Abstract
Aim: To evaluate the utility of a novel nanocomposite biomaterial consisting of poly-L/D-lactide, and hydroxyapatite bioceramics, enriched with sodium alginate in articular cartilage defect treatment. Materials and Methods: The biomaterial was prepared using the method of solvent casting and particle leaching. The study was conducted on 20 New Zealand White rabbits. Experimental osteochondral defects were created in the femoral trochlear grooves and filled with biomaterials. In control groups, the defects were left to spontaneously heal. The quality of newly-formed tissue was evaluated on the basis of macroscopic and histological assessment. Additionally the level of osteogenic and cartilage degradation markers were measured. Results: The majority of the defects from the treatment group were covered with tissue similar in structure and colour to healthy cartilage, whereas in the control group, tissue was uneven, and not integrated into the surrounding cartilage. Conclusion: The results obtained validate the choice of biomaterial used in this study as well as the method of its application.
Keywords: Cartilage, biomaterial, rabbit, osteochondral defect, tissue regeneration, femoral trochlea, poly-L/D-lactide
One of the key directions in the development of regenerative medicine is related to studies into bone turnover and the broadly understood problem of degenerative joint lesions. Humanity has struggled with cartilage trauma for centuries. One of the problems that remain unsolved is the method of effective treatment of articular cartilage damage. Due to its unique qualities, that include the absence of vascularisation and innervation, as well as its so-called non-linearity, articular cartilage is subject to specific processes not observed in other tissues. Treatment of articular cartilage defects remains one of the greatest challenges in modern orthopaedics (1-7).
The cartilage is primarily composed of chondrocytes and the extracellular matrix they produce. The quality that distinguishes articular cartilage cells is their lack of cell junctions and their ability to exist in a low-oxygen environment (8). The space between the articular cartilage and cancellous bone of the epiphysis is occupied by subchondral bone. It consists of a large number of tightly woven collagen fibres and intracellular substance saturated with calcium and phosphorus minerals. The purpose of the subchondral bone is to transmit and distribute loads between the flexible articular cartilage and the stiff cancellous and cortical bone (9).
Recent decades have been a period of intensive development in the techniques of treatment and regeneration of damaged cartilage tissue, including preventive, pharmacological, and surgical methods, as well as tissue engineering. Despite these efforts, however, there is still no fully effective and generally accepted method of choice when attempting reconstruction of cartilage tissue defects (1,3,10-12).
Surgical treatment of cartilage defects can entail the application of reparative techniques, regenerative techniques, and palliative treatment. Reparative techniques such as subchondral bone drilling, microfracture, abrasion, spongio-lisation, and autologous matrix-induced chondrogenesis rely on the recruitment of mesenchymal bone marrow cells by interrupting the continuity of cancellous bone vessels (so-called ‘marrow stimulation techniques’) (13). The effect of stimulating the organism’s biological response is the formation of scar tissue known as fibrocartilage. These methods can be applied in the treatment of small (<2 cm2) as well as larger (up to 2.5 cm2) defects, depending on the lifestyle and expectations of the patient. The mentioned methods are among the most commonly used techniques (8).
Tissue regeneration entails the replacement of damaged cells and intracellular substance while preserving their micro-architecture and biomechanical function. Regenerative methods include: periosteal and perichondral grafts, autologous and allogenic osteochondral transplants (mosaicplasty), autologous chondrocyte implantation, autologous chondrocyte transplantation, matrix-induced autologous chondrocyte implantation, and cartilage defect treatment using cellular engineering techniques and biomaterials (10,12).
Cellular engineering is yet another area of study that can contribute to the treatment of chondral defects by combining knowledge derived from surgery, physiology, cellular and molecular biology with biotechnology and material engineering, including polymer chemistry. Its goal is to obtain biological substitutes capable of restoring, maintaining or improving tissue or organ function (14,15). The concept of tissue engineering covers three primary areas of development: the search for adequate sources of cells potentially capable of tissue regeneration, the creation of suitable scaffolds and matrices as bases for tissue growth, and influencing the proliferation and diversification of cells using adequate regulators and bioreactors. The above triad of tissue engineering was first proposed by Marx and then reiterated by Lynch in the 1990s (14,16-18). In 1994, Freed et al. proposed criteria for characterising a biomaterial ideal for the purposes of tissue engineering (19). The primary quality of biomedical materials is their biocompatibility (biotolerance), i.e. harmonious interaction with biological material. It is most commonly defined as lack of toxicity, irritative influence on tissues, and immunogenicity (20,21). The base should also provide a temporary scaffold for the newly formed tissue and be fully resorbable once the tissue develops. Moreover, the scaffold’s degradation should match the pace of tissue development (22). The purpose of such a scaffold is to create an optimum microenvironment for the migrating cells, a stable supporting structure in the early stages of new tissue formation, and to allow transfer of intercellular signals (20,23,24). Additonally, such a material should be characterised by adequate micro- and macroporosity, stability in a tissue environment, biodegradability, and proper structural integrity to withstand mechanical loads related to the joint’s physiological functions (2,21).
The goal of this study was to evaluate the applicability of a specific nanocomposite biomaterial of poly-L/D-lactide (PLDLA)/hydroxyapatite (HAp) enriched with sodium alginate in articular cartilage treatment. Studies were performed after experimentally drilling osteochondral lesions into rabbit femoral trochlea and introducing implants into the same. The process of implant fixation was monitored, as was the similarity of the newly formed tissue to healthy articular cartilage.
Materials and Methods
The study was conducted using a three-component nanocomposite biomaterial PLDLA/HAp modified superficially and internally with sodium alginate. Implant materials were prepared using an innovative method developed at the Faculty of Materials Science and Ceramics of Stanisław Staszic AGH University of Science and Technology in Cracow (25). Apart from a number of necessary tests on materials and degradation in an artificial biological environment, a rated cytotoxicity test (in accordance with ISO 10933) was performed as well as a study of material biocompatibility in in vivo conditions. Nanocomposite implants showed full biocompatibility and their toxicity class was established at zero (25). The material was obtained using the method of solvent casting and particle leaching.
