Summary
Aims
There is a large population of people with type 2 diabetes mellitus (T2DM) who are Muslim and fast during Ramadan. Changes in the pattern and amount of meal and fluid intake during Ramadan, in addition to the long fasting hours, may increase the risk of hypoglycaemia, hyperglycaemia, and dehydration. The Canagliflozin in Ramadan Tolerance Observational Study (CRATOS) evaluated the tolerability of canagliflozin, a sodium glucose co‐transporter 2 inhibitor, compared with sulphonylureas among patients with T2DM who fast during Ramadan.
Methods
This non‐randomised, parallel‐cohort, prospective, comparative, observational study was conducted in the Middle East during Ramadan and enrolled patients who were taking canagliflozin (n=162) or any sulphonylurea (n=159) added to metformin±dipeptidyl peptidase‐4 inhibitor. The proportion of patients who experienced hypoglycaemia events was assessed as the primary end‐point. Between‐cohort comparisons were adjusted using propensity score analysis.
Results
During Ramadan, fewer patients experienced symptomatic hypoglycaemia with canagliflozin vs sulphonylurea (adjusted odds ratio: 0.273 [95% CI: 0.104, 0.719]). Of hypoglycaemia events for which blood glucose was measured, two of six with canagliflozin and 27 of 37 with sulphonylurea were confirmed by blood glucose <3.9 mmol/L. More patients treated with canagliflozin experienced volume depletion events compared with sulphonylurea (adjusted odds ratio: 3.5 [95% CI: 1.3, 9.2]). Missed fasting days were few and medication adherence was high in both groups. No patients treated with canagliflozin and 9.4% treated with sulphonylurea adjusted their medication dose near the beginning of Ramadan. Both treatments were generally well tolerated, with low rates of adverse events and no serious adverse events in either group.
Conclusions
Overall, these findings support the use of canagliflozin for the treatment of adults with T2DM who fast during Ramadan.
ClinicalTrials.gov Identifier: NCT02737657
What's known
There are approximately 116 million Muslims with T2DM who fast each year during the month of Ramadan. During this time, food and water intake patterns are drastically altered, which can affect T2DM treatment; therefore, the safety and tolerability of T2DM medications in fasting patients must be established. Treatment with the SGLT2 inhibitor dapagliflozin was reported to result in lower rates of hypoglycaemia when compared to sulphonylurea during Ramadan fasting.
What's new
This study is the first to evaluate the SGLT2 inhibitor canagliflozin in patients with T2DM fasting an average of 15 hours daily during Ramadan; it shows that patients have a lower risk of hypoglycaemia with canagliflozin compared with sulphonylureas. Canagliflozin was generally well tolerated, with low rates of adverse events and higher rates of medication and fasting adherence, but was associated with an increased risk of volume depletion events.
1. INTRODUCTION
Globally, approximately 116 million Muslims with type 2 diabetes mellitus (T2DM) elect to fast during the holy month of Ramadan.1 Long periods of fasting and drastic changes in food and water intake increase the risk of hypoglycaemia, hyperglycaemia, and dehydration in these individuals. Patients with T2DM who fast during Ramadan have a 7.5‐fold increased risk of severe hypoglycaemia during Ramadan compared with non‐fasting months.2 Generally, insulin‐independent treatments for T2DM are associated with a lower risk of hypoglycaemia than treatments that increase insulin secretion,3 so these treatments may be more attractive options for patients who intend to fast during Ramadan, or at other times throughout the year. Studies of newer insulin‐independent treatments, such as sodium glucose co‐transporter 2 (SGLT2) inhibitors, during Ramadan are limited, but have shown promising results. In a study of fasting T2DM patients treated with the SGLT2 inhibitor dapagliflozin during Ramadan, a reduced incidence of hypoglycaemia was observed with dapagliflozin compared with sulphonylureas4; more patients had volume depletion–related adverse events (AEs) of postural hypotension with dapagliflozin vs sulphonylureas, but this was not statistically significant.
Canagliflozin is an SGLT2 inhibitor approved for the treatment of T2DM in adults.5 Canagliflozin lowers plasma glucose by promoting urinary glucose excretion, which results in a mild osmotic diuresis that may be associated with a reduction in intravascular volume.6, 7, 8, 9 While the insulin‐independent mechanism of canagliflozin leads to a low inherent risk of hypoglycaemia, the mild osmotic diuresis it causes may be associated with an increased risk of volume–depletion events, including dehydration. Across Phase 3 studies in a broad range of patients, canagliflozin provided reductions in HbA1c, body weight, and systolic blood pressure (BP) and was generally well tolerated, with a low risk of hypoglycaemia when not used in conjunction with insulin or sulphonylureas.10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22 An analysis of T2DM patients living in hot climates found that canagliflozin treatment was generally well tolerated, with a low incidence of volume depletion–related AEs.23
The Canagliflozin in Ramadan Tolerance Observational Study (CRATOS) is the first study to evaluate the tolerability of canagliflozin in combination with metformin with or without a dipeptidyl peptidase‐4 (DPP‐4) inhibitor for the treatment of T2DM in patients fasting during Ramadan.
2. PATIENTS AND METHODS
2.1. Study design and patient population
This was a multicentre, open‐label, observational study conducted in the Middle East (Lebanon, Kuwait, and the United Arab Emirates [UAE]) during Ramadan (6 June‐5 July 2016) where fasting lasted for approximately 15 hours each day and temperatures reached up to 45‐50°C in Kuwait and the UAE. Patients underwent pre‐ and post‐Ramadan examinations within approximately 8 weeks of the Ramadan fasting period. On enrolment in the study, patients entered one of two parallel treatment cohorts based on their ongoing T2DM therapy: patients treated with canagliflozin and metformin with or without a DPP‐4 inhibitor and patients treated with any sulphonylurea and metformin with or without a DPP‐4 inhibitor. Eligible participants were adults between the ages of 18 and 65 years who had a confirmed diagnosis of T2DM for at least 12 months before enrolment; had been treated with canagliflozin or any sulphonylurea, each on a background therapy of metformin with or without a DPP‐4 inhibitor for at least 12 weeks before enrolment; had an HbA1c measurement of ≤8.5% within 8 weeks of the beginning of Ramadan; and intended to fast during Ramadan.
