ABSTRACT
Multidisciplinary care is considered the standard of care for both adult and pediatric neuromuscular disorders and has been associated with improved quality of life, resource utilization, and health outcomes. Multidisciplinary care is delivered in multidisciplinary clinics that coordinate care across multiple specialties by reducing travel burden and streamlining care. In addition, the multidisciplinary care setting facilitates the integration of clinical research, patient advocacy, and care innovation (e.g., telehealth). Yet, multidisciplinary care requires substantial commitment of staff time and resources. We calculated personnel costs in our ALS clinic in 2015 and found an average cost per patient visit of $580, of which only 45% was covered by insurance reimbursement. In this review, we will describe classic and emerging concepts in multidisciplinary care models for adult and pediatric neuromuscular disease. We will then explore the financial impact of multidisciplinary care with emphasis on sustainability and metrics to demonstrate quality and value. Muscle Nerve 56: 848–858, 2017
Keywords: advocacy, disease outcomes, genetics, healthcare costs, multidisciplinary care, telehealth
Abbreviations
- AAN
American Academy of Neurology
- AANEM
American Association of Neuromuscular & Electrodiagnostic Medicine
- ALS
amyotrophic lateral sclerosis
- CMD
congenital muscular dystrophy
- CPAP
continuous positive airway pressure
- DMD
Duchenne muscular dystrophy
- DXA
dual‐energy X‐ray absorptiometry
- FTE
full‐time equivalent
- G‐tube
gastrostomy tube
- HIPAA
Health Insurance Portability and Accountability Act
- NGS
next generation sequencing
- VUS
variants of unknown significance
- WES
whole exome sequencing
- WGS
whole genome sequencing
Multidisciplinary care is considered to be the “gold standard” and state‐of‐the‐art model of care for many neuromuscular diseases, particularly those that are profoundly debilitating, and has been endorsed by several professional societies, expert panels, and advocacy groups.1, 2, 3, 4, 5, 6, 7, 8 Because patients with neuromuscular diseases typically find themselves grappling with the manifold consequences of weakness, as well as myriad extramuscular manifestations of their disorders, a multidisciplinary care model allows for coordinated management across multiple specialties, making it possible for complex clinical presentations to be addressed in one visit. This model also eases the stresses and burdens on patients that would be otherwise scheduled to attend multiple hospital or clinic visits to many different specialists. Patients and clinicians in multidisciplinary clinics believe that the model is preferable to uncoordinated care.9 Furthermore, studies have shown that the quality of care delivered in multidisciplinary care is high and that both quality of life and survival are better in neuromuscular populations treated in multidisciplinary clinics than in isolated neurological clinics.10, 11, 12, 13, 14, 15
The evolution of the health care system in the United States presents both an opportunity and a challenge to neuromuscular clinicians as they strive to establish and run multidisciplinary clinics. The recent focus on improving the quality of care through patient‐centered care and care coordination aligns with both the goals and demonstrable results of multidisciplinary care. The impact of a renewed focus on healthcare value is more complex. Value is defined as outcomes relative to costs16 and describes the relationship of the quality of care delivered with the cost of delivering that care (Fig. 1). However “value” can be a relative term in healthcare: the patient, insurer, healthcare provider, and society may each define value differently.16 Multidisciplinary care may reduce acute hospitalizations12, 17 and prevent redundancies in care.14, 18 This is hard to measure, but, if true, then insurers might see value in multidisciplinary clinics, particularly because for the time being, reimbursement for care delivered in a multidisciplinary clinic is no higher than traditional care. Patients likely see great value in multidisciplinary clinics.9, 19, 20 Yet, multidisciplinary care clinics are expensive to run,21 and the burden of these costs falls on the institution. Thus, potential cost‐savings are decoupled from the additional expense, threatening the sustainability of multidisciplinary clinics. This disconnect is rarely discussed, perhaps in part because of difficulty defining and measuring the concepts of quality and value for these purposes. A formal definition and assessment of the quality and value of multidisciplinary care for neuromuscular disease would be useful to cement its role in providing care and/or highlight areas in need of change.
Figure 1.
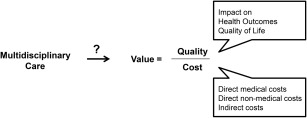
Framework for assessing the impact of multidisciplinary care in neuromuscular medicine.
In this review we describe classic concepts in multidisciplinary care models for adult and pediatric neuromuscular disease, opportunities for innovation, and integration of clinical research into the multidisciplinary clinic environment. We will then explore the financial impact of multidisciplinary care with emphasis on sustainability and metrics to demonstrate quality and value.
MULTIDISCIPLINARY CARE MODELS FOR ADULT NEUROMUSCULAR DISORDERS
Multidisciplinary neuromuscular clinics are often organized around specific neuromuscular patient populations to best gather the requisite specialists and expertise [e.g., amyotrophic lateral sclerosis (ALS) clinic, pediatric neuromuscular clinic, muscular dystrophy clinic]. In many cases, disease‐specific guidelines for multidisciplinary clinics have been published.1, 2, 3, 4, 5, 6, 7
The multidisciplinary clinical team consists of a core of healthcare professionals led by a neuromuscular‐trained physician (Fig. 2). Centralizing the location of these providers to streamline patients' visits with multiple providers is one function of the multidisciplinary clinic—perhaps the easiest to achieve. Improving communication among providers, treating people broadly across individual disciplines, and improving quality and efficiency of care delivery by developing partnerships is the more important, and more challenging, goal of multidisciplinary care. In fact, a dedicated team may spend a good deal of time communicating about complicated patients and coordinating care, but this time is well spent, leading to fewer inefficiencies, higher quality care, and, ultimately, better value.
Figure 2.
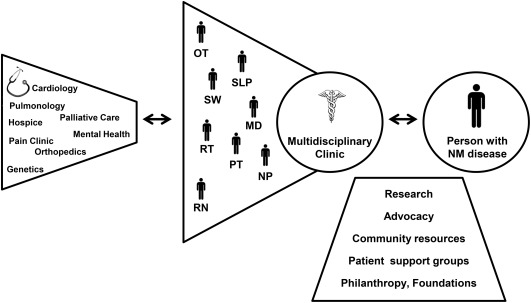
Multidisciplinary network of care for people with neuromuscular disease.
Abbreviations: MD, medical doctor; NM, neuromuscular; NP, nurse practitioner; PT, physical therapist; OT, occupational therapist; RN, registered nurse; RT, respiratory therapist; SLP, speech and language pathologist; SW, social worker.
This dedicated multidisciplinary team can provide a dynamic, individualized diagnosis, anticipatory guidance, and care plan. The multidisciplinary clinic staff provides the nexus between the patient, their family and caregivers, and the medical community (Fig. 2). The most successful multidisciplinary care teams are patient‐focused, aiming not only to address medical needs (e.g., prescriptions and diagnostic tests), but also to improve quality of life and assist in achieving life goals. The ideal clinic provides patient‐centered care and opportunities for clinical research, respect for patient autonomy, and an environment suffused with hope.