5% Hydroxyapatite nanofiller supplied by Sigma-Aldrich (Sigma-Aldrich Sp. z.o.o, Poznan, Poland) and 10% PLDLA were used in the production of two optical isomers - levorotary and levo/dexorotary – combined at a molar ratio of 80:20 (Purasorb PLDL 8038®, FDA-certified; Corbion PURAC, Gorinchem, the Netherlands). The manufactured biomaterials were sterilised with radiation.
Studies were conducted on 20 New Zealand white rabbits, male, aged between 6 and 8 months, with body mass within the range from 3.5 to 4.1 kg. The animals were obtained from the Central Animal Lab of the Faculty of Medicine at the Medical University of Silesia, Poland. All rabbits were vaccinated, dewormed, and placed in separate cages in a dedicated room for a 2-month adaptation period.
Next, the animals were divided into two groups: experimental and control groups of 12 and 8 animals, respectively. The experimental group comprised rabbits that underwent biomaterial implantation. In the control group, the drilled osteochondral lesions were left without implantation and allowed to heal spontaneously. Due to the adoption of two observation periods, the groups were subsequently divided into sub-groups of six animals each for the experimental group and four animals each for the control group. The animals from the sub-groups were euthanized after 1 and 6 months from surgery, respectively.
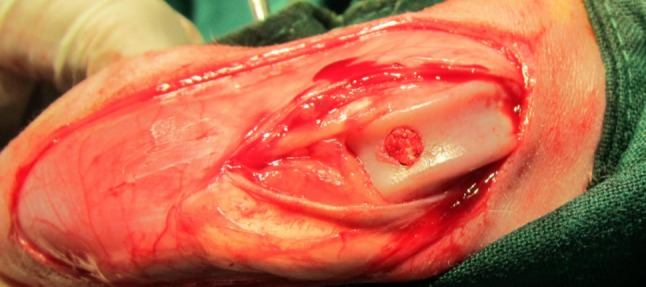
On the day of the surgery, the animals were put under general anaesthesia using a mixture of ketamine (30 mg/kg, Vetaketam; Vet-Agro, Lublin, Poland) and xylazine (5 mg/kg; Sedazin; Biowet Puławy, Puławy, Poland) injected intramuscularly. After approximately 15 minutes, an intravenous cannula (0.9×25 mm) was introduced into the marginal auricular vein. Anaesthesia was maintained intravenously using the mixture mentioned above administered adequately. Once the sterile field was prepared, lateral approach to the knee joint was performed. Arthrotomy and medial patella dislocation allowed access to the femoral trochlea. A bone bur was used to drill a cylindrical hole into the patellar sulcus, 4 mm in diameter and approximately 5 mm in depth, reaching the subchondral bone (26). The bottom border of the defect was determined on the basis of bleeding from the bone surface. Next, in six rabbits from each research sub-group, the created defect was filled with the previously described biomaterial by manually press-fitting it into place (Figure 1). An analogous procedure was conducted on the remaining animals comprising the control group but the defect was left to heal naturally, without implantation. After reducing the patella dislocation, a standard anastomosis procedure was performed on the articular capsule and the fascia lata, and then the subcutaneous tissue and skin. Once the animals recovered from anaesthesia, they were placed back in their cages. For 5 days after the surgery, antibiotics were administered subcutaneously to prevent bacterial infection (Sul-Tridin 24%, sulfadiazine with trimethoprim; Scanvet, Warszawa, Poland), dosed at 30 mg/kg. Fixed dressing was not applied to allow the animals to burden the affected limb as early as possible.
Figure 1. Intraoperative view of osteochondral defect filled with the biomaterial.
Animals from the respective study and control sub-groups were anaesthetised after 1 or 6 months of observation. Premedication was administered by intravenous injection of xylazine (Sedazin; Biowet Puławy) dosed at 5 mg/kg. Next, blood was drawn from each animal for biochemical testing. The euthanasia was conducted using sodium pentobarbital (Morbital; Biowet Puławy, Poland). During the autopsy, distal femoral epiphyses of the relevant bones were collected along with popliteal lymphatic nodes and single fragments of healthy femoral bone samples from each of the sub-groups to provide a point of reference for subsequent histological tests and material studies.
The conditions of the experiment were approved by the Local Ethics Committee No. II in Lublin (permit no. 19/2011 to conduct experiments on animals).
The qualities of the tissue formed at the locations of the defects were analysed using macroscopic assessment of the defect location, histological assessment and biochemical blood tests.
Macroscopic assessment. The range and potential deformations of the operated knee joints was assessed intravitally. The femoral trochleas collected during the autopsies were photographed and evaluated in terms of the potential presence of degenerative changes, adhesion to surrounding tissues, and synovitis in the knee joint capsule. The tissue formed at the location of the drilled hole was assessed in terms of surface colour, surface structure (smooth/rough), and integrity at the cartilage-implant phase border.
Histological assessment. Femoral trochleas were placed in 4% buffered formalin. Next, they were decalcified with a 20% solution of ethylenediaminetetra-acetic acid and submerged in paraffin. Each trochlea was cut laterally so that the section plane passed through the centre of the drilled hole. The specimens were then cut into sections of 5 μm in diameter. The specimens were stained with haematoxylin and eosin as well as toluidine blue. Analogous procedures were performed on specimens of healthy rabbit cartilage to provide a point of reference for the histological assessment.
Histological specimens were prepared at the Department of Histopathology of the Orthopaedics Clinic at the Medial University in Lublin. The histopathological assessment of the specimens was performed at the Medical University in Lublin. The specimens were evaluated according to the modified histological cartilage assessment scale proposed by O’Driscoll et al. (27) and (28): the scale ranges from 1 to 26, the higher number, the better the quality of the repaired tissue.
