Abstract
Aim: To evaluate the role of rotating gamma system (RGS) radiosurgery for low-grade brainstem gliomas. Patients and Methods: Thirty-seven patients undergoing RGS radiosurgery at the Bach Mai Hospital Hanoi for low-grade brainstem glioma were included in this prospective interventional study. The median RGS dose was 12 Gy (range=8-16 Gy). Endpoints included response to RGS radiosurgery given as change in glioma size (maximum diameter), survival and adverse events. Follow-up was performed for 36 months. Three dose-groups (<13, 13-14 and >14 Gy) were compared for survival. Results: Mean glioma size decreased from 1.87 cm before RGS irradiation to 1.15 cm at 36 months. Mean survival was 39.5 months. Mean survival after <13, 13-14 and >14 Gy were 22.7, 66.7 and 49 months, respectively (p<0.05). Adverse events, mainly reduced appetite, sleep disturbances, headache and edema, were not associated with RGS dose and were easily managed. Conclusion: RGS radiosurgery led to promising results with acceptable toxicity in patients with low-grade brainstem gliomas.
Keywords: Brain stem gliomas, radiosurgery, rotating gamma system, treatment outcomes, adverse events
Brainstem gliomas account for about 20% of primary brain tumors in children and for <2% of gliomas in adult patients (1-3). Gliomas of the brainstem are most commonly located in the pons, but are also found in the mesencephalon, the cerebellar peduncles or the medulla oblongata (4). A tumor located in the brainstem can be associated with severe clinical or even life-threatening symptoms, since this part of the brain regulates motion functions, as well as respiratory and circulatory functions. Such tumors include low- and high-grade lesions. The median survival time for patients with a brainstem glioma is about 10 months in children and 30-40 months in adult patients (5). The prognosis depends on the grade according to the World Health Organization (WHO) (6). In a retrospective study from the United States, median survival was 77 months for patients with WHO grade II lesions, 21 months for those with grade III lesions and 15 months for those with grade IV lesions (5). A great amount of research has been carried out on the treatment of higher grade (III or IV) lesions, whereas fewer data are available for patients with low-grade (grade II) gliomas (7-11). Therefore, the present study focused on low-grade gliomas of the brainstem and aimed to provide additional data for this less common situation.
Since complete surgical resection is not safely possible in many of these patients, radiotherapy is the most common treatment. In order to allow for optimal sparing of normal tissues and structures surrounding the tumor, radiotherapy of brainstem gliomas should ideally be performed with high-precision techniques such as intensity-modulated radiotherapy, fractionated stereotactic radiation therapy or stereotactic radiosurgery (SRS). SRS is defined as single-fraction treatment, mostly performed with a linear accelerator or a gamma system.
Two gamma systems are used for radiosurgery of cerebral lesions, including those located in the brainstem. The classic Gamma-Knife was already introduced about 50 years ago by Lars Leksell (12). The other system, the rotating gamma system (RGS), was developed in the late 1990s (13). The latter system includes technical features of the classic Gamma-Knife (Cobalt-60 sources) and linear accelerator-based radiosurgery (a rotating gantry). Although radiosurgery with a gamma system appears to be a reasonable option for treating brainstem gliomas, in particular low-grade tumors, only few data are available. Few retrospective studies with 20 or less patients have used classic Gamma-Knife radiosurgery for low-grade brainstem gliomas (14-21). We present here the first study, to our knowledge that used an RGS system for radiosurgery of low-grade brainstem gliomas. Moreover, it is the first study that prospectively evaluated the role of radiosurgery with a gamma system for these uncommon tumors.
Patients and Methods
Thirty-seven patients with a low-grade glioma of the brainstem who were treated with RGS radiosurgery at the Nuclear Medicine and Oncology Center of the Bach Mai Hospital in Hanoi, Vietnam, between from July 2007 and December 2013, were included in this prospective interventional study. To be included in this study, the patients were required to have a symptomatic tumor of the brainstem showing typical characteristics of a low-grade glioma on computed tomography (CT), magnetic resonance imaging (MRI) and magnetic resonance spectroscopy (MRS) (22-24). In addition, further criteria included the presence of only one intracerebral lesion, lesion size (maximum diameter) of ≤3 cm, no severe comorbidity, age 5-90 years and no pregnancy or lactation. Furthermore, the patients were to have been identified as suitable candidates for treatment with RGS irradiation by the Hospital Medical Council and to have given their informed consent to participate in the study.
