Abstract
Background
Nail‐bed injuries are the most common hand injury in children. Surgical dogma is to replace the nail plate after repairing the nail bed. Recent evidence suggests this might increase infection rates and returns to clinic. The aim of this feasibility trial was to inform the design and conduct of a definitive trial comparing replacing or discarding the nail plate after nail‐bed repair.
Methods
This study recruited participants from four hand units in the UK between April and July 2015. Participants were children under the age of 16 years with a nail‐bed injury requiring surgery. They were randomized to either having the nail plate replaced or discarded after nail‐bed repair. The follow‐up method was also allocated randomly (postal versus clinic). Information was collected on complications at 2 weeks and 30 days, and on nail‐plate appearance at 4 months using the Zook classification. Two possible approaches to follow‐up were also piloted and compared.
Results
During the recruitment phase, there were 156 potentially eligible children. Sixty were randomized in just over 3 months using remote web‐based allocation. By 2 weeks, there were two infections, both in children with replaced nail plates. The nail‐replaced group also experienced more complications. There was no evidence of a difference in return rates between postal and clinic follow‐up.
Conclusion
Recruitment was rapid and nail‐bed repair appeared to have low complication and infection rates in this pilot trial. The findings have led to revision of the definitive trial protocol, including the mode and timing of follow‐up, and modification of the Zook classification.
Short abstract
Informs the RCT
Introduction
Nail‐bed injury is the most common paediatric hand injury requiring surgery. Typically, the injury is sustained when a child catches their fingertip in a closing door. This results in a laceration across the nail bed, which sits beneath the hard nail plate (fingernail). A tertiary hand surgery unit will expect to treat approximately four children each week. This equates to approximately 10 000 children treated annually across the UK1. In the UK, 96 per cent of plastic surgery and orthopaedic hand surgeons replace the nail plate after repairing the nail‐bed injury (Fig. 1)1. The rationale most often given for replacing the nail plate is that it protects the repaired nail bed and splints open the nail fold to enable normal nail growth. Recent evidence challenges surgical dogma by suggesting that replacing the nail plate results in higher infection rates, delayed wound healing and increased outpatient visits2. A recent Cochrane review3 highlighted the lack of high‐level evidence in the management of these injuries.
Figure 1.
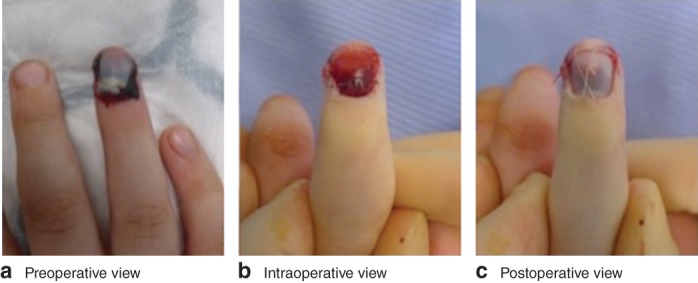
Preoperative view of a fingertip injury with suspected nail‐bed laceration. b The nail plate has been removed and the nail bed repaired with sutures. c The cleaned nail plate replaced into the nail fold and secured in position with a suture
A consensus meeting was held at the Royal College of Surgeons of England in February 2014 to discuss this clinical uncertainty. The meeting led to development of a trial protocol, but there were key methodological uncertainties that needed to be addressed. In light of this, a feasibility trial was undertaken with the aim of informing a large definitive trial to address the question of whether the nail plate should be replaced or discarded after nail‐bed repair in children.
Methods
This study received ethical approval from the National Research Ethics Service committee (15/LO/0067). It was carried out according to the principles of the Declaration of Helsinki and in accordance with the International Council for Harmonization Good Clinical Practice guidelines. The study was registered in the ISRCTN registry (ISRCTN16571591) and the full protocol has been published4. The study was on the UK Clinical Research Network Portfolio (ID 18516).
Study design
The Nail bed INJury Analysis (NINJA) Pilot was an external feasibility study for a large pragmatic, multicentre RCT (NINJA Trial). Trial participants were prospectively recruited in the UK from the four participating National Health Service (NHS) Trusts: Guy's and St Thomas' Hospitals NHS Trust, London; Mid Essex Hospitals NHS Trust, Chelmsford; Hull and East Yorkshire Hospitals NHS Trust, Hull; and Oxford University Hospitals NHS Trust, Oxford. Recruitment was undertaken between April and July 2015. All parents or guardians of children undergoing nail‐bed repair were screened for the inclusion criteria and, if appropriate, approached to participate in the study.