Biochemical blood serum tests. The following osteogenic markers were analysed using commercially available ELISA sets according to the protocol provided by the producer: N-terminal propeptide of type I collagen (Rat/Mouse PINP EIA: sensitivity=0.7 ng/ml; assay range=0.7-75 ng/ml), osteocalcin (N-MID osteocalcin ELISA: sensitivity=0.5 ng/ml; assay range=0-100 ng/ml) bone alkaline phosphatase (Ostase BAP EIA, sensitivity=0.7 μg/l; assay range=7-90 μg/l) (all from Immunodiagnostic Systems Holdings PLC, London, UK) and cartilage degradation marker C-terminal telopeptide of collagen type II [ELISA kit for cross-linked C-telopeptide of type II collagen (CTX-II), assay range=123.5-10,000 pg/ml; USCN Life Science Inc., USCN Business Co., Ltd., Wuhan, Hubei, P.R. China). Blood was drawn from all study and control group animals during each study period to obtain blood serum for immuno-enzymatic essays, and popliteal lymphatic nodes were collected for histopathological analysis.
As the point of reference for the obtained biochemical results blood was also drawn from four healthy rabbits before subjecting to any surgical procedures.
Statistical analysis. Basic statistical measures were calculated for assayed parameters, including mean values, standard deviation (SD), as well as minimum and maximum values, by way of characterising the samples. Normal distribution of the numerical data was verified by employing the Shapiro–Wilk test; as the above test confirmed normal distribution (Gauss curve) of the obtained values, the results were compared using Student’s t-test for data of equal variance, and the Cochran–Cox test for data with unequal variance. A 5% risk of error was assumed, which meant that a given hypothesis would be refuted for p>0.05, thus confirming the validity of the alternative hypothesis. Calculations were performed with the Statistica 10.0 statistical software package (StatSoft Polska Sp.z o. o., Cracow, Poland).
Results
Macroscopic assessment. Knee joints of operated rabbits from the study group showed physiological range of mobility. Implant migration was not observed in any of the experimental cases. In one rabbit of the control sub-group at 1 month after the surgery and in two control group animals 6 months after surgery reduced range of motion of the stifle joint was observed, accompanied by increased levels of synovial fluid and palpable deformations of the operated knee joints. No animal was diagnosed with lameness or tendon contracture in the operated limbs. After incising the articular capsule, no signs of adhesion or synovitis were observed in any of the cases. In four cases of the experimental group, the macroscopic assessment after the first month revealed clearly visible implant material covered with yellowish, glossy tissue of uneven surface, clearly separate from the surrounding cartilage. The edges of the defect were also rounded and reduced. In another study case, after 1 month the implant surface was completely overgrown with cartilage-like, smooth, yellowish tissue tightly adhering to the healthy cartilage. In all animals from the control group, whitish, uneven tissue was observed at the site of the defect, completely filling the hole and expanding beyond the surrounding articular cartilage. In two cases of the control group, bone erosion was observed at the trochlea crest.
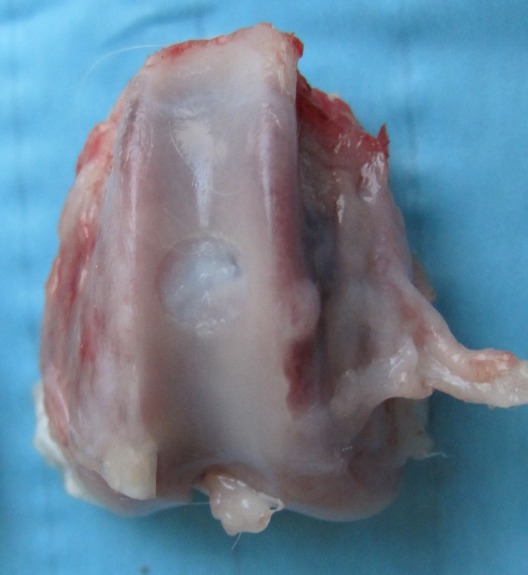
In four cases of the experimental group, after 6 months of observation, the implant was found to be completely covered with cartilage-like, whitish, glossy tissue which was well integrated into the surrounding cartilage and was of similar colour and surface characteristics (Figure 2). In two experimental cases after 6 months of observation, the area not covered with newly formed tissue corresponded to approximately 1/5 of the drilled hole and was located in its centre. In three study cases, erosion and unevenness was observed at the trochlea crest.
Figure 2. Macroscopic postmortem view of trochlea 6 months after implantation.
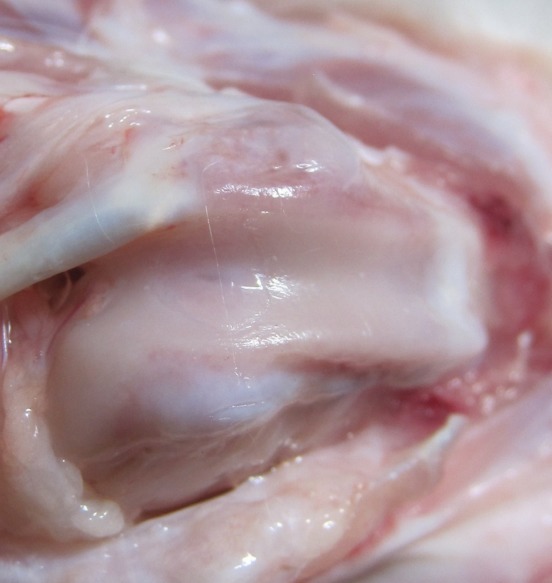
In control group cases, after 6 months of observation numerous spots of trochlea crest erosion were observed. In two control cases, the drilled hole was completely filled with whitish, uneven tissue which was clearly delimited and different from healthy cartilage (Figure 3); in one control case, the defect was filled with glossy, smooth tissue similar in surface structure and colour to the surrounding cartilage. In the last control case, deformation of the femoral trochlea was also observed as well as osteochondral exostoses on both sides of the trochlea; the defect site was filled with uneven, granulation tissue.
Figure 3. Control group defect after 6 months of observation. The defect can be seen to be filled with newly formed tissue.
Histological assessment. The scale proposed by O’Driscoll et al. was used for the spot histological analysis of selected parameters in the newly-formed tissue: surface regularity, matrix composition, thickness of neo-formed cartilage, bonding to adjacent cartilage, chondrocyte distribution, cell population viability, subchondral bone composition, type-II collagen staining of the matrix, degenerative changes in adjacent cartilage, presence of inflammation (27,28). The mean results of the histological assessment are presented in Table I. Significantly higher values in experimental sub-groups after 1 and 6 months of observation compared to control groups demonstrate the better quality of the newly- formed tissue on the basis of biomaterials.