Irradiation was performed as single-fraction radiosurgery with an RGS system (Gamma ART 6000; American Radiosurgery, San Diego, CA, USA). The median dose was 12 Gy (range=8-16 Gy) prescribed to the outer margin of the tumor representing the 50% isodose.
Investigated endpoints included response to RGS radiosurgery, survival and adverse events. Follow-up was performed at 6, 12, 24 and 36 months following RGS irradiation and included the patient’s history, a clinical examination as well as CT and MRI scans. In a subgroup analysis regarding survival, the potential impact of the RGS dose (<13 Gy vs. 13-14 Gy vs. >14 Gy) was investigated.
The data were coded and analyzed by STATA SE 10 (StataCorp LLC, College Station, TX, USA) using statistical logarithm. The overall survival rates were calculated with the Kaplan–Meier method. The Kaplan–Meier curves of the different RGS doses were compared with the log-rank test. A p-value of less than 0.05 was considered significant.
Results
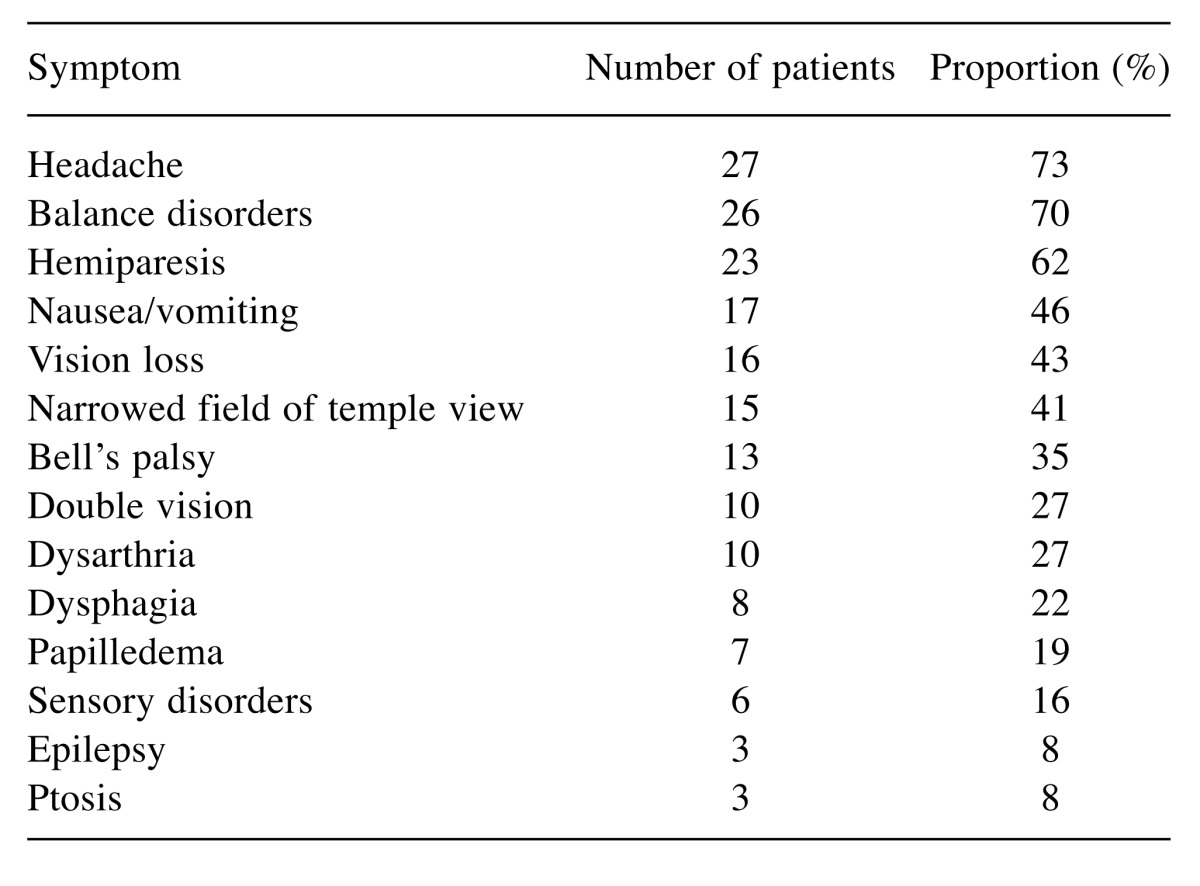
Thirty-seven patients, 17 women (46%) and 20 men (54%), met the criteria for inclusion in this study. The median age was 30 years (range: 5-63 years). The Karnofsky performance score was 40-50 in 19 patients (51%), 60-70 in nine patients (24%) and 80-100 in nine patients (24%), respectively. The tumor was located in the pons in 21 patients (57%), in the cerebral peduncle in 10 patients (27%) and in the medulla oblongata in six patients (16%), respectively. The clinical symptoms of the 37 patients are summarized in Table I.
Table I. Clinical symptoms prior to rotating gamma system radiosurgery.
