammer MJ, Williams SCR, Murray RM, McGuire PK. Severity of ‘positive’ formal thought disorder in schizophrenia is inversely correlated with activation in left superior temporal cortex. NeuroImage 2001;13:S1067‐S. [Google Scholar]
- 21. McGuire PK, Quested D, Spence S, Murray R, Frith C, Liddle P. Distinct neural correlates of ‘positive’ and ‘negative’ thought disorder. Schizophr Res 1998;2:111. [Google Scholar]
- 22. Kircher T, Liddle P, Brammer M, Murray R, McGuire P. Neural correlates of “negative” formal thought disorder. Der Nervenarzt 2003;74:748–754. [DOI] [PubMed] [Google Scholar]
- 23. Walther S, Schappi L, Federspiel A et al. Resting‐state hyperperfusion of the supplementary motor area in catatonia. Schizophr Bull 2016. pii: sbw140. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry 2003;64:663–667. [DOI] [PubMed] [Google Scholar]
- 25. Sheehan DV, Lecrubier Y, Sheehan KH et al. The Mini‐International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM‐IV and ICD‐10. J Clin Psychiatry 1998;59(Suppl 20):22–33; quiz 4‐57. [PubMed] [Google Scholar]
- 26. Ritter N, Kilinc E, Navruz B, Bae Y. Test of nonverbal intelligence‐4 (TONI‐4). J Psychoeducat Assessment 2011;29:484–488. [Google Scholar]
- 27. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 1987;13:261–276. [DOI] [PubMed] [Google Scholar]
- 28. Andreasen NC. Scale for the assessment of thought, language, and communication (TLC). Schizophr Bull 1986;12:473–482. [DOI] [PubMed] [Google Scholar]
- 29. Goldman HH, Skodol AE, Lave TR. Revising axis‐V for Dsm‐Iv – a review of measures of social functioning. Am J Psychiat 1992;149:1148–1156. [DOI] [PubMed] [Google Scholar]
- 30. Mausbach BT, Harvey PD, Goldman SR, Jeste DV, Patterson TL. Development of a brief scale of everyday functioning in persons with serious mental illness. Schizophr Bull 2007;33:1364–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Deichmann R, Schwarzbauer C, Turner R. Optimisation of the 3D MDEFT sequence for anatomical brain imaging: technical implications at 1.5 and 3 T. NeuroImage 2004;21:757–767. [DOI] [PubMed] [Google Scholar]
- 32. Dai WY, Garcia D, de Bazelaire C, Alsop DC. Continuous flow‐driven inversion for arterial spin labeling using pulsed radio frequency and gradient fields. Magn Reson Med 2008;60:1488–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wu WC, Fernandez‐Seara M, Detre JA, Wehrli FW, Wang J. A theoretical and experimental investigation of the tagging efficiency of pseudocontinuous arterial spin labeling. Magn Reson Med 2007;58:1020–1027. [DOI] [PubMed] [Google Scholar]
- 34. Federspiel A, Muller TJ, Horn H, Kiefer C, Strik WK. Comparison of spatial and temporal pattern for fMRI obtained with BOLD and arterial spin labeling. J Neural Transm 2006;113:1403–1415. [DOI] [PubMed] [Google Scholar]
- 35. Jann K, Orosz A, Dierks T, Wang DJ, Wiest R, Federspiel A. Quantification of network perfusion in ASL cerebral blood flow data with seed based and ICA approaches. Brain Topogr 2013;26:569–580. [DOI] [PubMed] [Google Scholar]
- 36. Walther S, Federspiel A, Horn H et al. Resting state cerebral blood flow and objective motor activity reveal basal ganglia dysfunction in schizophrenia. Psychiatry Res 2011;192:117–124. [DOI] [PubMed] [Google Scholar]
- 37. Smith SM, Nichols TE. Threshold‐free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage 2009;44:83–98. [DOI] [PubMed] [Google Scholar]
- 38. Rorden C, Karnath HO, Bonilha L. Improving lesion‐symptom mapping. J Cogn Neurosci 2007;19:1081–1088. [DOI] [PubMed] [Google Scholar]
- 39. Brett M, Anton JL, Valabregue R, Poline JB. Region of interest analysis using an SPM toolbox [abstract] Presented at the 8th International Conference on Functional Mapping of the Human Brain, Sendai: June 2‐6, 2002 Vol 16, No 2, 2002.
