Summary
Background
Significance of monitoring adalimumab trough levels and anti‐adalimumab antibodies (AAA) for disease outcome in Crohn's disease (CD) patients remained unclear.
Aim
To evaluate the association of adalimumab trough levels and AAA at week 26 with clinical remission at week 52, the effect of azathiopurine on AAA and factors influencing trough levels in CD patients in the DIAMOND trial.
Methods
We performed this study using adalimumab trough levels, AAA at week 26 and 6‐thioguanine nucleotide (TGN) in red blood cells at week 12. A multiple regression model and receiver operating analysis was performed to identify factors influencing adalimumab trough levels and AAA, and adalimumab thresholds for predicting disease activity.
Results
There was a significant difference of adalimumab trough level at week 26 between patients with disease remission and without at week 52 (7.7 ± 3.3 μg/mL vs 5.4 ± 4.3 μg/mL: P <.001). Adalimumab trough level of 5.0 μg/mL yielded optimal sensitivity and specificity for remission prediction (80.2% and 55.6%, respectively). AAA development at week 26 significantly affected remission at week 52 (P = .021), which was strongly associated with adalimumab trough levels. Female gender and increasing body weight were independently associated with low adalimumab trough levels, and female gender was associated with AAA development. A cut‐off 6TGN level of >222.5 p mol/8 ×108 RBCs yielded sensitivity (100%) and specificity (60.6%) for AAA negativity.
Conclusion
Adalimumab trough levels and AAA occurrence were significantly associated with clinical remission. Higher 6TGN affected AAA negativity. The combination therapy is beneficial in some relevant aspects for CD patients. (UMIN Registration No. 000005146)
1. INTRODUCTION
Monoclonal antibodies that target tumour necrosis factor (TNF) are highly effective therapies for inflammatory bowel diseases.1, 2 Adalimumab is a recombinant, fully human, subcutaneously delivered immunoglobulin G1 monoclonal antibody with proven efficacy for the treatment of Crohn's disease (CD).3, 4, 5 In several clinical trials, 40%‐50% of patients with CD who responded to an anti‐TNF agent lost the response within 6 months‐12 months. Although the exact mechanism underlying antibody production is unknown, the development of antibodies to anti‐TNF drugs and the associated low trough serum drug concentrations have been implicated as predisposing factors to anti‐TNF treatment failure in IBD patients.
Currently, we report the results of a randomised clinical trial to compare the clinical efficacy of adalimumab monotherapy with the combination of adalimumab with azathioprine (AZA) in the induction of remission in Japanese patients with CD naive to TNF antagonists (Deep Remission of Immunomodulator and Adalimumab Combination Therapy for Crohn's Disease [DIAMOND] trial) to investigate the effect of simultaneous IM administration to CD patients treated with adalimumab.6 In this study, the proportion of patients who achieved remission with adalimumab monotherapy was similar to those who achieved remission with immunomodulatory combination therapy, while the combination of AZA and adalimumab was superior to adalimumab monotherapy in obtaining mucosal healing at week 26. Recently, a post hoc analysis of six randomised controlled trials demonstrated no efficacy benefits with immunomodulator/adalimumab combination therapy compared with adalimumab monotherapy in patients with CD.7 However, whether adalimumab trough levels and anti‐adalimumab antibodies (AAA) were relevant to disease outcome remained unclear. The aim of this study is to evaluate the impact of adalimumab trough levels and AAA at week 26 on clinical activity at week 52 and to examine the effect of AZA on AAA in CD patients enrolled in the DIAMOND trial.
2. MATERIALS AND METHODS
2.1. Study design
Data in this analysis are from the preceding DIAMOND trial (UMIN registration No. 000005146). The methods in the DIAMOND study have been described in detail by Matsumoto et al6 In brief, DIAMOND was a multicentre, randomised, prospective, open‐labelled study in patients with moderately to severely active CD (defined as a Crohn's disease activity index [CDAI] of 220‐450).8 Enrolled patients were assigned to receive either a combination of adalimumab and AZA (combination group) or monotherapy with adalimumab (monotherapy group). All patients received subcutaneous administrations of adalimumab at doses of 160 mg at week 0, 80 mg at week 2, and 40 mg at every other week thereafter up to 52 weeks. Patients in the combination group were initially treated with 25 mg or 50 mg/day of AZA and the dose could be increased to a maximum of 100 mg during the initial four weeks under careful observation. The clinical efficacy was evaluated at week 26 and 52. Clinical remission was defined as a CDAI score of <150 points.
2.2. Patients
Data from randomised patients (DIAMOND) who continued monotherapy or combination therapy at week 26 were analysed. Therefore, we excluded patients discontinued either monotherapy or combination therapy by week 26 and in whom we could not obtained an adalimumab trough or 6‐thioguanine nucleotide (TGN) level.
2.3. Serum collection and analysis
Blood samples were collected from patients in the combination group at week 12, and processed to measure 6‐TGN in red blood cells (RBCs). Whole‐blood samples were collected in heparinised tubes and centrifuged. After the removal of plasma, RBCs were hydrolysed with acid and extracted with phenylmercuric acetate/ethyl acetate. 6‐TGN levels were measured via high‐performance liquid chromatography.9 We also collected serum samples from the patients in both groups at week 26 and measured the trough levels of adalimumab and antibodies to adalimumab (AAA).10, 11 Blood samples obtained just before or one day prior to, the next scheduled adalimumab injection, were included in our study.
2.4. Measurement of Adalimumab concentrations
Trough serum adalimumab concentrations were measured by enzyme‐linked immunosorbent assay (ELISA) based on the principle that adalimumab is captured via its ability to bind TNF‐alpha. Adalimumab was quantified as described previously for infliximab measurement with one modification.12 Adalimumab binding was assessed by incubation with biotinylated rabbit immunoglobulin directed to the adalimumab idiotype. Detection limit of the assay is approximately 0.001 mg/L.
2.5. Measurement of AAA
A radio immunoassay (the Netherlands, Sanquin) was used to detect the presence of AAA. After dilution of 1 μL of serum in phosphate‐buffered saline/0.3% bovine serum albumin (pro analysis buffer), overnight incubation followed with 1 mg Sepharose‐immobilised protein A (GE Health Care, Giles, England) in a final volume of 800 μL. Then, the samples were washed with phosphate‐buffered saline 0.005% polysorbate. The anti‐adalimumab binding was determined by overnight incubation with 20 000 disintegrations per minute (dpm [1 ng]) iodine 125‐labelled F(ab)2 adalimumab diluted in Freeze buffer (Sanquin). Unbound label was removed by washing, and protein A‐bound radioactivity was measured. Serum samples were further diluted if binding was more than 25% of the input. For determining antibody levels, a standard serum containing anti‐adalimumab was used for comparison. Anti‐adalimumab levels were expressed in arbitrary units (AU [1 AU = 12 ng]). The mean cut‐off value was derived from 100 healthy donors and set at 12 AU/mL. The specificity and validity of the radio immunoassay have been confirmed in a bioassay. The validation procedures of the assays for determining anti‐drug antibodies have been accredited. All baseline samples before the start of treatment were negative for AAA. Patients were defined as positive for AAA if titre were greater than 12 AU/mL on at least 1 occasion in combination with serum adalimumab levels of less than 5.0 mg/L.11
2.6. Assessment
To investigate the value of adalimumab levels and AAA positivity, 2 major analyses were performed. The first analysis was the impact of adalimumab trough levels and AAA positivity at week 26 on clinical activity at week 52. The second analysis was the effect of AZA on adalimumab trough levels in CD patients.