The mean size of the gliomas prior to RGS irradiation was 1.87 cm [standard deviation (SD)=0.51 cm]. Following RGS treatment, mean glioma sizes were 1.99 cm (SD=0.50 cm) at 6 months, 1.60 cm (SD=0.47 cm) at 12 months, 1.33 cm (SD=0.59 cm) at 24 months and 1.15 cm (SD=0.48 cm) at 36 months. Thus, at 36 months, the mean decrease in tumor size was 38.5%.
At 6 months after treatment, the rates of complete response, partial response, stable disease and progressive disease were 8.1%, 32.4%, 56.8% and 2.7%, respectively. The corresponding response rates were 23.5%, 47.1%, 23.5% and 5.9%, respectively, after 1 year, 37.1%, 29.6%, 22.2% and 11.1%, respectively, after 2 years, and 37.5%, 12.5%, 37.5% and 12.5%, respectively, after 3 years.
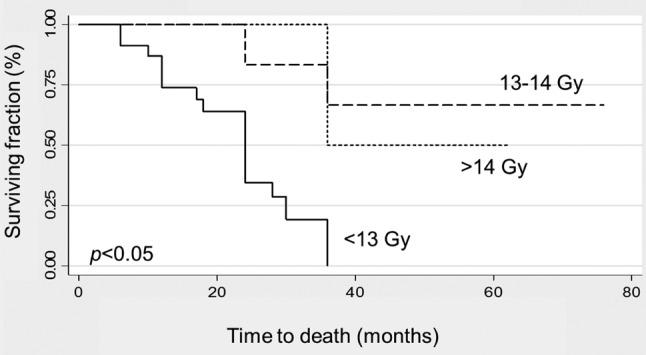
For the entire cohort, the mean and median survival times following RGS radiosurgery were 39.5 months and 30 months, respectively. The mean survival times of the <13 Gy, 13-14 Gy and >14 Gy dose groups were 22.7, 66.7 and 49 months, respectively (p<0.05) (Figure 1). Median survival was 30 months in the 13 Gy group and not reached in the other two groups.
Figure 1. Comparison of survival (Kaplan–Meier curves) of patients according to dose of rotating gamma system (RGS) radiosurgery (<13 Gy, 13-14 Gy and >14 Gy). The p-value was obtained from the log-rank test.
Adverse events related to RGS irradiation included reduced appetite in 46%, sleep disturbances in 32%, increasing or new headache in 27%, increasing or new cerebral edema in 22%, xerostomia in 19%, hair loss in 14% and radiation dermatitis in 5% of the patients. All these adverse events were transient and easily managed with appropriate medication. The RGS dose had no significant impact on the occurrence of adverse events (p<0.05).