Table I. Mean results of the histological assessment on the scale of O’Driscoll et al. (27) and (28). Higher values represent better outcome.
After 1 month of observation in the experimetal sub-group, the defect was mostly filled with fibrous tissue with individual, scattered chondrocytes. Only in two experimental cases was fibrocartilage present. In all cases, the surface was irregular with the presence of deep fissures. In the corresponding control sub-group, the defect was mostly filled with fibrocartilage-like tissue, closely adhering to the edge of the defect. In three control rabbits, tissue with irregular surface was observed, reaching beyond the level of the adjacent cartilage. Reconstruction of cartilage tissue continuity was not observed in any of the control cases after 1 month, although there were instances of scattered chondrocytes in mixed column-islet arrangement.
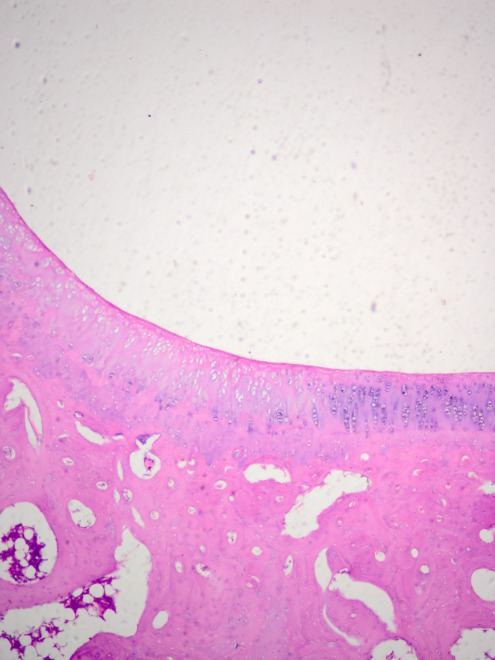
After 6 months, in five animals of the experimental sub-group, the defect location was covered with a smooth and continuous surface of newly formed cartilage, fully integrated into the adjacent tissue (Figure 4). The matrix of the reconstructed tissue was described as hyaline, monochromatically coloured, with a thickness nearly identical to that of the surrounding cartilage. Staining with toluidine blue revealed an even distribution of the stain, which evidenced comparable glycosaminoglycan content in the newly formed tissue compared to the healthy cartilage.
Figure 4. Smooth and continuous surface of cartilage-like tissue covering the defect in a rabbit of the experimental group 6 months after implantation. Haematoxylin-eosin staining, original magnification: ×200.
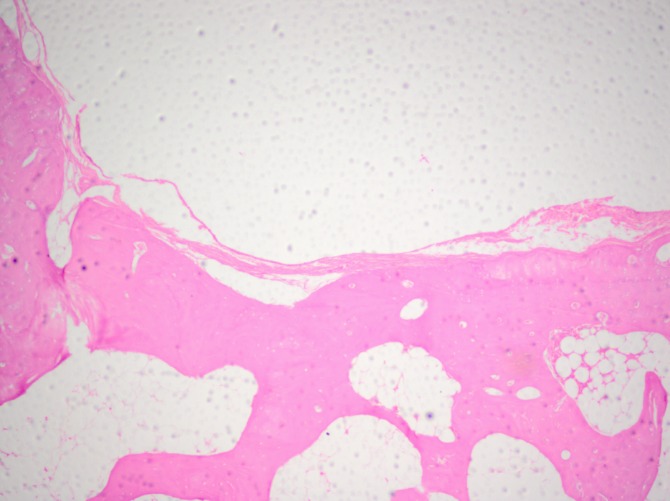
The distribution of the same feature in the control group after 6 months was chaotic and uneven. In one case, reconstruction of cartilage-like tissue with a thickness similar to that in the surrounding cartilage was observed, whereas in another case, total absence of cartilage and subchondral bone was noted. In most controls, the surface of the reconstructed tissue was irregular and fissured (Figure 5). After 6 months, in the experimental sub-group, type II collagen was detected in all specimens, whereas in the control group it was present in only in half of the cases.
Figure 5. Surface of reconstructed tissue in a rabbit of the control subgroup after 6 months of observation. Haematoxylin-eosin staining, original magnification: ×200.
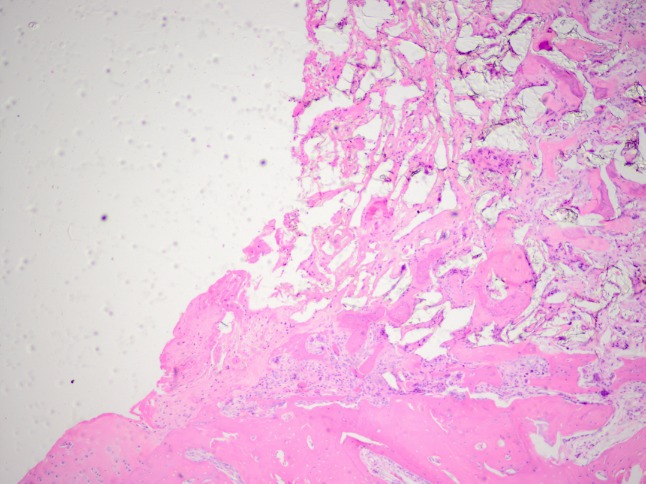
A positive correlation between the degree of biomaterial integration with the surrounding tissue and duration of the experiment was observed. After 1 month of observation, none of the 6 study subgroup rabbits showed full bonding with defect walls (Figure 6 ). Indeed, in two animals a complete lack of border integration was observed. However, after 6 months, full integration of the two phases was observed in all 6 study subgroup animals. A considerably more positive assessment of the newly formed tissue’s integration with its surroundings was reported for the control groups where implantation was not used. Significant integration of the edges was visible already after the 1st month of observation.
Figure 6. Cross section of osteochondral defect 1 month after implantation of the test material in a rabbit of the experimental group, showing the border between the scaffold and walls of the defect. Haematoxylin-eosin staining, original magnification: ×200.