As team leader, the neuromuscular specialist (generally a neurologist or physiatrist with neuromuscular training) acts as the physician of record, and can leverage the expertise of every team member to address myriad patient concerns (Fig. 2). A clinic administrator can play a key role in the coordination of care and support services. When available, a medical social worker provides psychosocial support and evaluates environmental, financial, and support needs. Patient advocacy groups can play a critical role in supporting the clinic's mission (Fig. 2). Finally, the multidisciplinary clinic interfaces with multiple medical specialists as warranted by the specific disease, including pulmonary medicine, cardiology, orthopedic surgery, pain medicine, endocrinology, psychiatry, psychology, anesthesia, genetics, palliative care, and hospice (Fig. 2). These specialists may or may not sit within the multidisciplinary care clinic. The lynchpin to delivering coordinated multidisciplinary care is team cohesion and strong communication. This approach requires the establishment and ongoing reassessment of interdepartmental workflows related to specific procedures and care delivery. As an example, in our experience having close ties with specific providers in interventional radiology has been key to ensure coordinated care around feeding tube placement and subsequent management. Similarly, we have developed a close relationship with home care providers and meet with them periodically to maintain these ties, which help to manage complex therapy needs in the home setting. Successful multidisciplinary teams build this teamwork approach and emphasis on communication into their clinic ethos.
This type of multidisciplinary care primarily occurs in the outpatient setting. There are many elective hospital admissions for treatments such as feeding tube placement or scheduled infusions. As the underlying diseases progress, acute presentations to the emergency department can become more frequent, often due to respiratory distress, traumatic falls, or dysphagia.22, 23, 24 The multidisciplinary clinic team can recognize disease progression, initiate palliative care discussions, and perhaps even reduce the number of these emergent presentations to the hospital.14, 17
PEDIATRIC CONSIDERATIONS IN MULTIDISCIPLINARY CARE MODELS FOR NEUROMUSCULAR DISORDERS
Multidisciplinary pediatric neuromuscular clinics offer coordinated care to infants, children, adolescents, and young adults with neuromuscular disorders and integrate that care across ages (Fig. 3).
Figure 3.
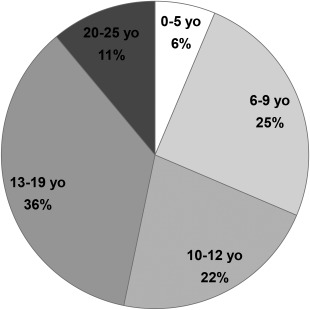
Age distribution of patients seen at the Massachusetts General Hospital pediatric multidisciplinary neuromuscular clinic in 2015. Emerging opportunities, such as newborn screening programs and disease‐modifying treatments, are likely to impact the age distribution of pediatric neuromuscular patients in the near future.
Age of presentation varies widely with disease type. Newborns often present with hypotonia, children may present with falls and reduced stamina or athleticism relative to peers, while teenagers may present with non‐specific muscle cramps or weakness. The team approach provides long‐term care to the children, as well as psychosocial support to the family and caregivers. Ongoing collaboration with the primary general pediatrician is essential, especially for families who live outside of the area and are at highest risk of miscommunication between physicians. With improved survival, children grow into young adults followed in the pediatric clinic, although they do ultimately require continued care in adult neuromuscular clinics.25, 26 A key aspect of providing comprehensive care to pediatric neuromuscular patients is to establish a transition of care team for young adults with neuromuscular disease.26, 27 This is especially necessary during hospital admissions when the needs of young adult patients can be better addressed on an adult inpatient unit.
Consensus statements have provided a framework for guiding care in specific pediatric populations.3, 4, 5 The core clinical team is usually similar to the one described in previous sections (Fig. 2), although the relative contribution of each team member varies based on patient's age, family needs, and specific disease. Table 1 provides an example of the type of medical appointments and their frequency for a child with muscular dystrophy.
Table 1.
Typical schedule of multidisciplinary assessments and possible interventions for a child with muscular dystrophy.
| Specialty | Frequency | Assessment | Interventions |
|---|---|---|---|
| Neurology | Twice a year | Diagnosis; medications; anticipatory guidance; coordination of care | Corticosteroids; anti‐epileptic drugs |
| Pulmonary | Twice a year | Pulmonary function tests; chest X‐ray; sleep study | Flu vaccine; nebulizer/inhalers; cough assist device; non‐invasive and invasive ventilation |
| Cardiology | Once a year, PRN | Echocardiogram; electrocardiogram | Medications for cardiomyopathy and/or arrhythmia |
| Endocrinology | Once a year, PRN | Growth; bone health; steroid withdrawal/stress dose | Calcium; vitamin D; bisphosphonates |
| Orthopedic surgery | Once a year | Spine films; bone X‐rays; MRI | Scoliosis management; ankle/joint surgery |
| Physical therapy and occupational therapy | Twice a year in clinic and PRN in the community | Functional evaluation; ongoing treatment | Stretching; strengthening; mobility evaluation; equipment need assessment |
| Wheelchair/mobility clinic and DME providers | PRN | Assistive/adaptive device assessment | Stroller; power wheelchair; shower chair; transfer devices and lifts; hospital bed |
| Brace clinic (orthotist) | PRN | Bracing needs evaluation | AFOs; back brace; cervical collar |
| Gastroenterology, speech therapy and nutrition | Once a year | Weight; swallowing; constipation/bowel function; GERD | Swallow evaluation; bowel regimen; GI prophylaxis; feeding tube |
| Genetics | At diagnosis and PRN | Consultation; genetic tests | Genetic counseling |
| Psychiatry and neuropsychology | At baseline and PRN | Consultation | Individualized education and behavioral plan; stimulants; SSRIs |
| Social work | Twice a year | Psychosocial support | Counseling; care coordination |
| Anesthesia | PRN | Pre‐procedural assessment of malignant hyperthermia risk | Prevent and treat malignant hyperthermia; pain management |
| Palliative care and hospice | PRN | Consultation | Pain management; advanced directives; end‐of‐life care; bioethics |
AFO, ankle–foot orthosis; DME, durable medical equipment; GERD, gastroesophageal reflux disease; GI, gastrointestinal; MRI, magnetic resonance imaging; PRN, pro re nata (i.e., “as needed”); SSRI, selective serotonin reuptake inhibitor.
