Abstract
Scope
A high salt (HS) diet is detrimental to cognitive function, in addition to having a role in cardiovascular disorders. However, the method by which an HS diet impairs cognitive functions such as learning and memory remains open.
Methods and results
In this study, we found that mice on a 7 week HS diet demonstrated disturbed short‐term memory in an object‐place recognition task, and both 4 week and 7 week HS treatments impaired long‐term memory, as evidenced in a fear conditioning test. Mechanistically, the HS diet inhibited memory‐related long‐term potentiation (LTP) in the hippocampus, while also increasing the levels of reactive oxygen species (ROS) in hippocampal cells and downregulating the expression of synapsin I, synaptophysin, and brain‐derived neurotrophic factor in specific encephalic region.
Conclusion
This suggests that oxidative stress or synaptic protein/neurotrophin deregulation was involved in the HS diet‐induced memory impairment. Thus, the present study provides novel insights into the mechanisms of memory impairment caused by excessive dietary salt, and underlined the importance of controlling to salt absorb quantity.
Keywords: High salt, Memory, Oxidative stress, Synaptic plasticity, Synaptic protein
Abbreviations
- ROS
reactive oxygen species
- LTP
long‐term potentiation
- BDNF
brain‐derived neurotrophic factor
- SYS
synapsin I
- SYP
synaptophysin
- ERK
extracellular signal‐regulated kinase
- OPR
object‐place recognition task
- FC
fear conditioning
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- TG
triglycerides
- TC
total cholesterol
- SOD
superoxide dismutase
- CAT
catalase
- GPx
glutathione peroxidase
- GST
glutathione S‐transferases
- GR
glutathione reductase
- CS
citrate synthase
- CamK
Calcium‐calmodulin dependent protein kinases
1. Introduction
Salt (sodium chloride) is a major source of sodium in human nutrition. In general, the dietary intake of sodium chloride around the world has increased considerably over the last few decades 1, 2. In many countries, daily salt intake has considerably exceeded the recommended guidelines 3 and is often underestimated by both hypertensive and normotensive subjects 4. Increased dietary salt intake is positively associated with the risk of many diseases, such as hypertension 5, hypertension‐related cardiovascular complications 6, and other health problems 7. Additionally, a high salt (HS) diet has also been found to correlate with impaired cognitive functioning 8 and changes in emotional state 9, although the underlying mechanisms remain largely unknown.
It is well documented that excessive dietary salt intake has adverse physiological consequences 10, with numerous studies on experimental animals having demonstrated that increased oxidative stress is associated with HS‐induced endothelial dysfunction 11, anxiety 12, and metabolic disturbance 13. Most of these adverse consequences share a common pathological condition: the generation of reactive oxygen species (ROS) and a decrease in antioxidative capacity. Another consequence of oxidative stress is cognitive impairment. Chugh et al. 9 demonstrated that excess salt‐induced anxiety‐like behavior and memory impairment are associated with a redox imbalance in the brain of aging rats. The high‐energy demands of synapses, together with their high production of ROS, place them at risk during conditions of increased stress in neurodegenerative disorders such as Alzheimer's and Parkinson's diseases 14, 15.
Synapses are capable of undergoing stable and long‐lasting changes in synaptic strength referred to as long‐term potentiation (LTP), changes that play a central role in nearly all models of learning and memory. Synaptic plasticity is induced at appropriate synapses during memory formation, and is highly suggestive of an information storage device in the brain 16, 17. A recent study found that oxidative stress can interact with brain‐derived neurotrophic factor (BDNF) to modulate synaptic plasticity and cognitive function. The oxidative damage was associated with decreased expression of synaptic markers, such as BDNF, synapsin I (SYS), synaptophysin (SYP), calcium‐calmodulin dependent protein kinases II (CamK‐II), CamK‐IV and Calcineurin A 18, 19, 20, 21, 22, 23. Other signaling mechanisms involved in synaptic plasticity, including the extracellular signal‐regulated kinase (ERK) pathway, could also be affected by oxidative stress 24. Whereas emerging evidence indicates that diet‐induced cognitive impairment seems to be a consequence of enhanced oxidative stress 8, the significance of the systemic environment in brain function needs to be explored by determining how diet‐induced systemic oxidative stress can influence synaptic transmission in the brain. The hippocampus plays a prominent role in the consolidation of declarative memories 25, and we therefore assessed the effects of excessive salt consumption on brain function, oxidation resistance, and synaptic plasticity in the mouse hippocampus.
2. Materials and methods
2.1. Animals
Male C57BL/6J mice (6–8‐week‐old, weighing 18–22 g) were purchased from the Experimental Animal Center of Xi'an Jiaotong University (permit No. 16013) (Shaanxi, China) and housed four or five per cage in a temperature‐controlled (22 ± 2°C) room with a 12:12 h light dark cycle (lights on 08:00–20:00), humidity of 50–60%, and food and water available ad libitum. Mice were randomly assigned to control or HS groups. The HS groups included HS intake for 4 weeks (HS‐4wk) and HS intake for 7 weeks (HS‐7wk), with both being fed chow with 8% NaCl. Mice in the control group were fed chow containing 0.4% NaCl. The mean arterial pressure (MAP) was measured using tail‐cuff plethysmography with a computerized system.
2.2. Tissue and blood sample preparation
After a HS diet for either 4 or 7 weeks, mice were tested with an object‐place recognition task (OPR) and a fear conditioning (FC) memory test, before being sacrificed by decapitation. The brain was then immediately removed and washed with ice‐cold normal saline, and the hippocampus was dissected. Tissues were then stored at −80°C for further analyses. Meanwhile, blood was rapidly collected and the serum was separated by centrifugation. Serum biochemistry parameters including blood glucose, alanine aminotransferase (ALT), aspartate aminotransferase (AST), triglycerides (TG), and total cholesterol (TC) were measured using commercial kits (Nanjing Jiancheng Bio, Nanjing, China).
2.3. Anxiety behavior tests
Anxiety‐like behavior was evaluated using the open‐field test following previously described procedures 26, with at least 10 mice per group. In this open‐field test, the percentage of time spent in the center of the open‐field area and the total distance traveled were examined.
2.4. Learning and memory function test
The OPR test to evaluate short‐term memory was conducted according to previously published details 27. On the day of training, the mice were firstly exposed to a context habituation period without objects in the arena. During the next three sessions, mice were placed in the training arena with two objects. The objects used were both wooden bricks (2 × 2 × 8 cm). Each session lasted for 10 min with an inter‐session interval of 3 min, during which the mice were returned to their home cages. The arenas were cleaned before each session, and the objects were wiped with 30% ethanol before each mouse. The tests were performed at 3 min after the last training session. Mice were placed in the original training arena for 10 min with one object displaced to a new location and the other object unmoved. A digital camera recorded activity during training and testing for subsequent scoring of the time spent exploring objects. Exploration of the objects was defined as the amount of time a mouse spent oriented towards an object with its nose within 1 cm of it. The preference index for the object displaced to a new location was calculated as the percentage of object exploration time dedicated to this object over the total exploration time in the last training session or the testing session.
