Abstract
Background
The objective of this study was to quantify the sensitivity of very low concentrations of high‐sensitivity cardiac troponin T (hsTnT) at ED arrival for acute myocardial infarction (AMI) in a large cohort of chest pain patients evaluated in real‐world clinical practice.
Methods
This retrospective study included consecutive ED patients with suspected cardiac chest pain evaluated in four urban EDs, excluding those with ST‐elevation AMI, cardiac arrest or abnormal kidney function. The primary outcomes were AMI at 7, 30, and 90 days. Secondary outcomes included major adverse cardiac events (MACE; all‐cause mortality, AMI, and revascularization) and the individual MACE components. Test characteristics were calculated for hsTnT values from 3 to 200 ng/L .
Results
A total of 7,130 patients met inclusion criteria. AMI incidences at 7, 30, and 90 days were 5.8, 6.0, and 6.2%. When the hsTnT assay was performed at ED arrival, the limit of blank of the assay (3 ng/L) ruled out 7‐day AMI in 15.5% of patients with 100% sensitivity and negative predictive value (NPV). The limit of detection of the assay (5 ng/L) ruled out AMI in 33.6% of patients with 99.8% sensitivity and 99.95% NPV for 7‐day AMI. The limit of quantification (the Food and Drug Administration [FDA]‐approved cutoff for lower the reportable limit) of 6 ng/L ruled out AMI in 42.2% of patients with 99.8% sensitivity and 99.95% NPV. The sensitivities of the cutoffs of <3, <5, and <6 ng/L for 7‐day MACE were 99.6, 97.4, and 96.6%, respectively. The NPVs of the cutoffs of <3, <5, and <6 ng/L for 7‐day MACE were 99.8, 99.5, and 99.4%, respectively. A secondary analysis was performed in a subgroup of 3,549 higher‐risk patients who underwent serial troponin testing. In this subgroup, a cutoff of 3 ng/L ruled out 7‐day AMI in 9.6% of patients with 100% sensitivity and NPV, a cutoff of 5 ng/L ruled out 7‐day AMI in 23.3% of patients with 99.7% sensitivity and 99.9% NPV, and a cutoff of 6 ng/L ruled out 7‐day AMI in 29.8% of patients with 99.7 and 99.9% NPV. In the higher‐risk subgroup, the sensitivities of cutoffs of <3, <5, and <6 ng/L for 7‐day MACE were 99.8, 97.4, and 96.6%, respectively. In this higher‐risk subgroup, the NPV of cutoffs of <3, <5, and <6 ng/L for 7‐day MACE were 99.7, 98.5, and 98.4%, respectively.
Conclusions
When used in real‐world clinical practice conditions, hsTnT concentrations < 6 ng/L (below the lower reportable limit for an FDA‐approved assay) at the time of ED arrival can rule out AMI with very high sensitivity and NPV. The sensitivity for MACE is unacceptably low, and thus a single‐troponin rule‐out strategy should only be used in the context of a structured risk evaluation.
Chest pain of potential cardiac origin accounts for up to 6% of emergency department (ED) presentations1, 2 and 25% of admissions.1 However, less than 15% of these patients actually have an acute coronary syndrome (ACS).2 Because of the risks associated with diagnostic error, emergency physicians strive for ACS miss rates of 1% or less.3, 4 The desire for near‐perfect sensitivity has led to high diagnostic utilization that involves provocative testing, advanced imaging, prolonged observation, or hospitalization for many low‐risk patients,5 generating excessive cost and potential iatrogenic harm.
High‐sensitivity troponin assays may identify low‐risk patients suitable for early discharge. Recent studies show that, in ED patients with chest pain, a high‐sensitivity cardiac troponin T (hsTnT) result below the assay's limit of blank (LoB, <3 ng/L) or limit of detection (LoD, <5 ng/L) at the time of ED arrival can rule out acute myocardial infarction (AMI) with high sensitivity and negative predictive value (NPV).6, 7 The 2015 European Society for Cardiology guidelines for non‐ST‐elevation ACS state that a single hsTnT level below 5 ng/L taken greater than 3 hours after symptom onset is sufficient to rule out AMI.8 The ability to rule out AMI without serial testing is a paradigm‐changing innovation that has profound implications for ED efficiency and diagnostic utilization.
In January 2017, the U.S. Food and Drug administration (FDA) approved hsTnT for clinical use. Although Europe and Canada have years of experience with a <5 ng/L LoD, the FDA has specified that U.S. laboratories will report a limit of quantification (LoQ) of <6 ng/L. To date, there are no published data describing the test characteristics of this proposed LoQ for ruling out AMI at the time of ED arrival.
Previous studies evaluating the sensitivity of an undetectable hsTnT were single site or used a common central laboratory, thus reducing variation in assay performance. There is concern that, when translated into real‐world practice, assay imprecision and bias may yield higher variation than observed in prospective observational studies.9, 10, 11, 12, 13, 14 This is especially true for hospitals using multiple analyzers and different reagent lots. The U.K. National Institute for Health and Care Excellence has emphasized the importance of verifying test performance in real‐world practice settings.9
The objective of this study was to quantify the test characteristics of very low concentrations of hsTnT drawn at the time of ED arrival in chest pain patients at four different North American EDs using different analyzers. The test characteristics of the manufacturer's stated and FDA‐approved LoQ will be also assessed in this study population.
Methods
Study Setting and Population
This observational study retrospectively analyzed one year of prospectively collected administrative data and registry data from four adult urban EDs in Calgary, Alberta, Canada (population 1.2 million), which have combined annual ED census of 325,000 visits, including 13,000 visits for chest pain. These four hospitals share a common, linked ED information system and administrative database. One of the four hospitals is the regional percutaneous coronary intervention site, while the other three have coronary care units.
