Abstract
Objective
In disease‐modifying antirheumatic drug–naive patients with early rheumatoid arthritis (RA) who had achieved sustained low disease activity (a Disease Activity Score in 28 joints using the erythrocyte sedimentation rate of ≤3.2 at both week 40 and week 52) after 1 year of treatment with certolizumab pegol (CZP) at a standard dose (200 mg every 2 weeks plus optimized methotrexate [MTX]), we evaluated whether continuation of CZP treatment at a standard dose or at a reduced frequency (200 mg every 4 weeks plus MTX) was superior to stopping CZP (placebo plus MTX) in maintaining low disease activity for 1 additional year.
Methods
A total of 293 patients from period 1 of our study were re‐randomized 2:3:2 in period 2 to CZP at a standard dose (n = 84), CZP at a reduced frequency (n = 127), or placebo plus MTX (CZP stopped) (n = 82). The primary end point was the percentage of patients who maintained low disease activity throughout weeks 52–104 without flares. We used a hierarchical testing scheme, comparing CZP at a standard dose with CZP stopped. If P < 0.05 was achieved, then CZP at a reduced frequency was compared with CZP stopped (nonresponder imputation).
Results
The 293 patients from period 1 represented 36% fewer patients than projected, yielding a smaller number of patients eligible for period 2. Higher proportions of patients treated with the standard and reduced frequency regimens maintained low disease activity than those who had stopped CZP (48.8% and 53.2%, respectively, versus 39.2% [P = 0.112 and P = 0.041, respectively; nominal P value, first hierarchical test not significant]). Similar trends were observed for radiographic nonprogression (change from baseline of ≤0.5 in modified Sharp/van der Heijde score; 79.2% and 77.9% of patients, respectively, versus 70.3%) and normative physical function (Health Assessment Questionnaire disability index score of ≤0.5; 71.4% and 70.6% of patients, respectively, versus 57.0%). Safety profiles were similar between all groups, with no new safety signals identified for continuing CZP to week 104. No deaths were reported.
Conclusion
The study failed to meet its primary end point. However, there were no clinically meaningful differences between the standard and reduced frequency doses of CZP plus MTX; both controlled RA more effectively than stopping CZP.
With the current armamentarium of treatments for rheumatoid arthritis (RA), targeting achievement of low disease activity or disease remission is recommended and achievable in many patients. While there is agreement that the clinical response induced by biologic disease‐modifying antirheumatic drugs (DMARDs) should ideally be sustained, there is currently no consensus regarding the length of time that defines a sustained response. Based on a state of enduring disease control, clinicians must also consider the possibility of successfully withdrawing or tapering therapy (i.e., decreasing dose or dosing frequency), a treatment concept referred to as the “induction‐maintenance” approach.
Tapering therapies after achieving a desired treatment target has therefore become a topic of considerable interest; results of several recent trials have suggested that this may indeed be possible with several biologic agents, with some of these trials also focusing on patients with newly diagnosed RA 1. Applying such treatment strategies may confer significant benefits to individual patients through the reduction of medication dosage and the associated risks while maintaining a state of disease control, as well as through easing of the economic burden of the disease as a result of increased cost effectiveness of the treatment 2, 3, 4, 5. Current recommendations issued by the American College of Rheumatology (ACR) and the European League Against Rheumatism (EULAR) 2, 6 suggest continuation of therapy with only conditional recommendations for tapering, thereby implying that RA patients will thus spend long periods taking biologic agents.
The aim of the C‐EARLY study was to advance potential care options with regard to the induction‐maintenance concept by evaluating the early initiation of certolizumab pegol (CZP) in combination with optimized methotrexate (MTX), as well as subsequent continuation, tapering, or withdrawal of CZP, in a population of DMARD‐naive patients with early RA at high risk of progressive disease. CZP is an Fc‐free, PEGylated, anti–tumor necrosis factor (anti‐TNF) biologic DMARD used to treat both established and early RA in combination with MTX 7, 8, 9. Period 1 of the C‐EARLY study (NCT01519791) assessed the efficacy and safety of 1 year of CZP in combination with optimized MTX versus optimized MTX alone. Results from period 1 (9) showed that significantly higher proportions of patients treated with CZP plus MTX achieved clinical treatment targets such as sustained remission (28.9% had a Disease Activity Score in 28 joints 10 using the erythrocyte sedimentation rate [DAS28‐ESR] of <2.6 at both week 40 and week 52; odds ratio [OR] 2.3 [95% confidence interval {95% CI} 1.50–3.47], P < 0.001) and sustained low disease activity (43.8% had a DAS28‐ESR of ≤3.2 at both week 40 and week 52; OR 2.0 [95% CI 1.38–2.78], P < 0.001) than did their counterparts treated with placebo plus optimized MTX (15.0% had sustained remission and 28.6% had sustained low disease activity). There was also significant inhibition of the progression of articular structural damage and marked improvements in the physical function of the CZP‐treated patients in comparison with the patients treated with optimized MTX alone 9.