- 40. Pinkham A, Loughead J, Ruparel K et al. Resting quantitative cerebral blood flow in schizophrenia measured by pulsed arterial spin labeling perfusion MRI. Psychiat Res‐Neuroim 2011;194:64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kindler J, Jann K, Homan P et al. Static and dynamic characteristics of cerebral blood flow during the resting state in schizophrenia. Schizophr Bull 2015;41:163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Liu JC, Qiu ML, Constable RT, Wexler BE. Does baseline cerebral blood flow affect task‐related blood oxygenation level dependent response in schizophrenia? Schizophr Res 2012;140:143–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ota M, Ishikawa M, Sato N et al. Pseudo‐continuous arterial spin labeling MRI study of schizophrenic patients. Schizophr Res 2014;154:113–118. [DOI] [PubMed] [Google Scholar]
- 44. Kircher T. Neurobiological foundations of thought and language disorder in schizophrenia. Fortschr Neurol Psychiatr 2008;76(Suppl 1):S24–S32. [DOI] [PubMed] [Google Scholar]
- 45. Sabri O, Erkwoh R, Schreckenberger M, Owega A, Sass H, Buell U. Correlation of positive symptoms exclusively to hyperperfusion or hypoperfusion of cerebral cortex in never‐treated schizophrenics. Lancet 1997;349:1735–1739. [DOI] [PubMed] [Google Scholar]
- 46. Lahti AC, Weiler MA, Holcomb HH, Tamminga CA, Carpenter WT, McMahon R. Correlations between rCBF and symptoms in two independent cohorts of drug‐free patients with schizophrenia. Neuropsychopharmacology 2006;31:221–230. [DOI] [PubMed] [Google Scholar]
- 47. Kaplan RD, Szechtman H, Franco S et al. Three clinical syndromes of schizophrenia in untreated subjects: relation to brain glucose activity measured by positron emission tomography (PET). Schizophr Res 1993;11:47–54. [DOI] [PubMed] [Google Scholar]
- 48. Pinkham AE, Liu P, Lu H, Kriegsman M, Simpson C, Tamminga C. Amygdala hyperactivity at rest in paranoid individuals with schizophrenia. Am J Psychiatry 2015;172:784–792. [DOI] [PubMed] [Google Scholar]
- 49. Horn H, Jann K, Federspiel A et al. Semantic network disconnection in formal thought disorder. Neuropsychobiology 2012;66:14–23. [DOI] [PubMed] [Google Scholar]
- 50. Chaika E. Thought disorder or speech disorder in schizophrenia? Schizophr Bull 1982;8:587–594. [DOI] [PubMed] [Google Scholar]
- 51. Tan EJ, Thomas N, Rossell SL. Speech disturbances and quality of life in schizophrenia: differential impacts on functioning and life satisfaction. Compr Psychiatry 2014;55:693–698. [DOI] [PubMed] [Google Scholar]
- 52. Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain 2006;129:564–583. [DOI] [PubMed] [Google Scholar]
- 53. Leeson VC, Laws KR, McKenna PJ. Formal thought disorder is characterised by impaired lexical access. Schizophr Res 2006;88:161–168. [DOI] [PubMed] [Google Scholar]
- 54. Grove WM, Andreasen NC. Language and thinking in psychosis – is there an input abnormality. Arch Gen Psychiatry 1985;42:26–32. [DOI] [PubMed] [Google Scholar]
- 55. Harinen K, Rinne T. Acoustical and categorical tasks differently modulate activations of human auditory cortex to vowels. Brain Lang 2014;138:71–79. [DOI] [PubMed] [Google Scholar]
- 56. Desai R, Liebenthal E, Waldron E, Binder JR. Left posterior temporal regions are sensitive to auditory categorization. J Cogn Neurosci 2008;20:1174–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chang EF, Rieger JW, Johnson K, Berger MS, Barbaro NM, Knight RT. Categorical speech representation in human superior temporal gyrus. Nat Neurosci 2010;13:1428–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fu CH, Vythelingum GN, Brammer MJ et al. An fMRI study of verbal self‐monitoring: neural correlates of auditory verbal feedback. Cereb Cortex 2006;16:969–977. [DOI] [PubMed] [Google Scholar]
