Abstract
Background/Aim: Antrodia cinnamomea is found with polysaccharides, lipids, vitamins, fibers and ash (minerals) and is well known in Taiwan as a traditional Chinese medicine. Its biological activities have been reported to have anti-inflammatory, anti-fatigue, anti-tumor and immunomodulatory effects, but its protective effects on liver function are still unclear. Materials and Methods: We determined if Antrodia cinnamomea was hepatoprotective against carbon tetrachloride (CCl4) toxicity in Wistar rats. Six groups were used in the study: 1) control (no induction by CCl4); 2) negative control (CCl4-induction and no treatment); 3) positive control (silymarin treatment); 4) groups 4-6 were treated with CC14 and different concentrations (350 mg/kg, 1,400 mg/kg, 3,150 mg/kg) of Antrodia cinnamomea. Blood and liver samples of rats were harvested and then detected by biochemical and tissue histochemical analysis. Activity of the antioxidative enzymes glutathione peroxidase, superoxide dismutase and catalase in the liver were also monitored. Results: Only the high-dose treatment was able to decrease serum glutamic-oxaloacetic transaminase (GOT) and glutamic-pyruvic transaminase (GPT) levels and improve liver function. High and medium doses increased total liver protein and reduced hydroxyproline. It was also observed that the high dose treatment reduced lipid peroxidation. Liver sections of CC14 treated animals receiving Antrodia cinnamomea showed less fibrosis compared to the CCl4 control group. Conclusion: This finding suggested that Antrodia cinnamomea can either enhance liver recovering from CCl4 damage or attenuate CCl4 toxicity in rats.
Keywords: Antrodia cinnamomea, carbon tetrachloride, hepatotoxicity, Wistar rats, hepatic fibrosis
Patients with chronic diseases are high utilizers of health care resources and/or the health care system throughout the world. Also they frequently seek Complementary and Alternative Medicine (CAM) services. Due to CAM is diverse and personal difference, the engagement in one’s own health and positive expectations of treatment efficacy with CAM are possible present. Approximately 80% of the world’s population uses traditional medicines for primary health needs, and most of these therapies involve herbal extracts (1,2). Although scientific studies have shown the uncertainty of CAM benefits and the lack of well controlled studies involving this type of medicine, cancer patients have increasingly selected complementary and alternative medicines for treatment (3). Recently, up to 50.4% of adults and 84.6 % of females in Taiwan use CAM to treat multiple cancers (4).
Mushrooms have been used in food since ancient times and with the oldest archaeological record regarding their profound flavor and distinct taste. Mushrooms contribute various nutrients in the human diet and are used as medicines in the oriental tradition. The use of mushrooms and/or their extracts have increased and many mushroom species are diminutive pharmaceutical factories with biological effects such as anti-oxidants, antitumor, anticarcinogenic, antiviral, anti-inflammatory, prebiotic, hypoglycemic, immunomodulating, anti-microbial, anti-diabetic and antihypertensive functions (5-9).
Antrodia camphorata (Syn. Antrodia cinnamomea), a popular medical mushroom well-known in Taiwan, is a natural fungal parasite on the inner trunk cavity of the endemic species Cinnamomum kanehirae (Bull camphor tree) Hayata (Lauraceae). The host plant is a large evergreen broad-leaf tree, that only grows in the central and northern parts of Taiwan, and is distributed over broad-leaf forests on hillsides at an altitude between 200 and 2,000 m (10).
Antrodia cinnamomea (AC) extract has be found to have a complex mixture of bioactive ingredients, such as triterpenoids, steroids, polysaccharides, and phenyl and biphenyl compounds (11) and possess health benefits (antioxidant, anti-itching and hepatoprotective effects) (12,13). A previous study has demonstrated its potential use as complementary and alternative therapeutic agent for the treatment of various cancers (14). Maleimide derivative isolated from AC suppresses breast cancer cell migration and invasion (15). In addition, AC fruiting body extract has cytotoxic effects on hepatoma HepG2 and PLC/PRF/5 cells (16). AC extract combined with anti-tumor agents has also antiproliferative effects on hepatoma cells in vitro and in vivo by inhibiting multi-drug resistance (MDR) gene expressions and COX-2-dependent phospho-AKT (p-AKT) signaling (17). In addition to its anticancer properties, it is also thought to be efficacious for musculoskeletal disorders, psychiatric conditions, influenza, cold, headache, fever and other conditions. AC has attracted great attention, and the available information show that it is able to reduce chronic CCl4-induced hepatic fibrosis resulting from chronic damage to the liver in conjunction with the progressive accumulation of fibrillar extracellular matrix protein (18). The main causes of hepatic fibrosis in humans include infection by hepatitis B and C, alcohol abuse and non-alcohol steatohepatitis; and experimentally, liver cirrhosis can be induced by carbon tetrachloride (CCl4), (19) which has been used widely to induce liver injury in animal models (20). In the current study, we aimed to increase the understanding of the effects of A. cinnamomea on liver damage anddetermine if AC would reduce chronic CCl4-induced liver injury in rats.