Eligibility criteria
Inclusion criteria were: aged below 16 years; acute nail‐bed injury (occurring within 48 h of presentation at trial centre) requiring surgical repair; injury type included sharp lacerations, stellate lacerations, crush and avulsion injuries of the nail bed, injuries involving the sterile and/or germinal matrix, and nail‐bed injuries with an associated pulp laceration and/or with an associated ‘tuft’ fracture of the distal phalanx; and children whose parent or legal guardian consented to their inclusion in the trial and were willing to return for follow‐up.
Children presenting with a nail‐bed injury that was already infected were excluded, as were those with underlying nail disease or deformity before the injury; children with an associated distal phalanx fracture requiring fixation with a Kirschner wire (this was considered to be another potential source of infection and therefore a confounding variable); children with complete amputation of the distal fingertip including all or part of the nail bed, which required repair as a composite graft or replantation; and children with loss of part or all of the nail bed requiring a nail‐bed graft or flap reconstruction.
Randomization
Children whose parents gave informed consent were randomized for the surgeon to either replace or discard the nail plate after nail‐bed repair. Randomization was undertaken by a web‐based system provided by the Oxford Clinical Trials Research Unit, with allocation stratified by site and formed of random permuted blocks of varying size. Each participant received two allocations, one for treatment and one for method of follow‐up at 4 months (postal or clinic). Randomization was undertaken shortly before surgery when the child was in the anaesthetic room.
Outcome measures
The feasibility measures were: number of potentially eligible children; number of patients/parents and guardians approached to take part in the study; proportion of children for whom consent was sought who took part in the study; proportion of children who received the allocated treatment and reasons for any non‐compliance; proportion of participants with a valid response at each follow‐up point (for the 4‐month time point both overall response rate and by method of follow‐up).
The clinical outcome measures were: postoperative complications at 2 weeks and 30 days; pain experienced by the child at their first dressing change at 2 weeks (measured using the Wong–Baker FACES® Pain Rating Scale; Wong–Baker FACES Foundation, Oklahoma City, Oklahoma, USA); and the cosmetic (aesthetic) appearance of the nail at 4 months measured on a visual analogue scale using the Zook classification system5.
The secondary study objectives were to inform the design and conduct of the definitive NINJA Trial. Two blinded independent observers assessed the clinical photographs collected at the 4‐month time point to allow an assessment of interobserver agreement.
Statistical analysis
A total of 60 children were targeted for recruitment. A study of this size would be sufficient to generate a binary feasibility measure with a 95 per cent confidence interval width of between 15 and 25 per cent, depending on the event proportion based on the Wald interval6.
Descriptive analyses of outcome data were carried out using appropriate summary measures. Outcome data were grouped according to the allocated intervention, irrespective of the actual treatment received. No imputation of missing data was performed; no children withdrew from the study. Binomial exact confidence intervals were calculated for the follow‐up return rates, using the cii command across both treatment groups. Agreement between assessors was calculated for each component and the overall Zook appearance score. The overall score of assessors was also evaluated using weighted κ values with 95 per cent confidence intervals. The response rate between methods was compared according to the follow‐up allocation by calculating the difference in percentage returned, with 95 per cent confidence intervals calculated using Newcombe's method 107. All analyses were carried out in Stata release 14.1 (StataCorp, College Station, Texas, USA).
Results
During the recruitment interval, between 21 April 2015 and 27 July 2015, a total of 156 children were potentially eligible, of whom 52 were missed or did not meet the inclusion criteria. Of the 104 that did, 44 were excluded. A total of 60 children were randomized, 32 to have the nail plate replaced and 28 to have it discarded (Fig. 2). Data on the number of children for whom a parent/guardian was approached about trial participation, but who declined, were not collected reliably and are not reported. The target recruitment was met within 97 days.
Figure 2.
