Abstract
Background
Tumor‐derived antigens are captured by CD169+ (SIGLEC1+) sinus macrophages in regional lymph nodes (LNs), and are presented to effector cells inducing an anti‐tumor immune response. Reduced CD169 expression in pre‐metastatic regional LNs is associated with subsequent metastatic disease and a poor outcome in several tumor types, but if this is the case in prostate cancer has not been explored.
Methods
CD169 expression was measured with immunohistochemistry in metastasis‐free regional LNs from 109 prostate cancer patients treated with prostatectomy (January 1996 to April 2002). Possible associations of CD169 expression with PSA‐relapse, prostate cancer death, Gleason score, and other clinical data were assessed using Kaplan‐Meier survival‐ and Cox regression analysis. In addition, the Dunning rat prostate tumor model was used to examine CD169 expression in pre‐metastatic LNs draining either highly metastatic MatLyLu‐ or poorly metastatic AT1‐tumors.
Results
In patients with low CD169 immunostaining in metastasis‐free regional LNs, 8 of the 27 patients died from prostate cancer compared with only three of the 82 patients with high immunostaining (P < 0.001). CD169 expression in regional LNs was not associated with PSA‐relapse. Rats with highly metastatic tumors had decreased CD169 immunoreactivity in pre‐metastatic regional LNs compared with rats with poorly metastatic tumors.
Conclusion
Low expression of CD169 in metastasis‐free regional LNs indicates a reduced anti‐tumor immune response. If verified in other studies, CD169 expression in regional LNs could, in combination with other factors, potentially be used as a marker of prostate cancer aggressiveness.
Keywords: Dunning rat prostate tumors, immunohistochemistry, prostate cancer death, SIGLEC1, tumor‐draining lymph nodes
1. INTRODUCTION
Metastatic prostate tumors secrete factors that precondition, not only the tumor stroma but also the remaining benign parts of the prostate, in ways that facilitate subsequent tumor growth and spread.1, 2, 3, 4, 5 If metastatic prostate tumors secrete factors that also prepare regional lymph nodes (LNs) for metastatic growth is only limitedly explored. Such pre‐metastatic niches have been shown to exist in various organs, including regional LNs, in other tumor types.6, 7, 8, 9, 10, 11 For example, tumors with high metastatic capacity suppress anti‐tumor immune responses in LNs prior to metastatic colonization.9, 11, 12, 13, 14, 15, 16, 17 In tumor‐free regional LNs from prostate cancer patients, an increased number of cells expressing VEGFR1,18, 19 and IL‐3020 have been shown to predict subsequent metastatic disease after radical prostatectomy. Furthermore, tumor‐free LNs from prostate cancer patients contained more CD68+ and pSTAT3+ myeloid cells than LNs form individuals without prostate cancer.13 LNs containing prostate cancer metastases showed signs of immunosuppression and were smaller than normal, and these responses probably occurred prior to the arrival of metastatic cells.21 Collectively, this suggests that examination of regional LNs could give information on tumor aggressiveness that could potentially aid in the choice of therapy.
We recently compared the global gene expression in tumor‐draining LNs from rats implanted with two different orthotopic tumors − the poorly metastatic AT1‐tumors and the highly metastatic MatLyLu (MLL)‐tumors.5 The gene expression profile in pre‐metastatic MLL‐LNs was associated with decreased antigen presentation, increased immunosuppression, and a reduced density of T lymphocytes.5, 22 One gene with lower expression in pre‐metastatic MLL‐LNs was Cd169 (Siglec1) (fold change −1.7, P = 0.01 vs LNs from treatment naive animals, and fold change −1.5, P = 0.08 vs AT1‐LNs).5 CD169 is normally expressed by subsets of macrophages in secondary lymphoid organs, for example, by subcapsular‐ and medullary sinus macrophages in LNs.23 By capturing and presenting tumor‐derived antigens, subcapsular sinus macrophages promote anti‐tumor immune responses and could in this way retard tumor growth and spread.23, 24, 25, 26, 27 A high density of CD169+ macrophages in tumor‐free regional LNs was associated with a favorable prognosis in melanoma, colorectal‐, and endometrial cancer.24, 27, 28, 29
The aim of this study was to examine CD169 expression in pre‐metastatic LNs in more detail, both in the animal model and in prostate cancer patients. We demonstrate that rats implanted with highly metastatic prostate tumors have reduced numbers of CD169+ macrophages in tumor‐draining LNs compared with rats implanted with less metastatic tumors. In prostate cancer patients, reduction of CD169 expression in regional LNs was associated with an increased risk of prostate cancer death in patients treated with prostatectomy.