Period 2 of the C‐EARLY study (NCT01521923) was a continuation of period 1 for those patients who had achieved sustained low disease activity at both week 40 and week 52. We hypothesized that continuing the standard dose of CZP or reducing dosage frequency would be superior to withdrawal of CZP treatment in maintaining clinical response over an additional 52 weeks.
PATIENTS AND METHODS
Full details of patient eligibility and inclusion/exclusion criteria for the C‐EARLY period 1 study (NCT01519791) have been reported previously 9. Briefly, eligible patients for the C‐EARLY period 1 study had active RA according to the 2010 ACR/EULAR classification criteria 11, were DMARD‐naive with early disease (diagnosis <1 year prior to randomization, with 76% of patients randomized within 4 months of their diagnosis), and had poor prognostic factors for severe disease progression (positive at screening for rheumatoid factor or anti–citrullinated protein antibody). Use of intraarticular, intramuscular, or intravenous corticosteroids at any dose was prohibited in the C‐EARLY study. Patients who had used intraarticular corticosteroids within 28 days of baseline (in C‐EARLY period 1) were excluded from study enrollment. The maximum allowed dose of oral corticosteroids during the study was ≤10 mg/day prednisone or equivalent, with changes in dose allowed only between weeks 4 and 14 and between weeks 24 and 34 in period 1 of the study. Therefore, patients taking oral corticosteroids were to maintain their dosage during period 2.
Patients in C‐EARLY period 1 who had achieved sustained low disease activity at weeks 40 and 52 (a key secondary end point of period 1) after 52 weeks of CZP plus optimized MTX treatment were eligible for enrollment into C‐EARLY period 2. Sustained low disease activity was chosen as the entry criterion for period 2 as a clinically relevant criterion that can be used to identify patients who are proven responders to treatment.
Study design
C‐EARLY period 2 was a phase III, multicenter, double‐blind, placebo‐controlled, randomized study conducted in Europe, Australia, North America, and Latin America at 103 participating sites (of the 181 that participated in C‐EARLY period 1) from February 2013 to July 2015. Eligible patients from period 1 treated with 200 mg CZP every 2 weeks plus MTX were re‐randomized at week 52 at a ratio of 2:3:2 to receive the standard CZP dose of 200 mg every 2 weeks plus MTX (CZP standard group), the reduced frequency regimen of 200 mg CZP every 4 weeks plus MTX (reduced frequency group), or CZP stopped (placebo every 2 weeks plus MTX) (CZP stopped group) (Figure 1). Re‐randomization at week 52 was performed centrally using an Interactive Voice/Web Response System (IXRS) and stratified by time since RA diagnosis at baseline (≤4 months or >4 months) and week 52 sustained remission status. Week 0 (period 1) was considered the study baseline. Placebo plus MTX–treated patients from period 1 who had sustained low disease activity at both week 40 and week 52 entered period 2 as a separate group (“MTX responder” group), continued to receive placebo plus MTX only, and were not re‐randomized. These patients were analyzed post hoc as a descriptive arm only.
Figure 1.
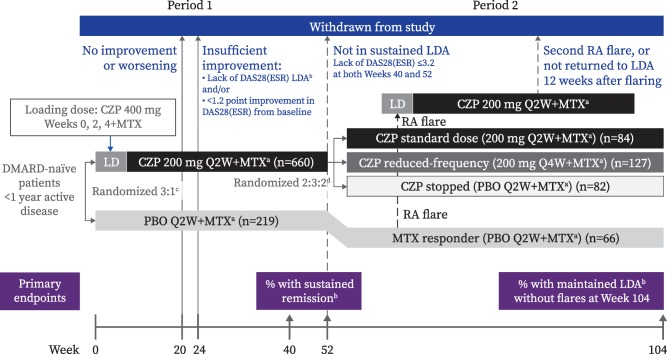
C‐EARLY study design, showing set of enrolled patients. a = optimized methotrexate (MTX), defined as dose titrated from 10 mg/week (week 0) and increasing by 5 mg every 2 weeks (Q2W) to a maximum dose of 25 mg/week by weeks 6–8. Patients unable to tolerate ≥15 mg/week by week 8 were withdrawn from the study. b = low disease activity (LDA), defined as a Disease Activity Score in 28 joints using the erythrocyte sedimentation rate (DAS28‐ESR) of ≤3.2. Low disease activity was considered to be maintained if continued throughout weeks 52–104 without disease flares. Patients reporting a flare had to meet the following 3 criteria at 2 consecutive visits 2 weeks apart: 1) an increase in the DAS28‐ESR of ≥0.6 above the DAS28‐ESR at week 52; 2) a DAS28‐ESR of >3.2; and 3) in the investigator's judgment, an increase in the patient's rheumatoid arthritis (RA) activity. Sustained remission was defined as a DAS28‐ESR of <2.6 at both week 40 and week 52. c = randomization stratified by time since RA diagnosis at baseline (≤4 months or >4 months). d = randomization stratified by time since RA diagnosis at baseline and by sustained remission status at week 52. CZP = certolizumab pegol; LD = loading dose; DMARD = disease‐modifying antirheumatic drug; PBO = placebo; Q4W = every 4 weeks. See Patients and Methods for descriptions of groups.