Patients who were treated with insulin or any T2DM therapy other than canagliflozin, any sulphonylurea, or metformin with or without a DPP‐4 inhibitor; changed their T2DM therapy within 12 weeks of enrolment; were being treated with loop diuretics; had a history of severe hypoglycaemia events (for which the patient required assistance from another person or that resulted in seizure or loss of consciousness) or severe volume depletion events within the 6 months prior to enrolment; had a history of diabetic ketoacidosis; had heart failure (New York Heart Association class 3‐4); had advanced cardiovascular disease; or had an estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2 were excluded from the study.
Patients in the study were required to keep a patient diary during Ramadan, where they recorded days when fast was broken; daily use of canagliflozin or sulphonylurea, metformin, and a DPP‐4 inhibitor, if applicable; reasons for any missed medication doses; hypoglycaemia and volume depletion events; and self‐monitored blood glucose measurements.
2.2. Study end‐points
The prespecified primary end‐point of this study was the proportion of patients with ≥1 hypoglycaemia episode during Ramadan. Hypoglycaemia episodes included symptomatic episodes (ie, symptoms of dizziness, visual blurring, palpitations, nausea, sweating, confusion, tremor, or intense hunger with or without biochemical confirmation), biochemically confirmed episodes (ie, self‐monitored blood glucose measurement of <3.9 mmol/L with or without symptoms), and severe episodes (ie, episodes for which the patient required assistance from another person or that resulted in seizure or loss of consciousness). Hypoglycaemia events for which blood glucose measurements were ≥3.9 mmol/L or no blood test was done were considered not confirmed. The 3.9 mmol/L threshold used to define hypoglycaemia was based on the American Diabetes Association (ADA) and Endocrine Society consensus report definition.24 In addition, hypoglycaemia events with blood glucose <3.0 mmol/L were reported in accordance with an ADA and European Association for the Study of Diabetes joint position statement, which defines these events as serious and clinically meaningful.25 Other parameters of interest included the proportion of patients with ≥1 volume depletion event (ie, symptoms of hypotension, orthostatic hypotension, postural dizziness, dehydration, syncope, or presyncope) during Ramadan, number of days that fasting was broken, and treatment adherence, based on the percentage of prescribed doses of canagliflozin or sulphonylurea taken by patients.
In addition to patient‐reported episodes, physician‐reported episodes of hypoglycaemia and volume depletion events were reported as AEs. The incidence of other AEs of interest potentially related to SGLT2 inhibition (including urinary tract infections, genital mycotic infections, and osmotic diuresis–related AEs) and metformin (gastrointestinal AEs) were also assessed. In addition, HbA1c, BP, body weight, and eGFR were measured at the pre‐ and post‐Ramadan visits.
2.3. Statistical analysis
Because of the exploratory nature of this observational study, sample size was based on the width of the 95% confidence interval (CI) of the observed proportion of patients with ≥1 episode of hypoglycaemia during the Ramadan period in the canagliflozin cohort. For an observed sample proportion of 0.15, the two‐sided Wilson 95% CI is 0.081 to 0.261 for a sample size of 60. To ensure that comparisons could be adjusted between the treatment cohorts for a non‐overlap in propensity score (PS), which was estimated to be 50%, the sample size was increased to 120 per cohort and subsequently increased to 150 to account for an expected rate of 20% unevaluable patients.
Analyses were conducted using the tolerability analysis set, which consists of all eligible patients who received ≥1 dose of canagliflozin or any sulphonylurea during the observation period, who fasted for at least 1 day, and for whom postbaseline tolerability data are available. For patients in the tolerability analysis set who switch to treatment with another T2DM therapy during the Ramadan period, only the observation period until the switch was included in the primary end‐point. For patients in the tolerability analysis set who stopped their fast entirely during Ramadan, only the observation period until the stop was included in the primary end‐point.
As this is an observational study, no direct statistical comparisons of the events of interest could be made between cohorts. Instead, the PS method was used to account for the probability that a patient in the treatment analysis subset received a treatment based on observed confounders. The following observed confounders were evaluated: baseline age, baseline body mass index (BMI), diabetes duration, treatment duration, baseline HbA1c, baseline eGFR, use of a DPP‐4 inhibitor, sex, current smoker status, presence of complicating diagnosis, home country, antihypertensive use, and diuretic use. The PS analysis was done in four steps. In step 1, the balance between treatment cohorts was investigated for each of the potential confounding factors by calculating the univariate odds ratio (OR) and 95% CI using logistic regression. In step 2, the predictive power was investigated separately for each potential confounder by means of a logistic regression analysis with the event (Y/N) as a dependent variable and the confounder as the predictor, and corrected for treatment group. In step 3, the confounders were selected and the PS model was created, which produced the PS, which represents the probability of being treated with canagliflozin. Finally, the adjusted treatment effect (OR and associated 95% Wilson CI) was estimated by a logistic regression analysis, with hypoglycaemia as the dependent and treatment as the independent variable of interest, while adjusting for the PS. The unadjusted and PS‐adjusted ORs and 95% Wilson CIs are presented for hypoglycaemia and volume depletion events.
3. RESULTS
3.1. Patients
Of the 379 patients enrolled in the study, 321 were included in the tolerability analysis (reasons for exclusion are shown in Figure 1). A total of 162 patients received canagliflozin and 159 received sulphonylurea; their baseline characteristics are shown in Table 1 . Of the patients taking canagliflozin (n=162), 134 (82.7%) were on a 100 mg daily dose, 1 (0.6%) was on a 200 mg daily dose, and 27 (16.7%) were on a 300 mg daily dose at the start of the study. Of the patients taking a sulphonylurea (n=159), 14 (8.8%) were on glibenclamide, 77 (48.4%) were on gliclazide, and 68 (42.8%) were on glimepiride at the start of the study; the median dose of glibenclamide was 10 mg, the median dose of gliclazide was 60 mg, and the median dose of glimepiride was 3 mg. The median daily dose of metformin at the beginning of the study was 2000 mg in both the canagliflozin and sulphonylurea groups. At the beginning of the study, 57.4% (93/162) of patients taking canagliflozin and 50.3% (80/159) of patients taking sulphonylurea were also taking a DPP‐4 inhibitor; sitagliptin was the most commonly used DPP‐4 inhibitor, and the median dose of DPP‐4 inhibitor was 100 mg in both groups. No patients treated with canagliflozin changed their canagliflozin dose, 1.9% decreased their metformin dose, and 3.2% decreased their DPP‐4 inhibitor dose at or just after the beginning of Ramadan. Among patients treated with sulfonylureas, 2.5% decreased their dose of sulfonylurea at the start of Ramadan, 6.9% decreased their dose of sulphonylurea during Ramadan, 3.1% decreased their dose of metformin, and no patients decreased their dose of DPP‐4 inhibitor at or just after the beginning of Ramadan.