Long‐term memory was evaluated using the FC task according to previously published reports 28 with minor modifications. On training day, mice were placed in the FC chamber located in the center of a sound attenuating cubicle. A background odor was provided by cleaning the conditioning chamber with 10% ethanol. Mice were allowed to explore the context for 3 min before being subjected to three tone‐foot shock pairings. The 85 dB 2 kHz tone lasted for 1 min with foot shocks of 0.75 mA administered for 2 s, with the shocks co‐terminating with the tone. Mice remained in the training chamber for another 30 s before being returned to their home cages. The total training session was 10 min, and was divided into four intervals by three foot shocks. In the context test, mice were placed back into the original conditioning chamber for 5 min without a tone. The behavior of the mice was recorded with Freezeframe software (Coulbourn Instruments, Holliston, MA, USA) and analyzed with Freezeview software (Actimetrics, Wilmette, IL, USA). Motionless bouts lasting more than 1 s were considered as freezing behavior. The percentage of freezing was calculated as the ratio of freeze time to observed time.
2.5. Electrophysiology
The field excitatory postsynaptic potentials (fEPSPs) from CA3‐CA1 synapses were recorded using previously described procedures 29, and in Supporting Information Materials and Methods 1. Briefly, test fEPSPs were evoked with single‐pulse stimulations at 0.33 Hz. Input/output (I/O) curves were generated by gradually increasing the stimulus intensities (input) and recording the fEPSPs generated (output). The stimulus intensity required to evoke half of the maximum fEPSP was selected as the baseline measurement. After recording a stable baseline fEPSP for at least 30 min, LTP was induced with high‐frequency stimulation (HFS).
2.6. Measurement of enzyme activities and ROS
Hippocampus samples were homogenized and extracted in HEPES (4‐[2‐hydroxyethyl]‐1‐piperazineethanesulfonic acid) solution (20 mM, pH 7.4). The protein concentrations of the extract were measured using a bicinchoninic acid colorimetric assay (Thermo Fisher). ROS production was measured using 2',7'‐dichlorodihydrofluorescein diacetate (20 μM H2DCFDA, Sigma, D6883) 30. Superoxide dismutase (SOD, A001), catalase (CAT, A007), H2O2 (A064), glutathione peroxidase (GPx, A005), glutathione S‐transferases (GST, A004), and glutathione reductase (GR, A062) were determined using commercial kits (Nanjing Jiancheng Bio, Nanjing, China) according to the manufacturer's instructions. Fluorescence intensity and absorbance were recorded using TECAN infinite M200 at 30°C and normalized to protein content. Hexokinase (HK) activity 31 was measured in 40 mM HEPES, 1 mM EDTA, 1 mM MgCl2, 2.5 mM ATP, 0.7 U glucose‐6‐phosphate dehydrogenase, and 0.1 mM NADP+ by addition of 8–24 μg of protein. Citrate synthase (CS) activity 32 was measured in 50 mM Tris (pH 7.5), 100 mM KCl, 1 mM EDTA, 83.3 μM acetyle‐CoA, 167.5 μM DTNB (5,5'‐Dithiobis‐[2‐nitrobenzoic acid]), and 0.67 mM oxaloacetate, and was started by addition of 8–24 μg of protein extract.
2.7. Western blot analyses
Protein levels were measured using western blot analysis, with β‐actin used to ensure equal loading of the samples, according to previously published details 33, and in Supporting Information Material and Methods 1. Primary antibody dilutions used were as follows; SYS, SYP, BDNF, CamK‐II, CamK‐IV, Calcineurin A (Abcam, USA), ERK1/2, p‐ERK1/2 (Cell Signaling Technology, MA, USA), and β‐actin (Santa Cruz).
2.8. Immunohistochemistry
Free‐floating sections were stained with a series of antibodies, namely rabbit polyclonal anti‐SYS (1:200) (Abcam, USA), rabbit polyclonal anti‐SYP (1:200) (Abcam, USA) and rabbit monoclonal anti‐BDNF (1:200) (Abcam, USA). All antibodies are routinely used by several laboratories. Immunohistochemistry for the hippocampus were performed as described previously and in Supporting Information Material and Methods 1.
2.9. Data analysis
Data are reported as the mean ± SD of at least three independent experiments. Significant differences between two groups were determined by Student's t‐tests. Significant differences between three or more groups were determined by a one‐way ANOVA followed by Tukey's post hoc tests. Means were considered to be statistically different according to a value of p < 0.05. Wherever appropriate, Pearson's correlation analysis was conducted to investigate correlations between the groups, with SPSS 20.0 (IBM Corp., Armonk, NY, USA) used for this purpose.
3. Results
3.1. High salt diet fails to elicit apparent physical disorders or anxiety
Mice were fed a HS diet and monitored for body weight and food and water consumption. Compared with the control group on a normal diet, the HS diet caused a significant reduction in body weight (p < 0.01; Fig. 1A), but increased food and water intake (p < 0.01; Fig. 1B, 1C). Blood biochemical examination revealed that the HS diet elicited a marked increase in blood glucose level (Table 1). Although slightly elevated ALT/AST and TC/TG concentrations were observed in HS‐fed mice, these alterations were not statistically significant in comparison with control mice, suggesting only a limited immediate influence of HS diet on the physical condition of the mice.
Figure 1.
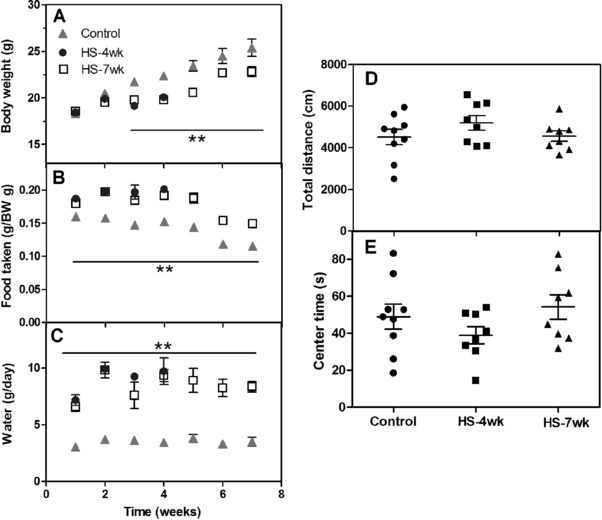
Anxiety‐like behavior and time courses of body weight, food intake, and water intake under high salt (HS) treatment. Mice were fed a HS or normal diet, and different parameters were monitored weekly. (A) Body weight; (B) food intake; (C) water intake. (D) Total distance traveled in an open‐field test, (E) percentage of time spent in the center arena. Data are presented as mean ± SEM, n ≥ 8 mice/group, ** p < 0.01 versus control group.
Table 1.