Respiratory insufficiency affects almost all pediatric neuromuscular disorders, although chronic and acute respiratory care can vary depending on the diagnosis and age of the child. As an example, a boy with Duchenne muscular dystrophy (DMD) may not need respiratory support until adolescence, whereas an infant with spinal muscular atrophy may require ventilation support in the first year of life.28, 29 Both conditions are marked by progressive respiratory insufficiency. In many cases, upper respiratory infection or pneumonia superimposed on chronic respiratory insufficiency may result in fulminant respiratory failure and admission to a pediatric intensive care unit. Preventive and proactive multidisciplinary care includes routine influenza vaccination and use of cough assist devices to mobilize mucous and clear the airways.30
Those patients with a high risk of malignant hyperthermia require coordination of care with anesthesiology before any procedure requiring sedation.31 Cardiovascular involvement can range from mild tachycardia, to fatal arrhythmias or cardiomyopathy.32, 33, 34 Screening for cardiac complications in pediatric neuromuscular disorders is recommended at baseline and at regular intervals in collaboration with cardiology.35
Another area of specific concern for pediatric neuromuscular disease is growth. Gastroenterology and nutrition services help guide management of swallowing and feeding issues, enteral feedings, and/or gastric hypomotility.36 Pediatric endocrinologists help assess and manage growth, delayed puberty/hypogonadism, and adrenal insufficiency, as well as bone health. Congenital bone fractures may occur and children treated with chronic corticosteroids are at increased risk for osteoporosis. Dual‐energy X‐ray absorptiometry (DXA) scans are obtained routinely in selected populations to screen for bone loss and guide treatment.37 Children treated with long‐term corticosteroids are also at risk for weight gain and hormonal imbalance, again highlighting the importance of an ongoing collaboration with pediatric endocrinologists.38, 39
Children with pediatric neuromuscular disorders may have varying degrees of cognitive impairment, and are at risk for social, emotional, and behavioral dysfunction.40 In addition, corticosteroids can exacerbate pre‐existing cognitive impairment and/or behavior problems. Thus, neuropsychology and psychiatry support is an integral component of pediatric neuromuscular care.
Multidisciplinary interventions can prolong survival in fatal conditions such as Duchenne muscular dystrophy and spinal muscular atrophy.41 Further, advances in drug discovery for pediatric neuromuscular disorders have led to clinical trials of candidate therapeutics42, 43, 44 and the recent approval of disease‐modifying treatments for selected populations.45, 46 These treatments will remain an adjunct to the current standard of care. As survival is hopefully extended by these and other novel treatments, additional care considerations will need to be established to optimally support these patients in later stages of their disease.47 Healthcare policies and health services considerations will be an important aspect of lifelong care for patients with pediatric neuromuscular disorders, given their dependence on caregivers (parent or guardian) as their survival extends well into adulthood. The prospect that pediatric neuromuscular patients may have children gives new meaning to continuity of care in light of the inheritance risk to their offspring. In addition, newborn screening for pediatric neuromuscular disorders is being piloted as emerging therapies are developed.47, 48 The identification of affected infants and their early treatment will significantly impact the volume of new referrals to pediatric neuromuscular clinics and, in conjunction with gene‐directed therapies, will affect the age distribution of children seen in pediatric neuromuscular clinics (Fig. 3).
NAVIGATING GENETICS/GENOMICS IN MULTIDISCIPLINARY NEUROMUSCULAR CLINICS
Diagnosis is central to providing care in any clinic, no less so a multidisciplinary clinic. Genetic testing has become a critical part of the diagnostic paradigm for neuromuscular disorders. The identification of novel disease‐causing mutations has expanded our understanding of underlying pathogenic mechanisms of neuromuscular and neurodegenerative disease.49 Also, the availability of genetic testing to provide definitive, rapid, non‐invasive, and specific diagnostic information has been welcomed by most neuromuscular physicians, although practices vary50 and clinicians face new challenging discussions about the ethical implications of genetic testing.51, 52
The impact of the broad adoption of genetic testing for diagnosis on the quality of clinical care is still being examined.53 Genetic testing can reduce the diagnostic burden on patients by reducing the need for invasive diagnostic procedures such as muscle biopsy and, at the same time, reduce diagnostic delay.53 Thus, it might facilitate entry into multidisciplinary clinics, access to treatment, and earlier research participation, maximizing the impact of multidisciplinary clinics. Genetic testing could potentially improve care value, if it reduces or eliminates the costs of expensive traditional testing. In addition, as the cost of high‐throughput genetic testing decreases, its potential value is likely to continue to increase. On the other hand, genetic testing remains costly and frequent discovery of variants of uncertain significance (VUS) can lead to expensive follow‐up testing. Also, beyond the diagnostic process, the impact of genetic testing on quality of care could be small, as so few targeted therapies exist. These arguments remain theoretical—more research into the impact of genetic testing on quality and value of neuromuscular care is clearly needed.
Single‐gene testing continues to be an important first tier for evaluation of neuromuscular disorders with a clear phenotype–genotype correlation. Both the American Academy of Neurology (AAN) and American Association of Neuromuscular & Electrodiagnostic Medicine (AANEM) practice guidelines recommend genetic testing, as guided by clinical phenotype and electrodiagnostic testing for neuromuscular disorders such as distal symmetric neuropathy54 and limb‐girdle muscular dystrophy.7 At the same time, high‐throughput methods, such as next generation sequencing (NGS), allow for timely evaluation of clinically heterogeneous neuromuscular disorders with high diagnostic yield, simultaneously examining several genes, although the exact list varies by laboratory.55 With NGS, there is less need to restrict testing by exact phenotype and the likelihood of shortening the diagnostic process is higher than with targeted genetic testing. Emerging high‐throughput methods include whole exome sequencing (WES, to analyze the entire coding portion of the genome) and whole genome sequencing (WGS, which spans the entire genome), although the latter has limited clinical utility due to the cost and complexity of bioinformatic analysis. Advantages of the NGS panel approach compared with WES and WGS include increased depth of coverage of genes (i.e., NGS panels are less likely to miss mutations) and decreased likelihood of identified (VUS).55
As we increasingly diagnose genetically mediated neuromuscular diseases, we also increase the number of asymptomatic “at‐risk” family members. This, in turn, may increase the pressure to screen asymptomatic at‐risk individuals.52, 56 This is viewed by some as a powerful opportunity to advance medicine and by others as a costly, gnarly, and unintended consequence of genetic testing.51, 52
EXTENDING MULTIDISCIPLINARY CARE BEYOND THE CLINIC WALLS
Multidisciplinary clinics for neuromuscular disease are generally located at major academic centers variably scattered across states and countries. Although multidisciplinary care reduces travel burden by bringing providers together within a medical center, patients and caregivers often travel great distances to reach the center. As we race toward new models for care delivery to improve quality and value, it is possible that multidisciplinary clinics can extend beyond the clinic walls to reach patients in their own environment. There may be many ways to extend impact beyond the clinic walls, each with a specific use or set of uses and benefits for care quality and/or value. Such means are listed in Table 2 and discussed in what follows.
Table 2.