Discussion
Brainstem gliomas are quite uncommon in adult patients (1-3). As complete removal of these lesions is not reasonable in many of these patients, they are assigned to radiotherapy rather than neurosurgical intervention. If radiotherapy is indicated, it is quite often performed as radiosurgery. Radiosurgery is a well-recognized treatment for patients with cerebral lesions such as metastases to the brain from cancer (25-27). Depending on the availability, radiosurgery is mostly performed as linear accelerator-based or Gamma-Knife radiosurgery. Besides the classic Gamma-Knife that has been used for cerebral lesions for almost five decades, a more recently developed device, the RGS, is also in use in several centers worldwide (12,13). However, it currently is much less common than the classic Gamma-Knife. Therefore, studies that investigated the value of the RGS are considerably rarer than studies using the classic Gamma-Knife. This applies particularly for rare cerebral lesions such as low-grade brainstem gliomas. The present study is the first, as far as we are aware, that evaluated the role of the RGS for this particular group of patients.
According to the results of this observational study, RGS radiosurgery resulted in a decrease in glioma size by 38.5% after 36 months. The effect of classic Gamma-Knife radiosurgery on tumor control varied in the previous studies from the literature. In 1995, Hirato et al. presented 10 patients with a brainstem malignancy; two of the patients had a glioma. Following Gamma-Knife treatment, one patient had stable disease and the other one progression of his glioma (15). In 2002, Fuchs et al. presented 21 patients (age=8-56 years) with a brainstem glioma, 12 with low-grade and nine with high-grade tumors (16). Fifteen patients were evaluable for response to irradiation; two had progression (13%), 10 had stable disease (67%) and only three patients (20%) showed a partial response. Hafez et al. reported two cases (10 years and 19 years of age, respectively) who underwent classic Gamma-Knife radiosurgery for a low-grade brainstem glioma (18). Both patients had a partial response following irradiation. High response rates were reported after classic Gamma-Knife treatment of low-grade brainstem gliomas by Yen et al. (17) and by El-Shehaby et al. (21). Of the 20 patients in the study of Yen et al., 16 (75%) responded to treatment (17); and of the 11 patients in the study of El-Shehaby et al., all patients experienced shrinkage of their brainstem glioma (21).
In the current study, the median survival time was 30 months, which was more favorable than the median survival of 21 months reported by Fuchs et al. (16). This difference may be explained by the dose of radiosurgery, which was a median of 12 Gy (range=9-20 Gy) in the study by Fuchs et al. (16). According to the present study, radiosurgery doses of 13 Gy or higher were associated with improved survival. Median survival in the present study was also better than the 26.4 months reported by Reithmeier et al. for 31 patients with grade II gliomas receiving different treatment approaches (28).
The adverse events related to RGS radiosurgery in the present study were transient and easily managed with appropriate medication and without surgical interventions, such as shunts for severe edema and aspiration or removal of cysts. In some of the studies from the literature that used classic Gamma-Knife radiosurgery, adverse events were stated. Kihlström et al. observed severe edema in two out of their seven patients (29%) (14). In the study of Fuchs et al., three out of 21 patients (14%) required surgical intervention due to radiosurgery-related adverse events (16). Yen et al. reported complications in two out of 20 patients (10%) (17). In the study of El-Shehaby, one out of 11 patients (9%) required aspiration of an intracerebral cyst (21).
In summary, radiosurgery with an RGS led to promising outcomes in terms of response and survival in patients with low-grade brainstem gliomas. Adverse events were easily managed. Doses of at least 13 Gy resulted in better outcomes than doses <13 Gy, without significantly increasing toxicity.
Conflicts of Interest
On behalf of all Authors, the corresponding Author states that there is no conflict of interest related to this study.
