Abstract
This work reports the synthesis of three multimeric RGD peptidomimetic‐paclitaxel conjugates featuring a number of αVβ3 integrin ligands ranging from 2 to 4. These constructs were assembled by conjugation of the integrin αVβ3 ligand cyclo[DKP‐RGD]‐CH2NH2 with paclitaxel via a 2′‐carbamate with a self‐immolative spacer, the lysosomally cleavable Val‐Ala dipeptide linker, a multimeric scaffold, a triazole linkage, and finally a PEG spacer. Two monomeric conjugates were also synthesized as reference compounds. Remarkably, the new multimeric conjugates showed a binding affinity for the purified integrin αVβ3 receptor that increased with the number of integrin ligands (reaching a minimum IC50 value of 1.2 nm for the trimeric), thus demonstrating that multivalency is an effective strategy to strengthen the ligand–target interactions.
Keywords: antitumor agents, click chemistry, integrins, multivalency, peptidomimetics
Nature makes widespread use of multivalency to create strong yet reversible interactions. In multivalent interactions, several covalently linked ligands bind to clustered receptors, with multiple simultaneous molecular recognition interactions. As a result, bond reinforcement occurs and strong overall binding is achieved even when the individual interactions are weak.1 In the last decade, multimeric ligands of cancer‐overexpressed receptors have been exploited for different kinds of tumor targeting, such as drug‐targeting,2 imaging,3 and the use of ′theranostic′ compounds.4 In this context, multivalency can be envisaged as a way to improve the tumor‐targeting performance of small molecule–drug conjugates (SMDCs), with the final goal of approaching the efficiency of the antibody–drug conjugates (ADCs).5 Indeed, SMDCs possessing multivalent ligands are expected to display enhanced affinity and selectivity for the corresponding tumor receptors, thus promoting more effectively drug accumulation at the diseased tissue.
In recent years, much research effort has been devoted to the development of SMDCs targeting integrin αVβ3,6 a transmembrane heterodimeric receptor that is overexpressed on the cell surface of various tumor types (e.g., melanoma, glioblastoma, ovarian, prostatic, and breast cancer).7 We entered this research field reporting a low‐nanomolar αVβ3 integrin ligand (compound 1 in Figure 1) featuring the Arg‐Gly‐Asp (RGD) sequence (i.e., the binding epitope of the endogenous ligand for this integrin) connected to a trans‐diketopiperazine (DKP) scaffold.8 Remarkably, ligand 1 was found to be 33 times more selective for integrin αVβ3 with respect to integrin αVβ5 in competitive binding assays with biotinylated vitronectin (IC50=4.5±1.1 nm vs. 149±25 nm).8 Later on, the functionalized ligand cyclo[DKP‐RGD]‐CH2NH2 (compound 2 in Figure 1), featuring a primary amino group, was prepared.9 The latter compound was conjugated to different payloads, such as the anticancer drug paclitaxel (PTX, compound 3 in Figure 1),9 a pro‐apoptotic SMAC (second mitochondria‐derived activator of caspases) mimetic compound10 and an anti‐angiogenic VEGFR‐targeting decapentapeptide,11 by means of ester and amide linkages. As a further step, to achieve selective release of PTX in the cancer cell environment, we synthesized conjugates of the cyclo[DKP‐RGD]‐CH2NH2 ligand 2 with paclitaxel (3) via a 2′‐carbamate with a self‐immolative spacer and the lysosomally cleavable linkers (Val‐Ala and Phe‐Lys dipeptide sequences).12 Notably, despite its remarkable size, the cyclo[DKP‐RGD]‐Val‐Ala‐PTX conjugate 4 (Figure 1) retained a very good affinity for the αVβ3 integrin receptor (IC50=13.3±3.6 nm in competitive binding assays with biotinylated vitronectin) and displayed fairly effective integrin targeting.12a
Figure 1.
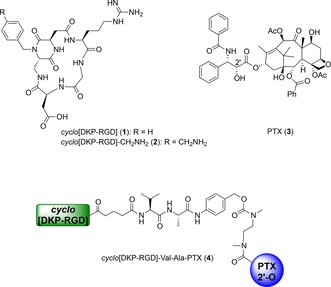
Molecular structures of the αVβ3 integrin ligand cyclo[DKP‐RGD] 1, its functionalized analogue 2, the cytotoxic drug paclitaxel (PTX) 3, and the SMDC cyclo[DKP‐RGD]‐Val‐Ala‐PTX 4.