Concentrations of glucose, AST, ALT, TC, and TG in mice fed normal and high salt diets
| Concentration | Control | HS‐4wk | HS‐7wk |
|---|---|---|---|
| MAP (mmHg) | 92 ± 3 | 92 ± 4 | 95 ± 4 |
| Glucose (mM) | 27.76 ± 3.83 | 20.24 ± 2.48** | 22.70 ± 2.96* |
| AST (U/L) | 16.93 ± 4.63 | 26.47 ± 3.04 | 23.37 ± 3.28 |
| ALT (U/L) | 6.00 ± 2.68 | 11.20 ± 1.52 | 13.02 ± 4.93 |
| TC (mmol/L) | 2.12 ± 0.41 | 2.13 ± 0.13 | 1.94 ± 0.25 |
| TG (mmol/L) | 0.72 ± 0.18 | 0.70 ± 0.12 | 0.66 ± 0.06 |
n ≥ 6 mice/group; *p < 0.05, **p < 0.01 versus control group.
We next examined the anxiety levels of mice using the open‐field test. The total distance traveled in a 10 min observation was similar across the three groups (Fig. 1D), with the mice subjected to a HS diet spending a comparable amount of time in the center area to that spent by the control mice (Fig. 1E), indicating that a 4 or 7 week HS diet was insufficient to induce anxiety in the mice.
3.2. High salt diet impairs short‐ and long‐term memory
We assessed the effect of a HS diet on the short‐term memory of the animals using an OPR paradigm 27, 34 (Fig. 2A). In the familiarity stage (training), the total explorative time (both of the objects) for all groups decreased with an increasing number of sessions, which was indicative of a decreased exploratory interest for familiar objects. Furthermore, the HS‐4wk mice exhibited a stronger desire to explore objects than those in the control and HS‐7wk groups (Fig. 2B). In the test stage 3 min after the last training session, mice were reintroduced to the same condition experienced in the training arena, with the exception that one of the two objects had been moved to a new location. The preference index increased significantly in both control and HS‐4wk groups, whereas the HS‐7wk mice did not show a difference of performance in test stage from that measured during the last training session (0.47 versus 0.51, p = 0.25, n = 11), suggesting that a 7 week HS diet impaired the short‐term memory of the mice (Fig. 2C).
Figure 2.
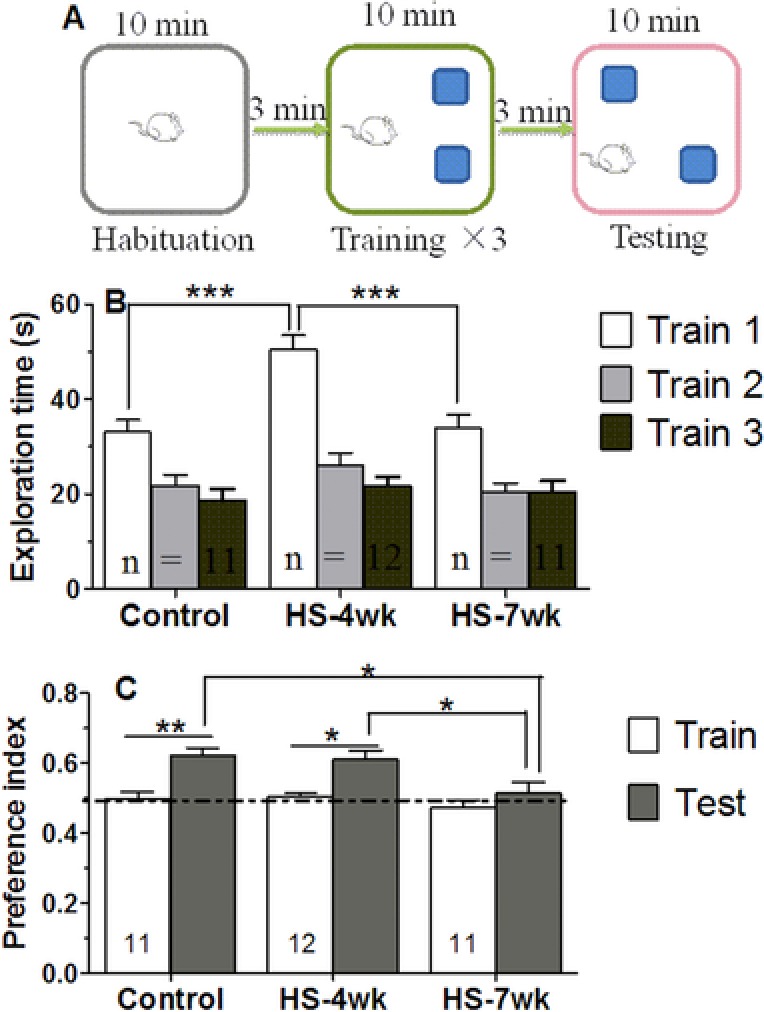
HS mice have impaired short‐term memory for object location. (A) Diagram illustrating the experimental design for object‐place recognition. (B) Bar graphs showing the total exploration times for the three groups. (C) Graphs showing the percentage preference for the displaced object after 3 min. Dotted line denotes 50% preference index. Bars are mean ± SEM, n ≥ 8 mice, * p < 0.05, ** p < 0.01, *** p < 0.001.
The influence of the HS diet on long‐term memory was next investigated using a FC memory task. In this task, the percentage of freezing time is considered to be indicative of the fear response. The freezing time increased similarly in the different groups of mice over the course of the four training stages divided by three foot shocks, suggesting a minimal immediate effect of HS diet treatment on fear response (Fig. 3B). Twenty‐four hours later, the animals were returned to the training box with no foot shock. In this stage of the experiment, the freezing during conditioning was significantly weaker in the HS mice than in the control mice (HS‐4wk: 27.5%, p = 0.013; HS‐7wk: 25.3%, p = 0.0014; control mice: 41.3%; Fig. 3C). Therefore, the HS diet was considered to cause a serious impairment to the long‐term memory in mice.
Figure 3.
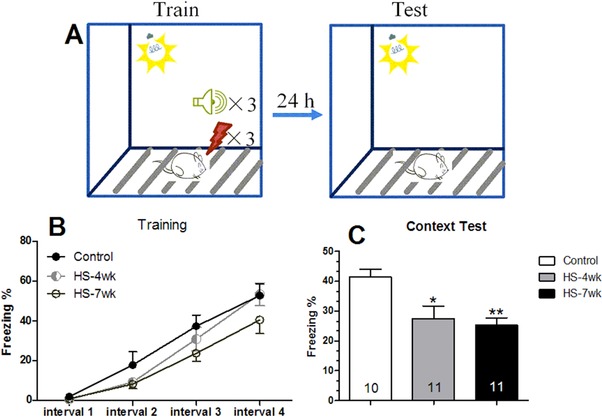
HS mice have impaired long‐term memory for contextual fear conditioning. (A) Diagram illustrating the experimental design for fear condition memory. (B) Percentage of freezing time during the acquisition training. (C) Percentage of freezing time during the contextual memory testing. Bars are mean ± SEM, n ≥ 10, * p < 0.05, ** p < 0.01 versus control group.