Beyond the clinic walls.
| Modality | Role |
|---|---|
| Telehealth | Replace in‐person visits, reduce travel efforts and costs, maintain connection with people who have lost ability to travel to clinic |
| Mobile health | Allow for real‐time access to clinic staff using relatively low‐cost technology, dedicated apps can provide patients with information or monitor function in the patient's environment |
| Remote monitoring platforms | Remote monitoring of well‐being based on information from treatment devices (e.g., data collected by non‐invasive or invasive ventilation machines, data collected from eye‐gaze or communication platforms, or other connected devices) |
| Patient support groups | Loaner closets, peer‐to‐peer support groups, funding for research and clinical care |
| Advocacy groups | Raise awareness about the disease, fundraising, advocate for policy changes |
| Philanthrophy (foundations, private donors) | Provide or help raise funding for research and clinical care |
| Newsletters/websites | Raise awareness about the disease and treatment and research options |
| Patient portal | Online access to one's own clinical and research information |
Telehealth
Patients and caregivers often devote an entire day to a clinic visit—for transit and clinical care. Some recover for days from the resulting fatigue. The burden is large for those who live a great distance from their multidisciplinary clinic, have advanced disease, are dependent on caregivers for transportation, or have cognitive‐behavioral changes.9 In our experience, as these diseases progress, patients stop visiting the clinic and are “lost to follow‐up.” At the most devastating point of the disease, consultation with experts is most inaccessible and patients and their loved ones are most disenfranchised from medical providers.
Advances in technology have sparked a growing emphasis on telehealth as a means to improve or maintain quality of care while also improving care value. Telehealth is a broad term describing medical care that takes place outside the traditional in‐person office visit.57 Emerging studies are evaluating the impact of telehealth on neuromuscular care. As an example, pilot studies in neuromuscular clinics in Europe have suggested that remote monitoring of non‐invasive ventilation in patients with ALS improves at least some aspects of quality of care (such as improved patient satisfaction) and value (reduced costs and hospitalizations).58, 59, 60
Patient–physician encounters via remote videoconferencing (often called “televisits” or “virtual visits”) are an emerging telehealth modality. Several platforms are available using Health Insurance Portability and Accountability Act (HIPAA)‐compliant video chat applications to provide multidisciplinary care to patients and caregivers in their homes.61, 62 Although not all patients or caregivers have technology in the homes to support videoconferencing, tablet computers and smartphones, now nearly ubiquitous, can be used by patients for video televisits. Adoption of televisits in neuromuscular medicine has begun, as has a searching evaluation of its impact on care quality and value.
In our experience, patients may be hesitant to substitute in‐person visits for video televisits, delaying their use until late in the disease and mitigating their impact on care quality, but acceptance is growing. The technology is improving ease of use, and integration of televisit technology with eye‐gaze devices is being explored. In our practice, even emotionally charged conversations, like end‐of‐life decisionmaking, have been remarkably successful via video televisit (unpublished observations). Perhaps this is because patients and their families are at home, or because facial expressions and tone of voice (both available via televisit) convey the majority of empathy and emotion. Or, perhaps it is because a larger group of family and caregivers can often be present for a video televisit than clinic visit. Regardless, in our experience, care quality is preserved for many clinical encounters, even unexpected ones.
Video televisits can also add to care quality by connecting the home care providers and the neuromuscular clinic team. They can be scheduled to coincide with times in which the home care providers (nursing, social work, physical, occupational, or speech therapy) are in the patient's home. These shared visits can improve communication and align efforts of the home care and multidisciplinary clinic teams.
The impact of video televists on care value is harder to predict. For patients, there can be considerable value—travel costs and overall duration of the clinic day can be dramatically shortened. From the provider perspective, there may be little time‐savings, because neuromuscular care requires a great deal of counseling and education, video televisits may be no briefer than in‐person visits. Furthermore, reimbursement of video televisits is in flux.57
Looking ahead, as mobile health technology grows, it is likely that we will begin to combine video televisits with mobile health and remote monitoring efforts. Sensors in peoples' homes may detect falls, respiratory decline, communication difficulty, or weight loss. This could improve care quality by providing timely medical interventions. The goal will be to also improve care value by preventing injury, predicting decline, and reducing use of expensive medical encounters like emergency room visits.
Interaction with Community Support Resources
An important task for multidisciplinary clinics is to leverage and integrate existing community support resources to facilitate the treatment and support plan established in clinic. There are several options to foster interaction with advocacy groups and community resources (Table 2). By building relationships with existing patient support groups and offering disease‐specific training to home care providers, the multidisciplinary clinic staff can impact the quality of home care services available to their patient population. To be sure, making time to open these lines of communication, provide training, and review patients with home care organizations results in substantial costs in personnel time. Yet, if these activities improve care at home and reduce unnecessary healthcare utilization, they will be viewed as having a high value to the patient and their families.
Patient Engagement
Emerging opportunities for enhancing patient engagement include the ever‐growing use and acceptance of social media, webinars, listservs along with the development of new assistive technology tools that allow people with disabilities to access computers. These tools can facilitate the development of patient communities and peer support groups, and increase patient engagement with the scientific community. Successful examples of the latter include the formation of coalitions of stakeholders that have drafted patient‐focused guidance documents to help guide the drug development process for Duchenne muscular dystrophy and ALS.63
Incorporating Research into Multidisciplinary Care
Recent advances in neuromuscular disease research, particularly in the field of genetics and gene therapy, hold the promise of not only yielding disease‐modifying treatments for neuromuscular diseases, but leading to treatments with marked and durable effect in the near future. Yet, neuromuscular research is best viewed as a concerted effort to study a grouping of rare diseases. The development of novel therapies depends heavily on patient involvement. Multidisciplinary clinics are ideally positioned to serve as a portal to clinical research. First, they are often located in academic centers where neuromuscular research occurs, putting the clinic staff in a unique position to recruit patients into the most potentially impactful research. Second, in our experience, people with neuromuscular disease are motivated to learn about and participate in research, and this is particularly true of those seeking multidisciplinary care.64 Third, incorporating research into multidisciplinary care can infuse the experience with the optimism of scientific discovery, an important source of hope for patients, loved ones, and caregivers. And, although multidisciplinary clinicians must manage expectations, this hope can be a powerful ally to patients as they proceed in their journey with a neuromuscular disease. The logistical challenges of recruiting for, or conducting, research in a busy multidisciplinary neuromuscular clinic (time and space constraints, throughput/clinical productivity) must be managed carefully to find a model that is both productive for research and sustainable for the clinic. Importantly, real or perceived conflicts of interest occur when the clinician is also an investigator enrolling participants into research studies. Ideally, multidisciplinary visits may be followed by separate research visits for enrollment into trials and other research projects. The efficiency of research visits may be enhanced by having a dedicated research access nurse or coordinator and by developing newsletters and web portals for information and communication with interested individuals.