2. MATERIALS AND METHODS
2.1. Animals
The local ethical committee for animal research approved all of the animal work (permit number A42‐15). The methods used for the animal experiments in this study have previously been used, and described.22
Briefly, poorly metastatic AT1‐ and highly metastatic MLL‐ Dunning rat prostate tumor cells30 were purchased from European Collection of Cell Cultures (ECACC, Salisbury, UK. MLL #94101454, AT1 #94101449) and were cultured as previously described.5 Immunocompetent and syngeneic adult Copenhagen rats (Charles River, Sulzfeld, Germany) were used in all experiments. Animals were anesthetized and 2 × 104 AT1 cells, or 1 × 103 MLL cells (suspended in 10 μL RPMI 1640) were carefully injected into one of the ventral prostate lobes as earlier described.5 Rats were sacrificed 3 (n = 8/treatment), and 10 days (n = 8/treatment) after tumor cell injection. The tumor‐containing prostates and the tumor‐draining regional LNs were removed, weighed, frozen in liquid nitrogen, and stored in −80°C. Tissues from treatment‐naïve rats (n = 8) and from vehicle‐injected rats (n = 10) were used as controls.
2.2. Patients
From Pathology archives at Umeå University Hospital we retrieved samples from 109 patients operated with radical prostatectomy between January 1996 and April 2002—and where the regional LNs (right and left obturatorius area) were also sampled. The tumor specimens were Gleason graded, and pathological stage and surgical margin status was determined. Gleason scores ranged from 6 (3 + 3) to 9 (4 + 5) and from stage pT2 (n = 45) to pT3 (n = 64), all cases were free from regional LN metastases (pN0).
Pre‐operative PSA levels and outcome data, in particular post‐operative PSA, PSA nadir value, dates of PSA‐relapse, detected bone metastases (bone scan), initiation of additional therapy, and cancer specific death, were retrieved from National Prostate Cancer Registry31 and patient records. Time from surgery to PSA‐relapse and time from surgery to death caused by prostate cancer was calculated. This study did not take into consideration which treatment the patients received after relapse.
PSA‐relapse was defined as an increase above nadir or, for patients with undetectable PSA (below 0.1 ng/mL) after surgery, when PSA was above 0.2 ng/mL. PSA‐relapse was detected in 43 patients (39%) and in nine of them metastases were also detected with bone scan. Living status was classified as either dead or alive, and for living patients with PSA‐relapse or without PSA‐relapse. At the end of 2016, 11 patients in this study had died from prostate cancer and 20 from other causes.
The ethical review board in Umeå approved the study (dnr 2010/366‐31M).
2.3. Immunohistochemistry
Four micron thick cryosections from rat regional LNs were fixed in formalin and immunostained using a mouse monoclonal anti‐rat CD169 antibody (diluted 1:100, #LS‐C124538, LSBio, Seattle, WA). The immunoreaction was detected using Mouse‐on‐rat HRP‐polymer (#MRT621, Biocare Pacheco, CA) and visualized using diaminobenzidine (Dako Glostrup, Denmark). The volume density of CD169+ cells was quantified by stereology. Using a light microscope equipped with a square‐lattice in the eyepiece, the fraction of grid‐intersections falling on CD169+ cells and reference space was counted.32
Five micron thick sections from paraffin embedded human LNs were stained with a primary mouse monoclonal antibody against human CD169 (diluted 1:25, sc53442, Santa Cruz Biotechnology, Dallas, TX). The immunoreaction was detected using Vectastain ABC‐kit (Vector labs, Burlingame, CA) and then visualized using diaminobenzidine (Dako, Glostrup, Denmark). As most patients had bilateral cancer foci, we assessed at least two LNs from each patient taken both from the left and right side. CD169 immunostaining was scored as either high (seen in most cases) or low (markedly reduced) by an experienced uropathologist (AB). LNs from the same patient had a similar staining pattern in the majority of patients and were in these cases evaluated together. In cases with heterogeneous staining, LNs were graded as having a low CD169 score if 50% or more of the LNs had absent or very low staining. At the time of scoring, the uropathologist was unaware of disease outcome. In order to examine the robustness of the staining and scoring procedure, tissue from all patients were sectioned, stained and scored twice, and the second score was compared to the first.
2.4. Statistical analysis
Fisher's exact test was used to compare CD169 staining score in relation to disease outcome in different patient subgroups. Possible association of CD169 staining and time to PSA‐relapse or time to prostate cancer death was assessed using Kaplan‐Meier survival analysis, and the log‐rank test was used to test for differences in survival distributions. The prognostic value of the CD169 score alone, and compared to traditional prognostic markers—such as pre‐operative PSA, PSA nadir, PSA‐relapse, tumor Gleason score, ISUP Gleason grade group,33 tumor stage, and surgical margins was examined by Cox regression analysis.
The CD169 score from the animal experiment is presented as mean ± SD, and Mann‐Whitney U test was used when comparing groups.
All statistics was computed in SPSS software (SPSS statistics 23, IBM, Armonk, NY), and a P‐value <0.05 was considered to be significant.