All four sites use a Roche Elecsys high‐sensitivity, fifth‐generation, troponin T assay performed on the cobas e601 instrument as per the manufacturer's specifications. This assay has a limit of blank of 3 ng/L, a LoD of 5 ng/L, a LoQ (of 20% coefficient of variation [CV]) of 6 ng/L, a 99th percentile of 14 ng/L in a healthy population outside the United States, and 19 ng/L in the United States. The assay is run on eight separate instruments across the four Calgary urban hospitals. Quality control material (Bio‐Rad Laboratories (Canada) Ltd) was run to monitor day to day test performance for all instruments (Concentration 1 = 15.5 ng/L [range = 13.8–17.0 ng/L], CV = 6.5% [range = 3.9%–11%]; Concentration 2 = 55.5 ng/L [range = 48.3–63.4 ng/L], CV = 4.2% [range = 2.2%–9.1%]; Concentration 3 = 474 ng/L [range = 445–506 ng/L], CV = 8.0% [range = 4.2%–12%]. Over the study period, three different lot numbers of reagent and two different lot numbers of calibrator were used. Results of hemolyzed samples of >100 mg/dL were not reported to the ED clinicians and samples were redrawn, and thus patients whose initial samples were hemolyzed were excluded from this analysis.
This assay has been in use at these sites since February 2012. Per local recommendations, AMI was considered ruled out if a patient's hsTnT concentration was less than 14 ng/L when measured more than 6 hours after onset of the patient's most significant symptoms.15
The study included patients age 18 years or older presenting to the participating EDs between January 1 and December 31, 2013, who were assigned a standardized triage code of “chest pain–cardiac features” or “cardiac‐type pain” (i.e., epigastric, neck, jaw or arm pain concerning for angina) by ED triage nursing staff and who had a serum hsTnT assay performed within 60 minutes of ED arrival. Patients were excluded if they presented with an ST‐elevation myocardial infarction or cardiac arrest in the ED or if they had abnormal kidney function (eGFR < 60 mL/min/m2 using the CKD‐EPI equation). The STARD patient flow diagram is shown in Figure 1. A subgroup analysis was performed including only patients who underwent serial hsTnT testing to evaluate test characteristics in a higher‐risk cohort clearly identified by treating physicians as being at risk for ACS.
Figure 1.
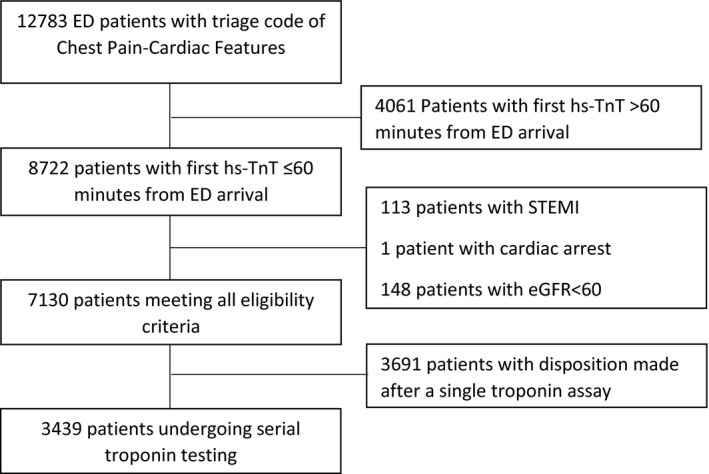
STARD patient flow diagram. hsTnT = high‐sensitivity cardiac troponin T.
Data Sources
Patients were identified using the participating EDs’ common administrative database. Outcome data were obtained by linking the ED and hospital administrative databases, Alberta provincial vital statistics, and the Alberta Provincial PRoject for Outcome Assessment in Coronary Heart disease (APPROACH) registry. APPROACH is a registry that prospectively collects data on all patients admitted with a cardiac diagnosis or who have a revascularization procedure in the province of Alberta.16 All data sources were linked using provincial personal health number, date of birth, and date of service, with a linkage success rate exceeding 99%.
The administrative databases include reliable electronic time stamps for all clinical encounters, including time of arrival, physician assessment, disposition decisions, and all diagnostic and therapeutic interventions. Diagnosis of AMI was made by clinicians based on clinical and electrocardiogram (ECG) features, hsTnT results, and results of cardiac catheterization and was ascertained using ICD‐10 codes for the primary diagnosis from hospital databases or as recorded in the APPROACH registry. Type and timing of revascularization procedures were ascertained from the APPROACH registry, and mortality was ascertained from Alberta provincial vital statistics.
The primary outcome was the incidence of AMI within 7, 30, and 90 days of ED arrival (including on the index visit). Secondary outcomes included major adverse cardiac events (MACE; all‐cause mortality, AMI, and revascularization) and MACE components. Patients whose initial hsTnT concentration was less than 15 ng/L, and who had a MACE outcome identified within 90 days in the APPROACH registry, had their outcomes adjudicated using an electronic medical record review.
Data Analysis
Descriptive statistics for the study cohort were generated. Sensitivity, specificity, NPVs, positive predictive values, and likelihood ratios for hsTnT concentrations ranging from 3 to 200 ng/L were generated. Differences in proportion of patients ruled out using different cutoffs were compared using confidence intervals (CIs) and Pearson's chi‐squared test. Statistical analyses were performed using SAS version 9.2 (SAS Institute) and R version 3.0.3 (www.r-project.org). The study was approved by the University of Calgary Conjoint Health Research Ethics Board without the need for informed consent.