Materials and Methods
Reagents. CCl4, olive oil and other reagents were purchased from Sigma-Aldrich Corp. (St. Louis, MO, USA). Alanine aminotransferase (ALT) kit, aspartate aminotransferase (AST) kit and albumin were purchased from Roche Cobas Mira (Roche Diagnostic Systems, Montclair, New Jersey, USA).
Preparation of AC test solution. AC and 2 ml distilled water were mixed thoroughly to provide a final solution with a concentration of 350, 1,400 and 3,150 mg/kg. AC was obtained from Chang Gung Biotechnology Corporation, Ltd. (Taipei, Taiwan).
Experimental animals. Male Wistar rats, specific pathogen-free, 6 weeks old and weighing 250-300 g, were obtained from the Animal Medicine Center, College of Medicine, National Taiwan University and were used for the chronic CCl4-induced liver injury model in all experiments. The rats were housed in plastic cages and maintained under standard conditions of controlled room temperature at 20±2˚C and relative humidity of 75±15% with a 12 h light/night cycle with free access to standard laboratory diet and water, and filtered laminar air flow controlled room in the animal facility of the Animal Medicine Center, College of Medicine, National Taiwan University, Taipei, Taiwan. The use of experimental animals in this study was conducted under the guidance of the basic standards in the care and use of laboratory animals, which has been prepared and published by the National Institutes of Health. The study protocol has been approved by the Research Ethics Committee, the Institutional Animal Care and are in agreement with the Helsinki declaration.
Experimental design and protocol. After 1 week of acclimatization, a total of sixty rats were randomly divided into six groups (each group consisted of 10 rats) with healthy rats (Only H2O, on-induction group; normal control), cirrhotic rat group with vehicle (olive oil) treatment only (CCl4 + H2O, negative control; non-treatment group), cirrhotic rat group with silymarin treatment (CCl4 + silymarin, positive treatment control), and cirrhotic rat groups with three different doses of AC treatment (experiment groups). As a non-induction group, 10 rats without CCl4 induction were fed a regular diet and double-distilled water. The other 50 rats treated with CCl4 were further divided into non-treatment group (n=10), silymarin treatment group (n=10) and three AC treatment groups (n=30). In these 50 rats, animals were fed 20% CCl4 in a 1:4 mixture with olive oil at a dose of 2 ml/kg, twice a week for 8 weeks to induce liver damage. During the 8-week induction period, negative control rats were treated only by vehicle and positive control rats were treated with silymarin. Rats in the three AC groups received AC at a low dose (350 mg/kg/day), medium dose (1400 mg/kg/day) or high dose (3150 mg/kg/day) daily for 8 weeks. The animals’ weights were monitored prior to the start of experiment and then checked 2 times per week over the 8 week period. After the last dose, blood from all of the rats was drawn and collected by SST tube via caudal artery under mild ether anesthesia. Blood was allowed to clot at room temperature and the serum was separated by centrifuging at 4000 rpm for 15 min and kept at –20˚C for further biochemical analysis. Rats were sacrificed, and the liver and spleen were dissected and used for histopathological (formalin fixed) and biochemical (frozen –80˚C) studies.
Analysis of plasma transaminase activities and albumin level in serum. Serum samples were prepared at the end of 8 weeks and were assayed for GOT, GPT activity and albumin concentration according to the manufacturer’s protocol for an estimation of liver function using a DxC 800 clinical chemistry analyzer with reagents (GOT lot number: M307050; GPT lot number: M312240; Albumin lot number: M310159) purchased from Beckman Coulter (Brea, CA, USA). These three tests were performed by the Animal Medicine Center, College of Medicine, National Taiwan University.
Assays for Superoxide Dismutase Assay (SOD), Glutathione Peroxidase (GSH-Px) and Catalase. Ten percent of homogenates of liver tissues were prepared separately in 100 mM KH2PO4 buffer containing 1 mM EDTA (pH 7.4) and centrifuged at 12,000 × g for 30 min at 4˚C. The supernatant was collected and used for the following experiments as described below.
In this method NADH (RANSOD manufactured by RANDOX Ltd. UK) was used as the substrate (21). Reaction mixture of this method contained 0.1 mL of phenazine methosulphate (186 μM), 1.2 mL of sodium pyrophosphate buffer (0.052 mM; pH 7.0), and 0.3 mL of supernatant after centrifugation (1500 × g for 10 min followed by 10000 × g for 15 min) of tissue homogenate was added to the reaction mixture. Enzyme reaction was initiated by adding 0.2 mL of NADH (780 μM) and stopped after 1 min by adding 1 mL of glacial acetic acid. Amount of chromogen formed was measured by recording color intensity at 560 nm. Results are expressed in units/mg protein.