3.3. High salt diet disturbed hippocampal synaptic plasticity
The current‐fEPSPs relationships in the I/O curves were examined immediately following the HS treatment. As delineated in Fig. 4A, the I/O curves exhibited a slight leftward shift in the HS‐7wk group, with the minimum output demonstrating a significant increase in comparison with the normal diet group. We examined the level of post‐tetanic potentiation produced immediately following the HFS measurement, and the data showed that the LTP of the fEPSP amplitude was induced by HFS in the control and HS‐4wk groups (Fig. 4B and 4C). By contrast, the normalized amplitude of the fEPSP was only slightly increased in the HS‐7wk group after HFS measurement, and this amplitude was less than that in the control group (control: 132.9%, HS‐4wk: 165.2%, HS‐7wk: 109.5%). Our results thereby demonstrated that a HS diet was responsible for eliciting disturbances in hippocampal LTP.
Figure 4.
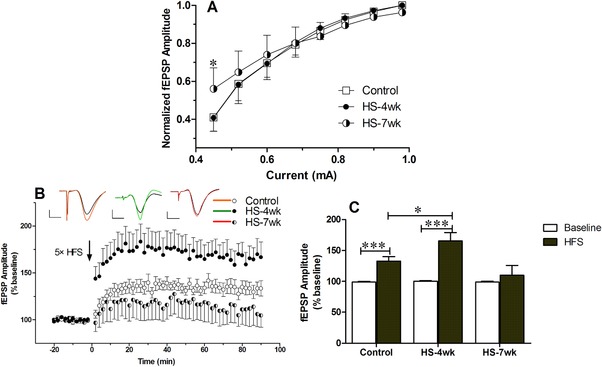
Effects of high salt on synaptic plasticity. Input/output (I/O) and long‐term potentiation (LTP) changes were measured in mice fed normal chow or a high salt diet. (A) I/O relationships after applying increasing stimulation to the stratum radiatum of the CA1 region in the hippocampus and recording the output. (B) LTP was induced at baseline intensity using high‐frequency stimulation. The amplitude of the field excitatory postsynaptic potential (fEPSP) was measured, and the results were normalized to the average value measured during the 20 min baseline period. (C) Recording continued for at least 90 min following high‐frequency stimulation (HFS), and the last 10 min was used to calculate the LTP and plot the histogram. Data are presented as mean ± SD, n ≥ 8 mice/group, * p < 0.05, *** p < 0.001.
3.4. High salt diet induces oxidative stress and metabolic reprogramming in the hippocampus
To further address the mechanisms of HS‐induced memory damage, we assessed the degree of oxidative stress by measuring ROS in the hippocampus. HS diet mice exhibited a higher level of ROS than control mice (Fig. 5). The antioxidant capacities, including the activities of CAT and SOD, were significantly reduced relative to those in control mice, whereas the levels of GST, GPx, and GR enzyme activities remained unchanged in all groups. Thus, our results demonstrated that the HS mice had higher oxidative stress due to either increased ROS production or decreased ROS scavenging capability. Furthermore, we also investigated the energy metabolism in hippocampal cells. Compared with control and HS‐4wk mice, HS‐7wk mice showed an obvious downregulation of hexokinase, a key enzyme in glycolysis. Concomitantly, a dramatic increase in the level of CS, a rate‐limiting enzyme for the tricarboxylic acid cycle, was observed in HS‐7wk mice. These findings suggest that a HS diet can trigger the metabolic reprogramming of hippocampal cells.
Figure 5.
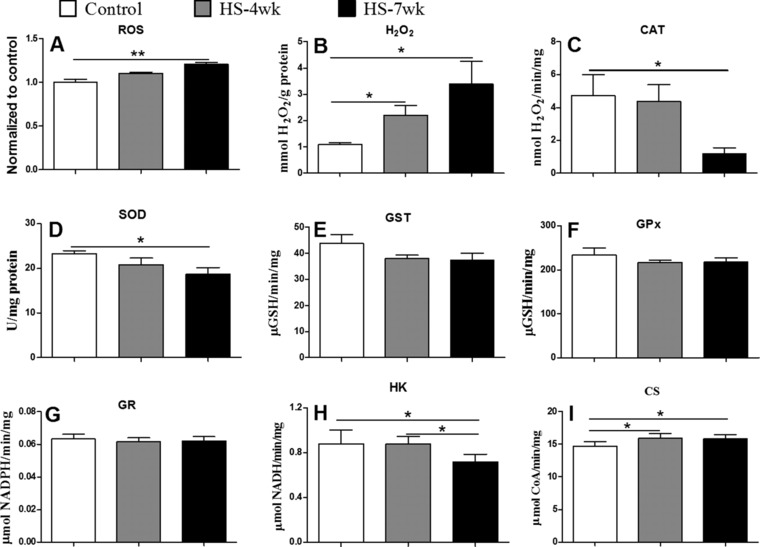
Markers of oxidative stress in mice fed normal or high salt diets. Concentrations of ROS (A), and H2O2 (B), and the enzyme activities of CAT (C), SOD (D), GST (E), GPx (F), GR (G), HK (H), and CS (I), were measured as described in the Materials and methods. Bars are mean ± SEM, n = 4–6, * p < 0.05, ** p < 0.01.
3.5. High salt diet suppresses synaptic protein expression
We examined the expression of the synaptic proteins and neurotrophins that probably underlie the aforementioned electrophysiological characters. As shown in Fig. 6, densitometry of western blotting analysis confirmed that the mice fed on a HS diet for 7 weeks exhibited a substantial decrease in SYS and SYP expression (32 and 40%, respectively) relative to the normal diet mice (Fig. 6A). Similarly, the expression of CamK‐II and CamK‐IV was reduced, whereas the level of calmodulin‐stimulated protein phosphatase, Calcineurin A, was slightly increased in HS diet mice compared with the control group (Fig. 6B). By contrast, we failed to detect a significant change in the levels of BDNF after treatment with a HS diet (Fig. 6A). Inhibition of phosphorylation of ERK1/2 occurred concurrently with the downregulation of SYS and SYP, which suggests attenuated activation of the Ras/MAPK pathway (Fig. 6A). Pearson's correlation showed a negative relationship between SYS or SYP expression and time on the HS diet (r = −0.777, p = 0.003; r = −0.696, p = 0.012, respectively). Thus, the HS diet suppressed proteins and intracellular signaling involved in synaptic plasticity.
Figure 6.