Results
Demographic details and event rates of the 7,130 patients who met inclusion criteria are found in Tables 1 and 2, along with characteristics of the 3,439 patients who underwent serial hsTnT testing. Among all 7,130 patients, the 7‐, 30‐, and 90‐day AMI incidences were 411 (5.8%), 427 (6.0%), and 444 (6.2%). The 7‐, 30‐, and 90‐day MACE incidences were 494 (6.9%), 551 (7.7%), and 610 (8.6%). The number of patients with an hsTnT concentration less than 3 ng/L was 1,103 (15.5%), the number of patients with an hsTnT concentration less than 5 ng/L was 2,399 (33.7%), and the number of patients with an hsTnT concentration less than 6 ng/L was 3,009 (42.2%,). The difference in proportion of patients with hsTnT < 5 and hsTnT < 6 was statistically significant (p < 0.0001).The distribution of initial hsTnT concentrations at ED arrival for all patients is shown in Figure 2. Seven‐, 30‐, and 90‐day event rates for MACE and its components below hsTnT cutoffs of <3, <5, and <6 ng/L are shown in Table 3.
Table 1.
Patient Demographics
| Eligible Patients | Total (N = 7,130) | Patients With Serial hsTnT (n = 3,439) |
|---|---|---|
| Age (y) | Median = 55.7, IQR = 45.1–66.7 | Median = 56.69, IQR = 50.75–70.38 |
| Sex | Male 55.1%, female 44.9% | Male 58.6%, female 41.4% |
| EMS arrival | 747 (10.5%) | 476 (13.8%) |
| Median time from triage to first hsTnT assay (min) | 26, IQR = 17–39 | 24, IQR = 16–36 |
| Patients with serial hsTnT assays, n (%) | 3,439 (48.2) | 3,439 (100) |
| Initial hsTnT (ng/L), n (%) | ||
| <3 | 1,103 (15.5) | 331 (9.62) |
| <5 | 2,399 (33.6) | 800 (23.26) |
| <6 | 3,009 (42.2) | 1,026 (29.83) |
hsTnT = high‐sensitivity cardiac troponin T; IQR = interquartile range.
Table 2.
Outcomes
| Outcome | Total (N = 7,130) | Patients With Serial hsTnT (n = 3,439) | ||||
|---|---|---|---|---|---|---|
| 7 days | 30 days | 90 days | 7 days | 30 days | 90 days | |
| Primary | ||||||
| AMI | 411 (5.8) | 427 (6.0) | 444 (6.2) | 386 (11.2) | 398 (11.6) | 413 (12.0) |
| Secondary | ||||||
| Any MACE | 494 (6.9) | 551 (7.7) | 610 (8.6) | 464 (13.5) | 508 (14.8) | 547 (15.9) |
| Death | 9 (0.13) | 23 (0.32) | 52 (0.73) | 6 (0.17) | 16 (0.47) | 34 (0.99) |
| Revascularization | 267 (3.7) | 328 (4.6) | 360 (5.0) | 256 (7.4) | 307 (8.9) | 330 (9.6) |
Data are reported as n (%).
AMI = acute myocardial infarction; hsTnT = high‐sensitivity cardiac troponin T; MACE = major adverse cardiac events.
Figure 2.
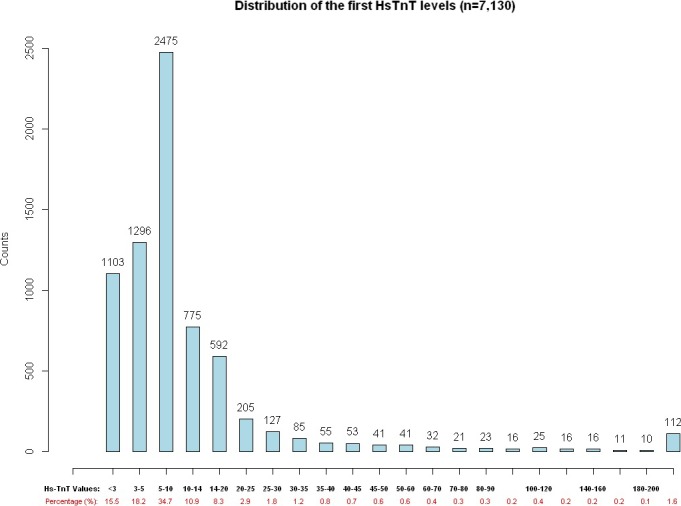
Distribution of initial hsTnT concentrations. hsTnT = high‐sensitivity cardiac troponin T.
Table 3.
Event Rates for MACE and Its Components for Patients With Initial hsTnT Concentrations Below the Cutoffs of <3, <5, and <6 ng/L (N = All 7,130 Patients)
| hsTnT Cutoff (ng/L) | Patients Ruled Out | Event | 7‐day Eventsa | 30‐day Eventsa | 90‐day Eventsa |
|---|---|---|---|---|---|
| <3 | 1,103 (15.4%; | AMI | 0 (0) | 0 (0) | 0 (0) |
| 95% CI = 14.7%–16.3%) | Death | 1 (0.09) | 1 (0.09) | 1 (0.09) | |
| Revascularization | 1 (0.09) | 2 (0.18) | 2 (0.18) | ||
| MACE | 2 (0.18) | 3 (0.27) | 3 (0.27) | ||
| <5 | 2,399 (33.6%; | AMI | 1 (0.04) | 2 (0.08) | 3 (0.13) |
| 95% CI = 32.6%–34.8%) | Death | 2 (0.08) | 2 (0.08) | 2 (0.08) | |
| Revascularization | 11 (0.46) | 16 (0.67) | 20 (083) | ||
| MACE | 13 (0.54) | 19 (0.79) | 22 (0.92) | ||
| <6 | 3,009 (42.2%; | AMI | 1 (0.03) | 2 (0.07) | 3 (0.10) |
| 95% CI = 41.1%–43.4%) | Death | 2 (0.06) | 2 (0.06) | 3 (0.10) | |
| Revascularization | 16 (0.53) | 23 (0.76) | 27 (0.90) | ||
| MACE | 17 (0.56) | 26 (0.86) | 30 (1.0) |
AMI = acute myocardial infarction; hsTnT = high‐sensitivity cardiac troponin T; MACE = major adverse cardiac events.