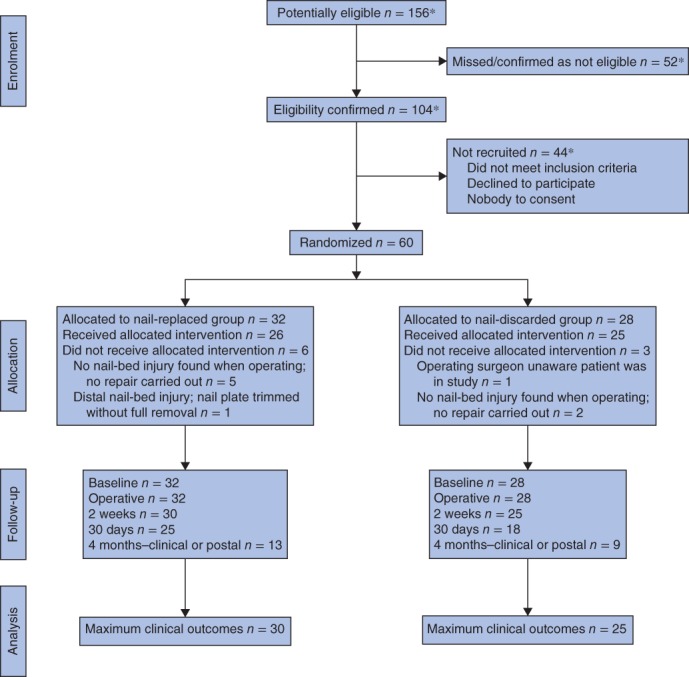
CONSORT diagram for the trial. *Full eligibility data were not available for one of the recruiting centres
The clinical details of the children and their injuries are reported in Table 1 and operative details in Table S1 (supporting information). The groups were well matched in terms of age, mechanism of injury and perioperative care.
Table 1.
Characteristics of the children and finger injuries
| Nail replaced | Nail discarded | |
|---|---|---|
| (n = 32) | (n = 28) | |
| Age (years)* | 5·8(3·8) (< 1 to 16) | 5·8(3·3) (1–14) |
| Sex ratio (F : M) | 14 : 18 | 15 : 13 |
| Right hand injured | 15 | 17 |
| Study digit | ||
| Thumb | 7 | 4 |
| Index | 4 | 5 |
| Middle | 12 | 8 |
| Ring | 4 | 8 |
| Little | 5 | 3 |
| Mechanism | ||
| Avulsion | 2 | 1 |
| Crush | 28 | 25 |
| Laceration | 2 | 2 |
| Other | 0 | 0 |
| Injury contaminated | 1 | 0 |
| Antibiotics started in emergency department | 24 | 20 |
| Medical condition | ||
| Diabetes | 0 | 1 |
| Psoriasis | 1 | 0 |
| Unrelated conditions | 2 | 5 |
| None | 29 | 22 |
| Regular preoperative medication | ||
| Insulin | 0 | 1 |
| Methotrexate | 0 | 1 |
| Antibiotic | 0 | 2 |
| Drug for related conditions | 4 | 3 |
| None | 28 | 21 |
Values are mean(s.d.) (range).
Compliance with the allocation was 85 per cent overall (Fig. 2). Six children in the nail‐replaced group and three in the nail‐discarded group did not receive the allocated intervention. The main reason for non‐compliance was that no nail‐bed injury was noted at the time of surgery.
Data completeness varied from excellent for baseline and operative data (100 (95 per cent c.i. 94 to 100) per cent for both), good for 2 weeks (92 (82 to 97) per cent), moderate for 30 days (72 (59 to 83) per cent) and poor for 4 months with either mode of follow‐up (37 (25 to 50) per cent). Thirty‐one children from the two groups were randomized to postal questionnaire follow‐up and 29 to clinic review. Nine postal questionnaires were returned and 13 patients came back for clinic review. There was no evidence of a difference in return rates between postal and clinic follow‐up (–16 (–38 to 8) per cent).
Clinical outcomes
During the first 2 weeks, two children were treated with antibiotics for wound infections. Although both children had the nail replaced, one was in the nail‐discarded group (Table 2). The first child had no nail‐bed laceration, received one additional course of antibiotics and was seen four times after surgery. The second child was randomized to have the nail discarded (protocol deviation), had two additional courses of antibiotics and was seen ten times after surgery.
Table 2.