Herein, we report our initial efforts to exploit multivalency for increasing the binding affinity of RGD ligands to integrin αVβ3.13 Thus, we set to synthesize a series of compounds (Figure 2) in which PTX is conjugated to one (compounds 5 and 6), two (compound 7), three (compound 8), and four cyclo[DKP‐RGD] ligands (compound 9), respectively. In this context, the new conjugates were designed to release PTX intracellularly14 by means of a self‐immolative spacer (PABC‐N,N′‐dimethylethylenediamine) and a lysosomally cleavable dipeptide linker (Val‐Ala),12 which connects PTX to a multivalent scaffold (Figure 2 A). The latter, in turn, is linked to the cyclo[DKP‐RGD] ligand(s) via triazole group(s) deriving from copper‐catalyzed azide‐alkyne cycloaddition (CuAAC “click” reaction).15 To connect the cyclo[DKP‐RGD] ligands to the scaffolds, tetraethylene glycol (PEG‐4) spacers were employed in order to make the conjugates more water‐soluble and flexible, which is reported to facilitate the binding to the receptor (Figure 2 A).16 The choice of short‐sized PEG spacers was made with the aim of minimizing the formation of bulky loops that can interfere with binding.17 With the exception of commercially available 4‐pentynoic acid (10) and of the previously reported acid 11,18 the alkyne scaffolds used for the synthesis of conjugates 5–9 (Figure 3) are new compounds, whose synthesis and characterization are described in the Supporting Information. The synthesis of conjugates 5–9 was carried out according to a common synthetic strategy, shown in Scheme 1. The bis‐protected compound 15, featuring the Val‐Ala linker connected to the para‐aminobenzyl carbamate (PABC)‐N,N′‐dimethylethylenediamine self‐immolative spacer, was prepared according to a methodology reported by our group.12a Compound 15 was Fmoc‐deprotected and the resulting crude free amine was coupled to scaffolds 10–14, affording the corresponding amides 16 a–e in good yields (71–92 %). Compounds 16 a–e were treated with trifluoroacetic acid for Boc removal and then reacted with 2′‐(4‐nitrophenoxycarbonyl)paclitaxel 17,12a affording carbamates 18 a–e again in satisfying yields (66–93 %). Finally, alkynes 18 a–b and polyalkynes 18 c–e were subjected to CuAAC reaction with cyclo[DKP‐RGD]‐PEG‐azide 19, prepared in two steps from cyclo[DKP‐RGD]‐CH2NH2 (2) as described in the Supporting Information. This reaction gave the target compounds 5–9 in good to excellent yields (62 %–quantitative).
Figure 2.
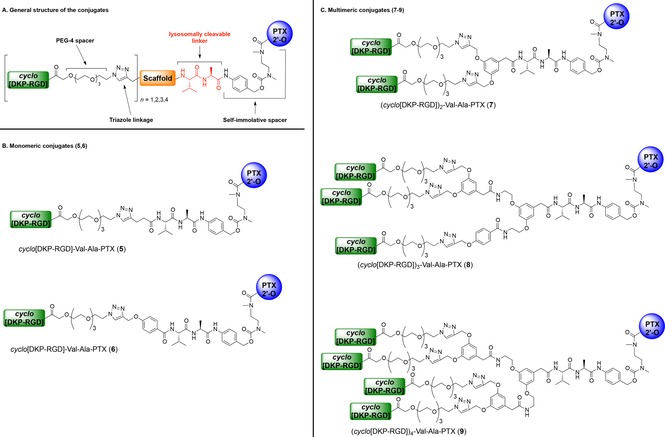
A) General structure of the conjugates. B) Molecular structures of monomeric conjugates (5, 6). C) Molecular structures of multimeric conjugates (7–9).
Figure 3.
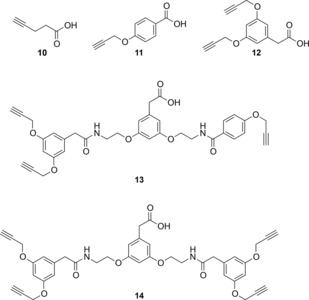
Mono‐ and polyalkyne scaffolds used for the preparation of conjugates 5–9.