Figure 1.
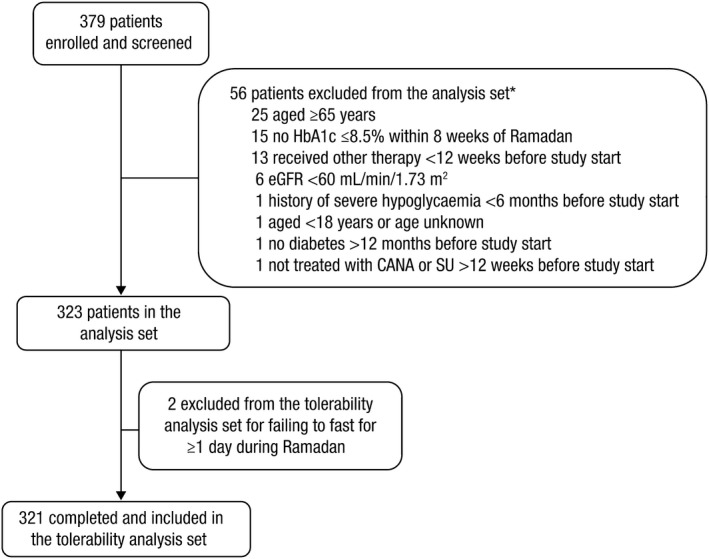
Patient enrolment. eGFR, estimated glomerular filtration rate; CANA, canagliflozin; SU, sulphonylurea.*There were seven patients with two reasons for exclusion. Patients were enrolled and screened at the pre‐Ramadan visit. Patients were excluded from the tolerability analysis set for failing to meet eligibility criteria or for failing to fast ≥1 day during Ramadan
Table 1.
Baseline demographic and disease characteristics
| Characteristica | SU (n=159) | CANA (n=162) |
|---|---|---|
| Country, n (%)b | ||
| Kuwait | 29 (18.2) | 26 (16.0) |
| Lebanon | 107 (67.3) | 112 (69.1) |
| UAE | 23 (14.5) | 24 (14.8) |
| Sex, n (%) | ||
| Male | 87 (54.7) | 100 (61.7) |
| Female | 72 (45.3) | 62 (38.3) |
| Age, y | 54.3 (7.4) | 52.3 (7.7) |
| BMI, kg/m2 | 29.6 (4.6) | 30.7 (4.7) |
| Weight, kg | 82.1 (14.1) | 87.1 (14.8) |
| Duration of T2DM, y | 7.6 (5.5) | 6.5 (5.9) |
| HbA1c, % | 7.2 (0.8) | 7.3 (0.8) |
| eGFR, mL/min/1.73 m2 | 88.7 (17.6) | 89.9 (19.6) |
| DPP‐4 inhibitor use, n (%) | 80 (50.3) | 93 (57.4) |
| Sitagliptinc | 44 (55.0) | 56 (60.2) |
| Vildagliptinc | 25 (31.3) | 23 (24.7) |
| Linagliptinc | 5 (6.3) | 4 (4.3) |
| Saxagliptinc | 6 (7.5) | 10 (10.8) |
SU, sulphonylurea; CANA, canagliflozin; UAE, United Arab Emirates; BMI, body mass index; T2DM, type 2 diabetes mellitus; eGFR, estimated glomerular filtration rate; DPP‐4, dipeptidyl peptidase‐4; SD, standard deviation.
Data are mean (SD) unless otherwise indicated.
Percentages may not total 100.0% due to rounding.
Percentages based on number of patients receiving DPP‐4 inhibitors (n=80 in the SU group and n=93 in the CANA group); percentages may not total 100.0% due to rounding.
3.2. Hypoglycaemia events
Six patients treated with canagliflozin (3.7%) reported ≥1 symptomatic hypoglycaemia episode compared to 21 patients treated with sulphonylurea (13.2%) during Ramadan (Figure 2A). The proportion of patients who experienced a hypoglycaemia event was related to treatment arms after adjustment for observed cofounders by means of the PS method. After adjustment for factors determined to be confounders (baseline age, baseline BMI, diabetes duration, treatment duration, baseline HbA1c, home country, DPP‐4 inhibitor use, sex, complicating diagnoses, and diuretic use), the adjusted OR was 0.273 (95% Wilson CI: 0.104, 0.719, P=.009), which indicates that the odds of a hypoglycaemia event were lower in patients treated with canagliflozin (unadjusted OR was 0.253 [95% Wilson CI: 0.099, 0.644, P=.004]). Of patients who reported a hypoglycaemia episode, two of six patients treated with canagliflozin and 11 of 21 patients treated with sulphonylurea had confirmed hypoglycaemia events with blood glucose <3.9 mmol/L (Figure 2A).
Figure 2.