The primary efficacy end point was the proportion of patients with sustained low disease activity (DAS28‐ESR of ≤3.2 at both week 40 and week 52) who maintained low disease activity (DAS28‐ESR of ≤3.2) for all 5 consecutive study visits to week 104 without flares. The key secondary end point was the proportion of patients with sustained remission (DAS28‐ESR of <2.6 at both week 40 and week 52) who maintained remission (DAS28‐ESR of <2.6) for all 5 consecutive study visits to week 104 without flares. Additional secondary end points included the overall proportions of patients with low disease activity (DAS28‐ESR of ≤3.2), remission (DAS28‐ESR of <2.6), and normative physical function (defined as a Health Assessment Questionnaire disability index [HAQ DI] score 12 of ≤0.5), as well as the proportion of patients achieving radiographic nonprogression during period 2 (defined as a change in the modified Sharp/van der Heijde score [SHS] 13 of ≤0.5 from week 52 to week 104) and over the 2 years of the study (defined as a change from baseline in the SHS of ≤0.5 at week 104).
Time to flare was also explored as a secondary end point. An exploratory end point was the proportion of patients with disease flares who underwent reinduction with CZP treatment and subsequently re‐achieved low disease activity. In this study, disease flares had to be self‐reported by the patient at a study visit; solicitation by the study investigators was not mandated by the protocol. In addition, patients reporting a flare also had to meet the following 3 criteria at 2 consecutive visits 2 weeks apart: 1) an increase in the DAS28‐ESR of ≥0.6 above the DAS28‐ESR at week 52; 2) a DAS28‐ESR of >3.2; and 3) in the investigator's judgment, an increase in the patient's RA activity. In the event of a confirmed disease flare, patients received a loading dose of CZP (400 mg at 3 subsequent visits) followed by the standard dose (200 mg every 2 weeks) until the end of the study. Patients who experienced flares twice or who did not re‐achieve low disease activity within 12 weeks after reintroduction of CZP were withdrawn from the study.
Study drug
Placebo was supplied as 0.9% saline, and CZP was supplied as a 200 mg solution. Both were in prefilled syringes for subcutaneous injection and were administered up to week 102. Additional details regarding the initiating CZP dose have been described previously 9.
Oral MTX was dose‐optimized throughout the study. MTX was initiated during period 1 (9) at 10 mg/week and escalated by 5 mg every 2 weeks to a maximum of 25 mg/week (with a minimum of 15 mg/week) by week 8, if tolerated. This maximum tolerated (“optimized”) dose was maintained and continued to be administered every week from week 52 to week 103.
Study procedures and evaluations
In period 2, patients were assessed every 8–12 weeks at weeks 52, 64, 76, 84, 92, and 104. All study personnel were blinded with regard to treatment, except for a separate group who supervised and administered the study medication and determined ESR but who otherwise had no involvement in the study. Safety analyses included all treatment‐emergent adverse events (AEs), serious AEs (SAEs), and severe treatment‐emergent AEs, as well as clinical laboratory measurements.
Ethical approval
This study was conducted in accordance with the current version of the applicable regulatory and International Conference on Harmonisation Guidelines for Good Clinical Practice, the ethical principles that have their origin in the principles of the Declaration of Helsinki, and the local laws of the countries involved.
Statistical analysis
Based on a priori assumptions of response during period 1, 455 CZP‐treated patients from period 1 were expected to be eligible for period 2, with 130, 195, and 130 patients randomly assigned to the CZP standard, reduced frequency, and CZP stopped groups, respectively. Given this sample size, power was calculated to be 99% and 92% to detect a statistically significant difference in maintaining low disease activity between the CZP standard and reduced frequency groups, respectively, compared with the CZP stopped group at a given α = 0.05. Response rates of 95%, 90%, and 75% were assumed for the CZP standard, reduced frequency, and CZP stopped groups, respectively.