3. RESULTS
3.1. Growth of metastatic PC in rats in relation to CD169+ macrophages in pre‐metastatic lymph nodes
To examine if the volume density of CD169+ macrophages were affected in regional LNs prior to metastasis we used a rat prostate tumor model.30 Highly metastatic MLL cells or poorly metastatic AT1 cells were injected into the prostate and the regional LNs (MLL‐LNs and AT1‐LNs) were examined 3 and 10 days post‐injection. LNs from vehicle‐injected rats were used as controls (vehicle‐LNs).
Detailed information about the tumors and LNs used in this study can be found in Strömvall et al.22 Briefly, the size of AT1‐tumors was similar to the size of MLL‐tumors at both day 3 and day 10. The first microscopically visible LN‐metastases in the MLL‐model were seen at 14 days post‐injection. AT1 rarely metastasize, however LN‐metastases have been seen occasionally, and the earliest at 28 days post‐injection. The weight of the ventral prostate was unaffected in vehicle‐injected rats compared to that in treatment‐naïve rats. However, abdominal surgery and vehicle injection increased the weight of the regional LNs. A similar increase in LN weight was also seen in animals with AT1‐ and MLL‐tumors. LNs from vehicle‐injected rats were therefore used as controls in this study.
The density of CD169+ macrophages was similar in all three groups at day 3 post‐injection (Figure 1). At day 10, the density of CD169+ macrophages was lower in MLL‐LNs compared to both AT1‐ and vehicle‐LNs (Figure 1), suggesting a reduction of CD169+ macrophages in tumor‐draining LNs prior to metastatic growth.
Figure 1.
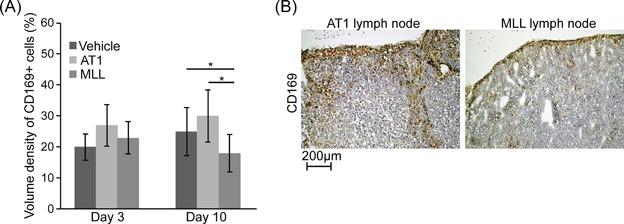
CD169 (brown) immunostaining of regional lymph nodes (LN) from rats with prostate cancer. (A) Volume density of CD169+ macrophages in regional LNs at 3 and 10 days post‐injection of vehicle, AT1‐, or MLL‐tumor cells into the prostate. Bars represent mean ± SD, n = 8‐10 animals/group, *P < 0.05, Mann‐Whitney U test. (B) Representative pictures of CD169 immunostaining of tumor‐draining regional LNs at day 10 post‐injection of AT1‐, or MLL‐tumor cells
3.2. CD169 expression in relation to prostate cancer death
To evaluate the clinical significance of CD169 in prostate cancer, LN tissue from 109 patients treated with radical prostatectomy was analyzed with immunohistochemistry and scored as having either low or high CD169 expression (Figure 2).
Figure 2.
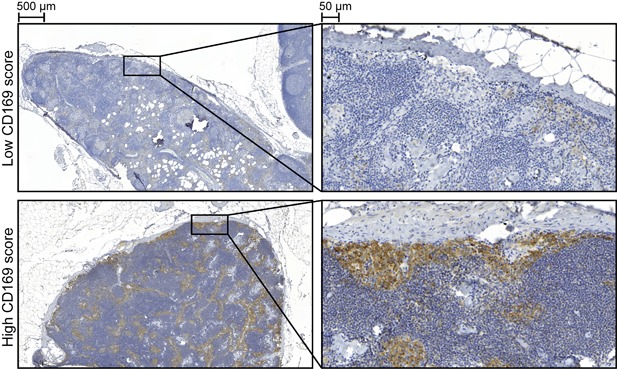
CD169 (brown) immunostaining of regional lymph nodes from prostate cancer patients. Representative pictures of low (upper panel) or high (lower panel) CD169 score
In most patients, CD169+ macrophages were observed in the subcapsular‐ and medullary sinuses of the LNs (Figure 2). However, in 27 patients the CD169 staining was markedly reduced or absent (Figure 2). In the group with low CD169 score, eight patients (30%) died due to prostate cancer, while in the group with high CD169 score, only three patients (3.7%) died of prostate cancer (P = 0.001, Table 1). We therefore further explored the CD169 score and other factors known to be associated with prostate cancer outcome (Table 1). High PSA nadir, PSA‐relapse, high Gleason score, and high ISUP Gleason grade group was significantly related to prostate cancer death (Table 1).
Table 1.