Scheme 1.
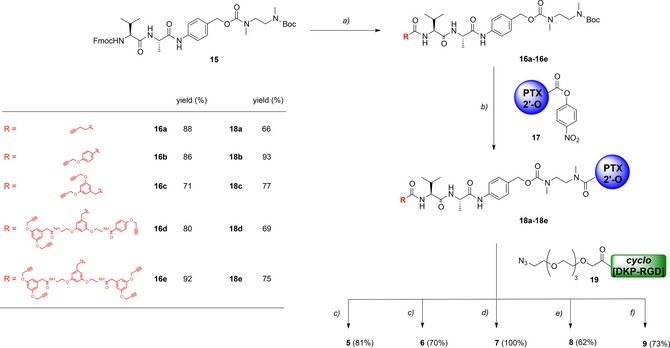
Synthesis of (cyclo[DKP‐RGD])n‐Val‐Ala‐PTX (n=1, 2, 3, or 4) conjugates 5 – 9. Reagents and conditions: a) 1) piperidine (5 equiv), DMF, RT, 2 h; 2) acids 10 – 14 (1.5 equiv), HATU (1.7 equiv), HOAt (1.7 equiv), iPr2NEt (4 equiv), DMF, RT, overnight (16 a – 16 e); b) 1) 1:2 TFA/CH2Cl2, 45 min; 2) 17 (1.5 equiv), iPr2NEt (4 equiv), DMF, RT, overnight; c) 19 (1 equiv) 18 a or 18 b (1.5 equiv), CuSO4 ⋅5 H2O (0.5 equiv), sodium ascorbate (0.6 equiv), 1:1 DMF/H2O, 30 °C, overnight; d) 18 c (1 equiv), 19 (3 equiv) CuSO4 ⋅5 H2O (1 equiv), sodium ascorbate (1.2 equiv), 1:1 DMF/H2O, 30 °C, overnight; e) 18 d (1 equiv), 19 (3.6 equiv) CuSO4 ⋅5 H2O (1.5 equiv), sodium ascorbate (1.8 equiv), 1:1 DMF/H2O, 30 °C, overnight; f) 18 e (1 equiv), 19 (4.8 equiv) CuSO4 ⋅5 H2O (2 equiv), sodium ascorbate (2.4 equiv), 1:1 DMF/H2O, 30 °C, overnight.
To assess the effect of ligand multipresentation on conjugates’ binding properties, (cyclo[DKP‐RGD])n‐Val‐Ala‐PTX (n=1–4) conjugates 5–9 were examined in vitro for their ability to inhibit biotinylated vitronectin binding to the purified αVβ3 receptor and were compared to the unconjugated ligand 1. The screening assays were performed by incubating the immobilized integrin receptors with solutions of the RGD‐PTX conjugates at different concentrations (10−12 to 10−5 m) in the presence of biotinylated vitronectin (1 μg mL−1) and measuring the concentration of bound vitronectin (Figure 4). The IC50 values are listed in Table 1.
Figure 4.
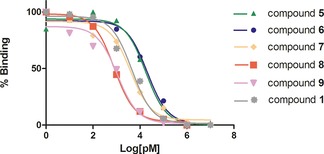
Inhibition of the binding of biotinylated vitronectin to αvβ3 integrin. A representative curve was selected for each compound. X‐axis shows the concentration of the tested compounds 1, 5–9 in logarithmic scale; Y‐axis shows the percentage of inhibition of the binding of biotinylated vitronectin in the presence of the tested compounds. Experimental data were fitted with the software, as described in the Supporting Information.
Table 1.
Inhibition of biotinylated vitronectin binding to the αvβ3 receptor.