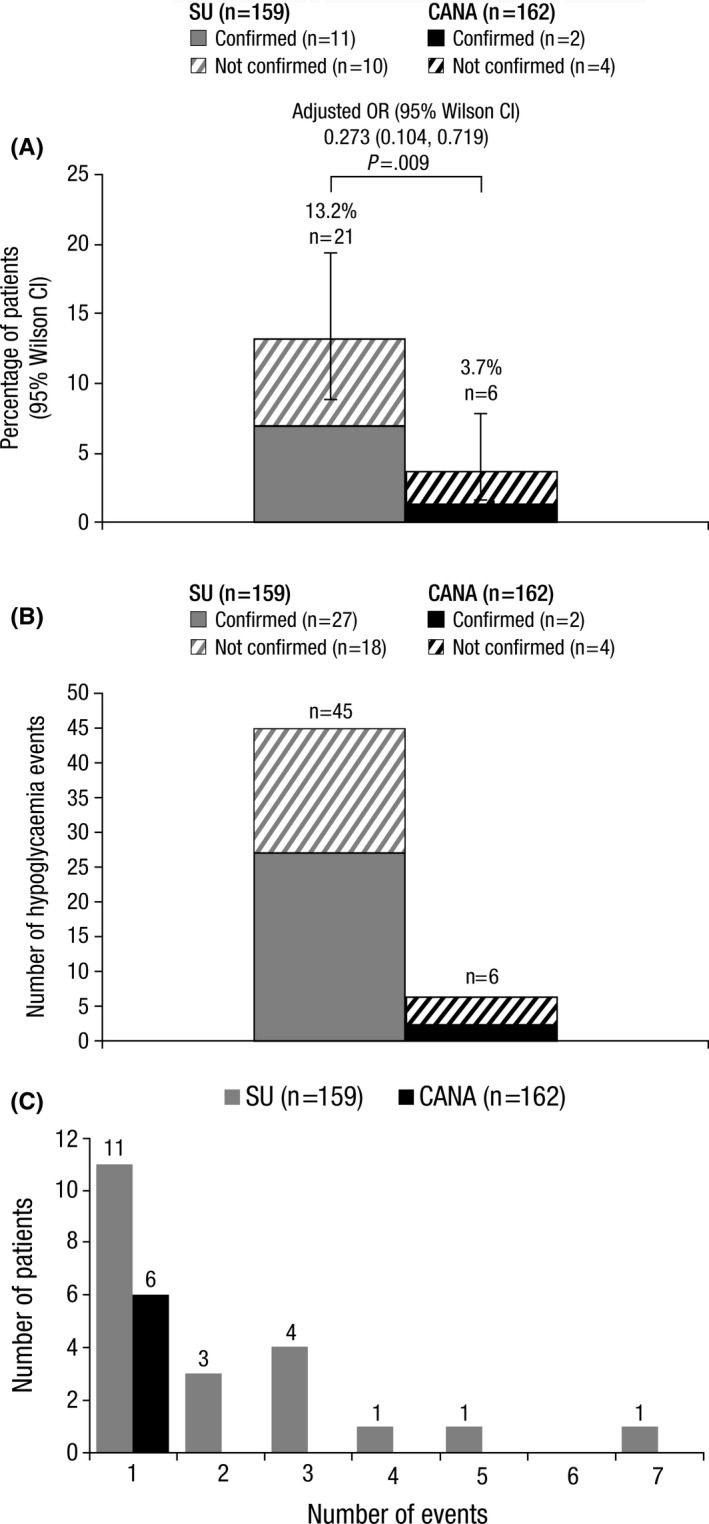
(A) Proportion of patients who experienced ≥1 hypoglycaemia event, (B) Number of hypoglycaemia events, and (C) Distribution of hypoglycaemia events among affected patients. SU, sulphonylurea; CANA, canagliflozin; OR, odds ratio; CI, confidence interval
There were a total of six hypoglycaemia episodes with canagliflozin and 45 hypoglycaemia episodes with sulphonylurea (Figure 2B). For individual hypoglycaemia events for which blood glucose was measured at the time of the event, two of six hypoglycaemia episodes with canagliflozin and 27 of 37 with sulphonylurea were confirmed by blood glucose measurement <3.9 mmol/L; blood glucose was not measured for eight hypoglycaemia events with sulphonylurea. There were no hypoglycaemia events in patients treated with canagliflozin for which blood glucose was <3.0 mmol/L, while blood glucose <3.0 mmol/L was seen in six of 37 events in patients treated with sulphonylurea.
Among patients who experienced a hypoglycaemia event, no (0/6) patients treated with canagliflozin reported >1 event, while 47.6% (10/21) of patients treated with sulphonylurea reported >1 event (Figure 2C). No patients in either treatment group experienced a severe hypoglycaemia event. Dizziness was the most commonly reported symptom of hypoglycaemia; two of six patients treated with canagliflozin reported dizziness and 19 of 45 patients treated with sulphonylurea reported dizziness (Table 2).
Table 2.
Summary of hypoglycaemia and volume depletion event signs and symptoms
| Signs and symptoms, n (%)a | SU | CANA |
|---|---|---|
| Number of hypoglycaemia events | 45 | 6 |
| Dizziness | 19 (42.2) | 2 (33.3) |
| Hunger | 10 (22.2) | 1 (16.7) |
| Weakness | 6 (13.3) | 0 |
| Headache | 4 (8.9) | 0 |
| Drowsiness | 1 (2.2) | 1 (16.7) |
| Sweating | 1 (2.2) | 1 (16.7) |
| No symptoms | 3 (6.7) | 1 (16.7) |
| Other | 1 (2.2) | 0 |
| Number of volume depletion events | 13 | 51 |
| Dehydration | 10 (76.9) | 44 (86.3) |
| Hypotension | 0 | 1 (2.0) |
| Postural dizziness | 3 (23.1) | 6 (11.8) |
SU, sulphonylurea; CANA, canagliflozin.
Percentages may not equal 100.0% due to rounding.
3.3. Volume depletion events
Twenty‐six patients treated with canagliflozin (16.1%) reported ≥1 volume depletion event compared with eight patients treated with sulphonylurea (5.0%, Figure 3A). The incidence of volume depletion events was related to treatment arms after adjustment for observed confounding factors by means of the PS method. After adjustment for factors determined to be confounders (baseline age, baseline BMI, diabetes duration, treatment duration, baseline HbA1c, baseline eGFR, DPP‐4 inhibitor use, sex, current smoker status, complicating diagnosis, and diuretic use), the adjusted OR was 3.5 (95% Wilson CI: 1.3, 9.2, P=.011), which indicates that the odds of a volume depletion event were lower in the sulphonylurea group (unadjusted OR was 3.6 [95% Wilson CI: 1.6, 8.2, P=.002]).
Figure 3.