Data are reported as n (%).
Sensitivities and NPVs for 7‐, 30‐, and 90‐day AMI for cutoffs of <3, <5, and <6 ng/L at the time of ED arrival for the entire cohort of 7,130 patients are shown in Table 4. The sensitivity, NPV, and negative likelihood ratio for 7‐day AMI of an hsTnT concentration < 3 ng/L were 100% (95% CI = 98.7%–100%), 100% (95% CI = 99.5%–100%), and 0. The sensitivity, NPV, and negative likelihood ratio for 7‐day AMI of an hsTnT concentration < 5 ng/L were 99.8% (95% CI = 98.7%–100%), 99.9% (95% CI = 99.8%–100%), and 0.007. The sensitivity, NPV, and negative likelihood ratio for 7‐day AMI of an hsTnT concentration < 6 ng/L were 99.8% (95% CI = 98.7%–100%), 99.9% (95% CI = 99.8%–100%), and 0.005. Test characteristics for 7‐day AMI across the range of initial hsTnT concentrations from <3 to 200 ng/L are illustrated in the Data Supplement S1 (Table S1 and Figures S1a–S1c, available as supporting information in the online version of this paper, which is available at https://doi.org/onlinelibrary.wiley.com/doi/10.1111/acem.13229/full).
Table 4.
Test Characteristics of Undetectable Concentrations of hsTnT at ED Arrival for AMI (N = All 7,130 Patients)
| hsTnT Cutoff (ng/L) | Patients Ruled Out | Outcome | Events Below Cutoff | Sensitivity (95% CI) | NPV (95% CI) | LR– (95% CI) |
|---|---|---|---|---|---|---|
| <3 | 1,103 (15.4%; | 7‐day AMI | 0 | 100 | 100 | 0 |
| 95% CI = 14.7%–16.3%) | (98.7–100) | (99.5–100) | (0–NA) | |||
| 30‐day AMI | 0 | 100 | 100 | 0 | ||
| (98.7–100) | (99.5–100) | (0–NA) | ||||
| 90‐day AMI | 0 | 100 | 100 | 0 | ||
| (98.7 –100) | (99.5–100) | (0–NA) | ||||
| <5 | 2,399 (33.6%; | 7‐day AMI | 1 | 99.8 | 99.9 | 0.007 |
| 95% CI = 32.6%–34.8%) | (98.7–100) | (99.8–100) | (0.001–0.048) | |||
| 30‐day AMI | 2 | 99.5 | 99.9 | 0.013 | ||
| (98.3–99.9) | (99.8–100) | (0.003–0.052) | ||||
| 90‐day AMI | 3 | 99.3 | 99.9 | 0.019 | ||
| (98.0–99.9) | (99.8–100) | (0.006–0.058) | ||||
| <6 | 3,009 (42.2%; | 7‐day AMI | 1 | 99.8 | 99.9 | 0.005 |
| 95% CI = 41.1%–43.4%) | (98.7–100) | (99.8–100) | (0.008–0.038) | |||
| 30‐day AMI | 2 | 99.5 | 99.9 | 0.010 | ||
| (98.3–99.9) | (99.8–100) | (0.026–0.412) | ||||
| 90‐day AMI | 3 | 99.3 | 99.9 | 0.015 | ||
| (98.0–99.9) | (99.8–100) | (0.005–0.046) |
AMI = acute myocardial infarction; hsTnT = high‐sensitivity cardiac troponin T; LR– = negative likelihood ratio; NPV = negative predictive value.
Sensitivity and NPVs for 7‐, 30‐, and 90‐day MACE for cutoffs of <3, <5, and <6 ng/L at the time of ED arrival for the entire cohort of 7,130 patients are shown in Table 5. The sensitivity, NPV, and negative likelihood ratio for 7‐day MACE of an hsTnT concentration < 3 ng/L were 99.6% (95% CI = 98.5%–99.9%), 99.8% (95% CI = 99.5%–100%), and 0.024. The sensitivity, NPV, and negative likelihood ratio for 7‐day MACE of an hsTnT concentration < 5 ng/L were 97.4 (95% CI = 95.6%–98.5%), 99.5 (95% CI = 99.1%–99.7%), and 0.073. The sensitivity, NPV, and negative likelihood ratio for 7‐day MACE of an hsTnT concentration < 6 ng/L were 96.6% (95% CI = 94.6%–97.8%), 99.4% (95% CI = 98.6%–99.4%), and 0.076. Sensitivity and specificity for 7‐day MACE across the range of initial hsTnT concentrations from <3 to 200 ng/L are illustrated in Data Supplement S1 (Table S2).
Table 5.