Histopathological examination confirmed the presence of numerous clusters of newly formed subchondral bone tissue in all study and control cases . Scattered islets of bone tissue were visible in the whole cross-section of the defect. Bone-forming trabecules were separated by wide lacunas filled with osteocytes, adipose tissue, and fibrovascular tissue. In rabbits selected for 1 month observation, a centrally located fibrous tissue core was visible. The density of the reconstructed subchondral bone islets was significantly higher in the experimental when compared to control subgroup animals. Only in one case, in a control subgroup rabbit euthanised after 6 months, was complete absence of new bone in the defect location observed. When comparing the mean histological assessment scores of specimens studied in our own research (Table I) it should be noted that values increased with time in the study subgroups - from 11.67 after the first month to 24.83 after 6 months. The maximum score possible on the scale used is 26 points (27,28). The obtained results are positive and evidence of progression of cartilage reparation stimulated by the presence of the implant and of the good quality of the newly formed tissue. Meanwhile, in the control subgroups the mean scores remained stable and the highest value observed after 6 months was 18.50.
Biochemical blood serum tests. In the presented study, BAP, CTX-II, osteocalcin, and PINP concentrations were measured. The study of the respective biomarker levels relevant to bone turnover and reconstruction cartilage provided information on the advancement of changes affecting the skeletal system (29,30). The elevated concentrations of particular markers reflected an increased resorption within bone tissue and its intensive synthesis. In comparison of BAP levels (Table II), at 1 month of observation, the value for the experimental group was significantly higher than that in the control group and exceeded that in healthy animals almost eightfold. After 6 months the mean value in the experimental subgroup was lower and comparable to that in the control and healthy animals.
Table II. Comparison of bone alkaline phosphatase (BAP) and osteocalcin levels between the experimental and the control groups.
The osteocalcin (OC) concentration measured after six months of observation remained at a similar level with no significant differences (between 4.642 ng/ml and 5.820 ng/ml) in the study and control subgroups alike. Whereas the highest and statistically significant increase – nearly two-fold –concentration of OC was observed after the first month in rabbits subjected to implantation, conversely to the observations made in the control group (Table II).
No statistically significant discrepancies in PINP and CTX-II concentrations were observed between the two groups were found at either of the time points.
Discussion
Experimental animal models provide the foundation for the assessment of the effectiveness and safety of new material solutions and the progression of disease processes (31). Rabbits are commonly used in experiments pertaining to articular cartilage healing due to their small size, easy upkeep and relatively low cost (32). Their femoral condyles and trochlea are large enough to easily accommodate osteochondral defects 3-4 mm in diameter (33). So far, based on the analysed literature, experimental reconstructions of osteochondral defects of femoral trochlea in rabbits have been performed with materials such as e.g. (4,5,8,21,34,35): porous hydroxyapatite with collagen at the molar ratio of 80/20, polyactide/polyglycolide copolymer (PLGA) modified with collagen and HAp, hyaluronate superficially coated with fibronectin, calcium phosphate covered with hyaluronate sponge, dacron (polyethylene terephthalate PET), PGA/PLA implants impregnated with demineralised bone matrix or TGF-β. In none of the studies was the resulting tissue identical in terms of characteristics to healthy hyaline cartilage (35).
Nowadays in tissue engineering, biomimetic biomaterials seem to show the most promise, i.e. composites comprising of natural and synthetic components, which allows them to provide the benefits of both (36). In the context of articular cartilage, a particular importance is attributed to chondroinductive and chondroconductive qualities, which allow the material to not only serve as a passive scaffold for the newly formed tissue, but also to actively stimulate adhesion, migration, proliferation, and differentiation of cells to the desired phenotypes (2). Moreover, a significant role is played by proper integration of the biomaterial with the host tissue. The interaction taking place at the point of contact between the implant and healthy tissue is the key to securing stability and functional integrity until the biomaterial is overgrown with the tissue itself (11). The biomaterial discussed here is an innovative nanocomposite combining the benefits of artificial polylactide and natural alginate and nanohydroxyapatite.
In our own research, the implant was manufactured using a bioresorbable lactic acid polymer called poly(L/LD)lactide 80/20 – Purasorb® PLDL 8038 – a copolymer of two optical isomers combined at the molar ratio of 80:20. Research conducted at the AGH University of Science and Technology in Cracow confirms that three-dimensional porous lactic-acid structures at ratio of 80:20 are characterised by better characteristics than e.g. 70:30 mixtures (25,37). It is believed that such isomers content allows optimal surface interaction with tissues, which translates into structural protein synthesis, osteoblast settlement and activity (38). On the other hand, Mueller et al. compared two types of polyactide membranes in the treatment of skull defects in rabbits, using L/DL 70:30 and L/DL 80:20 copolymers. The study revealed no significant differences in the speed and quality of healing processes (37).
The usability of sodium alginate as a homogeneous scaffold is limited primarily by its low mechanical strength and quick degeneration at the site of implantation (39-41). Therefore, it is most commonly used in combination with other biomaterials or additionally processed to improve its in vivo and in vitro stability (40,41). In our own research, pressurised soluble sodium alginate was introduced onto the ready PLA-HAp matrix. This allowed stable settlement of alginate molecules both superficially and inside the porous sponge. The form of sodium salt allowed the post-implantation formation of cross-bonds with calcium present in the tissue and alginate transfer to the gel form, which facilitated stable anchoring of the material on the defect walls.