Glutathione peroxidase activity was assayed by the method of Mohandas et al. (22). Liver was homogenized with GSHPx cold buffer (50 mM Tris-HCl containing 5 mM EDTA and 1 mM dithiothreitol (DTT), pH 7.5). GSHPx activity was measured using a GSHPx assay kit. The reaction was initiated by the mixing glutathione, glutathione reductase, NADPH with cumene (isopropylbenzene) hydroperoxide, and then detecting conversion of NADPH to NADP with a spectrophotometer at 340 nm and 25˚C for 5 min. The specific enzyme activity was measured as nanomoles of NADPH oxidized to NADP per minute per milligram protein (oxidized/min/mg protein).
CAT activities were determined according to manufacturer’s protocols of corresponding kits. Using H2O2 as a substrate (23), 0.1 mL of the supernatant was mixed with 2.5 mL of 50 mM phosphate buffer (pH 5.0) and 0.4 mL of 5.9 mM H2O2, and change in absorbance was recorded at 240 nm after one min. One unit of CAT activity was defined as an absorbance change of 0.01 units/min.
Hepatic protein, malondialdehyde (lipid peroxidation) and hydroxyproline assays. Total liver protein concentration in each sample was measured using Coomassie blue assay reagent (KENLOR Industries Inc., CA, USA) based on an absorbance at 540 nm. Bovine serum albumin was used as a standard. The amount of total protein was expressed as mg/g tissue. Lipid peroxidation was measured using the method of Ohkawa et al. (24) and 2-thiobarbituric acid. Lipid peroxidation was expressed as the amount of malondialdehyde/mg protein. Hydroxyproline determination used procedures reported previously (25). After hydrolysis, dried liver tissue was oxidized by H2O2 and colored by p-dimethylaminobenzoaldehyde (Sigma Chemical Co. St Louis, Mo., USA). Absorbance was determined at 540 nm, and the amount of hydroxyproline was expressed as μg/g tissue.
Histopathology. For liver histology, liver tissue was immediately fixed in 10% buffered formaldehyde and the blocks were incubated twice with 10% neutral buffered formalin at room temperature for 0.5 h each, 75% alcohol at room temperature for 1 h, 85% alcohol at room temperature for 1 h, 95% alcohol at room temperature for 1 h, twice; 100% alcohol at 40˚C for 1 h, twice; xylene at 40˚C for 1 h, twice; and in molten wax at 60˚C for 0.5 h and repeated 4 times. Blocks were then embedded in paraffin. Some paraffin sections were stained using Haematoxylin-Eosin stain solution (Muto Pure Chemicals, Co., Ltd., Tokyo, Japan). Other paraffin sections were stained using Sirius red stain (Direct Red 80) (Sigma Chemical Co. St Louis, Mo., USA). The stained sections were examined under a microscope for histopathological changes in liver architecture and photomicrographs taken.
Results
Analysis of GOT, GPT and Albumin in serum. Table I shows that administration of CCl4 caused an increase in glutamic-oxaloacetic transaminase (GOT) and glutamic-pyruvic transaminase (GPT) serum concentrations, that are markers of liver injury and dysfunction as compared with a non-treated control group. We also found that CCl4 was associated with low albumin levels. The high dose AC administration reduced effects of CCl4 on GOT and GPT levels but a high-dose treatment could not return serum markers to normal levels (Table I). AC treatment at a high dose inhibited effects of CC14 on albumin levels. Liver function was not significantly altered by silymarin which was considered a positive control treatment.
Table I. The Effect of Antrodia cinnamome on GOT, GPT and albumin in CCl4-treated mice.
All values are means±S.D. (n=10). ###p<0.001 compared to control group; *p<0.05; **p<0.01 compared to CCl4 + H2O group. $200 mg/kg.
SOD, Catalase and GSH-PX levels in CCl4 and AC treated rats. To determine if AC could inhibit effects of CC14 induced liver damage, we analyzed SOD, catalase and GSHPx levels. It can be seen in Table II that CCl4 reduced SOD, catalase and GSHPx activities. Generally, AC did not counteract effects of CC14 on activity of the three proteins. An exception was that the high AC dose reduced the effects of CC14 on catalase activity (Table II).
Table II. The Effect of Antrodia cinnamomea on SOD, catalase and GSH-PX activities.
All values are means±S.D. (n=10). ###p<0.001 compared to control group; *p<0.05; **p<0.01; ***p<0.001 compared to CCl4 + H2O group. $200 mg/kg.
Hepatic protein, malondialdehyde and hydroxyproline levels. A possible molecular mechanism involved in CCl4 hepatotoxicity is the disruption of the delicate oxidant/antioxidant balance, leading to liver injury via oxidative damage (24). Moreover, CCl4 is a prototypical lipid peroxidant that induces early lipid peroxidation in the liver (24). As a marker of lipid peroxidation, malondialdehyde (MDA) concentration was measured in all animal groups.