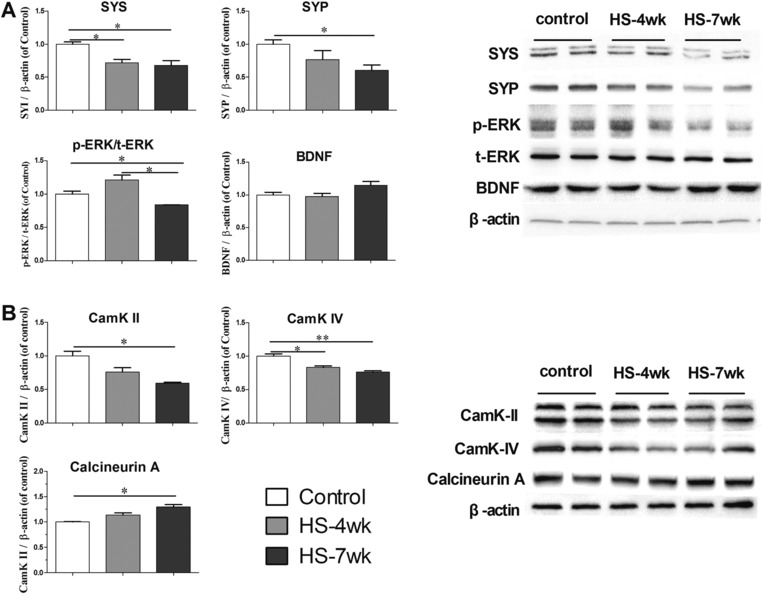
Protein levels in the hippocampus shown by western blotting. Bar graphs in panels are ratios of SYS, SYP, BDNF to β‐actin (A), p‐ERK to t‐ERK (A), and CamK‐II, CamK‐IV and Calcineurin A (B). The right panels are representative blots for SYS, SYP, p‐ERK, t‐ERK, BDNF, CamK‐II, CamK‐IV, and Calcineurin A as well as the protein loading control β‐actin. Bars are mean ± SEM, n = 3–4 mice, * p < 0.05.
To confirm the influence of HS on protein expression, we also performed immunohistochemical evaluations (Fig. 7). Similar to the previous results, the expression of SYS and SYP in the HS groups decreased significantly in a time‐dependent manner. In particular, these proteins were highly expressed in the CA1 area of the control mice, but not in the HS diet‐treated mice. However, the results were not exactly the same as those from the western blot analysis. Immunohistochemical analyses of BDNF protein performed after 4 or 7 weeks of feeding a HS diet showed slight declines (not significant) in comparison with controls fed a normal diet. Although western blotting revealed comparable total amounts of BDNF in the hippocampi of control and HS diet‐treated mice, immunohistochemical staining suggested that CA1 was the high expression area in control mice, whereas BDNF was localized mainly around the dentate gyrus nearby in the HS groups. These observations indicate that a HS diet may affect the expression of synaptic proteins and the source or production of neurotrophins.
Figure 7.
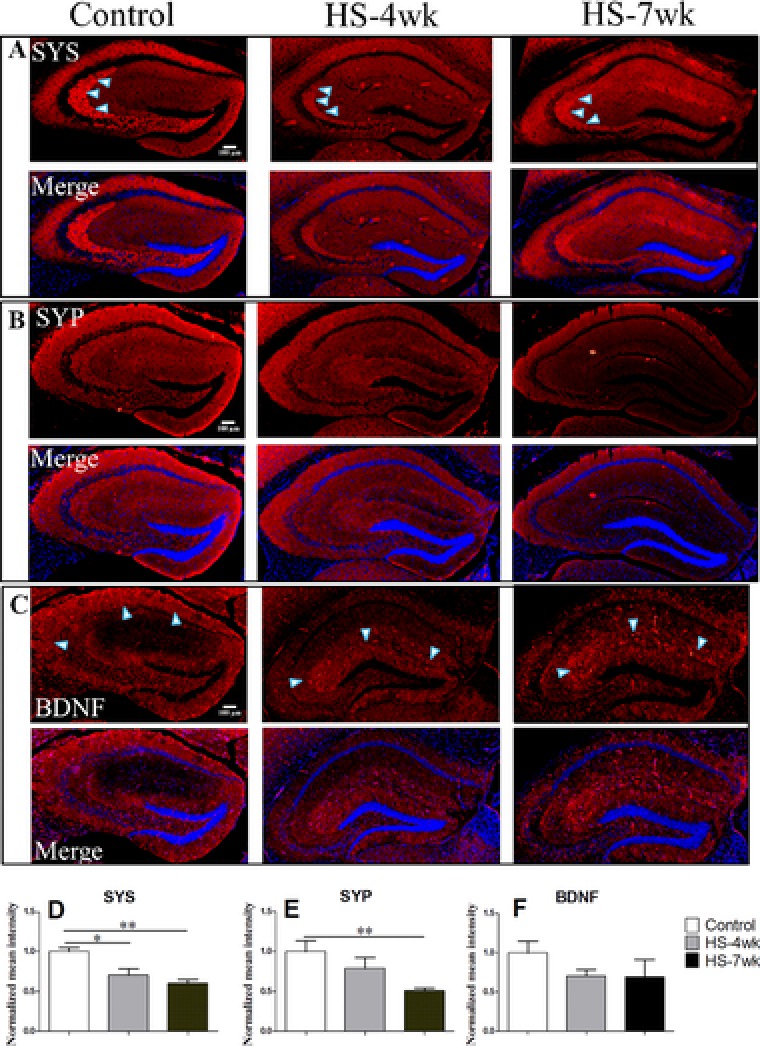
Immunohistochemistry of coronal sections of the hippocampus. (A, D) SYS, (B, E) SYP, (C, F), BDNF. The graphs compare the normalized mean intensity for the immunohistochemistry with DAPI intensity measured by Image J software (D–F).
4. Discussion
Excessive dietary salt intake is considered a risk factor for hypertension and other diseases 5, 35. It has been demonstrated that, in salt‐sensitive rats, the association of hypertension with poor cognitive performance is presumably due to sodium intake and blood pressure 36, 37, 38. Sodium intake is associated with high blood pressurein both animals and human 39, 40, which increases the risk of developing cardiovascular disease. However, we failed to observed a significant increase in blood pressure upon HS diet treatment of C57Bl/6J mice (Table 1), which is consistent with previous reports using the same strain of mice 8, 41. In addition, we found that the body weight, food and water intake, and blood glucose were significantly altered by HS diet. Our observation in combination with previous studies suggest a broad physiological and behavioral influence of HS diet on rodents, although these animals may develop mechanisms to circumvent blood pressure increase by high dietary salt 42, 43.
In the present study, we also evaluated the effects of a HS diet on short‐ and long‐term memory using different experimental paradigms. In the OPR, an equal time spent at the two objects by HS‐7wk mice indicated impairment to short‐term memory. Conversely, the performances of control and HS‐4wk mice were similar, which is consistent with a recently published study showing that short‐term memory in adult rats (tested using a radial arm water maze test) was not impaired after a short time on a HS diet (4 weeks) 9. In the FC task, we found that the freezing time in the condition was significantly lower after 4 weeks on a HS diet than it was on a normal diet. Additionally, we did not observe any difference in the time that the HS‐4wk and HS‐7wk mice spent in the context, suggesting that the impairments in long‐term memory were comparable in both groups. Therefore, it appears as though long‐term memory is more susceptible to HS than short‐term memory. Liu et al. 8 reported that 12 weeks on a HS diet (7% NaCl) impaired the retention of spatial memory in mice, but they did not find that 4 or 7 weeks of HS diet resulted in hypertension or cognitive dysfunction. We conjecture that, in comparison with the present study, the longer treatment times they required to detect memory changes were because of the lower salt concentration and a longer learning time. Nonetheless, the effects on learning and memory are selective and not generalized, given that impaired performance is not observed in the passive avoidance task and object recognition memory 36, 37.