Nanocomposites are considered to show particular promise in terms of reconstructions of osteochondral defects (4,27,34,42,43). They include composites wherein at least one component is nanometric, i.e. the size of its crystals does not exceed 200 nm. Nanomolecules modify the base material at a molecular level, which creates new possibilities for the applications of such composites (44). In our own study a nanohydroxyapatite was used as a nanometric component of the tested composite. The most important quality from the perspective of medical applications is its bioactivity, which is necessary for the facilitation of bone tissue regeneration (44). Hydroxyapatite is also an osteoinductive and osteoconductive substance with a unique ability to form direct bonds with bone tissue (36). Some authors believe that the bioactivity of polymer grafts modified with bioactive nanomollecules is low in the initial phase after application but increases in time due to its biodegradation in the tissular environment (44). This has been confirmed in our research where we observed a progression of the osteochondral defect reconstruction rate in the study subgroups relative to the time of observation. Numerous publications corroborate positive results in experiments conducted with the use of nanocomposites (4,27,34,45,46). In two studies conducted on rabbit and rat models, the authors observed more advanced reconstruction of osteochondral defects when using PLGA polymer modified with nano-HAp cells, compared to implants without such modification (27,34). Some researchers suggest that the development of osteons in the graft area is possible when the size of pores is approximately 200 μm (36). In the biomaterial tested in our study, the size of pores was between 200 and 300 μm, i.e. it was within the accepted range. On the other hand, it is noteworthy that increase in the overall porosity of the implant, i.e. the share of pores in the total volume, lowers the material’s mechanical strength. This parameter must be considered when planning the selection of porous HAp material for a given medical application. Literature provides discrepant information with regard to the optimum implant porosity (2,21,31,34,36,47,48).
In our own research a positive assessment of the newly formed tissue’s integration with its surroundings was reported for the control group where implantation was not used. This is inconsistent with results obtained by other authors studying the characteristics of osteochondral defects in rabbits without the use of biomaterials. In most animals operated on they reported absence of integration at the level of cartilage with the surrounding healthy tissue, although the longest observation period in these studies was 12 weeks (49). Histological assessment scores obtained in our own research evidence the poorer characteristics of the fibrocartilage spontaneously filling the defect when compared to tissue formed on implant scaffolds. Similar conclusions were reached by authors studying the fixation of similar biomaterials in osteochondral defects in rats and rabbits when compared to control groups without implantation (4,27). In another study of osteochondral defects in rabbits, in groups where defects were left to heal spontaneously, the histopathological and macroscopic assessment of the formed tissue was also unsatisfactory, despite additional oral supplementation with glucosamine sulphate and chondroitin preparations (50). Gradual changes in BAP concentrations observed in the conducted study are consistent with the results reported by Kim et al. who studied the behaviour of osteoblasts on porous bases of PLGA copolymer modified with nanohydroxyapatite. The highest increase in BAP concentrations in that study was also observed in the first 4 weeks, after which it gradually decreased over a 2-month period (51). PINP is a protein marker of osteogenesis. In the presented study, slight discrepancies in PINP concentration levels were observed between the study and control subgroups. Statistically significant differences were not observed, however, which might be a reflection of the uniformity of osteogenic processes. Osteocalcin (OC) is a basic noncollagenous protein of the bone matrix synthesised by osteoblasts, odontoblasts and hypertrophic chondrocytes, making it a protein specific to bone tissue and dentine. The results collected in our research reflect the intensive metabolic bone activity in the 1 month observation period. Similar observations were obtained by Stafford et al. by measuring osteocalcin production by chondrocytes and osteoblasts in a rabbit experimental study (52). Progressive decay of articular cartilage is one of the main degenerative changes observed in the course of osteoarthritis. Given the fact that the aim of the presented research was not to induce osteoarthritis but to study biomaterial implantation, the obtained constant CTX-II values in all study and control animals seem positive and evidence the absence of severe inflammation and osteoarthritis. This observation is consistent with results reported by other authors (29,53).
Some reports include the comment that recruitment of multipotent cells of cancellous bone marrow and the osteoconductive qualities of the porous implant stimulate the reconstruction of trabeculous bone structure (31,36,47,48). Simultaneously, factors produced by active bone tissue cells have a paracrine effect on chondroblasts (4). The above findings from literature formed the basis for the choice of biomaterial used in this study as well as the method of its application. The premise in the present research was that the presence of healthy cartilage at the edge of the defect would have a paracrine effect on non-diversified blood marrow cells stimulating chondrogenesis. The results obtained in our study were promising and confirm the possibility of reconstructing chondral tissue in situ on a scaffold made of the biomaterial used and using the patient’s own bone marrow cells.