Statistical testing for the primary and key secondary end points was performed in a hierarchical manner. CZP standard versus CZP stopped was tested first. If the difference was significant at the α = 0.05 level, then reduced frequency versus CZP stopped was tested. The full analysis set (those with sustained low disease activity at both week 40 and week 52 [per the IXRS] with valid post–week 52 efficacy measurements for DAS28‐ESR) was used for all efficacy data except for radiographic data, which used the radiographic data set (those with valid radiographs at baseline, week 52, and week 104/withdrawal visit). Where reported in radiographic analyses, the MTX responder patients treated with placebo plus MTX throughout the study fulfilled the same criteria for the radiographic data set. The safety set comprised all patients who received at least 1 dose of study medication, and was used for the safety data.
A logistic regression model with factors for treatment, region, time since RA diagnosis at baseline, and week 52 sustained remission status was used for the primary and key secondary end points, as well as for the proportions of patients achieving low disease activity, remission, normative physical function, and radiographic nonprogression during period 2. The Clopper‐Pearson method was used to generate 95% CIs for the logistic regression models.
Missing data from patients who entered period 2 but withdrew before the end of the study were imputed using nonresponder imputation for the primary and key secondary end points. Radiographic analyses used linear extrapolation. In post hoc analyses, last observation carried forward (LOCF) imputation was used for the proportions of patients achieving low disease activity, remission, and normative physical function.
RESULTS
Patient disposition and baseline characteristics
A total of 293 CZP‐treated patients from period 1 were re‐randomized at week 52 and enrolled in this study, with 84 patients in the CZP standard group, 127 patients in the reduced frequency group, and 82 patients in the CZP stopped group. These patients represent 44% of the 660 CZP‐treated patients in period 1. However, this was 36% fewer than the planned randomizations of 130, 195, and 130 patients, respectively, due to fewer patients than projected with sustained low disease activity at the end of period 1. Of those who were re‐randomized, 84, 126, and 79 patients comprised the full analysis set; 72, 113, and 75 patients comprised the radiographic set (see Supplementary Figure 1, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.40196/abstract).
In period 2, 14 of 84 patients (16.7%) in the CZP standard group, 15 of 127 patients (11.8%) in the reduced frequency group, 10 of 82 patients (12.2%) in the CZP stopped group, and 7 of 66 MTX responder patients (10.6%) discontinued treatment by week 104. The most common reason for discontinuation was treatment‐emergent AEs, in 4 of 84 patients (4.8%), 8 of 127 patients (6.3%), 7 of 82 patients (8.5%), and 2 of 66 patients (3.0%), respectively (see Supplementary Figure 1, http://onlinelibrary.wiley.com/doi/10.1002/art.40196/abstract). Patient demographic characteristics were generally well balanced across all treatment groups (Table 1) except for sex, with a higher percentage of women in the CZP standard group (66 of 84 [78.6%]) than in the reduced frequency group (86 of 126 [68.3%]) and CZP stopped group (58 of 79 [73.4%]). Disease characteristics at both baseline and week 52 (Table 1; also see Supplementary Table 1, http://onlinelibrary.wiley.com/doi/10.1002/art.40196/abstract) were also reasonably well balanced across all groups. The mean weekly optimized MTX dose was similar across all groups (mean 20–22 mg/week), with a maximum of 25 mg/week.
Table 1.