METRICS TO MEASURE QUALITY OF CARE AND VALUE AND FINANCIAL CONSIDERATIONS
People with neuromuscular diseases suffer increasing disability, and their rating of health utility (a measure of the relative value of a year of life in a given health state) falls over the course of the disease as disability increases. Furthermore, neuromuscular diseases exact high costs on those affected, their families,65 and society.66, 67, 68, 69 Direct costs can be divided into medical expenses (e.g., outpatient visits, physical therapy, hospitalizations) and non‐medical costs (e.g., cost of adaptive equipment and home renovation).66, 67, 68, 69, 70 Indirect costs include lost income due to personal illness or because of the need to act as a caregiver at home.66, 67, 68, 69, 70 The large negative impact on health state and substantial cost of neuromuscular diseases open the window to justify intense, impactful care, even if it is relatively expensive to administer. Intuitively, this equation has driven the development of multidisciplinary clinics. In today's data‐driven model of care delivery, a more formal cost‐effectiveness model may be required to demonstrate value (the quality improvements of multidisciplinary care relative to its costs) (Fig. 1).
MEASURING THE COSTS OF MULTIDISCIPLINARY CARE
There is an intuitive and widely accepted dogma that multidisciplinary care clinics cost more to deliver care than standard models (i.e., 1 patient–1 physician). However, one study comparing the cost of ALS multidisciplinary care to traditional care models suggested that the medical cost of multidisciplinary care is very similar to general care.71 Nevertheless, even if this is the case, the multidisciplinary care model shifts the burden of funding onto the shoulders of the multidisciplinary care team, rather than dispersing it across divisions in a healthcare system. In the United States, insurance reimbursement covers physician or nurse practitioner time during clinics. Although allied health providers, including physical therapists, speech therapists, occupational therapists, and respiratory therapists, may bill for services rendered during a clinic visit, this will result in multiple copayments for a single clinic visit. Furthermore, these services are typically not covered by insurance for patients receiving ongoing treatment from these specialties outside clinic. To ensure that all patients can visit with allied health providers during multidisciplinary clinic, clinics might need to use foundation support and/or philanthropy funding to cover the cost of these services.
A recent study examined the cost of delivering multidisciplinary care adhering to AAN practice parameters for ALS across 18 ALS clinics in the United States.21 The cost of running an ALS multidisciplinary clinic was estimated to range from $258 to $806 per patient per visit.21
We performed a study of the personnel costs of the ALS multidisciplinary clinic at our institution in 2015 and found an average cost per patient visit of $580. In our study, we reviewed 2015 annual budgeted personnel costs (salary, fringe, and overhead) to provide multidisciplinary care for 409 unique ALS patients in 1,285 ambulatory office patient encounters, consisting of 300 new patient visits and 985 follow‐up visits. The multidisciplinary clinic staff and percent full‐time effort was comprised of: physicians [0.58 full‐time equivalents (FTE)]; nurse practitioners (0.3 FTE); registered nurses (2.0 FTE); physical therapist (1.55 FTE); speech and language therapists (0.2 FTE); practice managers (0.05 FTE); and patient coordinators (1.1 FTE). We calculated the total insurance reimbursement revenue for billable physician or nurse practitioner encounters over the same time period (our clinic does not bill for allied health providers). Annual personnel costs to support 1,285 patient encounters with allocated staff in an ALS multidisciplinary clinic was $745,593, with an average cost per patient per clinic of $580, in line with findings by Boylan et al.21 Insurance reimbursement totaled $338,398 ($263 per patient per clinic) leaving a shortfall of $407,195 ($317 per patient per clinic). Only a portion (about 45%) of personnel costs to provide care for people with ALS in a multidisciplinary clinic were covered by insurance reimbursement.
Traditional insurance reimbursement covers physician time. Although some clinics bill for allied health provider services, this results in higher copayments for patients and may interfere with their ability to seek ongoing therapy from these specialties outside clinic or limit the accessibility of multidisciplinary clinic services themselves. It is common experience that support from healthcare professionals and nursing staff is critical to address the patients' complex follow‐up issues. Personnel costs to support clinic staff are the great majority of multidisciplinary clinic costs not covered by insurance. The type and amount of work performed by multidisciplinary clinic staff is rarely remunerated. We attempted to quantify and categorize the work done by our ALS multidisciplinary clinic nursing staff over a 2‐week period to support 3 physicians at our institution whose total cumulative FTE is 0.2 (i.e., 1 ALS clinic day per week). We prospectively monitored and collected the time that nursing staff spent in direct patient care and coordination of care for these physicians' patients over 2 weeks. Direct patient care was defined as any interaction with a patient or family member on the phone, by e‐mail, or in person; it encompassed assessment of symptoms, pulmonary function testing, clinic follow‐up, counseling, feeding tube teaching, and prescription refills, to name a few. Coordination of care was defined as interaction with hospital staff or outside entities, such as insurance companies, pharmacies, or home care companies, and included activities such as completion of forms, letters of necessity, or orders for outside entities. We only accounted for nursing staff time, not the time of the entire multidisciplinary clinic staff (e.g., we did not account for physical therapist or speech therapist's time). Still, the amount of follow‐up effort was substantial. Over a 2‐week period, our nursing staff spent 80 hours in direct patient care and coordination of care for 85 patients (Fig. 4). This effort corresponds to a full‐time nursing position to support 1 clinic day per week. These data can be helpful in estimating staff support needs and costs for ALS clinics, because, in general, clinic directors support clinic nurses and other allied health providers using funding from advocacy organizations and philanthropy.
Figure 4.
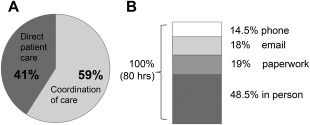
Time study of nursing activities in a multidisciplinary ALS clinic. Data on nursing staff efforts to support 3 physicians whose cumulative FTE is 0.2 (i.e., 1 clinic per week) were prospectively collected over a 2‐week period. Nursing staff spent 80 hours over a 2‐week period caring for 85 individual patients. The distribution of direct patient care and coordination of care is shown on the left. The modality of care (in‐person vs. non–in‐person activities are shown on the right).
As noted, until now, the additional costs of providing multidisciplinary care for neuromuscular diseases have been paid by patient advocacy organizations and philanthropy raised to support these clinics. However, with the tectonic shift in healthcare reimbursement underway, appropriate analysis of cost‐effectiveness may justify the additional expense of multidisciplinary care and lead to increased financial support from accountable care organizations (ACOs) and insurers. To support these changes, researchers will need to gather data to address both the cost and effect of multidisciplinary neuromuscular clinics.