CD169 score and traditional prognostic markers in relation to prostate cancer death
| Prostate cancer death | ||||
|---|---|---|---|---|
| No (mean ± SD) | Yes (mean ± SD) | MWU test, P‐value | ||
| Pre‐operative PSA | 9.3 ± 4.4 | 11 ± 6.8 | 0.310 | |
| Gleason score | 6.7 ± 0.78 | 7.3 ± 0.79 | 0.024 | |
| No (n) | Yes (n) | Total (n) | Fisher's exact test, P‐value | |
| CD169 score | ||||
| Low | 19 | 8 | 27 | |
| High | 79 | 3 | 82 | |
| Total | 98 | 11 | 109 | 0.001 |
| Pre‐operative PSA | ||||
| ≤10 | 72 | 6 | 78 | |
| >10 | 26 | 5 | 31 | |
| Total | 98 | 11 | 109 | 0.288 |
| PSA‐nadir | ||||
| ≤0.1 | 88 | 6 | 94 | |
| >0.1 | 10 | 5 | 15 | |
| Total | 98 | 11 | 109 | 0.007 |
| PSA‐relapse | ||||
| No | 65 | 1* | 66 | |
| Yes | 33 | 10 | 43 | |
| Total | 98 | 11 | 109 | <0.001 |
| ISUP Gleason grade group | ||||
| Low (ISUP 1‐2) | 84 | 6 | 90 | |
| High (ISUP 3‐5) | 14 | 5 | 19 | |
| Total | 98 | 11 | 109 | 0.022 |
| Tumor stage | ||||
| pT2 | 43 | 2 | 45 | |
| pT3 | 55 | 9 | 64 | |
| Total | 98 | 11 | 109 | 0.119 |
| Surgical margins | ||||
| Positive | 36 | 7 | 43 | |
| Negative | 62 | 4 | 66 | |
| Total | 98 | 11 | 109 | 0.108 |
MWU = Mann‐Whitney U; SD = Standard Deviation.
Notably one patient died from prostate cancer without documented PSA‐relapse. The diagnosis was in this case verified by autopsy and histological verification of prostate cancer liver metastases.
In the Kaplan‐Meier survival analysis, patients with low CD169 score had a significantly shorter survival time than patients with high CD169 score. The estimated mean survival time for those having a low CD169 score was 200 (95%CI 178‐221) months, while for those with a high CD169score it was 232 (95%CI 226‐239) months (Figure 3).
Figure 3.
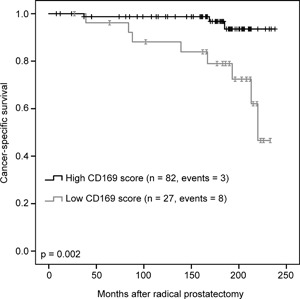
Kaplan‐Meier survival analysis showing cumulative cancer specific survival of patients with either high or low CD169 score. Patients with low CD169 score had a significantly shorter survival time than patients with high CD169 score, P = 0.002, log‐rank test
To further assess the significance of CD169 score compared to the other clinically established markers for prognosis, uni‐ and multivariable Cox regression analysis was performed. In univariable analysis, low CD169 score, Gleason score, high ISUP Gleason grade group, high PSA nadir, and PSA‐relapse, were all related to increased risk of prostate cancer death (Table 2). In multivariable stepwise elimination analysis, only CD169 score and PSA‐relapse remained significantly associated with an increased risk of dying from prostate cancer (Table 2).
Table 2.
Cox regression analysis of CD169 score and traditional prognostic markers in relation to prostate cancer death
| Variable | n | RR | 95%CI | P‐value |
|---|---|---|---|---|
| Univariable analysis | ||||
| CD169 score** | ||||
| High | 82 | 1* | ||
| Low | 27 | 6.6 | 1.7‐25.1 | 0.006 |
| Gleason score | ||||
| 6‐10 | 109 | 3.1 | 1.3‐7.2 | 0.010 |
| Pre‐operative PSA** | ||||
| ≤10 | 78 | 1* | ||
| >10 | 31 | 2.3 | 0.7‐7.5 | 0.180 |
| PSA‐nadir** | ||||
| ≤0.1 | 94 | 1* | ||
| >0.1 | 15 | 4.8 | 1.5‐15.8 | 0.010 |
| PSA‐relapse** | ||||
| No | 66 | 1* | ||
| Yes | 43 | 13 | 1.7‐101.7 | 0.015 |
| ISUP Gleason grade group** | ||||
| Low (ISUP 1‐2) | 90 | 1* | ||
| High (ISUP 3‐5) | 19 | 3.7 | 1.1‐12.1 | 0.031 |
| Tumor stage** | ||||
| pT2 | 45 | 1* | ||
| pT3 | 64 | 3.5 | 0.8‐16.1 | 0.111 |
| Surgical margins** | ||||
| Negative | 66 | 1* | ||
| Positive | 43 | 3 | 0.9‐10.1 | 0.084 |
| Multivariable analysis, stepwise elimination | ||||
| Step 1 | ||||
| CD169 score** low vs high | 4.8 | 1.1‐21.3 | 0.038 | |
| Gleason score (6‐10) | 3.2 | 0.6‐16.2 | 0.162 | |
| Pre‐operative PSA** >10 vs ≤10 | 0.5 | 0.1‐2.7 | 0.424 | |
| PSA‐nadir** >0.1 vs ≤0.1 | 4.4 | 0.9‐22.6 | 0.076 | |
| PSA‐relapse** yes vs no | 5.3 | 0.5‐57.5 | 0.168 | |
| ISUP Gleason grade group** 3‐5 vs 1‐2 | 1.1 | 0.2‐8.1 | 0.924 | |
| Tumor stage** pT3 vs pT2 | 3.3 | 0.4‐31.1 | 0.293 | |
| Surgical margins** positive vs negative | 0.4 | 0.05‐3.9 | 0.451 | |
| Step 7 | ||||
| CD169 score** low vs high | 4.8 | 1.3‐18.5 | 0.022 | |
| PSA‐relapse** yes vs no | 10.0 | 1.3‐79.2 | 0.029 | |
RR = relative risk; CI = confidence interval.