Demographic and clinical characteristics of patients enrolled in C‐EARLY period 2 at study baseline (entry to period 1) and week 52a
|
CZP standard dose (n = 84)b |
CZP reduced frequency (n = 126)b |
CZP stopped (n = 79)b |
MTX responders (n = 66)c |
|
|---|---|---|---|---|
| Baseline demographics | ||||
| Age, mean ± SD years | 49.1 ± 13.1 | 49.2 ± 12.5 | 47.6 ± 14.0 | 51.2 ± 13.7 |
| Women, no. (%) | 66 (78.6) | 86 (68.3) | 58 (73.4) | 54 (81.8) |
| BMI, mean ± SD kg/m2 | 28 ± 5.6d | 27 ± 6.0 | 26 ± 4.5 | 28 ± 5.0 |
| Time since RA diagnosis, mean ± SD months | 2.5 ± 2.5 | 2.6 ± 2.8 | 2.9 ± 3.1 | 2.7 ± 2.8 |
| Region, no. (%) | ||||
| Europe and Australia | 57 (67.9) | 85 (67.5) | 59 (74.7) | 42 (63.6) |
| Latin America and North America | 27 (32.1) | 41 (32.5) | 20 (25.3) | 24 (36.4) |
| Patient characteristics | ||||
| Oral corticosteroids, mean ± SD/median (range) mg/day | ||||
| Baseline | 1.9 ± 3.5/0 (0–10.0) | 1.6 ± 3.2/0 (0–10.0) | 2.0 ± 3.5/0 (0–10.0) | 1.4 ± 3.0/0 (0–10.0) |
| Taking oral corticosteroids, no. (%) | 22 (26.2) | 30 (23.8) | 22 (27.8) | 13 (19.7) |
| Week 52 | 1.3 ± 3.1/0 (0–10.0) | 1.3 ± 2.9/0 (0–10.0) | 1.5 ± 3.1/0 (0–10.0) | 1.0 ± 2.5/0 (0–10.0) |
| Taking oral corticosteroids, no. (%) | 13 (15.5) | 25 (19.8) | 18 (22.8) | 11 (16.7) |
| Oral MTX dose, mean ± SD/median (range) mg/weekc | 21.3 ± 4.3/24.1 (10.5–25.0)d | 20.3 ± 4.4/20.0 (10.0–25.0) | 20.9 ± 4.5/20.0 (10.0–25.0)e | 22.0 ± 3.9/25.0 (15.0–25.0) |
| Concomitant use of NSAIDs, no. (%)c | 52 (62.7)d | 77 (60.6)f | 46 (56.8)e | 43 (65.2) |
| RF positive (≥14 IU/ml) at baseline, no. (%) | 82 (97.6) | 120 (95.2) | 79 (100.0) | 63 (95.5) |
| ACPA positive (≥7 IU/ml) at baseline, no. (%) | 77 (91.7) | 112 (88.9) | 68 (86.1) | 59 (89.4) |
| TJC28, mean ± SD | ||||
| Baseline | 13.0 ± 6.0 | 15.4 ± 5.7 | 14.1 ± 6.3 | 15.5 ± 6.6 |
| Week 52 | 0.4 ± 0.7d | 0.7 ± 1.3 | 0.4 ± 0.8 | 0.6 ± 1.3g |
| SJC28, mean ± SD | ||||
| Baseline | 11.3 ± 5.3 | 12.4 ± 5.1 | 11.3 ± 4.8 | 11.9 ± 4.8 |
| Week 52 | 0.3 ± 1.2d | 0.5 ± 1.4 | 0.3 ± 0.7 | 0.7 ± 1.6g |
| ESR, mean ± SD mm/hour | ||||
| Baseline | 47.9 ± 23.6 | 46.6 ± 21.7 | 46.1 ± 19.3 | 43.5 ± 18.6 |
| Week 52 | 15.1 ± 13.2d | 12.3 ± 8.4 | 13.2 ± 9.4 | 15.7 ± 11.0g |
| High‐sensitivity CRP, mean ± SD/median (range) mg/liter | ||||
| Baseline | 20.8 ± 23.8/11.9 (0.2–131.9) | 21.0 ± 30.0/8.6 (0.2–171.0) | 17.3 ± 25.6/7.9 (0.4–156.7) | 16.0 ± 20.6/8.0 (0.3–97.2) |
| Week 52 | 3.1 ± 5.7/1.6 (0.2–41.3)d | 2.5 ± 4.7/1.3 (0.2–43.4) | 3.6 ± 9.5/1.6 (0.2–75.2) | 4.0 ± 7.2/1.6 (0.2–50.4)g |
| DAS28‐ESR, mean ± SD | ||||
| Baseline | 6.4 ± 1.0 | 6.6 ± 0.8 | 6.5 ± 0.8 | 6.6 ± 0.9 |
| Week 52 | 2.0 ± 0.7d | 2.0 ± 0.6 | 1.9 ± 0.7 | 2.2 ± 0.7g |
| SHS, mean ± SD/median (range) | ||||
| Baseline | 3.1 ± 5.4/1 (0–34) | 4.5 ± 9.2/1.5 (0–64) | 5.1 ± 8.5/1.5 (0–40) | 6.0 ± 12.4/2.0 (0–70) |
| Week 52 | 3.3 ± 5.1/1.8 (0–34) | 4.5 ± 8.9/1.5 (0–64)h | 5.0 ± 7.4/2.5 (0–38) | 6.8 ± 12.7/2.5 (0–70) |
| HAQ DI score, mean ± SD/median (range) | ||||
| Baseline | 1.6 ± 0.6/1.6 (0.0–2.9) | 1.6 ± 0.6/1.6 (0.1–3.0) | 1.5 ± 0.5/1.5 (0.4–2.5) | 1.5 ± 0.6/1.6 (0.0–2.6) |
| Week 52 | 0.3 ± 0.4/0.1 (0.0–1.6)d | 0.3 ± 0.5/0.1 (0.0–2.0) | 0.3 ± 0.5/0.1 (0.0–1.6) | 0.4 ± 0.5/0.1 (0.0–1.4)g |
Nonsteroidal antiinflammatory drugs (NSAIDs) were defined by the preferred term M01A from the World Health Organization Drug Dictionary. CZP = certolizumab pegol; MTX = methotrexate; BMI = body mass index; RA = rheumatoid arthritis; RF = rheumatoid factor; ACPA = anti–citrullinated protein antibody; TJC28 = tender joint count in 28 joints; SJC28 = swollen joint count in 28 joints; ESR = erythrocyte sedimentation rate; CRP = C‐reactive protein; DAS28‐ESR = Disease Activity Score in 28 joints using the ESR; SHS = modified Sharp/van der Heijde score; HAQ DI = Health Assessment Questionnaire disability index.