References
- 1.Hu J, Western S, Kesari S. Brainstem glioma in adults. Front Oncol. 2016;6:180. doi: 10.3389/fonc.2016.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salmaggi A, Fariselli L, Milanesi I, Lamperti E, Silvani A, Bizzi A, Maccagnano E, Trevisan E, Laguzzi E, Rudà R, Boiardi A, Soffietti R. Natural history and management of brainstem gliomas in adults. A retrospective Italian study. J Neurol. 2008;255:171–177. doi: 10.1007/s00415-008-0589-0. [DOI] [PubMed] [Google Scholar]
- 3.Reyes-Botero G, Mokhtari K, Martin-Duverneuil N, Delattre JY, Laigle-Donadey F. Adult brainstem gliomas. Oncologist. 2012;17:388–397. doi: 10.1634/theoncologist.2011-0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kesari S, Kim RS, Markos V, Drappatz J, Wen PY, Pruitt AA. Prognostic factors in adult brainstem gliomas: a multicenter, retrospective analysis of 101 cases. J Neurooncol. 2008;88:175–183. doi: 10.1007/s11060-008-9545-1. [DOI] [PubMed] [Google Scholar]
- 5.Theeler BJ, Ellezam B, Melguizo-Gavilanes I, de Groot JF, Mahajan A, Aldape KD, Bruner JM, Puduvalli VK. Adult brainstem gliomas: correlation of clinical and molecular features. J Neurol Sci. 2015;353:92–97. doi: 10.1016/j.jns.2015.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han IB, Kim M, Lee SH, Kim JK, Kim SH, Chang JH, Teng YD. Down-regulation of microRNA-126 in glioblastoma and its correlation with patient prognosis: a pilot study. Anticancer Res. 2016;36:6691–6697. doi: 10.21873/anticanres.11280. [DOI] [PubMed] [Google Scholar]
- 7.Yount G, Soroceanu L, Wang HJ, Singer E, Yount R, Luu T, Yang LX. Selective toxicity of a highly potent camptothecin analogue: a pilot study with glioblastoma multiforme cells. Anticancer Res. 2016;36:5845–5848. doi: 10.21873/anticanres.11169. [DOI] [PubMed] [Google Scholar]
- 8.Förnvik K, Zolfaghari S, Salford LG, Redebrandt HN. ITPP Treatment of RG2 glioblastoma in a rat model. Anticancer Res. 2016;36:5751–5755. doi: 10.21873/anticanres.11158. [DOI] [PubMed] [Google Scholar]
- 9.Shah BK, Bista A, Sharma S. Survival trends in elderly patients with glioblastoma in the United States: a population-based study. Anticancer Res. 2016;36:4883–4886. doi: 10.21873/anticanres.11052. [DOI] [PubMed] [Google Scholar]
- 10.Torrens M, Malamitsi J, Karaiskos P, Valotassiou V, Laspas F, Andreou J, Stergiou C, Prassopoulos V. Although non-diagnostic between necrosis and recurrence, FDG PET/CT assists management of brain tumours after radiosurgery. In Vivo. 2016;30:513–520. [PubMed] [Google Scholar]
- 11.Leksell L. Stereotactic radiosurgery. J Neurol Neurosurg Psychiatry. 1983;46:797–803. [Google Scholar]
- 12.Goetsch SJ, Murphy BD, Schmidt R, Micka J, De Werd L, Chen Y, Shockley S. Physics of rotating gamma systems for stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 1999;43:689–696. doi: 10.1016/s0360-3016(98)00428-3. [DOI] [PubMed] [Google Scholar]
- 13.Kihlström L, Lindquist C, Lindquist M, Karlsson B. Steerotactic radiosurgery for tectal low-grade gliomas. Acta neurochir Suppl. 1994;62:55–57. doi: 10.1007/978-3-7091-9371-6_11. [DOI] [PubMed] [Google Scholar]
- 14.Hirato M, Nakamura M, Inoue HK, Ohye C, Hirato J, Shibazaki T, Andou Y. Stereotact Funct Neurosurg 64. (Suppl 1) 1995;1:32–41. doi: 10.1159/000098762. [DOI] [PubMed] [Google Scholar]