CCl4-induced liver fibrosis in rats resulted in a significant decrease in hepatic protein content (p<0.001) and accompanied by a marked elevation of malondialdehyde (p<0.001) and hydroxyproline (p<0.001) compared to the control group. Medium or high-dose treatment of Antrodia cinnamomea attenuated the decrease of hepatic protein level induced by CCl4 but silymarin did not. Antrodia cinnamomea could not lower the elevation in malondialdehyde except with a high dose treatment. Only medium or high-dose treatment of Antrodia cinnamomea could lower the increase in hepatic hydroxyproline content (Table III).
Table III. Effect of Antrodia cinnamomea on hepatic protein, Lipid Peroxidation and hydroxyproline content in CCl4-treated rats.
All values are means±S.D. (n=10). ###p<0.001 compared with control group; *p<0.05; **p<0.01; ***p<0.001 compared to CCl4+ H2O group. $200 mg/kg.
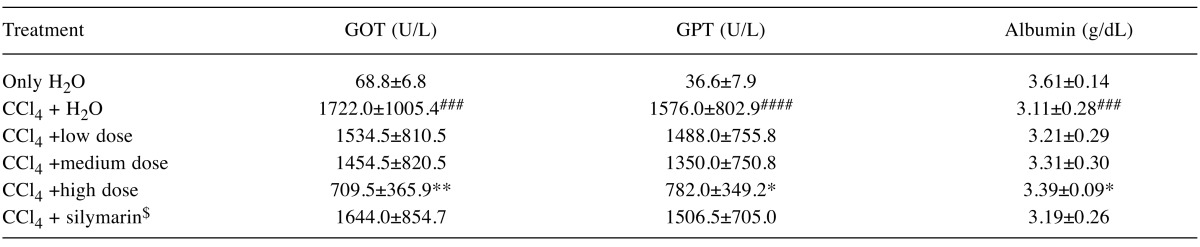
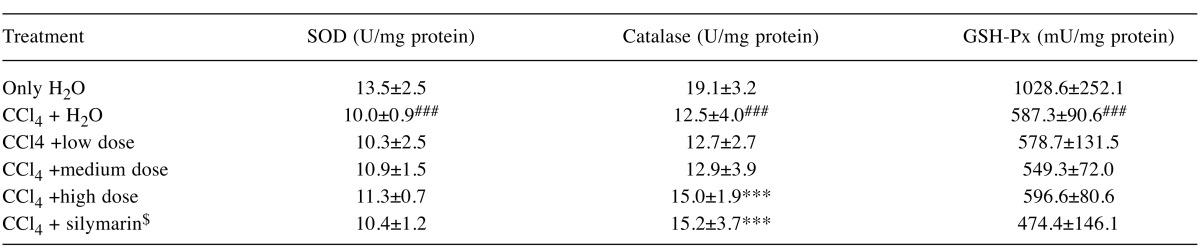
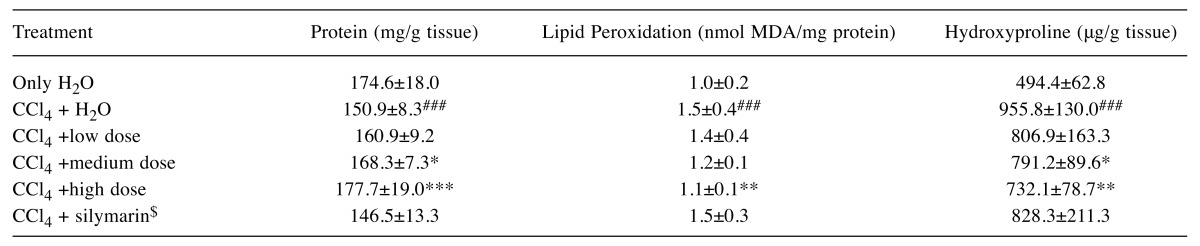
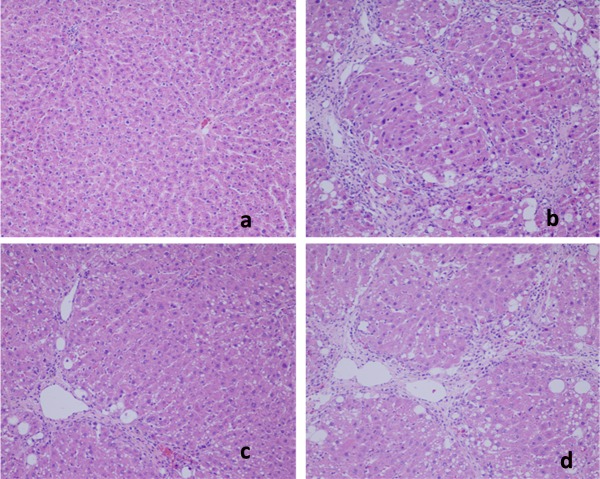
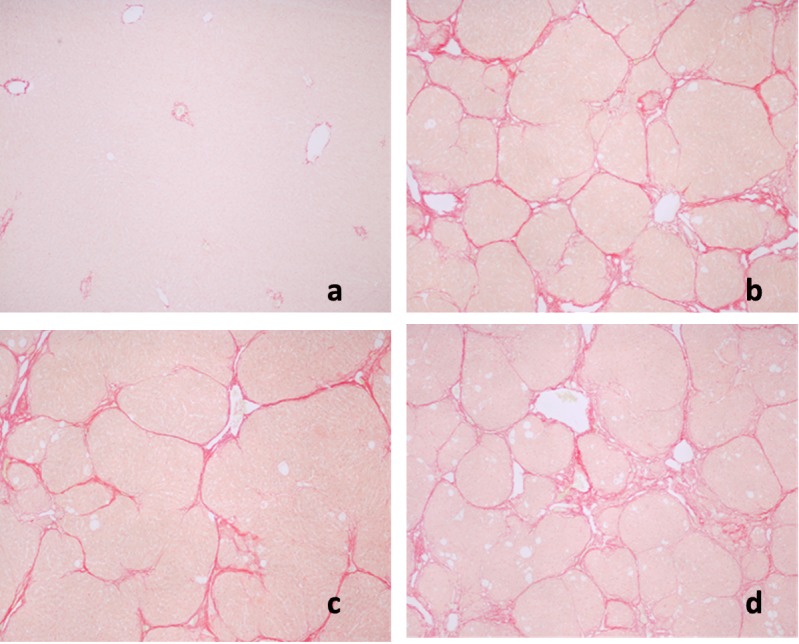
Effects of AC on liver pathology. The pathological changes of liver tissues by HE staining were observed. In the negative control group, liver tissues were radiated around the central veins, and liver cells were arranged neatly without degeneration, steatosis, cavitation, fibrosis or necrosis. Negative control tissue sections exhibited no apparent pathological changes (Figure 1a). In contrast, Figure 1b shows that in the model group, sections from CCl4-only treated rats displayed cavitations in broad areas and fatty changes like ballooning of cells, inflammatory cell infiltration, and dilation of central vein, cellular hypertrophy, necrosis, and degeneration of the lobular architecture. AC treatment attenuated CC14-induced liver pathology as seen in Figure 1c Silymarin was not effective in reducing effects of CC14 on liver injury (Figure 1d). Figure 2 shows that CCl4 induced liver lesions in rats. Sirius red stain indicated obvious nodular fibrosis (Figure 2b). AC at a high dose reduced CC14 induced-collagen accumulation in contrast to silymarin (Figure 2c and d).
Figure 1. Microphotograph of rat liver (H&E stain). (a) Representative section of liver from the control group showing normal histology. Normal hepatic cells are characterized by well-defined cell linings, prominent nucleus, and prominent central vein surrounded by reticular fibers. (b) Massive necrosis formation, hepatocytes ballooning, distortion of hepatocytes, shrinkage of nucleus, clear cell foci formation, loss of cellular