| Entry | Cpd | Structure | αvβ3 IC50 [nm][a] | Rp/n [b] |
|---|---|---|---|---|
| 1 | 5 | cyclo[DKP‐RGD]‐Val‐Ala‐PTX (aliphatic scaffold) | 14.8±3.9 | – |
| 2 | 6 | cyclo[DKP‐RGD]‐Val‐Ala‐PTX (aromatic scaffold) | 27.3±9.8 | – |
| 3 | 7 | (cyclo[DKP‐RGD])2‐Val‐Ala‐PTX | 4.0±0.1 | 3.4 |
| 4 | 8 | (cyclo[DKP‐RGD])3‐Val‐Ala‐PTX | 1.2±0.5 | 7.6 |
| 5 | 9 | (cyclo[DKP‐RGD])4‐Val‐Ala‐PTX | 1.3±0.3 | 5.3 |
| 6 | 1 | cyclo[DKP‐RGD] | 4.5±0.1 | – |
[a] IC50 values were calculated as the concentration of compound required for 50 % inhibition of biotinylated vitronectin binding, as estimated by GraphPad Prism software. All values are the arithmetic mean ± the standard deviation (SD) of triplicate determinations. [b] The relative potency Rp is obtained by dividing the IC50 of the monovalent reference 6 by the IC50 of each multivalent conjugate. Rp/n values were calculated by dividing Rp of the multivalent conjugates by the valency (n) of each conjugate.22
As can be observed in Table 1, conjugates 5 (entry 1) and 6 (entry 2), featuring only one cyclo[DKP‐RGD] ligand moiety, displayed slightly reduced binding ability (3‐fold and 6‐fold increase of IC50, respectively) compared to the free ligand 1 (entry 6). To our delight, when the number of cyclo[DKP‐RGD] ligand moieties in the conjugates increases from 1 to 3, a clear trend of IC50 decrease can be observed (entries 1–2→3→4), to reach an IC50 lower than that of the free ligand 1 (entry 4 vs. entry 6). However, with the trimeric conjugate 8 a plateau is reached (entry 4, Rp/n=7.6), and no further improvement is obtained when an additional cyclo[DKP‐RGD] ligand is present (conjugate 9, entry 5, Rp/n=5.3). These data demonstrate that multiple presentation of the integrin ligand leads to a significant improvement of the binding affinity,13 although this effect seems to be partially balanced by the increasing steric bulk.
In conclusion, five new conjugates (5–9), featuring a number of cyclo[DKP‐RGD] αVβ3 integrin ligands ranging from 1 to 4 have been synthesized using a straightforward modular approach. Binding tests carried out with the purified receptor of integrin αVβ3 (displacement of biotinylated vitronectin) show that the IC50 decrease with increasing number of ligand moieties, down to a plateau reached with the trimeric conjugate 8 (IC50=1.2 nm, Rp/n=7.6). These results demonstrate that multivalency is a valuable tool to enhance the integrin targeting performance of this kind of conjugates, and may represent a possible way to improve the in vivo tumor‐targeting properties of RGD conjugates, which are often suboptimal.3b,3d,3h, 6e Moreover, it should be noted that the new ligands are also suitable for conjugation to different kinds of ′smart′ linkers such as those amenable to extracellular cleavage19 (for example, by matrix metalloproteinases20 or elastases21).
Experimental Section
Cyclo[DKP‐RGD]‐CH2NH2 (2),9 Fmoc‐Val‐Ala‐N‐[4‐[[[(N‐(Boc)‐N,N′‐dimethylethylenediamine)carbonyl]oxy]methyl]phenyl] (15)12 and 2′‐(4‐nitrophenoxycarbonyl)paclitaxel (17),12 were prepared according to literature procedures, and their analytical data were in agreement with those already published. The synthetic procedures for the preparation of compounds 5–9 and 11–14 are reported in the Supporting Information, along with the 1H NMR and 13C NMR spectra, the HPLC traces and HRMS spectra. The inhibition assays of biotinylated vitronectin binding to the αvβ3 receptor for compounds 1 and 5–9 are reported in the Supporting Information.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary
Acknowledgements
We thank the University of Milan for PhD fellowships (to A.P. and A.D.C.) and the European Commission (Marie Skłodowska‐Curie ITN MAGICBULLET 642004) for a PhD fellowship (to A.R.M.D.) and financial support. We also gratefully acknowledge Ministero dell′Università e della Ricerca (PRIN 2015 project 20157WW5EH) for financial support.
A. Raposo Moreira Dias, A. Pina, A. Dal Corso, D. Arosio, L. Belvisi, L. Pignataro, M. Caruso, C. Gennari, Chem. Eur. J. 2017, 23, 14410.
Contributor Information
Dr. Luca Pignataro, Email: luca.pignataro@unimi.it.
Prof. Cesare Gennari, Email: cesare.gennari@unimi.it.
References
- 1.