2.7. Statistical analysis
Students's t test and Fisher's exact test were used to examine the association between disease activity (clinical remission [CR] vs active disease) and adalimumab trough level or AAA positivity, respectively. The relationship between the initial dose of AZA or 6‐TGN level at week 26 and AAA positivity at week 26 was examined via Students's t test. In addition, since 6‐TGN value is distributed as long‐normal distribution, it was compared by Student's t test after log‐transformation and calculated geometric mean for its summary statistics.
Receiver operating characteristic (ROC) analysis was performed to examine the discriminatory ability of 6 ‐TGN levels for development of AAA and to find optimised drug thresholds for predicting disease activity. A multiple linear and logistic regression analysis with backward elimination (excluded when P >.1) was performed to identify factors independently related to trough levels of adalimumab and AAA at week 26, respectively. Covariates included in the model were age, sex, body weight, disease duration, disease location, previous surgery, the presence of an internal fistula, the presence of an anal fistula, smoking status, elemental diet, medication, CDAI, CRP, SESCD and allocation (combination therapy or monotherapy). ANOVA with post hoc Tukey's comparison was used for the comparison of adalimumab trough level between body weight categories. All statistics were performed using IBM spss Statistics 23 (IBM). A P value of <.05 was considered significant. All statistical tests were two‐sided.
2.8. Ethical consideration
This study was approved by the IRB of each hospital. All patients gave verbal and written informed consent for blood testing and clinical data collection.
3. RESULTS
3.1. Study population
Demographics and characteristics of patients in the DIAMOND trial have been reported previously.6 Briefly, during the predetermined period of recruitment from 1 June 2011 until 31 June 2014, 176 patients were randomly assigned to either the combination group (91) or the monotherapy group (85). Only one patient was excluded from the study because of a diagnosis of intestinal tuberculosis after enrolment in the study.
In the combination group, 22 patients discontinued the study due to adverse events and seven patients due to other reasons (dose escalation of AZA after 4 weeks for two patients, consent withdrawal for three patients and loss to follow‐up for two patients). In the monotherapy group, 19 patients discontinued the study owing to adverse events, and three patients discontinued the study for other reasons (CDAI not available for two patients and consent withdrawal for one patient). Finally, 62 patients in the combination group and 63 patients in the monotherapy group completed the study through week 52.
3.2. The correlation between adalimumab trough level and AAA positivity at week 26 and clinical activity
One hundred and fifty‐one serum samples were analysed from 176 patients enrolled in the study (Figure 1). Adalimumab trough levels and AAA were measured in 75 patients from the combination group and in 76 patients from monotherapy group. The preceding DIAMOND trial showed that AAA positivity rates in the combination group and the monotherapy group were 4% and 13.2%, respectively (P = .078). Furthermore, the adalimumab trough levels at week 26 in the combination group and the monotherapy group were 7.6 ± 3.6 μg/mL and 6.5 ± 3.9 μg/mL, respectively (P = .084). These data suggested that there were trends towards a higher adalimumab trough level and a lower positive rate of AAA in the combination group compared with the monotherapy group. In this study, we further analysed whether the adalimumab trough level and AAA positivity at week 26 could affect clinical outcome at week 52 in patients in the combination or monotherapy group who completed the study.
Figure 1.
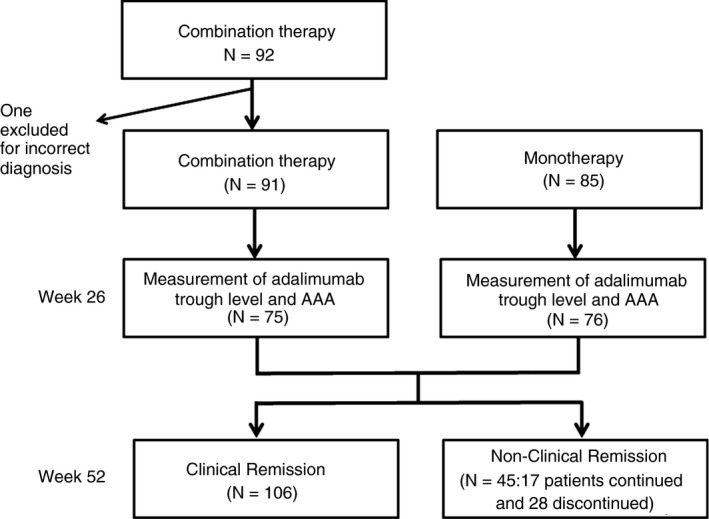
Flow chart of this study
Among 151 patients whose adalimumab trough level and AAA were measured, 106 achieved CR at week 52 and 45 (17 patients completed study and 28 discontinued it) did not. Patients with CR at week 52 had significantly higher trough level of adalimumab (7.7 ± 3.3 μg/mL) at week 26 than those with active disease (5.4 ± 4.3 μg/mL: P <.001) (Figure 2A).
Figure 2.