MEASURING THE IMPACT OF MULTIDISCIPLINARY CARE ON DISEASE OUTCOMES AND QUALITY OF LIFE
A nascent literature has already begun to document that multidisciplinary care is more effective than the traditional alternative as it has been associated with increased adherence to clinical care guidelines and more efficient resource utilization.10, 11, 12, 13, 14, 15, 17, 18 There is also some evidence to suggest that multidisciplinary care is associated with reduced hospital admissions/emergency‐room visits, increased survival, and improved quality of life.10, 11, 12, 13, 14, 15, 17, 18
Ideally, one would measure and track patient‐centered outcome data to document the impact of multidisciplinary care on health utility.16 Unfortunately, there is no consensus yet on what metrics best reflect improved function in this population. Furthermore, it is challenging to capture this information even when there is consensus about what metrics to capture. Also, finally, one must not only assess metrics about patient survival, function, and health utility, but also about healthcare resource utilization outside the clinic (to assess impact of multidisciplinary care on overall medical resource utilization).16
Still, advocacy groups that sponsor neuromuscular clinics have recently begun to systematically collect data on these topics. As an example, the Muscular Dystrophy Association has launched a national registry to track resource utilization and patient outcomes in clinic. The AAN has related initiatives across neurological diseases. These efforts will generate concrete data with which to begin to calculate the impact of multidisciplinary clinics. In combination with emerging evidence about the costs of these clinics, it will soon be possible to assess the cost‐effectiveness of this model of care.
SUMMARY
For a variety of reasons, multidisciplinary care has become established as the best care model for people with neuromuscular diseases. The multidisciplinary team members may vary slightly from center to center and between neuromuscular disease–focused clinics, but the core principles remain the same: provide expert diagnosis, holistic assessment, and comprehensive care, and access to research. This care can be expensive, and is largely funded by philanthropy and patient advocacy organizations. Clearly, additional research is urgently needed to answer pressing questions about quality and cost‐effectiveness of multidisciplinary clinics for neuromuscular disease, and to develop a long‐term sustainability plan for this care model. The benefits of multidisciplinary clinics for neuromuscular patients are well accepted by providers, patients, and families. Although the costs of providing this care are high, studies examining the cost‐effectiveness of these clinics are urgently needed, particularly as such studies may provide a basis for influencing healthcare policy and clinic reimbursement. As novel disease‐modifying therapies and newborn screening programs emerge, further growth in clinic volumes is likely to occur and will be driven by increased numbers of newly diagnosed infants and extended survival of pediatric and adult patients. This shift will represent both an opportunity and a challenge, and will require strategic health services planning. The need for multidisciplinary care for neuromuscular patients is likely to continue to grow.
ACKNOWLEDGEMENTS
The authors thank the members of our nursing team at the Massachusetts General Hospital (MGH) ALS clinic (Melissa Arnold, Jennifer Scalia, Sarah Luppino, Ashley Robichaud, and Katie Tee) and MGH pediatric neuromuscular clinic (Ijeoma Nwanko) for data collection.
Funding: National Institutes of Health (Career Development Award 2K12HD001097‐16 to S.P., and 5K12 NS066225‐04 to F.L.) and the National Institutes of Child Health and Development (R01‐HD69045 to K.S.); Target ALS (to S.P.); ALS Association (to S.P.); ALS Finding a Cure (to S.P., and Anne B. Young Fellowship to K.N.); Amylyx (to S.P.); the Salah Foundation (to S.P.); the Spastic Paraplegia Foundation; and Biogen (to K.N.).
Conflicts of Interest: K.S. receives or has received funding from Biogen as a consultant and as site principal investigator for the SMA clinical trials. She serves as a scientific advisory board member for Cure SMA, the AHC Foundation, and the PURA Foundation. F.L. has provided consulting to Sarepta and Summit Therapeutics. M.C. has provided consulting for Cytokinetics, Astra Zeneca, Lilly, Genentech, Biogen‐IDEC, Voyager, and Biohaven. J.D.B. has consulted with Biogen‐IDEC, Denali Therapeutics, and Neuraltus Pharmaceuticals, and has received research support from Voyager Therapeutics, GSK, Cytokinetics, Brainstorm Cell Therapeutics, Novartis, ALS Therapy Development Institute, ALS Association, the Muscular Dystrophy Foundation, and the National Institutes for Health.
REFERENCES
- 1. Miller RG, Brooks BR, Swain‐Eng RJ. Quality improvement in neurology: amyotrophic lateral sclerosis quality measures: report of the quality measurement and reporting subcommittee of the American Academy of Neurology. Neurology 2013;81:2136–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Miller RG, Jackson CE, Kasarskis EJ. Practice parameter update: the care of the patient with amyotrophic lateral sclerosis: multidisciplinary care, symptom management, and cognitive/behavioral impairment (an evidence‐based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2009;73:1227–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bushby K, Finkel R, Birnkrant DJ. Diagnosis and management of Duchenne muscular dystrophy, part 2: implementation of multidisciplinary care. Lancet Neurol 2010;9:177–189. [DOI] [PubMed] [Google Scholar]
- 4. Wang CH, Finkel RS, Bertini ES. Consensus statement for standard of care in spinal muscular atrophy. J Child Neurol 2007;22:1027–1049. [DOI] [PubMed] [Google Scholar]
- 5. Kang PB, Morrison L, Iannaccone ST. Evidence‐based guideline summary: evaluation, diagnosis, and management of congenital muscular dystrophy: report of the Guideline Development Subcommittee of the American Academy of Neurology and the Practice Issues Review Panel of the American Association of Neuromuscular & Electrodiagnostic Medicine. Neurology 2015;84:1369–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tawil R, Kissel JT, Heatwole C. Evidence‐based guideline summary: evaluation, diagnosis, and management of facioscapulohumeral muscular dystrophy: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology and the Practice Issues Review Panel of the American Association of Neuromuscular & Electrodiagnostic Medicine. Neurology 2015;85:357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Narayanaswami P, Weiss M, Selcen D. Evidence‐based guideline summary: diagnosis and treatment of limb‐girdle and distal dystrophies: report of the guideline development subcommittee of the American Academy of Neurology and the practice issues review panel of the American Association of Neuromuscular & Electrodiagnostic Medicine. Neurology 2014;83:1453–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Paganoni S, Karam C, Joyce N, Bedlack R, Carter GT. Comprehensive rehabilitative care across the spectrum of amyotrophic lateral sclerosis. NeuroRehabilitation 2015;37:53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stephens HE, Young J, Felgoise SH, Simmons Z. A qualitative study of Multidisciplinary ALS clinic use in the United States. Amyotroph Lateral Scler Frontotemporal Degener 2015;17:55–61. [DOI] [PubMed] [Google Scholar]