- 1a. Barnard A., Smith D. K., Angew. Chem. Int. Ed. 2012, 51, 6572–6581; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2012, 124, 6676–6685; [Google Scholar]
- 1b. Fasting C., Schalley C. A., Weber M., Seitz O., Hecht S., Koksch B., Dernedde J., Graf C., Knapp E.-W., Haag R., Angew. Chem. Int. Ed. 2012, 51, 10472–10498; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2012, 124, 10622–10650; [Google Scholar]
- 1c. Mahon E., Barboiu M., Org. Biomol. Chem. 2015, 13, 10590–10599. [DOI] [PubMed] [Google Scholar]
- 2.
- 2a. Janssen M., Oyen W. J. G., Massuger L. F. A. G., Frielink C., Dijkgraaf I., Edwards D. S., Radjopadhye M., Corstens F. H. M., Boerman O. C., Cancer Biother. Radiopharm. 2002, 17, 641–646; [DOI] [PubMed] [Google Scholar]
- 2b. Thumshirn G., Hersel U., Goodman S. L., Kessler H., Chem. Eur. J. 2003, 9, 2717–2725; [DOI] [PubMed] [Google Scholar]
- 2c. Gillies E. R., Fréchet J. M. J., Drug Discovery Today 2005, 10, 35–43; [DOI] [PubMed] [Google Scholar]
- 2d. Garanger E., Boturyn D., Coll J. L., Favrot M. C., Dumy P., Org. Biomol. Chem. 2006, 4, 1958–1965; [DOI] [PubMed] [Google Scholar]
- 2e. Deyev S. M., Lebedenko E. N., BioEssays 2008, 30, 904–918; [DOI] [PubMed] [Google Scholar]
- 2f. Welsh D. J., Smith D. K., Org. Biomol. Chem. 2011, 9, 4795–4801; [DOI] [PubMed] [Google Scholar]
- 2g. Choi D. S., Jin H.-E., Yoo S. Y., Lee S.-W., Bioconjugate Chem. 2014, 25, 216–223; [DOI] [PubMed] [Google Scholar]
- 2h. Krall N., Pretto F., Neri D., Chem. Sci. 2014, 5, 3640–3644; [Google Scholar]
- 2i. Bianchi A., Arosio D., Perego P., De Cesare M., Carenini N., Zaffaroni N., De Matteo M., Manzoni L., Org. Biomol. Chem. 2015, 13, 7530–7541. [DOI] [PubMed] [Google Scholar]
- 3.
- 3a. Boturyn D., Coll J. L., Garanger E., Favrot M. C., Dumy P., J. Am. Chem. Soc. 2004, 126, 5730–5739; [DOI] [PubMed] [Google Scholar]
- 3b. Shi J., Wang L., Kim Y.-S., Zhai S., Liu Z., Chen X., Liu S., J. Med. Chem. 2008, 51, 7980–7990; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3c. Sancey L., Garanger E., Foillard S., Schoehn G., Hurbin A., Albiges-Rizo C., Boturyn D., Souchier C., Grichine A., Dumy P., Coll J. L., Mol. Ther. 2009, 17, 837–843; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3d. Wang L., Shi J., Kim Y.-S., Zhai S., Jia B., Zhao H., Liu Z., Wang F., Chen X., Liu S., Mol. Pharm. 2009, 6, 231–245; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3e. Liu S., Bioconjugate Chem. 2015, 26, 1413–1438; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3f. Lopez-Rodriguez V., Galindo-Sarco C., García-Pérez F. O., Ferro-Flores G., Arrieta O., Ávila-Rodríguez M. A., J. Nucl. Med. 2016, 57, 404–409; [DOI] [PubMed] [Google Scholar]
- 3g. Zhai C., Franssen G. M., Petrik M., Laverman P., Summer D., Rangger C., Haubner R., Haas H., Decristoforo C., Mol. Imaging Biol. 2016, 18, 758–767; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3h. Imberti C., Terry S. Y. A., Cullinane C., Clarke F., Cornish G. H., Ramakrishnan N. K., Roselt P., Cope A. P., Hicks R. J., Blower P. J., Ma M. T., Bioconjugate Chem. 2017, 28, 481–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.