boundaries, and reticular fibers were observed in CCl4-intoxicated rat liver section thus indicative of extensive liver injuries. (c) High-dose treatment of Antrodia cinnamomea partly prevented hepatoprotective activity. The histopathological changes such as necrosis, ballooning, clear cell foci formation, and structural loss of hepatic lobules were moderate recovery. (d) However, the histological architecture of liver sections of the rats treated with silymarin still showed some cavities and necrosis.
Figure 2. Sirius red staining of rat liver sections. a: Control; b: CCl4 + H2O, showing micronodular formation and complete septa interconnection with each other; c: CCl4 + high-dose treatment of Antrodia cinnamomea; d: CCl4 + silymarin.
Discussion
Medicinal plant extracts and their bioactive metabolites have been shown to induce the prevention of oxidative damages which especially from CCl4 induced hepatic injuries animals studies (26). Antrodia, one of the widely known medicinal mushrooms, belongs to the family Fomitopsidaceae which is valued in Taiwan for its medicinal effects. Yang et al. described some of his early experimental findings that the fermented culture broth of Antrodia camphorata was associated with inducing cell cycle arrest and apoptosis of human estrogen-non-responsive breast cancer (MDA-MB-231) cells and also demonstrated that non-cytotoxic concentrations (20-80 μg/ml) of Antrodia camphorata markedly inhibited the invasion/migration via inhibition of the MAPK signaling pathway (27). Antroquinonol, a ubiquinone derivative isolated from Antrodia camphorata, was capable of inducing anti-cancer activity involving G1 arrest of the cell cycle and subsequent apoptosis in human pancreatic cancers through asuppression of PI3-kinase/Akt/mTOR pathways. Moreover, antroquinonol induced the down-regulation of several cell cycle regulators and mitochondrial anti-apoptotic proteins but caused the up-regulation of p21(Waf1/Cip1) and K-ras (28). Peng et al. demonstrated that treatment with Antrodia camphorata crude extract inhibited both the superficial cancer cell line RT4 through overexpression of p21 and the metastatic cell lines (TSGH-8301 and T24) via down-regulation of Cdc2 and Cyclin B1 (29). Hseu et al. reported that Antrodia camphorata induced apoptosis and suppressed cyclooxygenase-2 (COX-2) in MDA-MB-231 cancer cells (30). Hseu et al. also demonstrated that cell-cycle arrest was related to a decrease in cyclin D1, cyclin E, CDK4 and cyclin A levels, and increased CDK inhibitor p27/KIP and p21 in Antrodia camphorata-treated MDA-MB-231 cells (31).