- 10. Rooney J, Byrne S, Heverin M. A multidisciplinary clinic approach improves survival in ALS: a comparative study of ALS in Ireland and Northern Ireland. J Neurol Neurosurg Psychiatry 2015;86:496–501. [DOI] [PubMed] [Google Scholar]
- 11. Traynor BJ, Alexander M, Corr B, Frost E, Hardiman O. Effect of a multidisciplinary amyotrophic lateral sclerosis (ALS) clinic on ALS survival: a population based study, 1996–2000. J Neurol Neurosurg Psychiatry 2003;74:1258–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chio A, Bottacchi E, Buffa C, Mutani R, Mora G, Paral S. Positive effects of tertiary centres for amyotrophic lateral sclerosis on outcome and use of hospital facilities. J Neurol Neurosurg Psychiatry 2006;77:948–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van den Berg JP, Kalmijn S, Lindeman E. Multidisciplinary ALS care improves quality of life in patients with ALS. Neurology 2005;65:1264–1267. [DOI] [PubMed] [Google Scholar]
- 14. Vry J, Gramsch K, Rodger S. European cross‐sectional survey of current care practices for Duchenne muscular dystrophy reveals regional and age‐dependent differences. J Neuromuscul Dis 2016;3:517–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Otto C, Steffensen BF, Hojberg AL. Predictors of health‐related quality of life in boys with Duchenne muscular dystrophy from six European countries. J Neurol 2017;264:709–723. [DOI] [PubMed] [Google Scholar]
- 16. Porter ME. What is value in health care? N Engl J Med 2010;363:2477–2481. [DOI] [PubMed] [Google Scholar]
- 17. Cordesse V, Sidorok F, Schimmel P, Holstein J, Meininger V. Coordinated care affects hospitalization and prognosis in amyotrophic lateral sclerosis: a cohort study. BMC Health Serv Res 2015;15:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Corr B, Frost E, Traynor BJ, Hardiman O. Service provision for patients with ALS/MND: a cost‐effective multidisciplinary approach. J Neurol Sci 1998;160(suppl 1):S141–145. [DOI] [PubMed] [Google Scholar]
- 19. Pucillo EM, Christensen‐Mayer N, Poole SD. Same‐day physical therapy consults in an outpatient neuromuscular disease physician clinic. J Multidiscip Healthc 2016;9:493–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Majmudar S, Wu J, Paganoni S. Rehabilitation in amyotrophic lateral sclerosis: why it matters. Muscle Nerve 2014;50:4–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Boylan K, Levine T, Lomen‐Hoerth C. Prospective study of cost of care at multidisciplinary ALS centers adhering to American Academy of Neurology (AAN) ALS practice parameters. Amyotroph Lateral Scler Frontotemporal Degener 2015;17:119–127. [DOI] [PubMed] [Google Scholar]
- 22. Pisa FE, Logroscino G, Giacomelli Battiston P, Barbone F. Hospitalizations due to respiratory failure in patients with Amyotrophic Lateral Sclerosis and their impact on survival: a population‐based cohort study. BMC Pulm Med 2016;16:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lechtzin N, Wiener CM, Clawson L, Chaudhry V, Diette GB. Hospitalization in amyotrophic lateral sclerosis: causes, costs, and outcomes. Neurology 2001;56:753–757. [DOI] [PubMed] [Google Scholar]
- 24. Dubinsky R, Chen J, Lai SM. Trends in hospital utilization and outcome for patients with ALS: analysis of a large U.S. cohort. Neurology 2006;67:777–780. [DOI] [PubMed] [Google Scholar]
- 25. Rahbek J, Werge B, Madsen A, Marquardt J, Steffensen BF, Jeppesen J. Adult life with Duchenne muscular dystrophy: observations among an emerging and unforeseen patient population. Pediatr Rehabil 2005;8:17–28. [DOI] [PubMed] [Google Scholar]
- 26. Schrans DG, Abbott D, Peay HL. Transition in Duchenne muscular dystrophy: an expert meeting report and description of transition needs in an emergent patient population (Parent Project Muscular Dystrophy Transition Expert Meeting 17‐18 June 2011, Amsterdam, The Netherlands). Neuromuscul Disord 2013;23:283–286. [DOI] [PubMed] [Google Scholar]
- 27. Lue YJ, Chen SS, Lu YM. Quality of life of patients with Duchenne muscular dystrophy: from adolescence to young men. Disabil Rehabil 2016:1–6. [DOI] [PubMed] [Google Scholar]
- 28. Panitch HB. Respiratory issues in the management of children with neuromuscular disease. Respir Care 2006;51:885–893. [PubMed] [Google Scholar]
- 29. Schroth MK. Special considerations in the respiratory management of spinal muscular atrophy. Pediatrics 2009;123(suppl 4):S245–249. [DOI] [PubMed] [Google Scholar]
- 30. Birnkrant DJ, Bushby KM, Amin RS. The respiratory management of patients with duchenne muscular dystrophy: a DMD care considerations working group specialty article. Pediatr Pulmonol 2010;45:739–748. [DOI] [PubMed] [Google Scholar]
- 31. Romero A, Joshi GP. Neuromuscular disease and anesthesia. Muscle Nerve 2013;48:451–460. [DOI] [PubMed] [Google Scholar]
- 32. Dellefave L, McNally E. Cardiomyopathy in neuromuscular disorders. Pediatr Cardiol 2007;27:35–46. [Google Scholar]
- 33. Hsu DT. Cardiac manifestations of neuromuscular disorders in children. Paediatr Respir Rev 2010;11:35–38. [DOI] [PubMed] [Google Scholar]
- 34. Palladino A, Passamano L, Taglia A. Cardiac involvement in patients with spinal muscular atrophies. Acta Myol 2011;30:175–178. [PMC free article] [PubMed] [Google Scholar]
- 35. McNally EM, Kaltman JR, Benson DW. Contemporary cardiac issues in Duchenne muscular dystrophy. Working Group of the National Heart, Lung, and Blood Institute in collaboration with Parent Project Muscular Dystrophy. Circulation 2015;131:1590–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tilton AH, Miller MD, Khoshoo V. Nutrition and swallowing in pediatric neuromuscular patients. Semin Pediatr Neurol 1998;5:106–115. [DOI] [PubMed] [Google Scholar]
- 37. Ness K, Apkon SD. Bone health in children with neuromuscular disorders. J Pediatr Rehabil Med 2014;7:133–142. [DOI] [PubMed] [Google Scholar]