- 4a. Lee B. C., Moon B. S., Kim J. S., Jung J. H., Park H. S., Katzenellenbogen J. A., Kim S. E., RSC Adv. 2013, 3, 782–792; [Google Scholar]
- 4b. Lee M. H., Sessler J. L., Kim J. S., Acc. Chem. Res. 2015, 48, 2935–2946; [DOI] [PubMed] [Google Scholar]
- 4c. Jin Z.-H., Furukawa T., Degardin M., Sugyo A., Tsuji A. B., Yamasaki T., Kawamura K., Fujibayashi Y., Zhang M.-R., Boturyn D., Dumy P., Saga T., Mol. Cancer Ther. 2016, 15, 2076–2085. [DOI] [PubMed] [Google Scholar]
- 5.
- 5a. Mahato R., Tai W., Cheng K., Adv. Drug Delivery Rev. 2011, 63, 659–670; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5b. Lammers T., Kiessling F., Hennink W. E., Storm G., J. Controlled Release 2012, 161, 175–187; [DOI] [PubMed] [Google Scholar]
- 5c. Krall N., Scheuermann J., Neri D., Angew. Chem. Int. Ed. 2013, 52, 1384–1402; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2013, 125, 1424–1443; [Google Scholar]
- 5d. Casi G., Neri D., J. Med. Chem. 2015, 58, 8751–8761; [DOI] [PubMed] [Google Scholar]
- 5e. Wong P. T., Choi S. K., Chem. Rev. 2015, 115, 3388–3432. [DOI] [PubMed] [Google Scholar]
- 6.For the use of RGD peptides and peptidomimetics as carriers of nanoparticles, imaging agents, and anticancer drugs, see:
- 6a. Danhier F., Le Breton A., Préat V., Mol. Pharm. 2012, 9, 2961–2973; [DOI] [PubMed] [Google Scholar]
- 6b. Arosio D., Casagrande C., Manzoni L., Curr. Med. Chem. 2012, 19, 3128–3151; [DOI] [PubMed] [Google Scholar]
- 6c. Gaertner F. C., Kessler H., Wester H.-J., Schwaiger M., Beer A. J., Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 126–138; [DOI] [PubMed] [Google Scholar]
- 6d. Anwar N., Rix A., Lederle W., Kuehne A. J. C., Chem. Commun. 2015, 51, 9358–9361; [DOI] [PubMed] [Google Scholar]
- 6e. Dal Corso A., Pignataro L., Belvisi L., Gennari C., Curr. Top. Med. Chem. 2016, 16, 314–329; [DOI] [PubMed] [Google Scholar]
- 6f. Arosio D., Casagrande C., Adv. Drug Delivery Rev. 2016, 97, 111–143; [DOI] [PubMed] [Google Scholar]
- 6g. Arosio D., Manzoni L., Corno C., Perego P., Recent Pat. Anti-Cancer Drug Discovery 2017, 12, 148–168. [DOI] [PubMed] [Google Scholar]
- 7.
- 7a. Schottelius M., Laufer B., Kessler H., Wester H.-J., Acc. Chem. Res. 2009, 42, 969–980; [DOI] [PubMed] [Google Scholar]
- 7b. Sutherland M., Gordon A., Shnyder S. D., Patterson L. H., Sheldrake H. M., Cancers 2012, 4, 1106–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Marchini M., Mingozzi M., Colombo R., Guzzetti I., Belvisi L., Vasile F., Potenza D., Piarulli U., Arosio D., Gennari C., Chem. Eur. J. 2012, 18, 6195–6207. [DOI] [PubMed] [Google Scholar]
- 9. Colombo R., Mingozzi M., Belvisi L., Arosio D., Piarulli U., Carenini N., Perego P., Zaffaroni N., De Cesare M., Castiglioni V., Scanziani E., Gennari C., J. Med. Chem. 2012, 55, 10460–10474. [DOI] [PubMed] [Google Scholar]
- 10. Mingozzi M., Manzoni L., Arosio D., Dal Corso A., Manzotti M., Innamorati F., Pignataro L., Lecis D., Delia D., Seneci P., Gennari C., Org. Biomol. Chem. 2014, 12, 3288–3302. [DOI] [PubMed] [Google Scholar]
- 11. Zanella S., Mingozzi M., Dal Corso A., Fanelli R., Arosio D., Cosentino M., Schembri L., Marino F., De Zotti M., Formaggio F., Pignataro L., Belvisi L., Piarulli U., Gennari C., ChemistryOpen 2015, 4, 633–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.