Full analysis set.
Safety set.
n = 83.
n = 81.
n = 127.
n = 65.
n = 123.
Efficacy
At week 104, 41 of 84 patients (48.8%) in the CZP standard group and 67 of 126 patients (53.2%) in the reduced frequency group had maintained low disease activity without flares, compared with 31 of 79 patients (39.2%) in the CZP stopped group (P = 0.112 and P = 0.041, respectively; nominal P value due to lack of significance in first hierarchical test). Of patients with sustained remission, 22 of 50 (44.0%) in the CZP standard group and 36 of 83 (43.4%) in the reduced frequency group maintained remission to week 104 without flares, compared with 17 of 51 patients (33.3%) in the CZP stopped group (P = 0.274 and P = 0.253, respectively) (Figure 2A).
Figure 2.
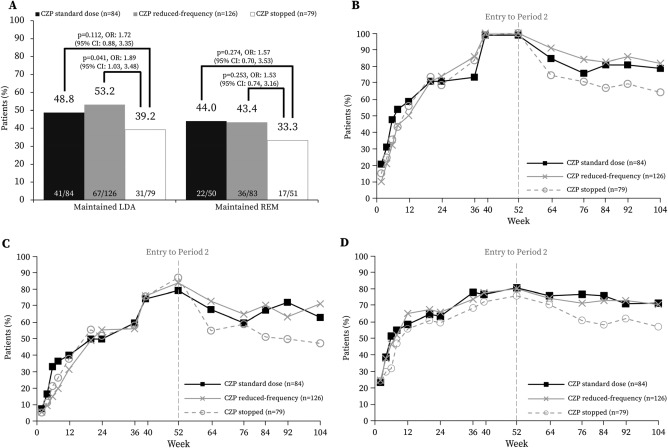
Clinical response in period 2 of the C‐EARLY study, showing the full analysis set. A, Proportion of patients in whom low disease activity (LDA) was maintained, and proportion in whom remission (REM) was maintained, at week 104. Odds ratios (ORs) with 95% confidence intervals (95% CIs) and corresponding P values are from a logistic regression model with factors for treatment, region, time since rheumatoid arthritis (RA) diagnosis, and sustained remission status at week 52. Only patients with disease in sustained remission at week 52 were included in the analysis for maintained remission at week 104. Nonresponder imputation was used for missing data. B, Proportion of patients with low disease activity, defined as a Disease Activity Score in 28 joints using the erythrocyte sedimentation rate (DAS28‐ESR) of ≤3.2. C, Proportion of patients with disease in remission, defined as a DAS28‐ESR of <2.6. D, Proportion of patients with normative physical function, defined as a Health Assessment Questionnaire disability index score of ≤0.5. B–D show results of post hoc analyses. The last observation carried forward approach was used to impute missing data. CZP = certolizumab pegol. See Patients and Methods for descriptions of groups.
Despite the failure to achieve the primary end point, the overall data demonstrate that patients continuing CZP at either the standard or reduced frequency doses were better able to maintain clinical response compared with those who stopped CZP, as illustrated by low disease activity, remission, and normative physical function. In post hoc analyses using LOCF imputation, higher proportions of patients in the CZP standard and reduced frequency groups achieved low disease activity (66 of 84 [78.6%] and 103 of 126 [81.7%], respectively), remission (53 of 84 [63.1%] and 90 of 126 [71.4%], respectively), and normative physical function (60 of 84 [71.4%] and 89 of 126 [70.6%], respectively) at week 104 compared with those who stopped CZP (50 of 78 [64.1%; 1 patient's data missing] achieved low disease activity, 37 of 78 [47.4%; 1 patient's data missing] achieved remission, and 45 of 79 [57.0%] achieved normative physical function) (Figures 2B–D). Furthermore, mean DAS28‐ESR and HAQ DI score values were also numerically lower throughout period 2 for patients continuing CZP treatment compared with those who stopped (see Supplementary Figure 2, http://onlinelibrary.wiley.com/doi/10.1002/art.40196/abstract).