Reference value.
Categorical variable.
3.3. CD169 expression in relation to PSA‐relapse
We tested if the CD169 score was associated to PSA‐relapse and compared the result with established prognostic markers of PSA‐relapse (Table 3). In line with multiple previous studies, pre‐operative PSA, PSA nadir, tumor Gleason score, ISUP Gleason grade group, tumor stage, and positive surgical margins, were all related to the risk of PSA‐ relapse, but CD169 score was not (Table 3).
Table 3.
CD169 score and traditional prognostic markers in relation to PSA‐relapse
| PSA‐relapse | ||||
|---|---|---|---|---|
| No (mean ± SD) | Yes (mean ± SD) | MWU test, P‐value | ||
| Pre‐operative PSA | 8.4 ± 3.9 | 10.9 ± 5.4 | 0.014 | |
| Gleason score | 6.5 ± 0.77 | 7.1 ± 0.66 | <0.001 | |
| No (n) | Yes (n) | Total (n) | Fisher's exact test, P‐value | |
| CD169 score | ||||
| Low | 13 | 14 | 27 | |
| High | 53 | 29 | 82 | |
| Total | 66 | 43 | 109 | 0.173 |
| Pre‐operative PSA | ||||
| ≤10 | 53 | 25 | 78 | |
| >10 | 13 | 18 | 31 | |
| Total | 66 | 43 | 109 | 0.017 |
| PSA‐nadir | ||||
| ≤0.1 | 63 | 31 | 94 | |
| >0.1 | 3 | 12 | 15 | |
| Total | 66 | 43 | 109 | 0.001 |
| ISUP Gleason grade group | ||||
| Low (ISUP 1–2) | 62 | 28 | 90 | |
| High (ISUP 3‐5) | 4 | 15 | 19 | |
| Total | 66 | 43 | 109 | < 0.001 |
| Tumor stage | ||||
| pT2 | 36 | 9 | 45 | |
| pT3 | 30 | 34 | 64 | |
| Total | 66 | 43 | 109 | 0.001 |
| Surgical margins | ||||
| Positive | 17 | 26 | 43 | |
| Negative | 49 | 17 | 66 | |
| Total | 66 | 43 | 109 | 0.001 |
MWU = Mann‐Whitney U; SD = Standard Deviation.
As CD169 expression appeared more strongly related to prostate cancer death than to PSA‐relapse, we grouped patient with PSA‐relapse into those that died from prostate cancer and those who did not (Table 4). In patients with PSA‐relapse, a low CD169 score was associated to an increased risk of dying from prostate cancer compared to those with a high CD169 score (Table 4). Interestingly, in patients with PSA‐relapse, only CD169 score was related to prostate cancer death.
Table 4.
CD169 score and traditional prognostic markers in relation to prostate cancer death within the subgroup of patients with PSA‐relapse
| Prostate cancer death | ||||
|---|---|---|---|---|
| No (mean ± SD) | Yes (mean ± SD) | MWU test, P‐value | ||
| Pre‐operative PSA | 10.6 ± 5.0 | 12.1 ± 6.5 | 0.580 | |
| Gleason score | 7.1 ± 0.61 | 7.3 ± 0.82 | 0.380 | |
| No (n) | Yes (n) | Total (n) | Fisher's exact test, P‐value | |
| CD169 score | ||||
| Low | 7 | 7 | 14 | |
| High | 26 | 3 | 29 | |
| Total | 33 | 10 | 43 | 0.007 |
| Pre‐operative PSA | ||||
| ≤10 | 20 | 5 | 25 | |
| >10 | 13 | 5 | 18 | |
| Total | 33 | 10 | 43 | 0.717 |
| PSA‐nadir | ||||
| ≤0.1 | 26 | 5 | 31 | |
| >0.1 | 7 | 5 | 12 | |
| Total | 33 | 10 | 43 | 0.110 |
| ISUP Gleason grade group | ||||
| Low (ISUP 1‐2) | 23 | 5 | 28 | |
| High (ISUP 3‐5) | 10 | 5 | 15 | |
| Total | 33 | 10 | 43 | 0.281 |
| Tumor stage | ||||
| pT2 | 7 | 2 | 9 | |
| pT3 | 26 | 8 | 34 | |
| Total | 33 | 10 | 43 | 1.00 |
| Surgical margins | ||||
| Positive | 19 | 7 | 26 | |
| Negative | 14 | 3 | 17 | |
| Total | 33 | 10 | 43 | 0.714 |
MWU = Mann‐Whitney U; SD = Standard Deviation.