Our results demonstrated that treatment with CCl4 promoted a marked increase in serum GPT and GOT activities that were due to liver damage in rats. The concentrations of GPT and GOT in the pathological model group significantly rose compared with the control group, indicating that the model is successful.
High-dose treatment with Antrodia camphorata protected the liver from damage by CCl4. Albumin produced by liver was also ameliorated only with a high dose treatment which means that liver function was improved only by high-dose treatment. We may conclude that Rats treated with high dose Antrodia cinnamomea showed a protection against CCl4-induced hepatotoxicity, with the levels of both plasma GOT and GPT being reduced. CCl4 treatment caused alteration in cholesterol profile. In this present investigation, we did not analyze the concentration of cholesterol.
Antrodia camphorata did not alter CC14-induced effects on SOD and GPX, but at a high concentration inhibited the reduction in catalase. Catalase significantly rose compared with the model control group. The liver synthesizes not only the protein it needs, but also produces numerous export proteins including albumin, globulin, fibrinogen, clotting factors and regulatory proteins (32). We found that CCl4 induced liver fibrosis in rats and it appeared to damage liver function and cause a decrease in both hepatic protein and serum albumin levels. High-dose treatment of Antrodia camphorata increase protein content in the lives (Table III) and albumin content in the serum (Table I). These results provide further support that there is a hepatoprotective effect.
Both free radical production and lipid peroxidation have been reported previously as major cellular mechanisms involved in CCl4 hepatoxicity (33). Furthermore, a dependent relationship has been proposed between lipid peroxidation and fibrogenesis in rats, in which fibrosis was induced by CCl4 administration (34). We also showed that liver lipid peroxidation was associated with increased hepatic fibrogenesis. Moreover, we observed that Antrodia camphorata inhibited CCl4-induced liver lipid peroxidation. CCl4 treatment significantly increased MDA levels, whereas pre-treatment with AC eliminated CCl4-induced MDA upregulation, suggesting that AC itself and/or AC-induced gene products may have antioxidant properties against reactive oxygen species (ROS) and free radical scavenging abilities.
Increased collagen synthesis contributes to liver fibrosis (35). Hydroxyproline is the characteristic component in collagen. The amount of collagen can be predicted by detecting hydroxyproline amounts and can be used to estimate the degree of liver fibrosis (36). We found that liver fibrosis was associated with hydroxyproline levels. Antrodia camphorata at medium or high doses was able to decrease hydroxyproline levels, indicating that it could lessen the actions of hepatic fibrosis caused by CCl4. In our CCl4-induced chronic liver injury model, we observed that livers of Antrodia camphorata-treated rats displayed less pathology as compared with the CCl4-only treated group. An overriding conclusion of this study is that Antrodia camphorata has recovery/reparative effects in liver injury induced by CCl4.
In conclusion, we demonstrated that treatment with Antrodia cinnamomea suppresses CCl4-induced anorexia and hepatic injuries and also proved that Antrodia cinnamomea has the ability to recover the metabolic enzymatic activities and repair cellular injuries, thus providing scientific evidence in favor of its pharmacological use in hepatic dysfunction. We hypothesize that the hepatoprotective effect of Antrodia cinnamomea is attributed to its antioxidant role. To our knowledge, this is the first evidence suggesting that Antrodia cinnamomea protects against CCl4 induced acute hepatotoxicity. Although further investigation is needed to clarify the active component within Antrodia cinnamomea, these findings are expected to improve our understanding of the protective effect of this herbal medicine against CCl4-induced organ injury and disease.
Conflicts of Interest
The Authors declare that there is no conflict of interest to disclose.
Acknowledgements
This work was supported by grant 102-49 from Cheng Hsin General Hospital, Taipei, Taiwan.