Learning and memory are complex events that depend on electrochemical processes. While it remains to be fully understood how a HS diet modulates cognitive behaviors, we established that a disturbance to synaptic plasticity may play a role in HS diet‐triggered memory impairment. Even though there was no significant difference in the measured I/O responses between control and HS groups, the HS‐7wk group expressed a substantially reduced LTP in the CA1 region of the hippocampus in comparison with the control group, which is indicative of the strength of synaptic plasticity. In an attempt to decipher the molecular basis of HS‐induced impairment of synaptic plasticity, we found that SYS, SYP, CamK‐II, CamK‐IV and phosphorylated ERK1/2 are downregulated by HS treatment. These molecules are thought to play a pivotal role in the ability to regulate synaptic plasticity via modulation of LTP 44. ERK mediates the phosphorylation of the cAMP‐response element‐binding protein (CREB), which is essential for several forms of learning and memory, and is induced by BDNF 45. Both SYS and SYP are associated with neurotransmitter release, and are responsible for the formation and anchoring of synaptic vesicles, as well as contributing to fast and efficient neurotransmission 46. A proportional relationship between the ability to remember and the synaptic vesicle proteins SYS and SYP was observed in many previous studies 47, 48, 49. Recent studies on rats also show that a systemic increase in plasma Na+ can stimulate some neurosecretory cells to release BDNF 50, and that BDNF is associated with the abolishment of GABAergic signaling inhibition due to a chronic HS intake, thereby suggesting that dietary salt can affect neurotrophin‐induced plasticity 39.
It is also documented that oxidative damage to the hippocampus contributes to HS‐induced cognitive dysfunction 8. Marked decreases in the levels of synapse associated proteins are also observed to result from oxidative damage to pre‐synaptic membranes and synaptic vesicles 51. In accord with this, we found that a HS diet resulted in an accumulation of ROS in hippocampal cells accompanied by a decreased antioxidant capacity. Conversely, antioxidant supplementation can improve antioxidant capacity and reverse cognitive decline completely 52. High‐salt intake increases superoxide (O2 −) formation by increasing NAPDH oxidase activity 53. Moreover, enhanced oxidative stress may underlie the formation of impaired Ca2+‐mediated signaling and reduced Na+/K+ATPase expression, which lead to perturbed synaptic plasticity underlying cognitive impairment 54. Increasing evidence indicates that oxidative stress synergizes with BDNF insufficiency in the hippocampus to induce behavioral dysfunction via neuronal death and neurogenesis impairment 55, 56. While we detected an apparently altered distribution of BDNF upon HS treatment, further studies are needed to determine whether BDNF is involved in HS‐induced memory impairment. Epidemiological studies also found that hypertension contributes to the impairment of cognitive function and the development of dementia 57, 58. Finally, the decrease in the level of the glycolysis key enzyme, hexokinase, and the concomitant upregulation of the tricarboxylic acid cycle rate‐limiting enzyme, citrate synthase, suggest a metabolic reprograming induced by HS diet, which has implications in determining the different levels of oxidative stress between normal and HS diet mice or ATP production previously reported to affect synaptic plasticity 59.
In summary, the present study demonstrates that a HS diet in mice could impair both short‐ and long‐term memory, probably due to disturbed synaptic plasticity in the hippocampus. The candidate mechanisms responsible for these neurological disorders may include oxidative stress and suppressed expression of synaptic proteins or neurotrophins. Considering that a HS diet is a key risk factor for hypertension, it would be interesting to determine whether increased blood pressure in the brain plays an indispensable role in HS‐triggered memory impairment. Although the exact mechanisms of action are still not certain, the present study provides novel insights into the mechanisms underlying the detrimental effects of excessive dietary salt on learning and memory.
Author Contributions: J.H., Z.J.W. and Q.G. designed the study; Q.G. and Z.J.W. performed the study; Z.J.W., Y.W.W., Q.H. and Z.Q.Q. performed some specific analysis analyzed the data; Q.G., Z.M.T., W.R., X.Z. and J.H. wrote the manuscript. All authors read and approved the final manuscript.
The authors have declared no conflict of interest.
Supporting information
Supplementary material
Acknowledgement
This work was supported by grants from the National Natural Science Foundation of China (31371137), Key Project of Chinese Ministry of Education (113056A), the Fundamental Research Funds for the Central Universities (GK261001183) and the “Foundation for Excellent Doctor Degree Dissertation” of Shaanxi Normal University (S2015YB02).
Ge Q., Wang Z., Wu Y., Huo Q., Qian Z., Tian Z., Ren W., Zhang X., Han J., Mol. Nutr. Food Res. 2017, 61, 1700134 https://doi.org/10.1002/mnfr.201700134
Colour Online: See the article online to view Figs. 2–4 in colour.
5 References
- 1. Nwanguma, B. C. , Okorie, C. H. , Salt (sodium chloride) content of retail samples of Nigerian white bread: implications for the daily salt intake of normotensive and hypertensive adults. J. Hum. Nutr. Diet 2013, 26, 488–493. [DOI] [PubMed] [Google Scholar]
- 2. Belz, M. C. , Ryan, L. A. , Arendt, E. K. , The impact of salt reduction in bread: a review. Crit. Rev. Food. Sci. Nutr. 2012, 52, 514–524. [DOI] [PubMed] [Google Scholar]
- 3. Johner, S. A. , Thamm, M. , Schmitz, R. , Remer, T. , Current daily salt intake in Germany: biomarker‐based analysis of the representative DEGS study. Eur. J. Nutr. 2015, 54, 1109. [DOI] [PubMed] [Google Scholar]
- 4. Zhang, J. , Guo, X. L. , Seo, D. C. , Xu, A. Q. et al., Inaccuracy of self‐reported low sodium diet among Chinese: findings from baseline survey for Shandong & Ministry of Health Action on Salt and Hypertension (SMASH) project 2015, 28, 161–167. [DOI] [PubMed] [Google Scholar]
- 5. Klein, A. V. , Kiat, H. , The mechanisms underlying fructose‐induced hypertension: a review. J. Hypertens. 2015, 33, 912–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baldo, M. P. , Rodrigues, S. L. , Mill, J. G. , High salt intake as a multifaceted cardiovascular disease: new support from cellular and molecular evidence. Heart Fail. Rev. 2015, 20, 461–474. [DOI] [PubMed] [Google Scholar]