Test Characteristics of Undetectable Concentrations of hsTnT at ED Arrival for MACE (N = All 7,130 Patients)
| hsTnT Cutoff (ng/L) | Patients Ruled Out | Outcome | Events Below Cutoff | Sensitivity (95% CI) | NPV (95% CI) | LR– (95% CI) |
|---|---|---|---|---|---|---|
| <3 | 1,103 (15.4%; | 7‐day MACE | 2 | 99.6 | 99.8 | 0.024 |
| 95% CI = 14.7%–16.3%) | (98.5–99.9) | (99.3–100) | (0.006–0.097) | |||
| 30‐day MACE | 3 | 99.4 | 99.7 | 0.033 | ||
| (98.2–99.8) | (99.2–99.9) | (0.011–0.101) | ||||
| 90‐day MACE | 3 | 99.5 | 99.7 | 0.029 | ||
| (98.6–99.8) | (99.2–99.9) | (0.009–0.090) | ||||
| <5 | 2,399 (33.6%; | 7‐day MACE | 13 | 97.4 | 99.5 | 0.073 |
| 95% CI = 32.6%–34.8%) | (95.6–98.5) | (99.1–99.7) | (0.043–0.125) | |||
| 30‐day MACE | 19 | 96.6 | 99.2 | 0.095 | ||
| (94.7–97.8) | (98.8–99.5) | (0.061–0.148) | ||||
| 90‐day MACE | 22 | 96.4 | 99.1 | 0.099 | ||
| (94.6–97.6) | (98.6–99.4) | (0.066–0.149) | ||||
| <6 | 3,009 (42.2%; | 7‐day MACE | 17 | 96.6 | 99.4 | 0.076 |
| 95% CI = 41.1%–43.4%) | (94.6–97.8) | (99.1–99.7) | (0.047–0.121) | |||
| 30‐day MACE | 26 | 95.3 | 99.1 | 0.103 | ||
| (93.2–96.8) | (98.7–99.4) | (0.071–0.150) | ||||
| 90‐day MACE | 30 | 95.1 | 99.0 | 0.107 | ||
| (93.1–96.5) | (98.6–99.3) | (0.075–0.151) |
hsTnT = high‐sensitivity cardiac troponin T; LR– = negative likelihood ratio; MACE = major adverse cardiac events; NPV = negative predictive value.
Among the 3,439 patients who underwent serial troponin testing, the 7‐, 30‐, and 90‐day AMI incidences were 386 (11.2%), 398 (11.6%), and 413 (12.0%). The 7‐, 30‐, and 90‐day MACE incidences were 464 (13.5%), 508 (14.8%), and 547 (15.9%). The number of patients with an hsTnT concentration less than 3 ng/L was 331 (9.6%), the number of patients with an hsTnT concentration less than 5 ng/L was 800 (23.3%), and the number of patients with an hsTnT concentration less than 6 ng/L was 1026 (29.8%; Table 1). Sensitivity for AMI and MACE was largely unchanged compared to the entire cohort (Tables 6 and 7). NPV and negative likelihood ratios for AMI were also similar between the overall cohort and the higher‐risk subgroup (Table 6). However, the NPV and negative likelihood ratios for MACE were worse in the higher‐risk subgroup, particularly for hsTnT concentrations of <5 and <6 ng/L (Table 7).
Table 6.
Test Characteristics of Undetectable Concentrations of hsTnT at ED Arrival for AMI Among 3,439 Patients With Serial Troponins Performed
| hsTnT Cutoff (ng/L) | Patients Ruled Out | Outcome | Events Below Cutoff | Sensitivity (95% CI) | NPV (95% CI) | LR– (95% CI) |
|---|---|---|---|---|---|---|
| <3 | 331 (9.6%; | 7‐day AMI | 0 | 100 | 100 | 0 |
| 95% CI = 8.7%–10.7%) | (98.6–100) | (98.3–100) | (0–NA) | |||
| 30‐day AMI | 0 | 100 | 100 | 0 | ||
| (98.6–100) | (98.3–100) | (0–NA) | ||||
| 90‐day AMI | 0 | 100 | 100 | 0 | ||
| (98.6–100) | (98.3–100) | (0–NA) | ||||
| <5 | 800 (23.33%; | 7‐day AMI | 1 | 99.7 | 99.9 | 0.009 |
| 95% CI = 21.9%–24.7%) | (98.6–99.9) | (99.3–100) | (0.001–0.070) | |||
| 30‐day AMI | 2 | 99.5 | 99.8 | 0.019 | ||
| (98.3–99.9) | (99.1–100) | (0.005–0.076) | ||||
| 90‐day AMI | 2 | 99.5 | 99.8 | 0.018 | ||
| (98.3–99.9) | (99.1–100) | (0.005–0.073) | ||||
| <6 ng/L | 1,026 (29.8%; | 7‐day AMI | 1 | 99.7 | 99.9 | 0.008 |
| 95% CI = 28.3%–31.4%) | (98.6–100) | (99.5–100) | (0.001–0.055) | |||
| 30‐day AMI | 2 | 99.5 | 99.8 | 0.014 | ||
| (98.2–99.9) | (99.3–100) | (0.014–0.011) | ||||
| 90‐day AMI | 2 | 99.5 | 99.8 | 0.015 | ||
| (98.3–99.9) | (99.3–100) | (0.014–0.016) |
AMI = acute myocardial infarction; hsTnT = high‐sensitivity cardiac troponin T; LR– = negative likelihood ratio; NPV = negative predictive value.
Table 7.