- 59. Desmond JE, Gabrieli JDE, Glover GH. Dissociation of frontal and cerebellar activity in a cognitive task: evidence for a distinction between selection and search. NeuroImage 1998;7:368–376. [DOI] [PubMed] [Google Scholar]
- 60. Konopka G, Roberts TF. Insights into the Neural and Genetic Basis of Vocal Communication. Cell 2016;164:1269–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Arcuri SM, Broome MR, Giampietro V et al. Faulty suppression of irrelevant material in patients with thought disorder linked to attenuated frontotemporal activation. Schizophrenia Res Treatment 2012;2012:176290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Fletcher PC, Frith CD, Grasby PM, Friston KJ, Dolan RJ. Local and distributed effects of apomorphine on fronto‐temporal function in acute unmedicated schizophrenia. J Neurosci 1996;16:7055–7062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wilcox J, Winokur G, Tsuang M. Predictive value of thought disorder in new‐onset psychosis. Compr Psychiatry 2012;53:674–678. [DOI] [PubMed] [Google Scholar]
- 64. Andreasen NC, Grove WM. Thought, language, and communication in schizophrenia – diagnosis and prognosis. Schizophr Bull 1986;12:348–359. [DOI] [PubMed] [Google Scholar]
- 65. Bzdok D, Hartwigsen G, Reid A, Laird AR, Fox PT, Eickhoff SB. Left inferior parietal lobe engagement in social cognition and language. Neurosci Biobehav Rev 2016;68:319–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Dunbar RI, Marriott A, Duncan ND. Human conversational behavior. Human Nature 1997;8:231–246. [DOI] [PubMed] [Google Scholar]
- 67. Goozee R, Handley R, Kempton MJ, Dazzan P. A systematic review and meta‐analysis of the effects of antipsychotic medications on regional cerebral blood flow (rCBF) in schizophrenia: association with response to treatment. Neurosci Biobehav R 2014;43:118–136. [DOI] [PubMed] [Google Scholar]
- 68. Gooding DC, Ott SL, Roberts SA, Erlenmeyer‐Kimling L. Thought disorder in mid‐childhood as a predictor of adulthood diagnostic outcome: findings from the New York High‐Risk Project. Psychol Med 2013;43:1003–1012. [DOI] [PubMed] [Google Scholar]
- 69. Kircher T, Krug A, Stratmann M et al. A rating scale for the assessment of objective and subjective formal Thought and Language Disorder (TALD). Schizophr Res 2014;160:216–221. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Frequency of overall FTD and FTD dimensions within patients.
Figure S2. Group comparison of schizophrenia patients and healthy controls.
Figure S3. Positive linear association of severity of positive FTD and resting state perfusion (rCBF) within a subgroup of schizophrenia patients with positive FTD (n = 25).
Figure S4. Linear association of social functioning after 6 month (n = 27) and resting state perfusion (rCBF) within the left supramarginal gyrus (left panel).
Table S1. Demographic and clinical characteristics of the sample grouped according to symptoms of FTD dimensions.
Table S2. Demographic and clinical characteristics of the sample baseline and follow‐up.
Table S3. Group comparison of resting state cerebral blood flow (rCBF) in schizophrenia patients compared to healthy controls.
Table S5. Linear association of severity of positive FTD within a subgroup of schizophrenia patients with positive FTD.
Table S6. Linear association of severity of positive FTD within a subgroup of schizophrenia patients with positive FTD independent of positive and linguistic FTD.
Table S7. Nonlinear association of severity of A: overall and B: negative FTD within schizophrenia patients.