- 7. Monteleone, I. , Marafini, I. , Dinallo, V. , Di Fusco, D. et al., Sodium chloride‐enriched diet enhanced inflammatory cytokine production and exacerbated experimental colitis in mice. J. Crohn's Colitis 2016, 11, 237–245. [DOI] [PubMed] [Google Scholar]
- 8. Liu, Y. Z. , Chen, J. K. , Li, Z. P. , Zhao, T. et al., High‐salt diet enhances hippocampal oxidative stress and cognitive impairment in mice. Neurobiol Learn Mem. 2014, 114, 10–15. [DOI] [PubMed] [Google Scholar]
- 9. Chugh, G. , Asghar, M. , Patki, G. , Bohat, R. et al., A high‐salt diet further impairs age‐associated declines in cognitive, behavioral, and cardiovascular functions in male Fischer brown Norway rats. J. Nutr. 2013, 143, 1406–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ernsberger, P. , Azar, S. , Iwai, J. , Open‐field behavior in two models of genetic hypertension and the behavioral effects of salt excess. Behav. Neural Biol. 1983, 37, 46–60. [DOI] [PubMed] [Google Scholar]
- 11. Cosic, A. , Jukic, I. , Stupin, A. , Mihalj, M. et al., Attenuated flow‐induced dilatation of middle cerebral arteries is related to increased vascular oxidative stress in rats on a short‐term high salt diet. J. Physiol 2016, 594, 4917–4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Banday, A. A. , Lau, Y. S. , Lokhandwala, M. F. , Oxidative stress causes renal dopamine D1 receptor dysfunction and salt‐sensitive hypertension in Sprague‐Dawley rats. Hypertension (Dallas, Tex.: 1979) 2008, 51, 367–375. [DOI] [PubMed] [Google Scholar]
- 13. Jin, K. , Vaziri, N. D. , Salt‐sensitive hypertension in mitochondrial superoxide dismutase deficiency is associated with intra‐renal oxidative stress and inflammation. Clin. Exp. Nephrol. 2014, 18, 445–452. [DOI] [PubMed] [Google Scholar]
- 14. Sultana, R. , Butterfield, D. A. , Oxidative modification of brain proteins in Alzheimer's disease: perspective on future studies based on results of redox proteomics studies. J. Alzheimer's Dis: JAD 2013, 33 Suppl 1, S243–S251. [DOI] [PubMed] [Google Scholar]
- 15. Chinopoulos, C. , Adam‐Vizi, V. , Mitochondria deficient in complex I activity are depolarized by hydrogen peroxide in nerve terminals: relevance to Parkinson's disease. J. Neurochem. 2001, 76, 302–306. [DOI] [PubMed] [Google Scholar]
- 16. Martin, S. J. , Grimwood, P. D. , Morris, R. G. M. , Synaptic plasticity and memory: an evaluation hypothesis. Annu. Rev. Neurosci. 2000, 23, 649–711. [DOI] [PubMed] [Google Scholar]
- 17. Silva, A. J. , Molecular and cellular cognitive studies of the role of synaptic plasticity in memory. J. Neurobiol. 2003, 54, 224–237. [DOI] [PubMed] [Google Scholar]
- 18. Wu, A. , Zhe, Y. , Ferno, G. P. , The interplay between oxidative stress and brain‐derived neurotrophic factor modulates the outcome of a saturated fat diet on synaptic plasticity and cognition. Eur. J. Neurosci. 2004, 19, 1699–1707. [DOI] [PubMed] [Google Scholar]
- 19. Ansari, M. A. , Roberts, K. N. , Scheff, S. W. , A time course of contusion‐induced oxidative stress and synaptic proteins in cortex in a rat model of TBI. J. Neurotrauma 2008, 25, 513–526. [DOI] [PubMed] [Google Scholar]
- 20. Kaneai, N. , Fukui, K. , Koike, T. , Urano, S. , Changes in the levels of CAM kinase II and synapsin I caused by oxidative stress in the rat brain, and its prevention by vitamin E. Adv. Biosci. Biotechnol. 2012, 03, 1199–1205. [Google Scholar]
- 21. Gault, V. A. , Lennox, R. , Flatt, P. R. , Sitagliptin, a dipeptidyl peptidase‐4 inhibitor, improves recognition memory, oxidative stress and hippocampal neurogenesis and upregulates key genes involved in cognitive decline. Diabetes, Obes. Metab. 2015, 17, 403–413. [DOI] [PubMed] [Google Scholar]
- 22. Kasahara, J. , Fukunaga, K. , Miyamoto, E. , Activation of calcium/calmodulin‐dependent protein kinase IV in long term potentiation in the rat hippocampal CA1 region. J. Biol. Chem. 2001, 276, 24044. [DOI] [PubMed] [Google Scholar]
- 23. Ahmed, B. Y. , Yamaguchi, F. , Tsumura, T. , Gotoh, T. et al., Expression and subcellular localization of multifunctional calmodulin‐dependent protein kinases‐I, ‐II and ‐IV are altered in rat hippocampal CA1 neurons after induction of long‐term potentiation. Neurosci. Lett. 2000, 290, 149–153. [DOI] [PubMed] [Google Scholar]
- 24. Barascu, A. , Le Chalony, C. , Pennarun, G. , Genet, D. et al., Oxidative stress induces an ATM‐independent senescence pathway through p38 MAPK‐mediated lamin B1 accumulation. EMBO J. 2012, 31, 1080–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Eichenbaum, H. , The hippocampus and declarative memory: cognitive mechanisms and neural codes. Behav. brain Res. 2001, 127, 199–207. [DOI] [PubMed] [Google Scholar]
- 26. Liu, Z. , Patil, I. Y. , Jiang, T. , Sancheti, H. et al., High‐fat diet induces hepatic insulin resistance and impairment of synaptic plasticity. PloS one 2015, 10, e0128274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wimmer, M. E. , Hernandez, P. J. , Blackwell, J. , Abel, T. , Aging impairs hippocampus‐dependent long‐term memory for object location in mice. Neurobiol. aging 2012, 33, 2220–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xu, W. , Sudhof, T. C. , A neural circuit for memory specificity and generalization. Science 2013, 339, 1290–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zheng, Q. , Liu, Z. , Wei, C. , Han, J. et al., Activation of the D1 receptors inhibits the long‐term potentiation in vivo induced by acute morphine administration through a D1‐GluN2A interaction in the nucleus accumbens. Neuroreport 2014, 25, 1191–1197. [DOI] [PubMed] [Google Scholar]
- 30. Kim, Y. H. , Hwang, J. H. , Noh, J. R. , Gang, G. T. et al., Prevention of salt‐induced renal injury by activation of NAD(P)H:quinone oxidoreductase 1, associated with NADPH oxidase. Free Radic. Biol. Med. 2012, 52, 880–888. [DOI] [PubMed] [Google Scholar]
- 31. Crane, R. K. , Sols, A. , The association of hexokinase with paniculate fractions of brain and other tissue homogenates. J. Biol. Chem. 1953, 203, 273–292. [PubMed] [Google Scholar]
- 32. Lemos, D. , Salomon, M. , Gomes, V. , Phan, V. N. et al. Citrate synthase and pyruvate kinase activities during early life stages of the shrimp Farfantepenaeus paulensis (Crustacea, Decapoda, Penaeidae): effects of development and temperature. Comp. Biochem. Physiol Part B Biochem. Mol. Biol. 2003, 135, 707–719. [DOI] [PubMed] [Google Scholar]