References
- 1.Patwardhan B, Warude D, Pushpangadan P, Bhatt N. Ayurveda and traditional Chinese medicine: a comparative overview. Evid Based Complement Alternat Med. 2005;2:465–473. doi: 10.1093/ecam/neh140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsao JC, Zeltzer LK. Complementary and Alternative Medicine Approaches for Pediatric Pain: A Review of the State-of-the-science. Evid Based Complement Alternat Med. 2005;2:149–159. doi: 10.1093/ecam/neh092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li X, Yang G, Li X, Zhang Y, Yang J, Chang J, Sun X, Zhou X, Guo Y, Xu Y, Liu J, Bensoussan A. Traditional Chinese medicine in cancer care: a review of controlled clinical studies published in chinese. PLoS One. 2013;8:e60338. doi: 10.1371/journal.pone.0060338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee YW, Chen TL, Shih YR, Tsai CL, Chang CC, Liang HH, Tseng SH, Chien SC, Wang CC. Adjunctive traditional Chinese medicine therapy improves survival in patients with advanced breast cancer: a population-based study. Cancer. 2014;120:1338–1344. doi: 10.1002/cncr.28579. [DOI] [PubMed] [Google Scholar]
- 5.Barros L, Baptista P, Estevinho LM, Ferreira ICFR. Bioactive properties of the medicinal mushroom Leucopaxillus giganteus mycelium obtained in the presence of different nitrogen sources. Food Chem. 2007;105:179–186. [Google Scholar]
- 6.Kim HG, Yoon DH, Lee WH, Han SK, Shrestha B, Kim CH, Lim MH, Chang W, Lim S, Choi S, Song WO, Sung JM, Hwang KC, Kim TW. Phellinus linteus inhibits inflammatory mediators by suppressing redox-based NF-kappaB and MAPKs activation in lipopolysaccharide-induced RAW 264.7 macrophage. J Ethnopharmacol. 2007;114:307–315. doi: 10.1016/j.jep.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 7.Sarikurkcu C, Tepe B, Yamac M. Evaluation of the antioxidant activity of four edible mushrooms from the Central Anatolia, Eskisehir - Turkey: Lactarius deterrimus, Suillus collitinus, Boletus edulis, Xerocomus chrysenteron. Bioresour Technol. 2008;99:6651–6655. doi: 10.1016/j.biortech.2007.11.062. [DOI] [PubMed] [Google Scholar]
- 8.Synytsya A, Míčková K, Synytsya A, Jablonský I, Spěváček J, Erban V, Kováříková E, Čopíková J. Glucans from fruit bodies of cultivated mushrooms Pleurotus ostreatus and Pleurotus eryngii: Structure and potential prebiotic activity. Carbohydr Polym. 2009;76:548–556. [Google Scholar]
- 9.Wang Z, Luo D, Liang Z. Structure of polysaccharides from the fruiting body of Hericium erinaceus Pers. Carbohydr Polym. 2004;57:241–247. [Google Scholar]
- 10.Chang TT, Chou WN. Antrodia cinnamomea sp. nov. on Cinnamomum kanehirai in Taiwan. Mycol Res. 1995;99:756–758. [Google Scholar]
- 11.Hsieh YH, Chu FH, Wang YS, Chien SC, Chang ST, Shaw JF, Chen CY, Hsiao WW, Kuo YH, Wang SY. Antrocamphin A, an anti-inflammatory principal from the fruiting body of Taiwanofungus camphoratus , and its mechanisms. J Agric Food Chem. 2010;58:3153–3158. doi: 10.1021/jf903638p. [DOI] [PubMed] [Google Scholar]
- 12.Ao ZH, Xu ZH, Lu ZM, Xu HY, Zhang XM, Dou WF. Niuchangchih (Antrodia camphorata) and its potential in treating liver diseases. J Ethnopharmacol. 2009;121:194–212. doi: 10.1016/j.jep.2008.10.039. [DOI] [PubMed] [Google Scholar]
- 13.Lee TH, Lee CK, Tsou WL, Liu SY, Kuo MT, Wen WC. A new cytotoxic agent from solid-state fermented mycelium of Antrodia camphorata. Planta Med. 2007;73:1412–1415. doi: 10.1055/s-2007-990232. [DOI] [PubMed] [Google Scholar]
- 14.Patel S, Goyal A. Recent developments in mushrooms as anti-cancer therapeutics: a review. 3 Biotech. 2012;2:1–15. doi: 10.1007/s13205-011-0036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar KJ, Vani MG, Chueh PJ, Mau JL, Wang SY. Antrodin C inhibits epithelial-to-mesenchymal transition and metastasis of breast cancer cells via suppression of Smad2/3 and beta-catenin signaling pathways. PLoS One. 2015;10:e0117111. doi: 10.1371/journal.pone.0117111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsu YL, Kuo YC, Kuo PL, Ng LT, Kuo YH, Lin CC. Apoptotic effects of extract from Antrodia camphorata fruiting bodies in human hepatocellular carcinoma cell lines. Cancer Lett. 2005;221:77–89. doi: 10.1016/j.canlet.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 17.Chang CY, Huang ZN, Yu HH, Chang LH, Li SL, Chen YP, Lee KY, Chuu JJ. The adjuvant effects of Antrodia Camphorata extracts combined with anti-tumor agents on multidrug resistant human hepatoma cells. J Ethnopharmacol. 2008;118:387–395. doi: 10.1016/j.jep.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Friedman SL. Seminars in medicine of the Beth Israel Hospital, Boston. The cellular basis of hepatic fibrosis. Mechanisms and treatment strategies. N Engl J Med. 1993;328:1828–1835. doi: 10.1056/NEJM199306243282508. [DOI] [PubMed] [Google Scholar]