References
- 1.Cai X, Lin Y, Ou G, Luo E, Man Y, Yuan Q, Gong P. Ectopic osteogenesis and chondrogenesis of bone marrow stromal stem cells in alginate system. Cell Biol Int. 2007;31:776–783. doi: 10.1016/j.cellbi.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 2.Danisovic L, Varga I, Zamborsky R, Bohmer D. The tissue engineering of articular cartilage: cells, scaffolds and stimulating factors. Exp Biol Med. 2011;237:10–17. doi: 10.1258/ebm.2011.011229. [DOI] [PubMed] [Google Scholar]
- 3.Haasper C, Zeichen J, Meister R, Krettek C, Jagodzinski M. Tissue engineering of osteochondral constructs in vitro using bioreactors. Injury. 2008;39:66–76. doi: 10.1016/j.injury.2008.01.037. [DOI] [PubMed] [Google Scholar]
- 4.Maehara H, Sotome S, Yoshii T, Torigoe I, Kawasaki Y, Sugata Y, Yuasa M, Hirano M, Mochizuki N, Kikuchi M, Shinomiya K, Okawa A. Repair of large osteochondral defects in rabbits using porous hydroxyapatite/collagen (HAP/COL) and fibroblast growth factor-2 (FGF-2) J Orthop Res. 2010;28:677–686. doi: 10.1002/jor.21032. [DOI] [PubMed] [Google Scholar]
- 5.Mano JF, Reis RL. Osteochondral defects: present situation and tissue engineering approaches. J Tissue Eng Regen Med. 2007;1:261–273. doi: 10.1002/term.37. [DOI] [PubMed] [Google Scholar]
- 6.Mollenhauer JA. Perspectives on articular cartilage biology and osteoarthritis. Injury. 2008;39S1:5–12. doi: 10.1016/j.injury.2008.01.044. [DOI] [PubMed] [Google Scholar]
- 7.Seung HH, Yun HK. Histological and biochemical properties of regenerated articular cartilage using chondrogenic bone marrow stromal cells with a PLGA scaffold in vivo. J Biomed Mater Res. 2008;87:850–861. doi: 10.1002/jbm.a.31828. [DOI] [PubMed] [Google Scholar]
- 8.Bhosale AM, Richardson JR. Articular cartilage: structure, injuries and review of management. Br Med Bull. 2008;87:77–95. doi: 10.1093/bmb/ldn025. [DOI] [PubMed] [Google Scholar]
- 9.Ding M, Dalstra M, Linde F, Hvid I. Mechanical properties of the normal human tibial cartilage-bone complex in relation to age. Clin Biomech. 1998;13:351–358. doi: 10.1016/s0268-0033(98)00067-9. [DOI] [PubMed] [Google Scholar]
- 10.Swieszkowski W, Ho Saey Tuan B, Kurzydlowski KJ, Hutmacher DW. Repair and regeneration of osteochondral defects in the articular joint. Biomol Eng. 2007;24:489–495. doi: 10.1016/j.bioeng.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 11.Ge Z, Li C, Heng BC, Cao G, Yang Z. Functional biomaterials for cartilage regeneration. J Biomed Mater Res. 2012;100A:2526–2536. doi: 10.1002/jbm.a.34147. [DOI] [PubMed] [Google Scholar]
- 12.Kalson NS, Gikas PD, Briggs TWR. Current strategies for knee cartilage repair. Int J Clin Pract. 2010;64:1444–1452. doi: 10.1111/j.1742-1241.2010.02420.x. [DOI] [PubMed] [Google Scholar]
- 13.Steinwachs MR, Guggi TH, Kreuz PC. Marrow stimulation techniques. Injury. 2008;39S1:26–31. doi: 10.1016/j.injury.2008.01.042. [DOI] [PubMed] [Google Scholar]
- 14.Pochwalski M, Urbanowska E, Wójtowicz A. Tissue engineering. Aplication in dentistry. Platelet rich plasma. Part I. Nowa Stomatol. 2000;1-2:17–22. [Google Scholar]
- 15.Widuchowski W, Tomaszewski W, Widuchowski J, Czamara A. Contemporary possibilities of treatment of cartilage lesions with special regard to the knee joint. Ortop Traumatol Rehabil. 2011;13:327–341. doi: 10.5604/15093492.955721. [DOI] [PubMed] [Google Scholar]
- 16.Chiang H, Jiang CC. Repair of articular cartilage defects: review and perspectives. J Formos Med Assoc. 2009;108:87–101. doi: 10.1016/S0929-6646(09)60039-5. [DOI] [PubMed] [Google Scholar]
- 17.Marx RE. Clinical applications of bone biology to mandibular and maxillary reconstruction. Clin Plast Surg. 1994;21:377–392. [PubMed] [Google Scholar]
- 18.Lynch SE, Marx RE, Nevins M, Wisner-Lynch LA. Tissue Engineering: Aplications in maxillofacial surgery and periodontitis. Quintessence Pub. Co. Inc. 2007;2:3–47. [Google Scholar]
- 19.Freed LE, Vunjak-Novakovic G, Biron RJ, Eagles DB, Lesnoy DC, Barlow SK, Langer R. Biodegradable polymer scaffolds for tissue engineering. Biotechnology. 1994;12:689–693. doi: 10.1038/nbt0794-689. [DOI] [PubMed] [Google Scholar]
- 20.Nowacki J, Dobrzański LA, Gustavo F. Intramedullary implants for osteosynthesis. Open Access Library. 2012;11:2–63. [Google Scholar]
- 21.Trzeciak T, Richter M. Biomaterials in articular cartilage lesions repair. Chir Narządów Ruchu Ortop Pol. 2008;73:107–111. [PubMed] [Google Scholar]
- 22.Nowacka M. Biomaterials for tissue engineering and regenerative Medicine. Chemical News. 2012;66:909–933. [Google Scholar]
- 23.Barone DTJ, Raquez JM, Dubois P. Bone-guided regeneration: from inert biomaterials to bioactive polymer (nano)composites. Polymer Adv Technol. 2011;22:463–475. [Google Scholar]
- 24.Kaźnica A, Joachimik R, Drewa T, Drewa G. Application of tissue engineering in selected branches of regenerative medicine. Uniwersytet Mikołaja Kopernika w Toruniu. Collegium Medicum im. Ludwika Rydygiera w Bydgoszczy, ISSN 1734-591X, Medical and Biological Sciences. 2007;XXI/4:9–15, Bydgoszcz, Poland. [Google Scholar]
- 25.Żylińska B, Stodolak-Zych E, Nowicka K, Silmanowicz P. Evaluation of repair of osteochondral defects in rabbits using new composite biomaterials during three-month observation. Engineering of Biomaterials. 2012;116-117:118–119. [Google Scholar]
- 26.PN-EN ISO 10993-6. Biological evaluation of medical devices. Tests for local effects after implantation. Polish Committee for Standardization, Warsaw, Poland. 2009; : – . [Google Scholar]
- 27.O’Driscoll SW, Keeley FW, Salter RB. Durability of regenerated articular cartilage produced by free autogenous periosteal grafts in major full-thickness defects in joint surfaces under the influence of continuous passive motion. A follow-up report at one year. J Bone Joint Surg Am. 1988;70:595–606. [PubMed] [Google Scholar]