- 33. Liu, T. , Zheng, Q. , Qian, Z. , Wang, H. et al., Cannabinoid‐elicited conditioned place preference in a modified behavioral paradigm. Biol. Pharmaceu. Bulletin 2016, 39, 747–753. [DOI] [PubMed] [Google Scholar]
- 34. Oliveira, A. M. , Hawk, J. D. , Abel, T. , Havekes, R. , Post‐training reversible inactivation of the hippocampus enhances novel object recognition memory. Learn. Mem. 2010, 17, 155–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Heerspink, H. L. , Ritz, E. , Sodium chloride intake: is lower always better? J. Am. Soc. Nephrol.: JASN 2012, 23, 1136–1139. [DOI] [PubMed] [Google Scholar]
- 36. Jr, T. A. , Hernandez, C. M. , Buccafusco, J. J. , Dahl salt‐sensitive and salt‐resistant rats: examination of learning and memory performance, blood pressure, and the expression of central nicotinic acetylcholine receptors. Neuroscience 2001, 103, 351–363. [DOI] [PubMed] [Google Scholar]
- 37. Ruiz‐Opazo, N. , Lopez, L. V. , Tonkiss, J. , Modulation of learning and memory in Dahl rats by dietary salt restriction. Hypertension (Dallas, Tex.: 1979) 2004, 43, 797–802. [DOI] [PubMed] [Google Scholar]
- 38. Herrera, V. L. , Pasion, K. A. , Tan, G. A. , Moran, A. M. et al., Sex‐Specific effects on spatial learning and memory, and sex‐independent effects on blood pressure of a <3.3 mbp rat chromosome 2 qtl region in dahl salt‐sensitive rats. PloS one 2013, 8, e67673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Choe, K. Y. , Han, S. Y. , Gaub, P. , Shell, B. et al., High salt intake increases blood pressure via BDNF‐mediated downregulation of KCC2 and impaired baroreflex inhibition of vasopressin neurons. Neuron 2015, 85, 549–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lava, S. A. , Bianchetti, M. G. , Simonetti, G. D. , Salt intake in children and its consequences on blood pressure. Ped. Nephrol. 2015, 30, 1389–1396. [DOI] [PubMed] [Google Scholar]
- 41. Chen, D. , La Greca, L. , Head, G. A. , Walther, T. et al., Blood pressure reactivity to emotional stress is reduced in AT1A‐receptor knockout mice on normal, but not high salt intake. Hypertens. Res.: official J. Japanese Soc. Hypertens. 2009, 32, 559–564. [DOI] [PubMed] [Google Scholar]
- 42. Jiang, Y. , Wang, H. Y. , Zheng, S. , Mu, S. Q. et al., Cardioprotective effect of valsartan in mice with short‐term high‐salt diet by regulating cardiac aquaporin 1 and angiogenic factor expression. Cardiovasc. pathol. 2015, 24, 224–229. [DOI] [PubMed] [Google Scholar]
- 43. Escano, C. S. , Armando, I. , Wang, X. , Asico, L. D. et al., Renal dopaminergic defect in C57Bl/6J mice. Am. J. Physiol. Regul., Integr. Comp. Physiol. 2009, 297, R1660‐R1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lp, V. D. H. , Ramakers, G. M. , Smidt, M. P. , Insulin signaling in the central nervous system: learning to survive. Prog. Neurobiol. 2006, 79, 205–221. [DOI] [PubMed] [Google Scholar]
- 45. Thomas, G. M. , Huganir, R. L. , MAPK cascade signalling and synaptic plasticity. Nat. Rev. Neurosci. 2004, 5, 173–183. [DOI] [PubMed] [Google Scholar]
- 46. Vaynman, S. S. , Ying, Z. , Yin, D. , Gomezpinilla, F. , Exercise differentially regulates synaptic proteins associated to the function of BDNF. Brain Res. 2006, 1070, 124–130. [DOI] [PubMed] [Google Scholar]
- 47. Schmitt, U. , Tanimoto, N. , Seeliger, M. , Schaeffel, F. et al., Detection of behavioral alterations and learning deficits in mice lacking synaptophysin. Neuroscience 2009, 162, 234–243. [DOI] [PubMed] [Google Scholar]
- 48. Gómez‐Pinilla, F. , So, V. , Kesslak, J. P. , Spatial learning induces neurotrophin receptor and synapsin I in the hippocampus. Brain Res. 2001, 904, 13–19. [DOI] [PubMed] [Google Scholar]
- 49. Qiao, S. , Peng, R. , Yan, H. , Gao, Y. et al., Reduction of phosphorylated synapsin I (Ser‐553) leads to spatial memory impairment by attenuating GABA release after microwave exposure in Wistar rats. PloS one 2014, 9, e95503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Arancibia, S. , Lecomte, A. , Silhol, M. , Aliaga, E. et al., In vivo brain‐derived neurotrophic factor release and tyrosine kinase B receptor expression in the supraoptic nucleus after osmotic stress stimulus in rats. Neuroscience 2007, 146, 864–873. [DOI] [PubMed] [Google Scholar]
- 51. Kaneai, N. , Arai, M. , Takatsu, H. , Fukui, K. et al., Vitamin E inhibits oxidative stress‐induced denaturation of nerve terminal proteins involved in neurotransmission. J. Alzheimers Dis. Jad 2012, 28, 183–189. [DOI] [PubMed] [Google Scholar]
- 52. Xia, S. F. , Xie, Z. X. , Qiao, Y. , Li, L. R. et al., Differential effects of quercetin on hippocampus‐dependent learning and memory in mice fed with different diets related with oxidative stress. Physiol.Behav. 2015, 138, 325–331. [DOI] [PubMed] [Google Scholar]
- 53. Lara, L. S. , Mccormack, M. , Semprumprieto, L. C. , Shenouda, S. et al., AT1 receptor‐mediated augmentation of angiotensinogen, oxidative stress, and inflammation in ANG II‐salt hypertension. Am. J. Physiol. Renal Physiol. 2012, 302, F85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Youn, S. C. , Chen, L. Y. , Chiou, R. J. , Lai, T. J. et al., Comprehensive application of time‐of‐flight secondary ion mass spectrometry (TOF‐SIMS) for ionic imaging and bio‐energetic analysis of club drug‐induced cognitive deficiency. Sci. Rep. 2015, 5, 18420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bhullar, K. S. , Rupasinghe, H. P. , Polyphenols: multipotent therapeutic agents in neurodegenerative diseases. Oxid. Med. Cell. Longev. 2013, 2013, 42–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tozuka, Y. , Wada, E. , Wada, K. , Diet‐induced obesity in female mice leads to peroxidized lipid accumulations and impairment of hippocampal neurogenesis during the early life of their offspring. Faseb J. 2009, 23, 1920–1934. [DOI] [PubMed] [Google Scholar]
- 57. Kilander, L. , Nyman, H. , Boberg, M. , Hansson, L. et al., Hypertension is related to cognitive impairment: a 20‐year follow‐up of 999 men. Hypertension (Dallas, Tex.: 1979) 1998, 31, 780–786. [DOI] [PubMed] [Google Scholar]
- 58. Fiocco, A. J. , Shatenstein, B. , Ferland, G. , Payette, H. et al., Sodium intake and physical activity impact cognitive maintenance in older adults: the NuAge Study. Neurobiol. aging 2012, 33, 829 e821‐e828. [DOI] [PubMed] [Google Scholar]
- 59. Gomez‐Pinilla, F. , Nguyen, T. T. , Natural mood foods: the actions of polyphenols against psychiatric and cognitive disorders. Nutr. Neurosci. 2012, 15, 127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material