- 12a. Dal Corso A., Caruso M., Belvisi L., Arosio D., Piarulli U., Albanese C., Gasparri F., Marsiglio A., Sola F., Troiani S., Valsasina B., Pignataro L., Donati D., Gennari C., Chem. Eur. J. 2015, 21, 6921–6929; [DOI] [PubMed] [Google Scholar]
- 12b. Zanella S., Angerani S., Pina A., López Rivas P., Giannini C., Panzeri S., Arosio D., Caruso M., Gasparri F., Fraietta I., Albanese C., Marsiglio A., Pignataro L., Belvisi L., Piarulli U., Gennari C., Chem. Eur. J. 2017, 23, 7910–7914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.For multivalent RGD conjugates targeting integrin αvβ3, see refs. [2a,b,d,f,g,i] and [3].
- 14. Nahrwold M., Weiß C., Bogner T., Mertink F., Conradi J., Sammet B., Palmisano R., Royo Gracia S., Preusse T., Sewald N., J. Med. Chem. 2013, 56, 1853–1864. [DOI] [PubMed] [Google Scholar]
- 15.
- 15a. 1,3-Dipolar Cycloadditions Chemistry, (Ed.: R. Huisgen), Wiley, Hoboken, 1984, Vol. 1, pp. 1–176; [Google Scholar]
- 15b. Huisgen R., Pure Appl. Chem. 1989, 61, 613–628; [Google Scholar]
- 15c. Kolb H. C., Finn M. G., Sharpless K. B., Angew. Chem. Int. Ed. 2001, 40, 2004–2021; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2001, 113, 2056–2075; [Google Scholar]
- 15d. Kolb H. C., Sharpless K. B., Drug Discovery Today 2003, 8, 1128–1137. [DOI] [PubMed] [Google Scholar]
- 16. Ordanini S., Varga N., Porkolab V., Thepaut M., Belvisi L., Bertaglia A., Palmioli A., Berzi A., Trabattoni D., Clerici M., Fieschi F., Bernardi A., Chem. Commun. 2015, 51, 3816–3819. [DOI] [PubMed] [Google Scholar]
- 17. Dal Pozzo A., Esposito E., Ni M., Muzi L., Pisano C., Bucci F., Vesci L., Castorina M., Penco S., Bioconjugate Chem. 2010, 21, 1956–1967. [DOI] [PubMed] [Google Scholar]
- 18. Gavrilyuk J. I., Wuellner U., Salahuddin S., Goswami R. K., Sinha S. C., Barbas C. F., Bioorg. Med. Chem. Lett. 2009, 19, 3716–3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.For the extracellular cleavage of non-internalizing conjugates, see:
- 19a. Cazzamalli S., Dal Corso A., Neri D., Mol. Cancer Ther. 2016, 15, 2926–2935; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19b. Cazzamalli S., Dal Corso A., Neri D., J. Controlled Release 2017, 246, 39–45; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19c. Dal Corso A., Cazzamalli S., Gebleux R., Mattarella M., Neri D., Bioconjugate Chem. 2017, 28, 1826–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.
- 20a. Crisp J. L., Savariar E. N., Glasgow H. L., Ellies L. G., Whitney M. A., Tsien R. Y., Mol. Cancer Ther. 2014, 13, 1514–1525; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20b. Peng Z. H., Kopeček J., J. Am. Chem. Soc. 2015, 137, 6726–6729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.
- 21a. Yamaguchi K., Shimada S., Tashima S., Ogawa M., Oncol. Rep. 2000, 7, 1017–1021; [DOI] [PubMed] [Google Scholar]
- 21b. Lapis K., Timar J., Semin. Cancer Biol. 2002, 12, 209–217; [DOI] [PubMed] [Google Scholar]
- 21c. Albers M. D., Baumgarten J. D., Lerchen H. G. D., Schoop A. D. (Bayer HealthCare AG), EP 1372732 A1, 2002.
- 22.A true multivalent effect is observed if Rp/n is higher than 1, see: Alvarez-Dorta D., King D. T., Legigan T., Ide D., Adachi I., Deniaud D., Désiré J., Kato A., Vocadlo D., Gouin S. G., Blériot Y., Chem. Eur. J. 2017, 23, 9022–9025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary