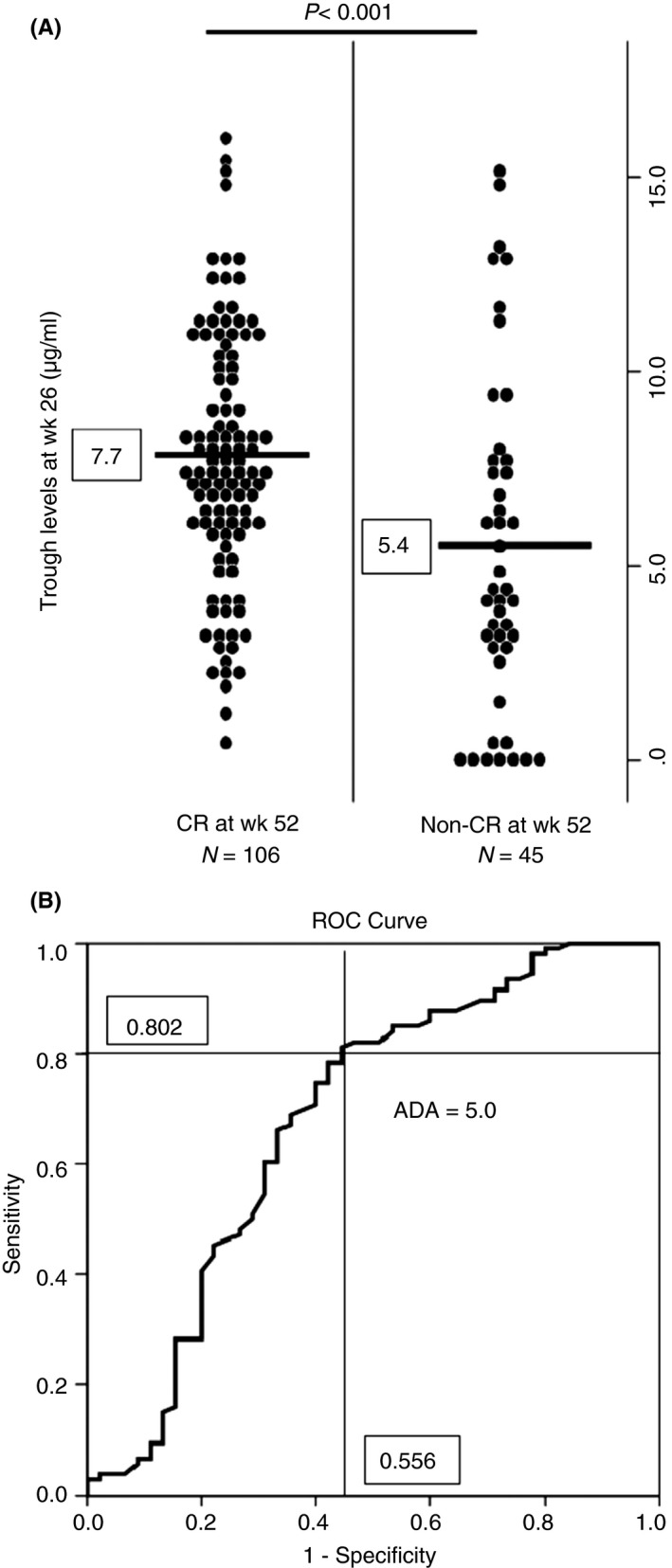
Analysis of disease activity at week 52 associated with ADA trough levels and AAA at week 26. A, There was a significant difference in ADA trough level at week 26 between patients with CR at week 52 and those with non‐CR. B, ROC curve of ADA trough level at week 26 and CR at week 52 (AUC [95% CI] 0.68 [0.577‐0.783])
We determined the closet point to the upper‐left corner of the ROC curve as the optimal cut‐off points for adalimumab trough level at week 26 to predict CR at week 52. A cut‐off drug level of 5.0 μg/mL yielded sensitivity of 80.0%, specificity of 55.6%, positive predictive values of 81.0%, and negative predictive value of 54.3% (Figure 2B).
As for AAA positivity, 13 patients were positive for AAA and 138 were negative. Eight of the 13 patients positive for AAA did not achieve CR. The development of AAA at week 26 was significantly and positively associated with disease activity at week 52 (P = .021) (Table 1). Moreover, patients with high titre of AAA showed low adalimumab trough level (Fig. S1).
Table 1.
Anti‐adalimumab antibodies (AAA) association with disease activity (P‐.021)
| Disease activity at 52 wk | |||
|---|---|---|---|
| AAA at week 26 | Active disease N (%) | Disease remission N (%) | Total |
| AAA | |||
| Negative | 37 (26.8) | 101 (73.2) | 138 |
| Positive | 8 (61.5) | 5 (38.5) | 13 |
| Total | 45 (29.8) | 106 (70.2) | 151 |
3.3. Potential factors associated with adalimumab trough values and the occurrence of AAA
A multivariable linear regression model with baseline information suggested that female and increasing body weight were independently associated with low trough level of adalimumab (Table 2 model 1). After including AAA detection, AAA was strongly associated with adalimumab trough levels and coefficients in the association of trough level with female sex or combination therapy became weakened because the effects of these factors could be expressed via AAA (model 2). Supporting the above interpretation, the multivariable logistic regression analysis revealed that female sex was associated with the occurrence of AAA (Table 3).
Table 2.
A multiple linear regression model to assess the association between adalimumab trough value and associated potential factors
| Model 1a | Model 2c | |||||||
|---|---|---|---|---|---|---|---|---|
| Parametera | P value | Difference for ADA through (beta) | 95%CI | P value | Difference for ADA through (beta) | 95%CI | ||
| Lower | Upper | Lower | Upper | |||||
| Constant | <.001 | 14.18 | 9.12 | 19.23 | <.001 | 13.08 | 8.56 | 17.60 |
| Female (vs Male) | .015 | −1.84 | −3.32 | −0.36 | .159 | −0.96 | −2.30 | 0.38 |
| Body weightb | .001 | −0.11 | −0.17 | −0.05 | .003 | −0.09 | −0.15 | −0.03 |
| Combination (vs monotherapy) | .085 | 1.09 | −2.32 | 0.15 | .286 | −.58 | −0.49 | 1.65 |
| Elemental diet ( ≥600) | .077 | −1.11 | −0.12 | 2.26 | .045 | −1.12 | −2.22 | −0.03 |
| AAA detection | <.001 | −5.61 | −7.55 | −3.67 | ||||
Backward elimination method was applied for model 1 after including factors listed in material and methods.
Per 1 kg increase CI, Confidential interval
AAA detection was added to the model 1.
Table 3.
The multivariate logistic regression of the association between the occurrence of AAA and associated potential factors
| Parametera | P value | OR | 95%CI | |
|---|---|---|---|---|
| Lower | Upper | |||
| Female (vs Male) | .009 | 6.92 | 1.64 | 29.24 |
| Body weightb | .051 | 1.06 | 1.00 | 1.12 |
| Combination (vs monotherapy) | .063 | 0.27 | 0.07 | 1.08 |
Backward elimination method applied for model 1 after including factors listed in material and methods.
Per 1 kg increase
CI, Confidence interval; OR, Odds ratio.
We investigated more in detail whether body weight categories had a differential effect on adalimumab trough levels (Fig. S2, Table. S1). We found that patients with higher body had a trend towards a lower trough level and there was a significant difference of mean trough level between patients with weight of less than 40 kg and those with more than 70 kg was observed. In addition, the significant association between body mass index (BMI) and trough level of adalimumab was observed as well (Table. S2).
3.4. The effect of AZA on adalimumab trough level and mucosal healing in CD patients
6‐TGN in RBCs was measured in 71 patients from the combination group at week 12. The patients were administered AZA at doses ranging from 25 mg to 100 mg per day with a mean ± the standard deviation (SD) of 0.86 ± 0.35 mg/kg. The 6‐TGN levels ranged from 50 to 1510 pmol/8 × 108 RBCs, with the median (interquartile ranges) of 257 (162‐426) pmol/8×108 RBCs.6
There was a trend towards a higher 6‐TGN level at week 12 in CD patients negative for AAA at week 26 (240.6 geometric SD (112.1‐523.7) pmol/8 × 108 RBCs) than in those positive for AAA (111.2 geometric SD (54.2‐248.4) pmol/8 × 108 RBCs) (Figure 3A), despite no significant difference in the mean dose of AZA between the AAA‐negative (34 ± 12 mg/day) and positive (42 ± 14 mg/day) groups. Moreover, we selected a marked cut‐off point with high sensitivity from the ROC curve for 6‐TGN level at week 12 to suppress AAA development. A cut‐off 6‐TGN level of >222.5 pmol/8 × 108 RBCs yielded sensitivity of 100% and specificity of 60.6%. Because of quite low incidence of AAA positivity, positive predictive value was very low (10.3%), but negative predictive value was remarkable (100%). (Figure 3B). Additionally, we investigated the effect of 6‐TGN level at week 12 on mucosal healing at week 26. There was no significant difference of mean 6‐TGN levels between patients with achievement of mucosal healing and without it (287.5 ± 213.6 vs 330.9 ± 263.7 pmol/8 × 108 RBCs, P = .588).
Figure 3.
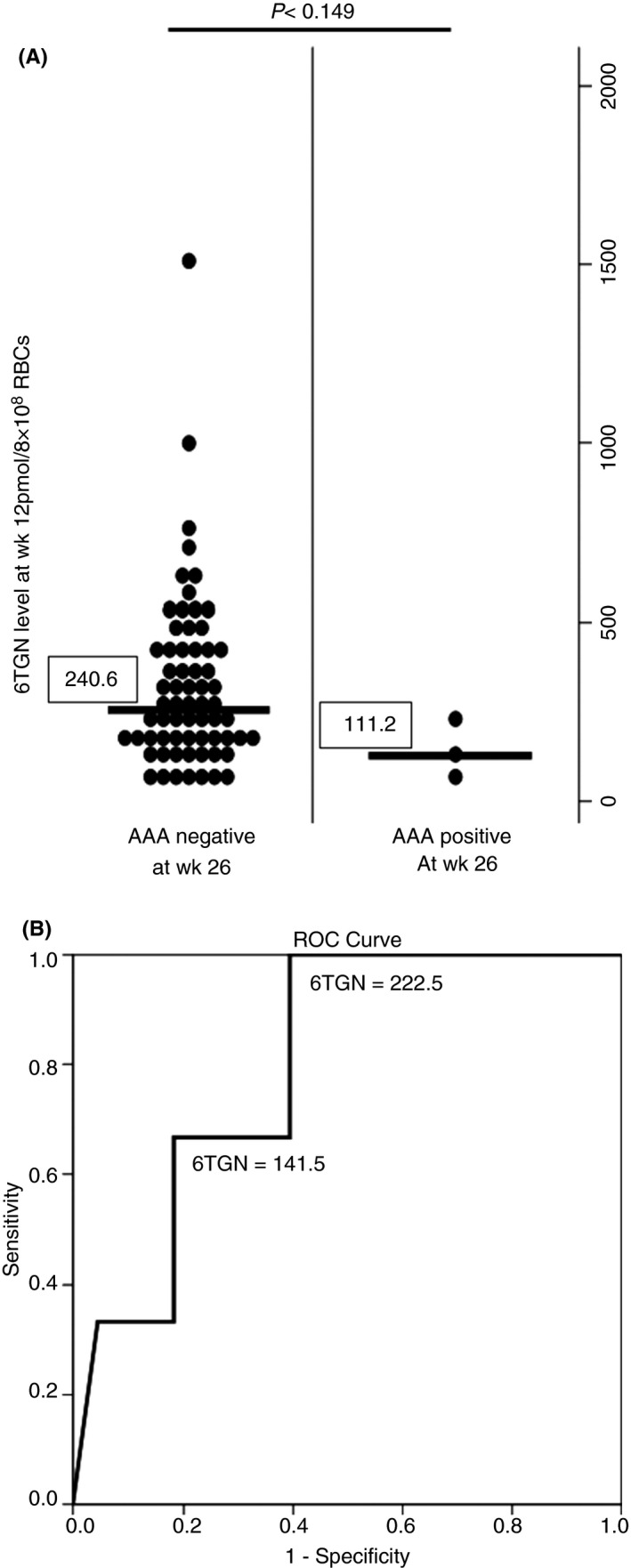
A, The association between 6‐TGN levels at 12 weeks and AAA positivity. CD patients negative for AAA at week 26 showed a higher 6‐TGN level at week 12 in comparison with those positive for AAA. The vertical line shows geometric data. B, ROC curve of 6‐TGN level at week 12 and development of AAA (AUC [95% CI] 0.801 [0.61‐0.988])
4. DISCUSSION
This study evaluated the impact of adalimumab trough level and AAA positivity at week 26 on clinical outcomes of patients enrolled in the DIAMOND trial. We found that higher trough levels of adalimumab and the absence of AAA at week 26 noticeably contributed to clinical activity at week 52 in CD patients treated with adalimumab monotherapy or the combination therapy, and a high 6‐TGN concentration might be required for the suppression of AAA.
Despite the proven efficacy of adalimumab in CD patients, the loss of response remains a common clinical problem, posing both evaluation and management dilemmas regarding the most appropriate intervention in the course of the treatment. Several reports have suggested that the trough levels of biologics and anti‐drug antibodies were correlated with a loss of response (LOR). Therefore, the concept of therapeutic drug monitoring (TDM) and the importance of individualised pharmacokinetics have been recognised in the management of IBD patients receiving biological therapy.13, 14 Several clinical trials suggested that early trough levels were predictive for short‐ and medium‐term clinical efficacy in IBD patients treated with biologics. In adalimumab‐treated patients with moderately to severely active CD, the serum adalimumab trough level was higher in patients who achieved clinical remission than those who did not.15 Mazor et al reported that the adalimumab trough level at, before, or 1 day prior to, the next scheduled injection was related to disease remission with an optimal cut‐off of 5.85 μg/mL.16 A recent report by Ward et al showed that a drug level of ≥4.9 μg/mL during the first 9 days predicts a therapeutic trough drug level with reasonable confidence.17 Roblin et al reported that the adalimumab trough level of ≥4.9 μg/mL was a predictor of clinical remission following adalimumab therapy optimisation.18 Retrospective data from Karmiris cohort showed that trough levels of adalimumab at week 4 <5.0 μg/mL significantly increased the future risk of AAA formation despite different time point of measuring trough level.19 In the present study, higher trough levels of adalimumab at week 26 resulted in favourable outcome in CD patients. Additionally, we identified a trough drug level of 5.0 μg/mL as an optimal cut‐off for predicting disease activity at week 52, which is in similar fashion to previous reports. Taken together, monitoring the adalimumab trough level at optimal points noticeably contributes to the prediction of the disease course and supports decision‐making for further treatment. However, it should be acknowledged that the cut‐off level might vary depending on the measurement time.
Among the factors we assessed, sex and low body weight were independently associated with high adalimumab trough levels, while the other factors were not. In addition, the multivariate logistic regression analysis revealed that female sex was associated with the occurrence of AAA. The reason why sex affected the adalimumab trough level and the occurrence of AAA remains unclear. Recent report suggested that higher body fat content at baseline was independently associated with a worse response to treatment with anti‐TNF inhibitors because adipose gain could conceivably play a role in the disparate serum drug levels.20 Also, females differ with respect to distribution of adipose tissues as follows: males tend to accrue more visceral fat, whereas females accrue more fats in the subcutaneous deposit.21 Thus, fat deposition might influence the lower trough level in female patients treated with subcutaneous administration of anti‐TNF inhibitors. In addition, considering that decreased serum concentrations of anti‐TNF‐inhibitors are accompanied by an increase in serum of anti‐drug antibody levels, lower trough level of adalimumab in female would result in the occurrence of AAA. In this regard, a more personalised sex‐specific approach for patients who receive treatment with biologics will be required in the future.22
A previous report suggested that obese (BMI >30 kg/m2) patients with CD who are beginning infliximab (IFX) therapy are more likely to have a flare than nonobese patients, and increased body weight is associated with an earlier time to a loss of response to IFX in CD.23 Patients with CD who require an adalimumab dose escalation had a higher BMI than patients who did not require a dose escalation.24 In the CLASSIC‐II post hoc analysis, patients with a BMI >29 kg/m2 were less often in clinical remission compared to patients with a lower BMI.25 Additionally, a recent report by Bond et al demonstrated that BMI did not differentially influence trough levels of adalimumab and IFX, but the investigators showed a trend towards a lower trough level in adalimumab‐treated patients with a BMI >30 kg/m2.26 Thus, BMI seems to influence the response to adalimumab. However, the effect of body weight on adalimumab trough level is controversial. Baert et al found a poor correlation between body weight and adalimumab concentration,27 while Ward et al described that weight was inversely related to drug levels.28 In this study, we found that body weight and BMI affected adalimumab trough level and analysis of a multiple linear regression model suggested that body weight might have a little stronger influence on adalimumab trough value than BMI. Although the involvement of body weight and BMI in the therapeutic effect of biologics is still unclear, data suggest that patients with body weight and BMI over a certain limit will require a dose escalation of adalimumab.
Another important issue in TDM is to check AAA during adalimumab treatment because several abstracts and reports have suggested that AAA levels are inversely associated with adalimumab levels and positively associated with disease activity.16, 29, 30 In the present study, we showed that the development of AAA at week 26 was positively associated with disease activity at week 52 and that higher 6‐TGN suppressed AAA. Also, we found that 8.6% (13/151) of serum samples were positive for AAA and patients with higher titre of AAA showed low adalimumab trough level. Until now, the immunogenicity of adalimumab was considered to be rare in the pivotal efficacy trials in CD. However, the incidence of AAA, which was higher than expected, varied from 2.6% to 44% of patients depending on the techniques applied for the detection of AAA.31, 32 In consistent with previous reports, our data suggest the significance of monitoring AAA during adalimumab treatment, however, the timing of AAA measurement might be optimised on the basis of demographic factors such as sex and body weight. Taken together, we should keep in mind that AAA occurs in a certain proportion of Japanese CD patients who are naive to biologics and monitor AAA in CD patients with an LOR to adalimumab under the concept of personalised medicine.
The published DIAMOND trial demonstrated that the clinical efficacy of a combination of adalimumab and azathioprine at week 26 did not differ from that of adalimumab monotherapy in patients with CD naive to both medications.6 A meta‐analysis of previous published data by Kopylov et al concluded that a combination of adalimumab and AZA was not superior to monotherapy with adalimumab in terms of the maintenance of remission at 1 year.33 A cross‐sectional study using trough sera from adalimumab‐treated CD patients demonstrated that concomitant immunosuppression did not influence adalimumab trough and AAA levels.16 Thus, with several retrospective and cohort studies, data from the DIAMOND trial suggested that AZA did not affect the enhancement of the efficacy of adalimumab. On the other hand, a retrospective analysis of a large tertiary centre cohort by Cosnes et al demonstrated that initial combination therapy with an immunomodulator improved all outcome measures.34 Recent meta‐analysis demonstrates that patients with IBD who are on anti‐TNF inhibitors with concomitant use of immunomodulators had a 51% reduced risk of developing anti‐drug antibodies compared with patients on anti‐TNF inhibitor monotherapy.35 Strik et al reported that the addition of immunomodulatory drugs resulted in undetectable anti‐drug antibody levels, increased serum drug concentrations and regained clinical response in IBD patients with loss of response due to immunogenicity.36
Another clinical issue is the optimisation of 6‐TGN levels for suppression of anti‐drug antibodies. Yarur et al recommended 6‐TGN >125 pmol/8×108 RBCs as an optimal cut‐off level to suppress antibodies to infliximab.37 Ward et al also described no correlation between 6‐TGN concentrations and anti‐TNF inhibitor levels.28 In this study, we found that CD patients who were negative for AAA at week 26 had a higher 6‐TGN level at week 12 than those positive for it and revealed a cut‐off 6‐TGN level of >222.5 pmol/8 × 108 RBCs to suppress development of AAA, which reflects the importance of immunomodulators for reducing immunogenicity of anti‐TNF inhibitors. As described in preceding DIAMOND trial, the mean value of 6‐TGN (257) was within the therapeutic threshold despite the lower doses of AZA applied to the combination group (25 mg/day‐100 mg/day) in comparison with those used in the West.6 Exactly, there was a possibility that some CD patients were treated with suboptimal level of 6‐TGN in the point of IQR (162‐426). In addition, the rate of AAA formation was low. Therefore, we must interpret a cut‐off 6‐TGN level for inhibiting AAA with an extreme caution because of the limited numbers of CD patients positive for AAA in this study. Nevertheless, adding AZA to adalimumab is useful for subpopulation of CD patients who presumably have AAA during adalimumab treatment, even if concomitant immunosuppression did not alter the overall clinical outcome. Based on a retrospective study that combination with immunosuppression may be beneficial during the first semester of initiating adalimumab,25 further investigation is required to determine an optimal 6‐TGN concentration to inhibit the development of AAA in patients who require immunomodulators.
There were some limitations in this study. First limitation is that we could not examine adalimumab trough level and AAA at week 4 and 12 in the DIAMOND trial, which might be available to identify CD patients requiring adalimumab intensification.15, 27 From the view of ensuring appropriately clinical decision‐making in an individual patient, adalimumab trough level should be checked at optimal time points. However, in real‐world practice, it may be difficult to obtain true trough level. Second, 52 weeks might be short in the assessment of secondary loss of response and immunogenicity. Third, the sample size may be too small to draw a definitive conclusion regarding the optimal baseline cut‐off adalimumab trough level and AAA for predicting a therapeutic response and our preliminary results await further confirmation by controlled and larger scale studies.
In conclusion, we demonstrated the impact of adalimumab trough level and AAA positivity at week 26 on the clinical outcome of CD patients under adalimumab treatment. Higher 6‐TGN levels could inhibit AAA occurrence. Therefore, the combination therapy is beneficial in some relevant aspects for CD patients. Findings from this study, similarly to previous studies, confirm the significance of TDM in the management of CD patients under treatment with adalimumab and the possible benefit of measuring AAA when triaging patients with LOR‐related immunogenicity for personalised anti‐TNF therapy in Crohn's disease in the future.
AUTHORSHIP
Guarantor of the article: Hiroshi Nakase.
Author contributions: Study concept and design (HN,TM, KW, TH, TK, YS, MW, TH), acquisition of data (SM, NY, TI, SK, TN, ME, MN, ToM, YN), analysis and interpretation of data (HN, TM, KW, HT, SM, MNo), drafting of the manuscript (HN, TM, KW, SM, MNo, TH), critical revision of the manuscript for important intellectual content (YS, MW, TH), statistical analysis (MNo) and study supervision (YS, MW, TH).
Supporting information
ACKNOWLEDGEMENTS
Declaration of personal interests: The authors have the following financial conflicts of interest regarding this manuscript. HN: Eisai Corporation (EC), Abbvie GK(AGK), Mitsubishi Tanabe Pharma (MTP), Astellas Pharma (AsP), Takeda Pharmaceutical Corporation (TPO), Kyorin Pharmaceutical Corporation(KPC). SM: EC, AGK, MTP, Jannsen Pharma. TM: EC, AGK, MTP. KW: EC, AGK, MTP. TH: EC, AGK, MTP, Ajinomoto Pharma (AP). NY: EC, AGK, MTP.
SK: AGK. ME: EC, AGK, MTP. MN: EC, AGK, MTP. ToM: EC, AGK, MTP. YNa: AsP, Otsuka Pharmaceutical Corporation (OPC), TPO, EC, MTP. TK: EC, AGK, MTP. YS: EC, AGK, MTP, Zeria Pharmaceutical Corporation (ZPO). MW: EC, AGK, MTP, KPC, Diichi Sankyo Corporation, Ono Pharmaceutical Corporation, Gene Care Research Institute, AsP, Asahi Kasei Kuraray Corporation, Chugai Pharmaceutical Corporation, TPO, AP, OPC, Kyowa Hakko Kirin Corporation, JIMRO Corporation, ZPO, UCB Japan Corporation, Dainippon Sumitomo Pharma, Toray Industries, Bristol‐Meyers KK. TH: EC, AGK, MTP.
APPENDIX 1.
1.1.
Members of the DIAMOND study group (name and affiliation) are Akira Andoh (Shiga University of Medical Science), Toshifumi Ashida (Sapporo Higashi Tokushukai Hospital), Katsuya Endo (Tohoku University), Yutaka Endo (Showa University Fujigaoka Hospital), Motohiro Esaki (Kyushu University), Hiroshi Fujita (Kagoshima University), Mikihiro Fujiya (Asahikawa Medical University), Ken Haruma (Kawasaki Medical School), Toshifumi Hibi (Kitasato University Kitasato Institute Hospital), Sakiko Hiraoka (Okayama University), Ichiro Hirata (Fujita Health University Hospital), Tadakazu Hisamatsu (Kyorin University), Yutaka Honda (Niigata University), Hideki Iijima (Osaka University), Bunei Iizuka (Tokyo Women's Medical University), Kentaro Ikeya (Hamamatsu South Hospital), Takuya Inoue (Osaka Medical College), Shuji Inoue (Kochi National Hospital), Tetsuya Ishida (Ishida Clinic of IBD and Gastroenterology), Yo Ishiguro (Hirosaki National Hospital), Shunji Ishihara (Shimane University), Hiroaki Ito (Kinshukai Infusion Clinic), Ryuichi Iwakiri (Saga University), Takashi Kagaya (Kanazawa University), Takanori Kanai (Keio University), Hiroshi Kashida (Kinki University), Shingo Kato (Saitama Medical University), Jun Kato (Wakayama Medical University), Takehiko Katsurada (Hokkaido University), Fukunori Kinjyo (Ryukyu University), Kiyonori Kobayashi (Kitasato University), Mayumi Kodama (Miyazaki Medical Center Hospital), Reiko Kunisaki (Yokohama City University Medical Center), Koichi Kurahara (Matsuyama Red Cross Hospital), Takafumi Kurokami (Shizuoka General Hospital), Lee Kyouwon (Moriguchikeijinkai Hospital), Koichiro Matsuda (Toyama Prefectural Central Hospital), Kazuhiro Matsueda (Kurashiki Central Hospital), Toshiyuki Matsui (FukuokaUniversity Chikushi Hospital), Takayuki Matsumoto (Iwate Medical University), Keiichi Mitsuyama (Kurume University), Yuji Mizokami (Tsukuba University), Satoshi Motoya (Sapporo Kosei General Hospital), Yuji Naito (Kyoto Prefectural University of Medicine), Tomoo Nakagawa (Chiba University), Shiro Nakamura (Hyogo College of Medicine), Hiroshi Nakase (Sapporo Medical University, School of Medicine), Masanori Nojima (The University of Tokyo), Masafumi Nomura (Teine Keijinkai Hospital), Atsuhiro Ogawa (Bellland General Hospital), Kazuichi Okazaki (Kansai Medical University), Kazuaki Otsuka (Showa University Northern Yokohama Hospital), Hirotake Sakuraba (Hirosaki University), Masayuki Saruta (The Jikei University School of Medicine), Makoto Sasaki (Aichi Medical University), Takayuki Shirai (Tokai University Hachioji Hospital), Tomoaki Suga (Shinshu University), Kazuhito Sugimura (Niigata city general hospital), Toshiro Sugiyama (Toyama University), Yasuo Suzuki (Toho University Sakura Medical Center), Fuminao Takeshima (Nagasaki University), Hiroyuki Tamaki (Takamatsu Red Cross Hospital), Shinji Tanaka (Hiroshima University), Satoshi Tanida (Nagoya City University), Keiichi Tominaga (Dokkyo Medical University), Taku Tomizawa (Gunma University), Kenji Watanabe (Hyogo College of Medicine), Mamoru Watanabe (Tokyo Medical and Dental University), Shojiro Yamamoto (Miyazaki University), Masaki Yamashita (St. Marianna University School of Medicine), Atsushi Yoshida (Ofuna Central Hospital), and Naoki Yoshimura (Tokyo Yamate Medical Center).
APPENDIX 2.
AUTHORS' COMPLETE AFFILIATIONS
Hiroshi Nakase: Department of Gastroenterology and Hepatology, Sapporo Medical University School of Medicine, Sapporo, Japan; Satoshi Motoya: Sapporo‐kosei General Hospital, Sapporo, Hokkaido, Japan; Takayuki Matsumoto: Division of Gastroenterology and Hepatology, Department of Internal Medicine, Iwate Medical University, Morioka, Iwate, Japan; Kenji Watanabe: Hyogo Ika Daigaku, Nishinomiya, Hyogo, Japan; Tadakazu Hisamatsu: Kyorin Daigaku, Mitaka, Tokyo, Japan; Naoki Yoshimura: Department of Medicine, Division of Gastroenterology, Tokyo Yamate Medical Center, Tokyo, Japan; Tetsuya Ishida: Department of Gastroenterology, Oita Red Cross Hospital, Oita, Japan; Shingo Kato: Saitama Medical Center, Department of Gastroenterology and Hepatology, Saitama Medical University, Saitama, Japan; Tomoo Nakagawa: Department of Gastroenterology, Chiba Daigaku Daigakuin Igaku Kenkyuin Igakubu, Chiba, Japan; Motohiro Esaki: Medicine and Clinical Science, Kyushu University, Fukuoka, Japan; Masakazu Nagahori: Department of Gastroenterology and Hematology, Tokyo Ika Shika Daigaku, Bunkyo‐ku, Tokyo Japan; Toshiyuki Matsui: Department of Gastroenterology, Fukuoka University Chikushi Hospital, Chikushino, Fukuoka, Japan; Yuji Naito: Medical Proteomics, Department of Medicine, Kyoto Prefectural University of Medicine, Kyoto, Japan; Takanori Kanai: Division of Gastroenterology and Hepatology, Department of Internal Medicine, School of Medicine, Keio University, Shinjuku‐ku, Tokyo, Japan; Yasuo Suzuki: Department of Internal Medicine, Toho Daigaku Iryo Center Sakura Byoin, Sakura, Chiba, Japan; Masanori Nojima: Center for Translational Research, Institute of Medical Science, University of Tokyo, Minato‐Ku, Tokyo, Japan; Mamoru Watanabe: Department of Gastroenterology and Hepatology, Tokyo Ika Shika Daigaku, Bunkyo‐ku, Tokyo Japan Toshifumi Hibi: Center for Advanced IBD Research And Treatment, Gakko Hojin Kitasato Kenkyujo, Minato‐Ku, Tokyo, Japan.
Nakase H, Motoya S, Matsumoto T, et al. Significance of measurement of serum trough level and anti‐drug antibody of adalimumab as personalised pharmacokinetics in patients with Crohn's disease: a subanalysis of the DIAMOND trial. Aliment Pharmacol Ther. 2017;46:873–882. https://doi.org/10.1111/apt.14318
The Handling Editor for this article was Professor Roy Pounder, and it was accepted for publication after full peer‐review.
Hiroshi Nakase and Satoshi Motoya contributed equally to this work.
The authors' complete affiliations are listed in Appendix 2.
REFERENCES
- 1. Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet. 2002;359:1541‐1549. [DOI] [PubMed] [Google Scholar]
- 2. Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462‐2476. [DOI] [PubMed] [Google Scholar]
- 3. Hanauer SB, Sandborn WJ, Rutgeerts P, et al. Human anti‐tumor necrosis factor monoclonal antibody (adalimumab) in Crohn's disease: the CLASSIC‐I trial. Gastroenterology. 2006;130:323‐333. [DOI] [PubMed] [Google Scholar]
- 4. Colombel JF, Sandborn WJ, Rutgeerts P, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn's disease: the CHARM trial.. Gastroenterology. 2007;132:52‐65. [DOI] [PubMed] [Google Scholar]
- 5. Watanabe M, Hibi T, Lomax KG, et al. Adalimumab for the induction and maintenance of clinical remission in Japanese patients with Crohn's disease. J Crohns Colitis. 2012;6:160‐173. [DOI] [PubMed] [Google Scholar]
- 6. Matsumoto T, Motoya S, Watanabe K, et al. Adalimumab monotherapy and a combination with azathioprine for Crohn's disease: a prospective randomized trial. J Crohns Colitis. 2016;10:1259‐1266. [DOI] [PubMed] [Google Scholar]
- 7. Colombel JF, Jharap B, Sandborn WJ, et al. Effects of concomitant immunomodulators on the pharmacokinetics, efficacy and safety of adalimumab in patients with Crohn's disease or ulcerative colitis who had failed conventional therapy. Aliment Pharmacol Ther. 2017;45:50‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Best WR, Beckrel JM, Singleton JW, Kern F. Development of a Crohn's disease activity index. Gastroenterology. 1976;70:439‐444. [PubMed] [Google Scholar]
- 9. Lennard L, Singleton HJ. High‐performance liquid chromatographic assay of the methyl and nucleotide metabolites of 6‐mercaptopurine. Quantification of red blood cell 6‐thioguanine nucleotide, 6‐thionosinic acid and 6‐methylmercaptopurine metabolites in a single sample. J Chromatogr. 1992;583:83‐90. [DOI] [PubMed] [Google Scholar]
- 10. Bartelds GM, Krieckaert CL, Nurmohamed MT, et al. Development of antidrug antibodies against adalimumab and association with disease activity and treatment failure during long‐term follow‐up. JAMA. 2011;305:1460‐1468. [DOI] [PubMed] [Google Scholar]
- 11. Bartends GM, Wijbrandts CA, Nurmohamed MT, et al. Clinical response to adalimumab. Relationship to anti‐adalimumab antibodies and serum adalimumab concentrations in rheumatoid arthritis. Ann Rheum Dis. 2007;66:921‐926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wolbink GJ, Voskuyl AE, Lems WF, et al. Relationship between serum trough infliximab levels, pretreatment C reactive protein levels, and clinical response to infliximab treatment in patients with rheumatoid arthritis. Ann Rheum Dis. 2005;64:704‐707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yarur AJ, Rubin DT. Therapeutic drug monitoring of anti‐tumor necrosis factor agents in patients with inflammatory bowel diseases. Inflamm Bowel Dis. 2015;21:1709‐1718. [DOI] [PubMed] [Google Scholar]
- 14. Ben‐Horin S, Chowers Y. Tailoring anti‐TNF therapy in IBD: drug levels and disease activity. Nat Rev Gastroenterol Hepatol. 2014;11:43‐55. [DOI] [PubMed] [Google Scholar]
- 15. Chiu YL, Rubin DT, Vermerire S, et al. Serum adalimumab consentration and clinical remission in patients with Crohn's disease. Inflamm Bowel Dis. 2013;19:1112‐1122. [DOI] [PubMed] [Google Scholar]
- 16. Mazor Y, Almog R, Kopylov U, et al. Adalimumab drug and antibody as predictors of clinical and laboratory response in patients with Crohn's disease. Aliment Pharmacol Ther. 2014;40:620‐628. [DOI] [PubMed] [Google Scholar]
- 17. Ward MG, Thwaites PA, Beswick L, et al. Intra‐patient variability in adalimumab drug levels within and between cycles in Crohn's disease. Aliment Pharmacol Ther. 2017;45:1135‐1145. [DOI] [PubMed] [Google Scholar]
- 18. Roblin X, Rinaudo M, del Tedesco E, et al. Development of algorithm incorporating pharmacokinetics of adalimumab in inflammatory bowel disease. Am J Gastroenterol. 2014;109:1250‐1256. [DOI] [PubMed] [Google Scholar]
- 19. Baert F, Kondragunta V, Lockton S, et al. Antibodies to adalimumab are associated with future inflammation in Crohn's patients receiving maintenance adalimumab therapy: a post hoc analysis of the Karmiris trial. Gut. 2016;65:1126‐1131. [DOI] [PubMed] [Google Scholar]
- 20. Ibáñez Vodnizza SE, Nurmohamed MT, Visman IM, et al. Fat mass lowers the response to tumor necrosis factor‐α blockers in patients with ankylosing spondylitis. J Rheumatol. 2017. pii: jrheum.170094. https://doi.org/10.3899/jrheum.170094 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 21. Palmer BF, Clegg DJ. The sexual dimorphism of obesity. Mol Cell Endocrinol. 2015;402:113‐119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lie MR, Kreijne JE, van der Woude CJ. Sex is associated with adalimumab side effects and drug survival in patients with Crohn's disease. Inflamm Bowel Dis. 2017;23:75‐81. [DOI] [PubMed] [Google Scholar]
- 23. Harper JW, Sinanan MN, Zisman TL. Increased body mass index is associated with earlier time to loss of response to infliximab in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:2118‐2124. [DOI] [PubMed] [Google Scholar]
- 24. Bultman E, de Haar C, van Liere‐Baron A, et al. Predictor of dose escalation of adalimumab in a prospective cohort of Crohn's disease patients. Aliment Pharmacol Ther. 2012;35:335‐341. [DOI] [PubMed] [Google Scholar]
- 25. Reenaers C, Louis E, Belaiche J, et al. Does co‐treatment with immunosuppressions improve outcome in patients with Crohn's disease treated with adalimumab? Aliment Pharmacol Ther. 2012;36:1040‐1048. [DOI] [PubMed] [Google Scholar]
- 26. Bond A, Asher R, Jackson R, et al. Comparative analysis of the influence of clinical factors including BMI on adalimumab and infliximab trough levels. Eur J Gastroenterol Hepatol. 2016;28:271‐276. [DOI] [PubMed] [Google Scholar]
- 27. Baert F, Vande Casteele N, Tops S, et al. Prior response to infliximab and early serum drug concentrations predict effects of adalimumab in ulcerative colitis. Aliment Pharmacol Ther. 2014;40:1324‐1332. [DOI] [PubMed] [Google Scholar]
- 28. Ward MG, Watner B, Unsworth N, et al. Infliximab and adalimumab drug levels in Crohn's disease: contrasting associations with disease activity and influencing factors. Aliment Pharmacol Ther. 2017;46:150‐161. [DOI] [PubMed] [Google Scholar]
- 29. Imaeda H, Bamba S, Takahashi K, et al. Relationship between serum infliximab trough levels and endoscopic activities in patients with Crohn's disease under scheduled maintenance treatment. J Gastroenterol. 2014;49:674‐682. [DOI] [PubMed] [Google Scholar]
- 30. Wolf DC, Scott H, Lockton S, Singh S. Mechanisms of loss of response to adalimumab in Crohn's disease. Gastroenterology. 2013;144:s775. [Google Scholar]
- 31. Sandborn WJ, Rutgeerts P, Enns R, et al. Adalimumab induction therapy for Crohn disease previously treated with infliximab: a randomized trial. Ann Intern Med. 2007;146:829‐838. [DOI] [PubMed] [Google Scholar]
- 32. Wang SL, Hauenstein S, Ohrmund L, et al. Monitoring of adalimumab and antibodies‐to‐adalimumab levels in patient serum by the homogeneous mobility shift assay. J Pharm Biomed Anal. 2013;78‐79:39‐44. [DOI] [PubMed] [Google Scholar]
- 33. Kopylov U, Al‐Taweel T, Yaghoobi M, et al. Adalimumab monotherapy versus combination therapy with immunomodulators in patients with Crohn's disease. A systematic review and meta‐analysis. J Crohns Colitis. 2014;8:1632‐1641. [DOI] [PubMed] [Google Scholar]
- 34. Cosnes J, Sokok H, Bourrier A, et al. Adalimumab or inflizximab as monotherapy, or in combination with an immunomodulatory, in the treatment of Crohn's disease. Aliment Pharmacol Ther. 2016;44:1102‐1113. [DOI] [PubMed] [Google Scholar]
- 35. Qiu Y, Mao R, Chen B, et al. Effects of combination therapy with immunomodulators on trough levels and antibodies against tumor necrosis factor antagonists in patients with inflammatory bowel diseases: a meta‐analysis. Clin Gastroenterol Hepatol. 2017;15:1359‐1372. [DOI] [PubMed] [Google Scholar]
- 36. Strik AS, van den Brink GR, Ponsioen C, et al. Suppression of anti‐drug antibodies to infliximab or adalimumab with the addition of an immunomodulatory in patients with inflammatory disease. Aliment Pharmacol Ther. 2017;45:1128‐1134. [DOI] [PubMed] [Google Scholar]
- 37. Yarur AJ, Kubilliun MJ, Czul F, et al. Concentrations of 6‐thioguanine nucleotide correlate with trough levels of infliximab in patients with inflammatory bowel disease on combination therapy. Clin Gastroenterol Hepatol. 2015;13:e3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