3.4. Robustness of CD169 staining and scoring
All lymph nodes were sectioned, stained, and scored twice without knowledge of the previous result. In total, the scoring differed in nine out of 110 cases. Eight low cases were re‐classified as high and one case initially scored high was re‐scored as low. Among the 11 patients dying from prostate cancer, one case with low score was re‐scored as high. This suggests that the scoring was relatively robust (Kendal Tau correlation coefficient 0.74) but also that sensitivity of the staining method may shift between staining sessions. Using the first or second data set in the statistical analysis gave the same general result. The reported results are calculated based on the first data set.
4. DISCUSSION
The principal observation in this paper is that reduced CD169 expression in tumor‐draining LNs is associated to aggressive prostate cancer. Highly metastatic rat prostate tumors induced down‐regulation of CD169 expression in pre‐metastatic regional LNs. In prostate cancer patients, a low number of CD169+ macrophages in metastasis‐free regional LNs was significantly associated to an increased risk of prostate cancer associated death.
Metastatic cancers suppress anti‐tumor immune responses in regional LNs prior to metastasis.8, 11, 12, 13, 15, 17, 24 In line with this, the global gene expression in regional LNs from rats carrying highly metastatic MLL‐prostate tumors indicated increased immunosuppression and reduced antigen presentation prior to metastasis.5 Cd169 (Siglec1) mRNA expression was shown to be decreased in highly metastatic MLL‐ versus poorly metastatic AT1‐LNs.5 Consistent with the previous study, we now demonstrate that this is accompanied by a reduced number of CD169+ macrophages in MLL‐LNs.
In lymph nodes, CD169 is normally expressed by macrophages in the subcapsular‐ and medullary sinuses.23 One function of the subcapsular sinus‐macrophages is to capture lymph borne particles, including tumor‐derived antigens and exosomes.25, 26, 34 Antigens sampled by the CD169+ macrophages are presented to effector cells like T‐cells and NK‐cells, which migrate to the tumor where they promote an anti‐tumor immune response.3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 35 In breast cancer, the density of CD169+ cells in regional LNs correlated with clinical stage, and also with numbers of cytotoxic CD8+ T cells in high grade tumors, but was not associated to cancer specific survival.36 In our experimental model, we found that rats carrying poorly metastatic AT1‐tumors had a higher density of CD169+ macrophages in the tumor‐draining LNs and in a previous study we also found a higher amount of tumor‐infiltrating CD3+ T‐cells,5 compared with rats carrying highly metastatic MLL‐tumors. Similarly, high levels of CD169+ cells in regional LNs correlated with high numbers of cytotoxic CD8+ T‐cells in melanoma, colon, and endometrial tumors, and this was associated with a favorable clinical outcome.24, 27, 28, 29 Our observation that highly metastatic rat prostate cancers down‐regulated CD169 expression in regional LNs, and that a low expression of CD169 in regional LNs from prostate cancer patients was associated with increased risk of prostate cancer death, are thus in line with the known biological role of CD169+ sinus macrophages.
PSA‐relapse occurs in a substantial number of men treated for prostate cancer, but in many cases this is not associated with clinical relapse and prostate cancer death.37 Among patients with PSA‐relapse, pre‐operative PSA, tumor grade, tumor stage, and positive surgical margins could not predict death in prostate cancer. However, in this group of patients, low levels of CD169 in regional LNs—a marker that can be measured already at the time of surgery—was related to an increased risk of prostate cancer death. This suggests that patients with systemic metastases (detected by raising PSA levels) hypothetically can be separated into two groups, one with an effective immune surveillance and another with a less effective immune response. The CD169 expression in regional LNs could be one marker of this difference in immune response. In our previous studies we found several immune‐related genes, in addition to Cd169, to be differentially expressed comparing pre‐metastatic MLL‐LNs to AT1‐LNs at different time points, for example, Cd3, Cd8a, Clec1b, Ctla4, Foxp3, Ido1, Il4, Il10, Il1r2, Lag3, Pla2g7, and Tgfb1.5, 22 Further studies should therefore explore whether these factors could be differentially expressed in pre‐metastatic LNs also in patients and if this difference is related to patient outcome. It is also of interest to examine whether other factors in pre‐metastatic LNs already found to predict prognosis in patients, like VEGFR1,18, 19 IL‐30,20 pSTAT3+ macrophages,13 and lymph node size,21 can be used in combination with CD169.