- 28.Xue D, Zheng Q, Zong C, Li Q, Qian S, Zhang B, Yu L, Pan Z. Osteochondral repair using porous poly(lactide-co-glycolide)/nano-hydroxyapatite hybrid scaffolds with undifferentiated mesenchymal stem cells in a rat model. J Biomedl Mater Res A. 2010;94:259–270. doi: 10.1002/jbm.a.32691. [DOI] [PubMed] [Google Scholar]
- 29.Duclos ME, Roualdes O, Cararo R, Rousseau JC, Roger T, Hartmann DJ. Significance of the serum CTX-II level in an osteoarthritis animal model: a 5-month longitudinal study. Osteoarthritis Cartilage. 2010;18:1467–1476. doi: 10.1016/j.joca.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 30.Matuszewska A. Bone turnover markers. Przegl Reumatatol. 2006;3:5–7. [Google Scholar]
- 31.Archer C, Ralphs J. Cartilage repair and regeneration. In: Regenerative medicine and biomaterials for the repair of connective tissues, First Edition. Woodhead Publishing, Cambridge, UK. 2010;1:83–344. [Google Scholar]
- 32.Wessely-Szponder J, Bobowiec R, Szponder T. The influence of porcine prophenin on neutrophils isolated from rabbit blood during implantation of calcium sulphate graft material into bone tissue. World Rabbit Sci. 2012;20:163–172. [Google Scholar]
- 33.Chu CR, Szczodry M, Bruno S. Animal Models for Cartilage Regeneration and Repair. Tissue Eng. 2010;16:105–115. doi: 10.1089/ten.teb.2009.0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pamuła E, Orzelski M, Malisz P, Rumian Ł, Menaszek E, Nowak B, Dobrzyński P, Silmanowicz P. Resorbable scaffolds modified with collage type I or hydroxyapatite: in vivo studies on rabbits. Eng Biomat. 2012;17:115–117. [Google Scholar]
- 35.Tyyni A, Karlsson J. Biological treatment of joint cartilage damage. Review article. Scand J Med Sci Sports. 2008;10:249–265. doi: 10.1034/j.1600-0838.2000.010005249.x. [DOI] [PubMed] [Google Scholar]
- 36.Błażewicz S, Stoch L. Biomaterials, vol. 4. Polish Academy of Sciences, 2003;Warszawa:Poland, 97–209. [Google Scholar]
- 37.Mueller AA, Rahn BA, Gogolewski S, Leiggener CS. Early dural reaction to polylactide in cranial defects of rabbits. Pediatr Neurosurg. 2005;41:285–291. doi: 10.1159/000088730. [DOI] [PubMed] [Google Scholar]
- 38.Gugala Z, Gogolewski S. Protein adsorption, attachment, growth and activity of primary rat osteoblasts on polylactide membrans with defined surface characteristics. Biomaterials. 2004;25:2341–2351. doi: 10.1016/j.biomaterials.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 39.Reinholz GG, Lu L, Saris DBF, Yaszemski MJ, O’Driscoll SW. Animal models for cartilage reconstruction. Biomaterials. 2004;25:1511–1521. doi: 10.1016/s0142-9612(03)00498-8. [DOI] [PubMed] [Google Scholar]
- 40.Stodolak E, Błażewicz M, Boguń M. Alginate fibers based compositesfor medical applications. Acta Bioch Pol 55. 2008;S4:79–83. [Google Scholar]
- 41.Lis A, Szarek D, Laska J. Biomaterials engineering strategies for spinal cord regeneration: state of the art. Polim Med. 2013;43:59–80. [PubMed] [Google Scholar]
- 42.Stoop R. Smart biomaterials for tissue engineering of cartilage. Injury. 2008;39:577–587. doi: 10.1016/j.injury.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 43.Tamai N, Myoui A, Hirao M, Kaito T, Ochi T, Tanaka J, Takaoka K, Yoshikawa H. A New biotechnology for articular cartilage rep air: subchondral implantation of a composite of interconnected porous hydroxyapatite, synthetic polymer (PLA-PEG), and bone morphogenetic protein-2 (rhBMP-2) Osteoarthritis Cartilage. 2005;13:405–417. doi: 10.1016/j.joca.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 44.Sołtysiak E, Długoń E, Dulnik J, Błażewicz M. Surface modification of polymer nanocomposites by electrophoretic deposition. Diag Mat Polim. 2011;6:511–514. [Google Scholar]
- 45.Chris Arts JJ, Verdonschot N, Schreurs BW, Buma P. The use of a bioresorbable nano-crystalline hydroxyapatite paste in acetabular bone impaction grafting. Biomaterials. 2006;27:1110–1118. doi: 10.1016/j.biomaterials.2005.07.024. [DOI] [PubMed] [Google Scholar]
- 46.Thorwarth M, Schultze-Mosgau S, Kessler P, Wiltfang J, Schlegel KA. Bone regeneration in osseous defects using a resorbable nanoparticular hydroxyapatite. J Oral and Maxillofac Surg. 2005;63:1626–1633. doi: 10.1016/j.joms.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 47.Grad S, Kupsik L, Górna K, Gogolewski S, Alini M. The use of biodegradable polyurethane scaffolds for cartilage tissue engineering: potential and limitations. Biomaterials. 2003;24:5163–5171. doi: 10.1016/s0142-9612(03)00462-9. [DOI] [PubMed] [Google Scholar]
- 48.Grande DA, Halberstadt C, Schwarz R, Manji R. Evaluation of matrix scaffolds for tissue engineering of articular cartilage grafts. J Biomed Mater Res. 1997;34:211–220. doi: 10.1002/(sici)1097-4636(199702)34:2<211::aid-jbm10>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 49.Wei X, Gao J, Messner K. Maturation-dependent repair of untreated osteochondral defects in the rabbit knee joint. J Biomed Mater Res. 1997;34:63–72. doi: 10.1002/(sici)1097-4636(199701)34:1<63::aid-jbm9>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 50.Kamarul T, Ab-Rahim S, Tumin M, Selvaratnam L, Ahmad TS. Preliminary study of the effects of glucosamine sulphate and Chondroitin sulphate on surgically treated and untreated focal cartilage damage. Eur Cell Mater. 2011;21:259–271. doi: 10.22203/ecm.v021a20. [DOI] [PubMed] [Google Scholar]
- 51.Kim SS, Sun Park M, Jeon O, Yong Choi C, Kim BS. Poly(lactide-co-glycolide)/hydroxyapatite composites scaffolds for bone tissue engineering. Biomaterials. 2006;27:1399–1409. doi: 10.1016/j.biomaterials.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 52.Stafford HJ, Roberts MT, Oni OOA, Hay J, Hay Gregg. Localisation of bone-forming cells during fracture healing by osteocalcin immunocytochemistry: An experimental study of the rabbit tibia. J Orthop Res. 1994;12:29–39. doi: 10.1002/jor.1100120105. [DOI] [PubMed] [Google Scholar]
- 53.Garvican ER, Vaughan-Thomas A, Clegg PD, Innes JF. Biomarkers of cartilage turnover. Part I: markers of collagen degradation and synthesis. Vet J. 2010;185:36–42. doi: 10.1016/j.tvjl.2010.04.011. [DOI] [PubMed] [Google Scholar]