Test Characteristics of Undetectable Concentrations of hsTnT at ED Arrival for MACE Among 3,439 Patients With Serial Troponins Performed
| hsTnT Cutoff (ng/L) | Patients Ruled Out | Outcome | Events Below Cutoff | Sensitivity (95% CI) | NPV (95% CI) | LR– (95% CI) |
|---|---|---|---|---|---|---|
| <3 | 331 (9.6%; | 7‐day MACE | 1 | 99.8 | 99.7 | 0.019 |
| 95% CI = 8.7%–10.7%) | (98.8,100) | (93.3–100) | (0.003–0.138) | |||
| 30‐day MACE | 2 | 99.6 | 99.4 | 0.035 | ||
| (98.6–100) | (97.8,100) | (0.009–0.140) | ||||
| 90‐day MACE | 2 | 99.6 | 99.4 | 0.032 | ||
| (98.6–100) | (97.8,100) | (0.008–0.129) | ||||
| <5 | 800 (23.33%; | 7‐day MACE | 12 | 97.4 | 98.5 | 0.098 |
| 95% CI = 21.9%–24.7%) | (95.5–98.7) | (99.1–99.7) | (0.056–0.171) | |||
| 30‐day MACE | 18 | 96.5 | 97.8 | 0.133 | ||
| (94.5–97.9) | (96.5–98.6) | (0.084–0.209) | ||||
| 90‐day MACE | 19 | 96.5 | 97.6 | 0.129 | ||
| (94.6–97.9) | (96.3–98.6) | (0.082–0.201) | ||||
| <6 | 1,026 (29.8%; | 7‐day MACE | 16 | 96.6 | 98.4 | 0.102 |
| 95% CI = 28.3%–31.4%) | (94.5–98.0) | (97.5–99.1) | (0.063–0.165) | |||
| 30‐day MACE | 25 | 95.1 | 97.6 | 0.144 | ||
| (92.8–96.9) | (96.4–98.4) | (0.098–0.212) | ||||
| 90‐day MACE | 26 | 95.3 | 97.5 | 0.138 | ||
| (93.1–96.9) | (96.3–98.3) | (0.094–0.201) |
hsTnT = high‐sensitivity cardiac troponin T; LR– = negative likelihood ratio; MACE = major adverse cardiac events; NPV = negative predictive value.
Two patients with an hsTnT concentration of <6 ng/L (0.1%) died of a noncardiac cause within 7 days of their index visit, and a third died of a noncardiac cause approximately 6 weeks after their index visit (Table 8). One patient with an hsTnT concentration < 6 ng/L (0.04%) had a diagnosis of AMI within 7 days of their index ED visit, while two additional patients with an initial hsTnT < 6 ng/L had an AMI greater than 30 days after their index visit. Characteristics and diagnoses of patients with an initial hsTnT < 6 ng/L who died or were diagnosed with AMI are shown in Table 6. Test characteristics of the assay were similar across the participating sites, as shown in Data Supplement S1 (Table S3).
Table 8.
Patients With 90‐Day MI or Death Missed by hsTnT < 6 ng/L at ED Arrivala
| Initial hsTnT (ng/L) | Outcome | Clinical Features |
|---|---|---|
| 4 | AMI < 90 d | ED visit for possible angina Sep 2, 2013; discharged after investigations with outpatient follow‐up. Return ED visit with NSTEMI and PCI on Oct 4, 2013. |
| 4 | AMI < 30 d | Chest pain presentation, serial hsTnT concentrations 4 and 5 ng/L. Discharged with outpatient follow‐up. Re‐presented 29 days after index ED visit with chest pain, hsTnT concentration 26 and 28 ng/L, maximum hsTnT 82 ng/L. Underwent PCI on Day 30 post‐ED index visit. |
| 4 | Acute MI < 7 d | Chest pain. Initial hsTnT level 4 ng/L. Serial TnT level 86 ng/L 4 h after ED arrival. Admitted and underwent PCI. |
| <3 | Noncardiac death < 7 d | Chest pain, dyspnea, and hypoxemia. Initial hsTnT < 3 ng/L. AMI ruled out. Died same day of pneumosepsis. |
| 4 | Noncardiac death < 7 d | Chest and neck pain suggestive of angina, initial hsTnT 4 ng/L. AMI excluded. Diagnosed with aneurysmal subarachnoid hemorrhage in ED and subsequently died. |
| 5 | Noncardiac death < 90 d | Known hepatocellular carcinoma and chest pain. Discharged after AMI ruled out. Readmitted 6 wk later with fulminant liver failure and hepatorenal syndrome. Died in hospital. |
AMI = acute myocardial infarction; hsTnT = high‐sensitivity cardiac troponin T; MI = myocardial infarction; PCI = percutaneous coronary intervention.
Twenty‐four other patients had an initial hsTnT < 6 ng/L on ED arrival and underwent revascularization within 90 days. None of these patients died or had a diagnosis of AMI.
Discussion
The findings of this large multisite study, using hsTnT results obtained in real‐world clinical practice, suggest that AMI can be ruled out in patients with very low concentrations of hsTnT at ED arrival. ED chest pain patients in this study cohort who have hsTnT concentration less than 6 ng/L at the time of ED arrival had a 90‐day AMI risk of 0.13%. In this large cohort, a single biomarker drawn shortly after ED arrival could have ruled out AMI in up to 40% of patients.
This finding is consistent with previous prospective studies evaluating the test characteristics of an undetectable hsTnT concentration at the time of ED arrival.6, 7 These studies found sensitivity of a cutoff of 5 ng/L between 97 and 100%. Sensitivity is further improved when applied only to patients with nonischemic ECG findings. These studies generally included patients who had experienced maximal symptoms at minimum 3 hours prior to the initial hsTnT assay. Our data do not include timing of symptoms or ECG findings. However, we support the view that a single‐troponin rule‐out strategy should be used only in patients whose time from maximal symptoms is 3 hours or more and who have nonischemic ECG findings.