Few patients exhibited radiographic progression in period 2 (change in SHS of >0.5 from week 52); these included 7 of 72 patients (9.7%) in the CZP standard group and 18 of 113 patients (15.9%) in the reduced frequency group, compared with 14 of 74 patients (18.9%) in the CZP stopped group. Over the 2 years of the study, patients continuing CZP treatment (in the CZP standard and reduced frequency groups) exhibited stabilization of structural damage, with numerically fewer patients (15 of 72 [20.8%] and 25 of 113 [22.1%], respectively) experiencing radiographic progression at week 104 (change in SHS of >0.5 from baseline) in comparison with patients who stopped CZP (22 of 74 [29.7%]) after week 52 (Figure 3).
Figure 3.
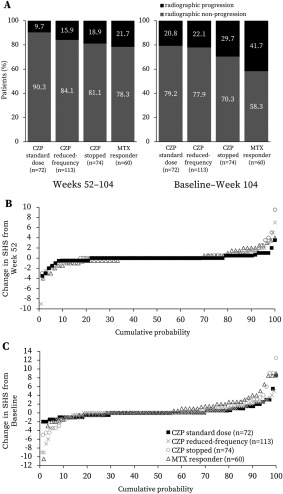
Radiographic progression in the C‐EARLY study. Shown is the radiographic data set with methotrexate (MTX) responder patients meeting the same criteria (those who had valid radiographs at baseline, week 52, and week 104/withdrawal visit). A, Radiographic progression and nonprogression rates. B, Cumulative probability of change in modified Sharp/van der Heijde score (SHS) from week 52 to week 104. C, Cumulative probability of change in SHS from baseline to week 104. Results for MTX responder patients were obtained using post hoc analyses. Radiographic nonprogression was defined as a change in the SHS of ≤0.5 from baseline or week 52. Radiographic progression was defined as a change in the SHS of >0.5 from baseline or week 52. One outlier in the certolizumab pegol (CZP) stopped group was excluded. Linear extrapolation was used for missing data. Symbols in B and C represent values for each patient. See Patients and Methods for descriptions of groups.
Relatively few disease flares were recorded in patients during this study, which was possibly due to the limitation of the patients having to self‐report flares to the investigator. Patients self‐reporting flares included 7 of 84 (8.3%) in the CZP standard group, 3 of 126 (2.4%) in the reduced frequency group, and 10 of 79 (12.7%) in the CZP stopped group. Most flares occurred in these patients by week 64 (i.e., 12 weeks after the change in treatment dosing) (Figure 4). Patients with flares underwent reinduction with the standard dose of CZP (including a loading dose), and 80% of them (16 of 20; 7 in the CZP standard group, 1 in the reduced frequency group, and 8 in the CZP stopped group) subsequently achieved low disease activity again within 12 weeks.
Figure 4.
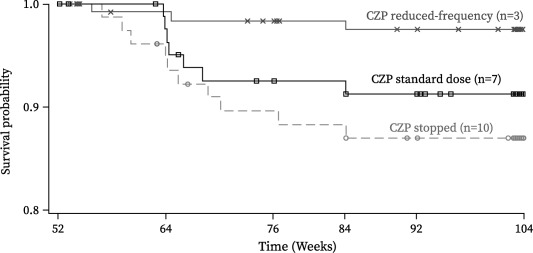
Kaplan‐Meier plot of time to rheumatoid arthritis (RA) disease flare, showing the full analysis set. Disease flares had to be self‐reported by the patient to the investigator at a study visit; solicitation by the study investigators was not mandated by the protocol. Patients reporting a flare had to meet the following 3 criteria at 2 consecutive visits 2 weeks apart: 1) an increase in the Disease Activity Score in 28 joints using the erythrocyte sedimentation rate (DAS28‐ESR) of ≥0.6 above the DAS28‐ESR at week 52; 2) a DAS28‐ESR of >3.2; and 3) in the investigator's judgment, an increase in the patient's RA activity. Time to flare was defined as the time from the date of the week 52 injection of study medication to the date of the first flare visit for a confirmed flare. Patients who did not have a flare were censored at the date of the latest assessment of the DAS28‐ESR. CZP = certolizumab pegol. See Patients and Methods for descriptions of groups.
MTX responders
Compared with the 44% of patients (293 of 660) in whom CZP treatment was initiated, a smaller proportion of patients who were only started on optimized MTX achieved sustained low disease activity at both week 40 and week 52 and continued to period 2, representing 30% (66 of 219) of the patients treated with placebo plus MTX. Sixty patients were included in the radiographic analyses as a separate group. The safety set included 83 patients in the CZP standard group, 127 in the reduced frequency group, 81 in the CZP stopped group, and 66 MTX responder patients.