- 38. Manzur AY, Kuntzer T, Pike M, Swan A. Glucocorticoid corticosteroids for Duchenne muscular dystrophy. Cochrane Database Syst Rev 2008:CD003725. [DOI] [PubMed] [Google Scholar]
- 39. Schara U, Mortier, Mortier W. Long‐term steroid therapy in Duchenne muscular dystrophy‐positive results versus side effects. J Clin Neuromuscul Dis 2001;2:179–183. [DOI] [PubMed] [Google Scholar]
- 40. D'Angelo MG, Bresolin N. Cognitive impairment in neuromuscular disorders. Muscle Nerve 2006;34:16–33. [DOI] [PubMed] [Google Scholar]
- 41. Passamano L, Taglia A, Palladino A. Improvement of survival in Duchenne muscular dystrophy: retrospective analysis of 835 patients. Acta Myol 2012;31:121–125. [PMC free article] [PubMed] [Google Scholar]
- 42. McDonald CM, Meier T, Voit T. Idebenone reduces respiratory complications in patients with Duchenne muscular dystrophy. Neuromuscul Disord 2016;26:473–480. [DOI] [PubMed] [Google Scholar]
- 43. Chiriboga CA, Swoboda KJ, Darras BT. Results from a phase 1 study of nusinersen (ISIS‐SMN(Rx)) in children with spinal muscular atrophy. Neurology 2016;86:890–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mendell JR, Goemans N, Lowes LP. Longitudinal effect of eteplirsen versus historical control on ambulation in Duchenne muscular dystrophy. Ann Neurol 2016;79:257–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hoy SM. Nusinersen: first global approval. Drugs 2017;77:473–479. [DOI] [PubMed] [Google Scholar]
- 46. Syed YY. Eteplirsen: first global approval. Drugs 2016;76:1699–1704. [DOI] [PubMed] [Google Scholar]
- 47. Farrar MA, Park SB, Vucic S. Emerging therapies and challenges in spinal muscular atrophy. Ann Neurol 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kwon JM, Abdel‐Hamid HZ, Al‐Zaidy SA. Clinical follow‐up for Duchenne muscular dystrophy newborn screening: a proposal. Muscle Nerve 2016;54:186–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pittman A, Hardy J. Genetic analysis in neurology: the next 10 years. JAMA Neurol 2013;70:696–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Vajda A, McLaughlin RL, Heverin M. Genetic testing in ALS: a survey of current practices. Neurology 2017;88:991–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Su X, Kang PB, Russell JA, Simmons Z. Ethical issues in the evaluation of adults with suspected genetic neuromuscular disorders. Muscle Nerve 2016;54:997–1006. [DOI] [PubMed] [Google Scholar]
- 52. Benatar M, Stanislaw C, Reyes E. Presymptomatic ALS genetic counseling and testing: experience and recommendations. Neurology 2016;86:2295–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kassardjian CD, Amato AA, Boon AJ, Childers MK, Klein CJ. The utility of genetic testing in neuromuscular disease: a consensus statement from the AANEM on the clinical utility of genetic testing in diagnosis of neuromuscular disease. Muscle Nerve 2016;54:1007–1009. [DOI] [PubMed] [Google Scholar]
- 54. England JD, Gronseth GS, Franklin G. Practice parameter: the evaluation of distal symmetric polyneuropathy: the role of laboratory and genetic testing (an evidence‐based review). Report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. PM R 2009;1:5–13. [DOI] [PubMed] [Google Scholar]
- 55. Warman Chardon J, Beaulieu C, Hartley T, Boycott KM, Dyment DA. Axons to exons: the molecular diagnosis of rare neurological diseases by next‐generation sequencing. Curr Neurol Neurosci Rep 2015;15:64. [DOI] [PubMed] [Google Scholar]
- 56. Simmons Z. Genetic Testing of presymptomatic individuals at risk for progressive myopathy. Continuum (Minneap Minn) 2016;22:2006–2011. [DOI] [PubMed] [Google Scholar]
- 57. Dorsey ER, Topol EJ. State of telehealth. N Engl J Med 2016;375:154–161. [DOI] [PubMed] [Google Scholar]
- 58. Pinto A, Almeida JP, Pinto S, Pereira J, Oliveira AG, de Carvalho M. Home telemonitoring of non‐invasive ventilation decreases healthcare utilisation in a prospective controlled trial of patients with amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 2010;81:1238–1242. [DOI] [PubMed] [Google Scholar]
- 59. Vitacca M, Assoni G, Pizzocaro P. A pilot study of nurse‐led, home monitoring for patients with chronic respiratory failure and with mechanical ventilation assistance. J Telemed Telecare 2006;12:337–342. [DOI] [PubMed] [Google Scholar]
- 60. Vitacca M, Bianchi L, Guerra A. Tele‐assistance in chronic respiratory failure patients: a randomised clinical trial. Eur Respir J 2009;33:411–418. [DOI] [PubMed] [Google Scholar]
- 61. Selkirk SM, Washington MO, McClellan F, Flynn B, Seton JM, Strozewski R. Delivering tertiary centre specialty care to ALS patients via telemedicine: a retrospective cohort analysis. Amyotroph Lateral Scler Frontotemporal Degener 2017;18:324–332. [DOI] [PubMed] [Google Scholar]
- 62. Henderson RD, Hutchinson N, Douglas JA, Douglas C. Telehealth for motor neurone disease. Med J Aust 2014;201:31. [DOI] [PubMed] [Google Scholar]
- 63. Parent Project Muscular Dystrophy. http://www.parentprojectmd.org/ and http://www.alsa.org/advocacy/fda/. Accessed May 17, 2017.
- 64. Chio A, Canosa A, Gallo S. ALS clinical trials: do enrolled patients accurately represent the ALS population? Neurology 2011;77:1432–1437. [DOI] [PubMed] [Google Scholar]
- 65. Gladman M, Dharamshi C, Zinman L. Economic burden of amyotrophic lateral sclerosis: a Canadian study of out‐of‐pocket expenses. Amyotroph Lateral Scler Frontotemporal Degener 2014;15:426–432. [DOI] [PubMed] [Google Scholar]
- 66. Ouyang L, Grosse SD, Kenneson A. Health care utilization and expenditures for children and young adults with muscular dystrophy in a privately insured population. J Child Neurol 2008;23:883–888. [DOI] [PubMed] [Google Scholar]
- 67. Larkindale J, Yang W, Hogan PF. Cost of illness for neuromuscular diseases in the United States. Muscle Nerve 2014;49:431–438. [DOI] [PubMed] [Google Scholar]
- 68. Schepelmann K, Winter Y, Spottke AE. Socioeconomic burden of amyotrophic lateral sclerosis, myasthenia gravis and facioscapulohumeral muscular dystrophy. J Neurol 2010;257:15–23. [DOI] [PubMed] [Google Scholar]
- 69. Gladman M, Zinman L. The economic impact of amyotrophic lateral sclerosis: a systematic review. Expert Rev Pharmacoecon Outcomes Res 2015;15:439–450. [DOI] [PubMed] [Google Scholar]
- 70. Connolly S, Heslin C, Mays I, Corr B, Normand C, Hardiman O. Health and social care costs of managing amyotrophic lateral sclerosis (ALS): an Irish perspective. Amyotroph Lateral Scler Frontotemporal Degener 2015;16:58–62. [DOI] [PubMed] [Google Scholar]
- 71. van der Steen I, van den Berg JP, Buskens E, Lindeman E, van den Berg LH. The costs of amyotrophic lateral sclerosis, according to type of care. Amyotroph Lateral Scler 2009;10:27–34. [DOI] [PubMed] [Google Scholar]