Postoperative outcomes at 2 weeks
| Nail replaced | Nail discarded | |
|---|---|---|
| (n = 30) | (n = 25) | |
| Postoperative problem† | 16 | 9 |
| Dressing change required | 8 | 9 |
| Fever | 1 | 0 |
| Pain | 7 | 0 |
| Rash | 1 | 0 |
| Allergic reaction to dressing | 0 | 1 |
| Infection | 1 | 1 |
| Light‐headedness | 0 | 1 |
| Additional treatment‡ | 13 | 9 |
| Analgesia | 3 | 0 |
| Oral antibiotics | 1 | 1 |
| Antihistamine | 1 | 0 |
| Antipyretics | 1 | 0 |
| Dressing change | 8 | 9 |
| Duration of antibiotics (days)* | 7 (5–7) | 7 (6–7) |
| Patient‐reported outcomes§ | ||
| No pain before dressing change | 23 of 24 | 16 of 24 |
| No pain during dressing change | 13 of 28 | 12 of 24 |
Values are median (i.q.r.).
Two participants in the nail‐replaced group had two problems, and one participant had two and one had three problems in the nail‐displaced group;
one participant in both groups had two treatments.
Assessed using the Wong–Baker FACES® Pain Rating Scale.
Between 2 weeks and 30 days there was little apparent difference between the two groups (Table 3). There were no additional infections and the two reported before 2 weeks had resolved. There were four additional unplanned dressing changes during this interval, two in each group.
Table 3.
Postoperative outcomes at 30 days
| Nail replaced | Nail discarded | |
|---|---|---|
| (n = 25) | (n = 18) | |
| Postoperative problem* | 4 | 3 |
| Dressing change required | 2 | 2 |
| Infection | 0 | 0 |
| Pain | 3 | 1 |
| Additional treatment | 2 | 2 |
| Antibiotic | 0 | 0 |
| Dressing change | 2 | 2 |
One participant had two problems (dressing change required and pain).
At 4 months, there were no additional treatments required by children in either group (Table 4). Of the children seen in the clinic, one complained of pain and another developed an unrelated rash that resolved with treatment from the family doctor.
Table 4.
Postoperative outcomes at 4 months
| Nail replaced | Nail discarded | |
|---|---|---|
| (n = 9) | (n = 4) | |
| Postoperative problem | 1 | 0 |
| Pain | 1 | 0 |
| Infection | 0 | 0 |
| Any additional treatment | 0 | 0 |
| VAS score for nail appearance* | ||
| Parent | 100 (70–100) | 100 (88–100) |
| Patient | 100 (60–100) (n = 5) | n.r. |
Values are median (i.q.r.). Of 16 patients in the nail‐replaced group and 13 in the nail‐discarded group randomized to follow‐up in the clinic, nine and four respectively returned for clinic review at 4 months. VAS, visual analogue scale (0, worst possible; 100, normal); n.r., not recorded.
During clinical assessment of nail‐plate growth in children returning to clinic at 4 months, the clinicians scored six of nine children as having an identical nail shape to that of the opposite uninjured finger in the nail‐replaced group and three of four in the nail‐discarded group (Table S2, supporting information).
The blinded independent observer scores showed no significant difference between the two groups (Table S3, supporting information). The limited parental assessment of nail‐plate growth, using a visual analogue scale, suggested normal growth for all children in both groups.
There was no evidence of a difference between assessor 1 and 2 summary Zook scores for returned photographs at 4 months (25 children in total). Nail‐shape and nail‐surface components had the lowest level of agreement (36 and 48 per cent), whereas adherence, eponychium and split components had a higher level of agreement (72, 88 and 100 per cent respectively). The overall score agreement between assessors was 40 per cent. The weighted κ value was 0·36 (95 per cent c.i. 0·09 to 0·68).
Discussion
The NINJA Pilot was a prospective multicentre RCT undertaken in paediatric nail‐bed repair, and the first delivered by the Reconstructive Surgery Trials Network. Surgeons within the network were able to recruit children successfully in a short time. The rate of recruitment suggested that a definitive multicentre trial recruiting across the network would be able to enrol sufficient participants for a definitive trial to be able to assess key outcomes.
Compliance was reasonably high, although the number of children who did not have a nail‐bed injury when examined during the operation was greater than anticipated. With regard to the definitive study, there are two main possible strategies to address this: to define the intervention as replacing or discarding the nail if a nail‐bed repair is appropriate when examined during surgery, or to randomize during the operation once the nail‐bed injury has been confirmed. The former is a more practical option. The latter is more desirable scientifically, but is not practical in an operating theatre.