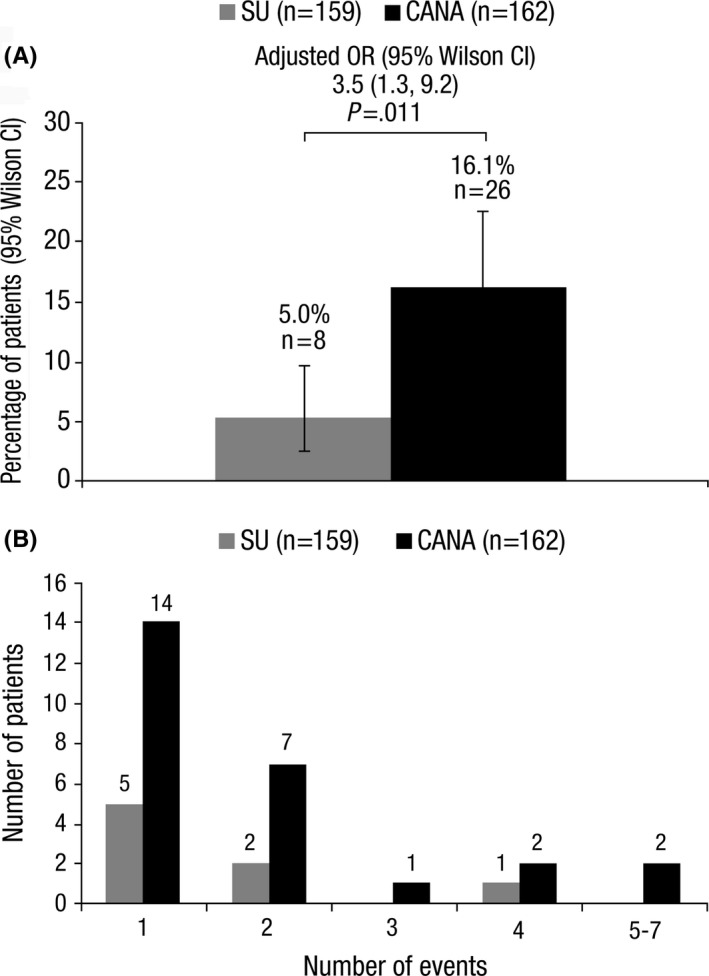
(A) Proportion of patients who experienced ≥1 volume depletion event and (B) Distribution of volume depletion events among affected patients. SU, sulphonylurea; CANA, canagliflozin; OR, odds ratio; CI, confidence interval
Among patients who reported a volume depletion event, 53.8% (14/26) of patients treated with canagliflozin and 62.5% (5/8) of patients treated with sulphonylurea had only one event (Figure 3B). Among the total of 64 volume depletion events reported, 86.3% (44/51) with canagliflozin and 76.9% (10/13) with sulphonylurea were based on symptoms of dehydration; this was explained most frequently for canagliflozin by thirst/low fluid intake (33.3%) or unknown cause (27.5%) and for sulphonylurea by diarrhoea (30.8%) or thirst/low fluid intake (23.1%) (Table 2).
3.4. Treatment adherence
Adherence to study treatments was high in both groups; 98.8% (160/162) of patients in the canagliflozin group and 96.2% (153/159) of patients in the sulphonylurea group reported no missed doses of canagliflozin and sulphonylurea, respectively (Table 3). In the canagliflozin group, one dose was missed because of excessive dehydration (reported as a volume depletion event) and one dose was missed because the patient forgot to take their medication. In the sulphonylurea group, four doses were missed to prevent hypoglycaemia, one dose was missed as a precaution, and one dose was missed because the patient forgot to take their medication. Adherence to metformin was also high in both study groups; 100.0% (162/162) of patients in the canagliflozin group and 98.7% (157/159) of patients in the sulphonylurea group reported no missed doses of metformin. In the sulphonylurea group, one dose was missed because the patient forgot to take their medication, and the reason one dose was missed was listed as “other.”
Table 3.
Medication and fasting adherence during Ramadan
| Patients, n (%)a | SU (n=159) | CANA (n=162) |
|---|---|---|
| Number of missed doses of SU or CANA | ||
| No missed doses | 153 (96.2) | 160 (98.8) |
| 1 missed dose | 3 (1.9) | 1 (0.6) |
| 3 missed doses | 2 (1.3) | 1 (0.6) |
| 5 missed doses | 1 (0.6) | 0 |
| Reason for missed dose of SU or CANA | ||
| Patients with missed doses | 6 | 2 |
| Forgot to take | 1 (16.7) | 1 (50.0) |
| Precaution | 1 (16.7) | 0 |
| Symptoms of excessive dehydration | 0 | 1 (50.0) |
| To prevent hypoglycaemia | 4 (66.7) | 0 |
| Number of missed doses of metformin | ||
| No missed doses | 157 (98.7) | 162 (100.0) |
| 1 missed dose | 1 (0.6) | 0 |
| 3 missed doses | 1 (0.6) | 0 |
| Reason for missed doses of metformin | ||
| Patients with missed doses | 2 | 0 |
| Forgot to take | 1 (50.0) | 0 |
| Other | 1 (50.0) | 0 |
| Number of missed fasting days during Ramadan | ||
| No missed days | 124 (78.0) | 133 (82.1) |
| 1 missed day | 4 (2.5) | 8 (4.9) |
| 2 missed days | 9 (5.7) | 11 (6.8) |
| 3 missed days | 4 (2.5) | 4 (2.5) |
| 4‐10 missed days | 14 (8.8) | 5 (3.1) |
| 11‐20 missed days | 0 | 1 (0.6) |
| 21‐29 missed days | 4 (2.5) | 0 |
SU, sulphonylurea; CANA, canagliflozin.
Percentages may not equal 100.0% due to rounding.
3.5. Fasting adherence
The number of missed fasting days was low in both study groups; 82.1% (133/162) of patients in the canagliflozin group and 78.0% (124/159) of patients in the sulphonylurea group reported no missed fasting days (Table 3). The mean (SD) number of fasting days was 29.5 (1.6) and 28.6 (4.1) days with canagliflozin and sulphonylurea, respectively.
A detailed list of reasons patients reported for breaking fast are detailed in Table S1. Among canagliflozin patients who broke fast (n=29), 24.1% reported symptoms of excessive dehydration, 20.7% reported fatigue/dizziness, and 13.8% reported diarrhoea/vomiting at least once as a reason for breaking fast. Among sulphonylurea patients who broke fast (n=35), 34.3% reported symptoms of low blood sugar, 31.4% reported “to prevent hypoglycaemia,” 17.1% reported symptoms of excessive dehydration, and 17.1% reported fatigue/dizziness at least once as a reason for breaking fast. A volume depletion event was reported on the same day as the first broken fast for four patients treated with canagliflozin and seven patients treated with sulphonylurea. No patients who reported a volume depletion event stopped fasting for Ramadan prematurely.