Previous studies, including multicenter investigations, have all used a central laboratory to perform their hsTnT assay and mostly on frozen samples, which are handled differently than fresh samples used in clinical practice. Thus, there is concern with respect to these studies that assay imprecision will be minimized, spuriously improving the assay's test characteristics.10, 11, 12, 13, 14 This study confirms that previous findings are robust when the assay is performed in real‐world clinical conditions on multiple analyzers in different hospitals, supporting the European Society for Cardiology recommendation that an hsTnT concentration < 5 ng/L at the time of ED arrival can be used to rule out AMI in patients presenting more than 3 hours from the onset of their symptoms.
These data also indicate that an hsTnT concentration below the LoQ value of 6 ng/L approved by the U.S. FDA has excellent sensitivity for AMI at the time of ED arrival. Compared to the LoD of the assay of <5 ng/L, the <6 ng/L LoQ endorsed by the FDA can rule out AMI in significantly more patients (42.2% vs. 33.6%, p < 0.0001) while maintaining similar sensitivity for AMI and having only a small incremental loss in sensitivity for MACE.
It is important to note that this single‐biomarker strategy had comparatively low sensitivity for MACE. The sensitivity for 7‐day MACE of an hsTnT concentration < 6 ng/L was only 96.6%, and the lower bound of the 95% CI for the NPV for MACE was only 99.1%. Test characteristics for 7‐day MACE were worse in the higher‐risk subgroup of patients who underwent serial troponin testing. Thus, the test characteristics of a single‐troponin strategy for MACE likely do not meet an often‐cited acceptable miss rate for ACS of less than 1%.3, 4 Our findings reinforce the notion that maximizing sensitivity for all patients with ACS should involve combining biomarker results with a clinical risk assessment and ECG findings. Indeed, the majority of patients in this cohort with a 7‐day MACE missed by the hsTnT assay were diagnosed clinically as unstable angina and admitted for further investigation and treatment. We therefore recommend that a single‐troponin rule‐out strategy only be used in conjunction with ECG findings and a structured risk assessment to identify low‐risk patients suitable for early discharge from the ED.17
Limitations
This is not a prospective research protocol. Rather, it is a retrospective, pragmatic study examining test characteristics of the hsTnT assay as used in clinical practice in multiple hospitals. This work gives an indication of how the assay performs in a real‐world conditions when multiple assay lots are used on several analyzers over 1 year. Patients were identified based on the triage code assigned by a triage nurse. Moreover, because a significant portion of patients had an initial troponin drawn as part of a nurse‐initiated protocol, it is possible that a slightly lower‐risk patient population was included in this study. However, the triage complaints of chest pain–cardiac features and cardiac‐type pain used to identify patients correspond to the AHA research definition of potential ACS symptoms and has been shown to have both construct and outcome validity.18 We are reassured that patient demographics and outcome rates are similar to other North American cohorts19, 20 and that the hsTnT assay's test characteristics are preserved in a higher‐risk subgroup. We caution that because the inclusion criteria focused on anginal‐type pain, these findings may not be generalizable to patients with atypical primary symptoms such as dyspnea or nausea.
Outcomes were ascertained using administrative and registry data, and the diagnosis of AMI was made clinically by attending physicians. Although outcomes were only adjudicated for patients with outcomes and an hsTnT < 15 ng/L, the administrative and registry data used for outcome ascertainment have been shown to be highly reliable for the diagnosis of recent AMI when compared to adjudicated data from medical records.21 Alberta Vital Statistics and the APPROACH registry capture all deaths, AMI, and revascularization outcomes in the province of Alberta, thereby minimizing the risk of missed outcomes.16 Overestimation of sensitivity and NPV because of false‐negative misclassification is unlikely given prior validation of AMI diagnosis in this administrative data.
As with any study evaluating the test characteristics of troponin assays for AMI, our findings may suffer from incorporation bias in that one outcome—AMI—is defined largely by a positive troponin result. This may bias test characteristic estimates upward and is a challenge affecting nearly all evaluations of high‐sensitivity troponin assays.6, 7
Conclusions
This study, in a large cohort of ED patients undergoing evaluation for possible acute chest syndrome, suggests that the Food and Drug Administration–approved limit of quantification for an high‐sensitivity cardiac troponin T assay has very high sensitivity for the diagnosis of acute myocardial infarction, and may be useful for the rapid rule‐out of acute myocardial infarction of up to 40% of ED chest pain patients. While this biomarker‐only testing strategy performs very well for ruling out acute myocardial infarction, it is less sensitive for all major adverse cardiac events. It is thus important to use a single‐troponin rule‐out strategy in combination with electrocardiogram findings and structured clinical evaluation to ensure patients at high risk of MACE are identified and appropriately investigated.
The authors acknowledge the assistance of Katrina Koger in the preparation of the manuscript.
Supporting information
Data Supplement S1. Supplementary appendix.
Academic Emergency Medicine 2017;24:1267–1277.
This study was funded by an operating grant from the Canadian Institutes of Health Research (MOP‐130316).
PK reports grants/honorariums/consultant/advisor fees from Abbott Laboratories, Abbott Point of Care, Beckman Coulter, Ortho Clinical Diagnostics, Roche Diagnostics, and Siemens Healthcare Diagnostics with respect to cardiac troponin testing. McMaster University has filed patents with PK as an inventor in the acute cardiac biomarkers area. JA, AM, GI, and EL have received research grants from Roche Diagnostics for an unrelated cardiac troponin study.