The overall follow‐up rate was good at 2 weeks (92 per cent), but only moderate at 30 days (72 per cent). At 4 months, both the clinic and questionnaire response rates were substantially lower than expected under either follow‐up method. Given the low complication rates at the early time points, it is likely the parents felt no need to return to the clinic. Subsequent public and patient engagement activities have provided ideas as to how this can be improved in the definitive trial using information technology and other approaches.
The agreement between assessors for the Zook classification‐based cosmetic appearance score was poor. Disagreement was mainly in two of the component scores. The definitive trial requires a modified version of the score, which will offer greater consistency between assessors. Additionally, training of assessors is required to reduce inter‐rater variability.
The present study suggests that surgery to repair nail‐bed injuries in children is generally successful, with a low complication rate. The current dogma is to replace the nail plate after nail‐bed repair. However, children with the nail replaced had both the reported infections and experienced complications typically requiring dressing changes and clinic visits.
Children in the nail‐replaced group were more likely to experience pain in the first 2 weeks, but registered pain less often immediately before the dressing change. Half of the children in each group had pain immediately after the dressing change at 2 weeks. By 30 days there were few additional problems and at 4 months the nails in both groups had regrown with minimal deformity.
The next step is to complete a definitive RCT; this pilot has been crucial in refining the protocol and ensuring that the next study is appropriately powered.
Collaborators
Members of the NINJA Pilot Collaborative who collaborated in this study: R. Agha, W. Albadry, Y. Cemal, P. Chadha, D. Collins, H. Creasy, A. Din, M. Ferreira, F. Maggiulli, A. Mitsakos, A. Molina, T. Pampiglione, M. Pywell, K. Richards, Y. Tavsanoglu, R. Tejero, S. Vamadeva, R. Wang, M. Wordsworth (Guy's and St Thomas' NHS Foundation Trust, London); W. Holmes, J. Haeney, N. Brierley, E. Pardoe, C. Naylor (Hull Royal Infirmary, Hull); T. Laing, I. Delikonstantinou, N. Whybro, H. Gerrish (Mid Essex Hospital Services NHS Trust, Chelmsford); E. Gammin (Oxford University Hospitals NHS Trust, Oxford).
Supporting information.
Additional supporting information may be found online in the supporting information tab for this article.
Supporting information
Table S1 Operative details
Table S2 Clinical assessment of postoperative nail‐plate growth at 4 months
Table S3 Assessment of clinical photographs at 4 months
Acknowledgements
The authors are grateful for the support of the Royal College of Surgeons of England, the British Society for Surgery of the Hand and the British Association of Plastic, Reconstructive and Aesthetic Surgeons.
Disclosure: The authors declare no conflict of interest.
Presented to the British Association of Plastic, Reconstructive and Aesthetic Surgeons Winter Scientific Meeting, London, UK, November 2016
References
- 1. Sierakowski A, Gardiner MD, Jain A, Greig AV; Nail bed INJury Analysis (NINJA) Collaborative Group. Surgical treatment of paediatric nail bed injuries in the United Kingdom: surgeon and patient priorities for future research. J Plast Reconstr Aesthet Surg 2016; 69: 286–288. [DOI] [PubMed] [Google Scholar]
- 2. Miranda BH, Vokshi I, Milroy CJ. Pediatric nailbed repair study. Plast Reconstr Surg 2012; 129: 394e–396e. [DOI] [PubMed] [Google Scholar]
- 3. Capstick R, Giele H. Interventions for treating fingertip entrapment injuries in children. Cochrane Database Syst Rev 2014; (4)CD009808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jain A, Sierakowski A, Gardiner MD, Beard D, Cook J, Cooper C et al Nail bed INJury Assessment Pilot (NINJA‐P) study: should the nail plate be replaced or discarded after nail bed repair in children? Study protocol for a pilot randomised controlled trial. Pilot Feasibility Stud 2015; 1: 1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zook EG, Guy RJ, Russell RC. A study of nail bed injuries: causes, treatment, and prognosis. J Hand Surg Am 1984; 9: 247–252. [DOI] [PubMed] [Google Scholar]
- 6. Wei L, Hutson AD. A comment on sample size calculations for binomial confidence intervals. J Appl Stat 2013; 40: 311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Newcombe RG. Interval estimation for the difference between independent proportions: comparison of eleven methods. Stat Med 1998; 17: 873–890. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Operative details
Table S2 Clinical assessment of postoperative nail‐plate growth at 4 months
Table S3 Assessment of clinical photographs at 4 months