3.6. Adverse events
Canagliflozin and sulphonylurea were generally well tolerated, with similar rates of AEs in both groups; no patients in either group experienced any serious AEs or AEs leading to discontinuation of study medication (Table 4). Ten patients had AEs of thirst in the canagliflozin group, and no other osmotic diuresis–related AEs (including pollakiuria, polyuria or polydipsia) were reported. At least one AE related to study treatment was reported in 9.3% (15/162) of patients treated with canagliflozin and 8.8% (14/159) of patients treated with sulphonylurea. At least one AE related to metformin was reported in 1.2% (2/162) of patients treated with canagliflozin and 0.6% (1/159) of patients treated with sulphonylurea, and no patients in either treatment group experienced an AE related to DPP‐4 inhibitor use. Only one patient (0.6%) in the canagliflozin group reported a urinary tract infection, and no genital mycotic infections were observed.
Table 4.
Summary of AEs
| Patients, n (%) | SU (n=159) | CANA (n=162) |
|---|---|---|
| Any AEs | 29 (18.2) | 37 (22.8) |
| AEs leading to discontinuation | 0 | 0 |
| AEs related to study drug | 14 (8.8) | 15 (9.3) |
| Serious AEs | 0 | 0 |
| Deaths | 0 | 0 |
| Genital mycotic infections | ||
| Male | 0 | 0 |
| Female | 0 | 0 |
| Urinary tract infections | 0 | 1 (0.6) |
| Osmotic diuresis–related AEsa | 0 | 10 (6.2) |
| Volume depletion–related AEsb | 6 (3.8) | 15 (9.3)c |
| Hypoglycaemia AEs | 17 (10.7) | 4 (2.5) |
AE, adverse event; SU, sulphonylurea; CANA, canagliflozin.
Includes thirst.
Includes dehydration, postural dizziness, and hypovolaemia.
One patient reported two volume depletion–related AEs.
3.7. HbA1c, body weight, BP and eGFR
Patients treated with canagliflozin and sulphonylurea had an average (standard deviation) of 87 (26) and 92 (26) days, respectively, between pre‐ and post‐Ramadan measurements. During this time, there were small reductions in HbA1c and body weight observed in both treatment groups, with numerically greater reductions in patients treated with canagliflozin (Table 5). Small changes were observed in systolic BP, diastolic BP and eGFR between pre‐ and post‐Ramadan measurements; small reductions from baseline in patients treated with canagliflozin and small increases from baseline in patients treated with sulphonylurea were observed.
Table 5.
Pre‐ and post‐Ramadan HbA1c, body weight, BP, and eGFR values
| SU (n=159) | CANA (n=162) | |||||
|---|---|---|---|---|---|---|
| Characteristic, mean (SD) | Pre‐Ramadan | Post‐Ramadan | Change from baseline | Pre‐Ramadan | Post‐Ramadan | Change from baseline |
| HbA1c, % | n=159 | n=125 | n=125 | n=162 | n=144 | n=144 |
| 7.2 (0.8) | 7.1 (0.7) | −0.2 (0.6) | 7.3 (0.8) | 6.9 (0.8) | −0.4 (0.7) | |
| Weight, kg | n=158 | n=150 | n=149 | n=162 | n=155 | n=155 |
| 82.1 (14.1) | 81.5 (14.0) | −0.5 (3.2) | 87.1 (14.8) | 85.3 (14.3) | −2.4 (3.1) | |
| Systolic BP, mmHg | n=158 | n=150 | n=149 | n=162 | n=155 | n=155 |
| 129.8 (12.0) | 130.7 (12.1) | 1.2 (12.5) | 129.0 (11.7) | 127.9 (10.2) | −1.5 (12.9) | |
| Diastolic BP, mmHg | n=158 | n=150 | n=149 | n=162 | n=155 | n=155 |
| 76.7 (8.0) | 77.0 (7.8) | 0.5 (9.0) | 78.2 (6.7) | 75.9 (7.3) | −2.9 (9.1) | |
| eGFR, mL/min/1.73 m2 | n=159 | n=96 | n=96 | n=162 | n=123 | n=123 |
| 88.7 (17.6) | 89.9 (19.9) | 3.1 (14.8) | 89.9 (19.6) | 88.4 (21.6) | −1.2 (19.4) | |
BP, blood pressure; eGFR, estimated glomerular filtration rate; SU, sulphonylurea; CANA, canagliflozin; SD, standard deviation.
4. DISCUSSION
Overall, the findings from this study showed that canagliflozin was generally well tolerated for the treatment of patients with T2DM fasting during Ramadan. Over the month of Ramadan, patients treated with canagliflozin had a lower risk of experiencing a hypoglycaemia episode compared to patients treated with sulphonylurea. Patients treated with canagliflozin also had a low number of missed fasting days and did not have to change canagliflozin dose for Ramadan.
The reduced risk of hypoglycaemia observed with canagliflozin is consistent with the results from a previous study that examined the safety and tolerability of the SGLT2 inhibitor dapagliflozin in patients with T2DM fasting during Ramadan.4 The consistency of these results suggests that the insulin‐independent mechanism of action of SGLT2 inhibitors may be associated with a lower risk of hypoglycaemia compared to insulin secretagogues, like sulphonylureas.
Because the mechanism of SGLT2 inhibitors causes mild osmotic diuresis, volume depletion events may be a concern for patients who are fasting for many hours each day and are at increased risk for dehydration. All patients taking canagliflozin should be informed of the potential risk of volume depletion and advised on how to avoid an event, especially those known to be at higher risk of volume depletion adverse events, including patients with impaired renal function, elderly patients, patients on diuretics or medications that interfere with the renin‐angiotensin‐aldosterone system, or patients with low systolic blood pressure.26 In this study, patients treated with canagliflozin were at a higher risk of experiencing volume depletion events (mostly dehydration) compared to patients treated with sulphonylurea. In addition, patients in the canagliflozin group experienced a greater number of volume depletion–related and osmotic diuresis–related AEs; the osmotic diuresis–related AEs were limited to thirst. In the majority of cases, volume depletion events did not lead to breaking of the fast, and no patients with a volume depletion event prematurely ended their Ramadan fast. In addition, there was only one report of a volume depletion event that led to a missed dose of canagliflozin. Overall adherence to study medications was high in both groups of patients. Patients should be aware that fasting during extremely hot conditions may lead to dehydration; therefore, appropriate hydration during Ramadan is advised.