- 19.Brattin WJ, Glende EA Jr., Recknagel RO. Pathological mechanisms in carbon tetrachloride hepatotoxicity. J Free Radic Biol Med. 1985;1:27–38. doi: 10.1016/0748-5514(85)90026-1. [DOI] [PubMed] [Google Scholar]
- 20.Lee KS, Buck M, Houglum K, Chojkier M. Activation of hepatic stellate cells by TGF alpha and collagen type I is mediated by oxidative stress through c-myb expression. J Clin Invest. 1995;96:2461–2468. doi: 10.1172/JCI118304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park SK, Qi XF, Song SB, Kim DH, Teng YC, Yoon YS, Kim KY, Li JH, Jin D, Lee KJ. Electrolyzed-reduced water inhibits acute ethanol-induced hangovers in Sprague-Dawley rats. Biomed Res. 2009;30:263–269. doi: 10.2220/biomedres.30.263. [DOI] [PubMed] [Google Scholar]
- 22.Mohandas J, Marshall JJ, Duggin GG, Horvath JS, Tiller DJ. Differential distribution of glutathione and glutathione-related enzymes in rabbit kidney. Possible implications in analgesic nephropathy. Biochem Pharmacol. 1984;33:1801–1807. doi: 10.1016/0006-2952(84)90353-8. [DOI] [PubMed] [Google Scholar]
- 23.Maehly A, Chance B. Assay of catalases and peroxidases. Methods Biochem Anal. 1954;1:357–424. doi: 10.1002/9780470110171.ch14. [DOI] [PubMed] [Google Scholar]
- 24.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 25.Neuman RE, Logan MA. The determination of hydroxyproline. J Biol Chem. 1950;184:299–306. [PubMed] [Google Scholar]
- 26.Tombolini A, Cingolani M. Fatal accidental ingestion of carbon tetrachloride: a postmortem distribution study. J Forensic Sci. 1996;41:166–168. [PubMed] [Google Scholar]
- 27.Yang HL, Kuo YH, Tsai CT, Huang YT, Chen SC, Chang HW, Lin E, Lin WH, Hseu YC. Anti-metastatic activities of Antrodia camphorata against human breast cancer cells mediated through suppression of the MAPK signaling pathway. Food Chem Toxicol. 2011;49:290–298. doi: 10.1016/j.fct.2010.10.031. [DOI] [PubMed] [Google Scholar]
- 28.Yu CC, Chiang PC, Lu PH, Kuo MT, Wen WC, Chen P, Guh JH. Antroquinonol, a natural ubiquinone derivative, induces a cross talk between apoptosis, autophagy and senescence in human pancreatic carcinoma cells. J Nutr Biochem. 2012;23:900–907. doi: 10.1016/j.jnutbio.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 29.Peng CC, Chen KC, Peng RY, Chyau CC, Su CH, Hsieh-Li HM. Antrodia camphorata extract induces replicative senescence in superficial TCC, and inhibits the absolute migration capability in invasive bladder carcinoma cells. J Ethnopharmacol. 2007;109:93–103. doi: 10.1016/j.jep.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 30.Hsieh C-C, Fang H-L, Lina W-C. Inhibitory effect of Solanum nigrum on thioacetamide-induced liver fibrosis in mice. J Ethnopharmacol. 2008;119:117–121. doi: 10.1016/j.jep.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 31.Hseu YC, Chen SC, Chen HC, Liao JW, Yang HL. Antrodia camphorata inhibits proliferation of human breast cancer cells in vitro and in vivo. Food Chem Toxicol. 2008;46:2680–2688. doi: 10.1016/j.fct.2008.04.036. [DOI] [PubMed] [Google Scholar]
- 32.Chance B, Maehly AC. Assay of catalases and peroxidases. In: Methods in. 1955;Enzymology:Academic Press, pp. 764–775. [Google Scholar]
- 33.Slater TF, Sawyer BC. The stimulatory effects of carbon tetrachloride on peroxidative reactions in rat liver fractions in vitro. Inhibitory effects of free-radical scavengers and other agents. Biochem J. 1971;123:823–828. doi: 10.1042/bj1230823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Pomerai DI, Pritchard DJ, Clayton RM. Biochemical and immunological studies of lentoid formation in cultures of embryonic chick neural retina and day-old chick lens epithelium. Dev Biol. 1977;60:416–427. doi: 10.1016/0012-1606(77)90139-7. [DOI] [PubMed] [Google Scholar]
- 35.Bissell DM, Friedman SL, Maher JJ, Roll FJ. Connective tissue biology and hepatic fibrosis: report of a conference. Hepatology. 1990;11:488–498. doi: 10.1002/hep.1840110322. [DOI] [PubMed] [Google Scholar]
- 36.Buko VU, Lukivskaya OY, Naruta EE, Belonovskaya EB, Tauschel HD. Protective Effects of Norursodeoxycholic Acid Versus Ursodeoxycholic Acid on Thioacetamide-induced Rat Liver Fibrosis. J Clin Exp Hepatol. 2014;4:293–301. doi: 10.1016/j.jceh.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]