- 15.Fuchs I, Kreil W, Sutter B, Papaethymiou G, Pendl G. Gamma Knife radiosurgery of brainstem gliomas. Acta Neurochir Suppl. 2002;84:85–90. doi: 10.1007/978-3-7091-6117-3_10. [DOI] [PubMed] [Google Scholar]
- 16.Yen CP, Sheehan J, Steiner M, Patterson G, Steiner L. Gamma Knife surgery for focal brainstem gliomas. J Neurosurg. 2007;106:8–17. doi: 10.3171/jns.2007.106.1.8. [DOI] [PubMed] [Google Scholar]
- 17.Hafez RF. Stereotaxic gamma knife surgery in treatment of critically located pilocytic astrocytoma: Preliminary results. World J Surg Oncol. 2007;5:39. doi: 10.1186/1477-7819-5-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liao CH, Pan DH, Yang HC, Wu HM, Ho DM, Wong TT, Shih YH. Gamma Knife radiosurgery as a treatment modality for low-grade pediatric brainstem gliomas: report of two cases. Childs Nerv Syst. 2012;28:175–178. doi: 10.1007/s00381-011-1620-9. [DOI] [PubMed] [Google Scholar]
- 19.Tuleasca C, Negretti L, Magaddino V, Maeder P, Lhermitte B, Borruat FX, Levivier M. Biphasic response of a tecto-mesencephalic pilocytic astrocytoma after Gamma Knife surgery – A case report. Neurochirurgie. 2015;61:275–278. doi: 10.1016/j.neuchi.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 20.El-Shehaby AM, Reda WA, Abdel Karim KM, Emad Eldin RM, Esene IN. Gamma Knife radiosurgery for low-grade tectal gliomas. Acta Neurochir. 2015;157:247–256. doi: 10.1007/s00701-014-2299-y. [DOI] [PubMed] [Google Scholar]
- 21.Bahary JP, Villemure JG, Choi S, Leblanc R, Olivier A, Bertrand G, Souhami L, Tampieri D, Hazel J. Low-grade pure and mixed cerebral astrocytomas treated in the CT scan era. J Neurooncol. 1996;27:173–177. doi: 10.1007/BF00177481. [DOI] [PubMed] [Google Scholar]
- 22.Yin L, Zhang L. Correlation between MRI findings and histological diagnosis of brainstem glioma. Can J Neurol Sci. 2013;40:348–354. doi: 10.1017/s0317167100014293. [DOI] [PubMed] [Google Scholar]
- 23.Yerli H, Ağildere AM, Ozen O, Geyik E, Atalay B, Elhan AH. Evaluation of cerebral glioma grade by using normal side creatine as an internal reference in multi-voxel 1H-MR spectroscopy. Diagn Interv Radiol. 2007;13:3–9. [PubMed] [Google Scholar]
- 24.Rades D, Huttenlocher S, Rudat V, Hornung D, Blanck O, Phuong PC, Khoa MT, Schild SE, Fischer D. Radiosurgery with 20 Gy provides better local control of 1-3 brain metastases from breast cancer than with lower doses. Anticancer Res. 2015;35:333–336. [PubMed] [Google Scholar]
- 25.Rades D, Dahlke M, Gebauer N, Bartscht T, Hornung D, Trang NT, Phuong PC, Khoa MT, Gliemroth J. A new predictive tool for optimization of the treatment of brain metastases from colorectal cancer after stereotactic radiosurgery. Anticancer Res. 2015;35:5515–5518. [PubMed] [Google Scholar]
- 26.Dziggel L, Dahlke M, Janssen S, Hornung D, Blanck O, Khoa MT, Schild SE, Rades D. Predicting the risk of new cerebral lesions after stereotactic radiosurgery (SRS) for brain metastases from breast cancer. Anticancer Res. 2015;35:6793–6797. [PubMed] [Google Scholar]
- 27.Reithmeier T, Kuzeawu A, Hentschel B, Loeffler M, Trippel M, Nikkhah G. Retrospective analysis of 104 histologically proven adult brainstem gliomas: clinical symptoms, therapeutic approaches and prognostic factors. BMC Cancer. 2014;14:115. doi: 10.1186/1471-2407-14-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW. The 2016 world health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]