Notably, no patients in either group discontinued study treatment because of AEs. Patients in this study reported missing few fasting days, and most patients reported no missed fasting days. These data suggest that although some patients in the canagliflozin group experienced thirst and volume depletion–related AEs, these AEs did not make them uncomfortable enough to discontinue treatment or miss many fasting days. Likewise, although patients treated with sulphonylureas experienced dizziness and hypoglycaemia AEs, these AEs generally did not lead to discontinuation of treatment or fasting. Not missing fasting days during Ramadan is important for Muslim patients with T2DM, because fasting days that are missed during Ramadan must be made up later in the year.1
In this study, both treatments were generally well tolerated, with low overall rates of AEs and no serious AEs reported in either group. Unlike the results of previous studies, canagliflozin was not associated with a higher incidence of genital mycotic infections, with no genital mycotic infections reported in males or females in either treatment group.27, 28
A limitation of this study was the geographic restriction to the Middle East, a region that may have different climate conditions than other countries where Muslims fast during Ramadan, which may affect the risk of hypoglycaemia and volume depletion. In addition, in the Middle East, fasting for Ramadan 2016 lasted approximately 15 hours each day, while in some far northern countries, fasting lasted for more than 20 hours each day. This study excluded several categories of patients at high risk of severe hypoglycaemia, including patients with a history of severe hypoglycaemia events, patients with renal impairment, and patients over the age of 65 years, which may have contributed to the lack of severe hypoglycaemia events observed and limit the generalisability of this study with regard to hypoglycaemia events. This study was also limited by its observational nature and its reliance on patient reports of medication adherence, which may not be an accurate representation of real‐world use. While PS analysis was performed to balance potential confounding factors in the patient population, bias due to demographic factors that were not considered cannot be ruled out.
Overall, canagliflozin was associated with a reduced risk of hypoglycaemia compared with sulphonylurea, an increased risk of volume depletion events that did not lead to breaking of the fast or discontinuation of treatment, and a low rate of AEs in patients with T2DM fasting for Ramadan. Therefore, the findings from this study support the use of canagliflozin for the treatment of these patients.
DISCLOSURE
M.H. has served as a speaker for Novo Nordisk, Lilly, Sanofi, Merck Sharpe & Dohme, Janssen, AstraZeneca, Servier, Novartis, Takeda, Abbott, and Johnson & Johnson. A.E. has served as a speaker for Novo Nordisk, Lilly, Sanofi, Merck, Sharpe & Dohme, Janssen, AstraZeneca, Servier, Novartis, Takeda, and Abbott and participated in clinical studies sponsored or supported by Novo Nordisk, Sanofi, Merck Sharpe & Dohme, Janssen, AstraZeneca, Servier, and Novartis. A.H. has served as a member of advisory boards for Novo Nordisk, Eli Lilly, MSD, AstraZeneca, Boehringer Ingelheim, Sanofi, and Servier. M.A. has served as a member of advisory boards for Sanofi, MSD, and Merck Serono. B.A. has served as a member of advisory boards for Novo Nordisk, Eli Lily, MSD, AstraZeneca, Boehringer Ingelheim, Sanofi, and Servier. R.P. has no disclosures. A.B. has served as a member of advisory boards for Sanofi, MSD, Servier, AstraZeneca, and Novo Nordisk. M.N. is a full‐time employee of Janssen‐Cilag Poland. P.B. is a full‐time employee of Janssen‐Cilag BV. S.K. is a full‐time employee of Janssen‐Cilag Portugal. G.H. is a full‐time employee of Janssen UK. S.T.A. has served as a speaker and advisor for Novo Nordisk, Lilly, Sanofi, Merck Sharpe & Dohme, Janssen, AstraZeneca, Servier, Novartis, Takeda, and Merck.
AUTHOR CONTRIBUTIONS
M.H., A.E., A.H., M.A., B.A., R.P., A.B., S.K and S.T.A. contributed to the conduct of the study, the interpretation of the data, and drafted, reviewed and approved the manuscript. P.B. contributed to the analysis and interpretation of the data, and drafted, reviewed, and approved the manuscript. M.N. and G.H. contributed to the design and conduct of the study; the acquisition, analysis, and interpretation of the data; and drafted, reviewed and approved the manuscript.
Supporting information
ACKNOWLEDGEMENTS
This study was supported by Janssen Pharmaceutica NV. The sponsor was involved in the study design, data collection, data analysis, manuscript preparation and publication decisions. Editorial support was provided by Dana Tabor, PhD, of MedErgy, and was funded by Janssen Pharmaceutica NV.
Canagliflozin has been developed by Janssen Research & Development, LLC, in collaboration with Mitsubishi Tanabe Pharma Corporation.
Hassanein M, Echtay A, Hassoun A, et al. Tolerability of canagliflozin in patients with type 2 diabetes mellitus fasting during ramadan: Results of the Canagliflozin in Ramadan Tolerance Observational Study (CRATOS). Int J Clin Pract. 2017;71:e12991 https://doi.org/10.1111/ijcp.12991
REFERENCES
- 1. International Diabetes Federation . Diabetes and Ramadan: Practical Guidelines. International Diabetes Federation (IDF), in collaboration with the Diabetes and Ramadan (DAR) International Alliance; 2016. April. [cited February 13, 2017]; Available from: http://www.idf.org/sites/default/files/IDF-DAR-Practical-Guidelines-Final-Low.pdf.