The reason why some tumors induce a decrease in numbers of CD169+ macrophages in regional LNs is unknown, but tumor‐derived factors could be involved.23, 25, 26, 27 CSF1, a survival factor for CD169+ macrophages, was down‐regulated in regional LNs from rats with highly metastatic MLL‐tumors, suggesting that CSF1 could be involved.5 Tumor‐derived exosomes is one key factor adapting pre‐metastatic sites to the subsequent arrival and growth of neoplastic cells.8, 11, 15, 38 Effects of tumor‐derived exosomes on regional LNs have not been examined in our model system. However, MLL‐derived exosomes injected into the prostate precondition it for accelerated growth of low‐malignant prostate cancer cells implanted 3 days later.3 It is likely that MLL‐exosomes also could influence the tumor‐draining LNs in a similar tumor‐promoting way.
One limitation of this study is the low number of patients examined. This makes our study underpowered to prove a robust relation between low CD169 expression and increased risk of prostate cancer death. Nevertheless, our data suggest that CD169 expression in pre‐metastatic regional LNs could be used, together with other markers, to evaluate prostate tumor aggressiveness. This however needs to be validated in larger studies using standardized staining and quantification methods. Evaluation of CD169 expression could be done either prior to choice of primary therapy, or to determine the need and choice of additional therapies.
5. CONCLUSIONS
In animals, and in patients with localized prostate cancer, reduced density of CD169+ sinus macrophages in pre‐metastatic regional LNs appear to be associated with subsequent metastatic colonization and a poor outcome.
ACKNOWLEDGMENTS
We thank Sigrid Kilter, Pernilla Andersson, and Susanne Gidlund who provided excellent technical assistance.
CONFLICTS OF INTEREST
The authors declare that no competing interests exist.
Strömvall K, Sundkvist K, Ljungberg B, Halin Bergström S, Bergh A. Reduced number of CD169+ macrophages in pre‐metastatic regional lymph nodes is associated with subsequent metastatic disease in an animal model and with poor outcome in prostate cancer patients. The Prostate. 2017;77: 1468–1477. https://doi.org/10.1002/pros.23407
REFERENCES
- 1. Adamo HH, Stromvall K, Nilsson M, Halin Bergstrom S, Bergh A. Adaptive (TINT) changes in the tumor bearing organ are related to prostate tumor size and aggressiveness. PLoS ONE. 2015; 10:e0141601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hagglof C, Bergh A. The stroma‐a key regulator in prostate function and malignancy. Cancers (Basel). 2012; 4:531–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Halin Berstrom S, Hagglof C, Thysell E, Bergh A, Wikstrom P, Lundholm M. Extracellular vesicles from metastatic rat prostate tumors prime the normal prostate tissue to facilitate tumor growth. Sci Rep. 2016; 6:31805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Halin S, Hammarsten P, Adamo H, Wikstrom P, Bergh A. Tumor indicating normal tissue could be a new source of diagnostic and prognostic markers for prostate cancer. Expert Opin Med Diagn. 2011; 5:37–47. [DOI] [PubMed] [Google Scholar]
- 5. Stromvall K, Thysell E, Halin Bergstrom S, Bergh A. Aggressive rat prostate tumors reprogram the benign parts of the prostate and regional lymph nodes prior to metastasis. PLoS ONE. 2017; 12:e0176679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hirakawa S. From tumor lymphangiogenesis to lymphvascular niche. Cancer Sci. 2009; 100:983–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jung T, Castellana D, Klingbeil P, et al. CD44v6 dependence of premetastatic niche preparation by exosomes. Neoplasia. 2009; 11:1093–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Peinado H, Zhang H, Matei IR, et al. Pre‐metastatic niches: organ‐specific homes for metastases. Nat Rev Cancer. 2017; 17:302–317. [DOI] [PubMed] [Google Scholar]
- 9. Psaila B, Lyden D. The metastatic niche: adapting the foreign soil. Nat Rev Cancer. 2009; 9:285–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rinderknecht M, Detmar M. Tumor lymphangiogenesis and melanoma metastasis. J Cell Physiol. 2008; 216:347–354. [DOI] [PubMed] [Google Scholar]
- 11. Sleeman JP. The lymph node pre‐metastatic niche. J Mol Med (Berl). 2015; 93:1173–1184. [DOI] [PubMed] [Google Scholar]
- 12. Cochran AJ, Huang RR, Su A, Itakura E, Wen DR. Is sentinel node susceptibility to metastases related to nodal immune modulation? Cancer J. 2015; 21:39–46. [DOI] [PubMed] [Google Scholar]