A related article appears on page 1278.
References
- 1. Goodacre S, Cross E, Arnold J, Angelini K, Capewell S, Nicholl J. The healthcare burden of acute chest pain. Heart 2005;91:229–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Buhuiya FA, Pitts SR, McCaig LF. Emergency department visits for chest pain and abdominal pain: United States, 1999–2008. NCHS Data Brief 2010;(43):1–8. [PubMed] [Google Scholar]
- 3. Than M, Herbert M, Flaws D, et al. What is an acceptable risk of major adverse cardiac event in chest pain patients soon after emergency department discharge from the emergency department? A clinical survey. Int J Cardiol 2013;166:752–4. [DOI] [PubMed] [Google Scholar]
- 4. MacGougan CK, Christenson J, Innes G, Raboud J. Emergency physicians’ attitudes toward a clinical prediction rule for the identification and early discharge of low risk patients with chest discomfort. CJEM 2001;3:89–94. [DOI] [PubMed] [Google Scholar]
- 5. Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 ACC/AHA Guideline for the management of patients with non‐ST‐elevation acute coronary syndromes. J Am Coll Cardiol 2014;64:e143–228. [DOI] [PubMed] [Google Scholar]
- 6. Zhelev Z, Hyde C, Youngman E, et al. Diagnostic accuracy of single baseline measurement of Elecsys troponin T high‐sensitive assay for diagnosis of myocardial infarction in the emergency department: a systematic review and meta‐analysis. BMJ 2015;350:h15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pickering JW, Than MP, Cullen L, et al. Rapid rule‐out of acute myocardial infarction with a single high‐sensitivity cardiac troponin T measurement below the limit of detection: a collaborative meta‐analysis. Ann Intern Med 2017;166:715–24. [DOI] [PubMed] [Google Scholar]
- 8. Roffi M, Patrono C, Collet JP, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation. Eur Heart J 2016;37:267–315. [DOI] [PubMed] [Google Scholar]
- 9. National Institute for Health and Care Excellence . Myocardial Infarction (acute): Early rule out using high‐sensitivity troponin tests (Elecsys Troponin T High‐Sensitive, ARCHITECT STAT High Sensitive Troponin I and AccuTnI+3 assays)(DG15). 7: Recommendations for Further Research. 2014. Available at: https://www.nice.org.uk/guidance/dg15/chapter/7-Recommendations-for-further-research Accessed Sep 20, 2016.
- 10. Kavsak P. High‐five for high‐sensitivity cardiac troponin T: depends on the precision and analytic platform. JAMA Intern Med 2013;173:477. [DOI] [PubMed] [Google Scholar]
- 11. Kavsak PA, Hill SA, McQueen MJ, Devereaux PJ. Implications of adjustment of high‐sensitivity cardiac troponin T assay. Clin Chem 2013;59:574–6. [DOI] [PubMed] [Google Scholar]
- 12. Kavsak PA, Don‐Wauchope AC, Hill SA, Worster A. Acceptable analytic variation may exceed high‐sensitivity cardiac troponin I cutoffs in early rule‐out and rule‐in myocardial infarction algorithms. Clin Chem 2016;62:887–9. [DOI] [PubMed] [Google Scholar]
- 13. Kavsak PA, Beattie J, Pickersgill R, Ford L, Caruso N, Clark L. A practical approach for the validation and clinical implementation of a high‐sensitivity cardiac troponin I assay across a North American city. Pract Lab Med 2015;1:28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lyon AW, Kavsak PA, Lyon OA, Worster A, Lyon ME. Simulation models of misclassification error for single thresholds of high‐sensitivity cardiac troponin I due to assay bias and imprecision. Clin Chem 2017;63:585–92. [DOI] [PubMed] [Google Scholar]
- 15. Crowder KR, Jones T, Lang ES, et al. The impact of high‐sensitivity troponin implementation on hospital operations and patient outcomes in 3 tertiary care centers. Am J Emerg Med 2015;33:1790–4. [DOI] [PubMed] [Google Scholar]
- 16. Ghali WA, Knudtson ML. Overview of the Alberta Provincial Project for Outcome Assessment in Coronary Heart Disease. On behalf of the APPROACH investigators. Can J Cardiol 2000;16:1225–30. [PubMed] [Google Scholar]
- 17. Carlton EW, Khattab A, Greaves K. Identifying patients suitable for discharge after a single‐presentation high‐sensitivity troponin result: a comparison of five established risk scores and two high‐sensitivity assays. Ann Emerg Med 2015;66:635–45. [DOI] [PubMed] [Google Scholar]
- 18. Bullard MJ, Thomas R, Villa Roel C, Vester M, Rowe BH. Construct and outcome validity of CTAS Chest Pain. CJEM 2012;14(S1):S17. [Google Scholar]
- 19. Mahler SA, Riley RF, Hiestand BC, et al. The HEART Pathway randomized trial: identifying emergency department patients with acute chest pain for early discharge. Circ Cardiovasc Qual Outcomes 2015;8:195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stopyra SA, Miller CD, Hiestand BC, et al. Performance of the EDACS accelerated discharge protocol in a cohort of US patients with acute chest pain. Crit Pathw Cardiol 2015;14:134–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Quan H, Parsons G, Ghali WA. Validity of information on comorbidity derived from ICD‐9‐CCM administrative data. Med Care 2002;40:675–85. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Supplement S1. Supplementary appendix.