- 2. Salti I, Benard E, Detournay B, et al. A population‐based study of diabetes and its characteristics during the fasting month of Ramadan in 13 countries: results of the epidemiology of diabetes and Ramadan 1422/2001 (EPIDIAR) study. Diabetes Care. 2004;27:2306‐2311. [DOI] [PubMed] [Google Scholar]
- 3. Almaatouq MA. Pharmacological approaches to the management of type 2 diabetes in fasting adults during Ramadan. Diabetes Metab Syndr Obes. 2012;5:109‐119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wan Seman WJ, Kori N, Rajoo S, et al. Switching from sulphonylurea to a sodium‐glucose cotransporter2 inhibitor in the fasting month of Ramadan is associated with a reduction in hypoglycaemia. Diabetes Obes Metab. 2016;18:628‐632. [DOI] [PubMed] [Google Scholar]
- 5. Rosenthal N, Meininger G, Ways K, et al. Canagliflozin: a sodium glucose co‐transporter 2 inhibitor for the treatment of type 2 diabetes mellitus. Ann N Y Acad Sci. 2015;1358:28‐43. [DOI] [PubMed] [Google Scholar]
- 6. Devineni D, Morrow L, Hompesch M, et al. Canagliflozin improves glycemic control over 28 days in subjects with type 2 diabetes not optimally controlled on insulin. Diabetes Obes Metab. 2012;14:539‐545. [DOI] [PubMed] [Google Scholar]
- 7. Rosenstock J, Aggarwal N, Polidori D, et al. Dose‐ranging effects of canagliflozin, a sodium‐glucose cotransporter 2 inhibitor, as add‐on to metformin in subjects with type 2 diabetes. Diabetes Care. 2012;35:1232‐1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sha S, Devineni D, Ghosh A, et al. Pharmacodynamic effects of canagliflozin, a sodium glucose co‐transporter 2 inhibitor, from a randomized study in patients with type 2 diabetes. PLoS ONE. 2014;9:e105638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Devineni D, Curtin CR, Polidori D, et al. Pharmacokinetics and pharmacodynamics of canagliflozin, a sodium glucose co‐transporter 2 inhibitor, in subjects with type 2 diabetes mellitus. J Clin Pharmacol. 2013;53:601‐610. [DOI] [PubMed] [Google Scholar]
- 10. Stenlöf K, Cefalu WT, Kim KA, et al. Efficacy and safety of canagliflozin monotherapy in subjects with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetes Obes Metab. 2013;15:372‐382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lavalle‐González FJ, Januszewicz A, Davidson J, et al. Efficacy and safety of canagliflozin compared with placebo and sitagliptin in patients with type 2 diabetes on background metformin monotherapy: a randomised trial. Diabetologia. 2013;56:2582‐2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wilding JP, Charpentier G, Hollander P, et al. Efficacy and safety of canagliflozin in patients with type 2 diabetes mellitus inadequately controlled with metformin and sulphonylurea: a randomised trial. Int J Clin Pract. 2013;67:1267‐1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Forst T, Guthrie R, Goldenberg R, et al. Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes on background metformin and pioglitazone. Diabetes Obes Metab. 2014;16:467‐477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stenlöf K, Cefalu WT, Kim KA, et al. Long‐term efficacy and safety of canagliflozin monotherapy in patients with type 2 diabetes inadequately controlled with diet and exercise: findings from the 52‐week CANTATA‐M study. Curr Med Res Opin. 2014;30:163‐175. [DOI] [PubMed] [Google Scholar]
- 15. Cefalu WT, Leiter LA, Yoon KH, et al. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA‐SU): 52 week results from a randomised, double‐blind, phase 3 non‐inferiority trial. Lancet. 2013;382:941‐950. [DOI] [PubMed] [Google Scholar]
- 16. Bode B, Stenlöf K, Sullivan D, et al. Efficacy and safety of canagliflozin treatment in older subjects with type 2 diabetes mellitus: a randomized trial. Hosp Pract. 2013;41:72‐84. [DOI] [PubMed] [Google Scholar]
- 17. Leiter LA, Yoon KH, Arias P, et al. Canagliflozin provides durable glycemic improvements and body weight reduction over 104 weeks versus glimepiride in patients with type 2 diabetes on metformin: a randomized, double‐blind, phase 3 study. Diabetes Care. 2015;38:355‐364. [DOI] [PubMed] [Google Scholar]
- 18. Bode B, Stenlöf K, Harris S, et al. Long‐term efficacy and safety of canagliflozin over 104 weeks in patients aged 55‐80 years with type 2 diabetes. Diabetes Obes Metab. 2015;17:294‐303. [DOI] [PubMed] [Google Scholar]
- 19. Schernthaner G, Gross JL, Rosenstock J, et al. Canagliflozin compared with sitagliptin for patients with type 2 diabetes who do not have adequate glycemic control with metformin plus sulfonylurea: a 52‐week, randomized trial. Diabetes Care. 2013;36:2508‐2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yale JF, Bakris G, Cariou B, et al. Efficacy and safety of canagliflozin in subjects with type 2 diabetes and chronic kidney disease. Diabetes Obes Metab. 2013;15:463‐473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yale JF, Bakris G, Cariou B, et al. Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes mellitus and chronic kidney disease. Diabetes Obes Metab. 2014;16:1016‐1027. [DOI] [PubMed] [Google Scholar]
- 22. Neal B, Perkovic V, de Zeeuw D, et al. Efficacy and safety of canagliflozin, an inhibitor of sodium glucose co‐transporter 2, when used in conjunction with insulin therapy in patients with type 2 diabetes. Diabetes Care. 2015;38:403‐411. [DOI] [PubMed] [Google Scholar]
- 23. John M, Cerdas S, Violante R, et al. Efficacy and safety of canagliflozin in patients with type 2 diabetes mellitus living in hot climates. Int J Clin Pract. 2016;70:775‐785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care. 2013;36:1384‐1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. International Hypoglycaemia Study Group . Glucose concentrations of less than 3.0 mmol/L (54 mg/dL) should be reported in clinical trials: a joint position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2017;40:155‐157. [DOI] [PubMed] [Google Scholar]
- 26. INVOKANA® (Canagliflozin) Tablets, for Oral Use [Package Insert]. Titusville, NJ: Janssen Pharmaceuticals; 2016. [Google Scholar]
- 27. Usiskin K, Kline I, Fung A, et al. Safety and tolerability of canagliflozin in patients with type 2 diabetes: pooled analysis of phase 3 study results. Postgrad Med. 2014;126:16‐34. [DOI] [PubMed] [Google Scholar]
- 28. Qiu R, Balis D, Xie J, et al. Longer‐term safety and tolerability of canagliflozin in patients with type 2 diabetes: a pooled analysis. Curr Med Res Opin. 2017;33:553‐562. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