- 13. Deng J, Liu Y, Lee H, et al. S1PR1‐STAT3 signaling is crucial for myeloid cell colonization at future metastatic sites. Cancer Cell. 2012; 21:642–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kaplan RN, Riba RD, Zacharoulis S, et al. VEGFR1‐positive haematopoietic bone marrow progenitors initiate the pre‐metastatic niche. Nature. 2005; 438:820–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McAllister SS, Weinberg RA. The tumour‐induced systemic environment as a critical regulator of cancer progression and metastasis. Nat Cell Biol. 2014; 16:717–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ogawa F, Amano H, Eshima K, et al. Prostanoid induces premetastatic niche in regional lymph nodes. J Clin Invest. 2014; 124:4882–4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sceneay J, Smyth MJ, Moller A. The pre‐metastatic niche: finding common ground. Cancer Metastasis Rev. 2013; 32:449–464. [DOI] [PubMed] [Google Scholar]
- 18. Fujita K, Nakayama M, Nakai Y, et al. Vascular endothelial growth factor receptor 1 expression in pelvic lymph nodes predicts the risk of cancer progression after radical prostatectomy. Cancer Sci. 2009; 100:1047–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pal SK, Vuong W, Zhang W, et al. Clinical and translational assessment of VEGFR1 as a mediator of the premetastatic niche in high‐risk localized prostate cancer. Mol Cancer Ther. 2015; 14:2896–2900. [DOI] [PubMed] [Google Scholar]
- 20. Di Meo S, Airoldi I, Sorrentino C, Zorzoli A, Esposito S, Di Carlo E. Interleukin‐30 expression in prostate cancer and its draining lymph nodes correlates with advanced grade and stage. Clin Cancer Res. 2014; 20:585–594. [DOI] [PubMed] [Google Scholar]
- 21. Gannon PO, Alam Fahmy M, Begin LR, et al. Presence of prostate cancer metastasis correlates with lower lymph node reactivity. Prostate. 2006; 66:1710–1720. [DOI] [PubMed] [Google Scholar]
- 22. Stromvall K, Lundholm M, Bergh M, Halin Bergström S. Highly metastatic rat prostate cancers rapidly precondition regional lymph nodes for subsequent metastatic growth. PLoS ONE. under review. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. O'Neill AS, van den Berg TK. Mullen GE. Sialoadhesin − a macrophage‐restricted marker of immunoregulation and inflammation. Immunology. 2013; 138:198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Komohara Y, Ohnishi K, Takeya M. Possible functions of CD169‐positive sinus macrophages in lymph nodes in anti‐tumor immune responses. Cancer Sci. 2017; 108:290–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kuka M, Iannacone M. The role of lymph node sinus macrophages in host defense. Ann N Y Acad Sci. 2014; 1319:38–46. [DOI] [PubMed] [Google Scholar]
- 26. Martinez‐Pomares L, Gordon S. CD169+ macrophages at the crossroads of antigen presentation. Trends Immunol. 2012; 33:66–70. [DOI] [PubMed] [Google Scholar]
- 27. Saito Y, Ohnishi K, Miyashita A, et al. Prognostic significance of CD169+ lymph node sinus macrophages in patients with malignant melanoma. Cancer Immunol Res. 2015; 3:1356–1363. [DOI] [PubMed] [Google Scholar]
- 28. Ohnishi K, Komohara Y, Saito Y, et al. CD169‐positive macrophages in regional lymph nodes are associated with a favorable prognosis in patients with colorectal carcinoma. Cancer Sci. 2013; 104:1237–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ohnishi K, Yamaguchi M, Erdenebaatar C, et al. Prognostic significance of CD169‐positive lymph node sinus macrophages in patients with endometrial carcinoma. Cancer Sci. 2016; 107:846–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Isaacs JT, Isaacs WB, Feitz WF, Scheres J. Establishment and characterization of seven Dunning rat prostatic cancer cell lines and their use in developing methods for predicting metastatic abilities of prostatic cancers. Prostate. 1986; 9:261–281. [DOI] [PubMed] [Google Scholar]
- 31. Tomic K, Sandin F, Wigertz A, Robinson D, Lambe M, Stattin P. Evaluation of data quality in the national prostate cancer register of Sweden. Eur J Cancer. 2015; 51:101–111. [DOI] [PubMed] [Google Scholar]
- 32. Weibel ER. Stereological methods. London; New York: Academic Press; 1979. [Google Scholar]
- 33. Pierorazio PM, Walsh PC, Partin AW, Epstein JI. Prognostic Gleason grade grouping: data based on the modified Gleason scoring system. BJU Int. 2013; 111:753–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Saunderson SC, Dunn AC, Crocker PR, McLellan AD. CD169 mediates the capture of exosomes in spleen and lymph node. Blood. 2014; 123:208–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chavez‐Galan L, Olleros ML, Vesin D, Garcia I. Much more than M1 and M2 macrophages, there are also CD169(+) and TCR(+) macrophages. Front Immunol. 2015; 6:263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shiota T, Miyasato Y, Ohnishi K, et al. The clinical significance of CD169‐positive lymph node macrophage in patients with breast cancer. PLoS ONE. 2016; 11:e0166680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cornford P, Bellmunt J, Bolla M, et al. EAU‐ESTRO‐SIOG guidelines on prostate cancer. part II: treatment of relapsing, metastatic, and castration‐resistant prostate cancer. Eur Urol. 2017; 71:630–642. [DOI] [PubMed] [Google Scholar]
- 38. Suchorska WM, Lach MS. The role of exosomes in tumor progression and metastasis (Review). Oncol Rep. 2016; 35:1237–1244. [DOI] [PubMed] [Google Scholar]