In this group of patients with an enhanced response to MTX therapy, exploratory, descriptive post hoc analyses were performed with LOCF imputation. At week 104, 81.3% (52 of 64; 2 patients' data missing), 59.4% (38 of 64; 2 patients' data missing), and 59.4% (38 of 64; 2 patients' data missing) of these patients had achieved good clinical response—low disease activity, remission, and normative physical function, respectively (see Supplementary Figure 3, http://onlinelibrary.wiley.com/doi/10.1002/art.40196/abstract). However, a higher proportion of MTX responders experienced radiographic progression over the 2 years of the study (41.7% [25 of 60]) compared with the 3 groups initially treated with CZP (Figure 3).
Safety
The incidence of treatment‐emergent AEs in period 2 was similar across all 3 re‐randomized groups: 63.9% of patients (53 of 83) in the CZP standard group, 63.8% (81 of 127) in the reduced frequency group, and 59.3% (48 of 81) in the CZP stopped group experienced treatment‐emergent AEs, with a lower rate observed in the MTX responder group (50.0% [33 of 66]). Infections were the most frequent treatment‐emergent AEs, with a higher proportion of patients continuing CZP treatment reporting infections (31.3% [26 of 83] in the CZP standard group and 38.6% [49 of 127] in the reduced frequency group), compared with 27.2% (22 of 81) of those who stopped CZP and 15.2% (10 of 66) of MTX responders. Serious infections and infestations were experienced by 1.2% of patients (1 of 83) in the CZP standard group, 0.8% (1 of 127) in the reduced frequency group, 2.5% (2 of 81) in the CZP stopped group, and 1.5% (1 of 66) of MTX responders. For patients exposed to CZP treatment at any time in period 2 (patients in the CZP standard and reduced frequency groups and any patients who received CZP after disease flares), the incidence rate of infections was 45.1 per 100 patient‐years. The rate of SAEs was similar between all groups (4.8% of patients [4 of 83] in the CZP standard group, 7.1% [9 of 127] in the reduced frequency group, 7.4% [6 of 81] in the CZP stopped group, and 6.1% [4 of 66] of MTX responders).
There were higher rates of drug‐related treatment‐emergent AEs in the CZP‐treated patients: 30.1% of patients (25 of 83) in the CZP standard group and 23.6% (30 of 127) in the reduced frequency group, compared with 17.3% (14 of 81) in the CZP stopped group and 15.2% (10 of 66) of MTX responders. Discontinuations due to treatment‐emergent AEs followed a similar pattern, being observed in 2.4% of patients (2 of 83) in the CZP standard group and 5.5% (7 of 127) in the reduced frequency group, compared with 4.9% (4 of 81) in the CZP stopped group and 1.5% (1 of 66) of MTX responders.
Continuing treatment at the standard dose was not associated with an increased risk of malignancies compared with that in the reduced frequency or CZP stopped groups. The overall incidence of malignant tumors was low, with 2.4% of patients (3 of 127) in the reduced frequency group (1 with breast cancer, 1 with lip squamous cell carcinoma, and 1 with myxoid liposarcoma) and 2.5% of patients (2 of 81) in the CZP stopped group (both with prostate cancer) reporting any malignant tumors. No patients developed malignancies in the CZP standard or MTX responder groups (Table 2), nor were there any reports of hematologic reactions or demyelinating disorders. No deaths were reported in the re‐randomized treatment groups. There was 1 death due to cardiac failure in the MTX responder group 11 days after the final dose of study medication. The event was not considered to be related to study medication. The safety profile of MTX responder patients was comparable to that of the patients who were re‐randomized.
Table 2.
Summary of AEsa
| CZP standard dose (n = 83), no. (%) | CZP reduced frequency (n = 127), no. (%) | CZP stopped (n = 81), no. (%) | MTX responders (n = 66), no. (%) | CZP at any time (n = 223), no. (%)/event rateb | |
|---|---|---|---|---|---|
| Any treatment‐emergent AEs (≥5% in any system organ class) | 53 (63.9) | 81 (63.8) | 48 (59.3) | 33 (50.0) | 145 (65.0)/163.5 |
| Gastrointestinal disorders | 5 (6.0) | 6 (4.7) | 8 (9.9) | 7 (10.6) | 13 (5.8)/6.9 |
| General disorders and administration site conditions | 4 (4.8) | 10 (7.9) | 4 (4.9) | 5 (7.6) | 16 (7.2)/6.9 |
| Infections and infestations | 26 (31.3) | 49 (38.6) | 22 (27.2) | 10 (15.2) | 82 (36.8)/57.8 |
| Injury, poisoning, and procedural complications | 5 (6.0) | 14 (11.0) | 7 (8.6) | 1 (1.5) | 20 (9.0)/9.0 |
| Investigations | 11 (13.3) | 12 (9.4) | 8 (9.9) | 8 (12.1) | 23 (10.3)/13.3 |
| Metabolism and nutrition disorders | 3 (3.6) | 14 (11.0) | 6 (7.4) | 5 (7.6) | 17 (7.6)/9.0 |
| Musculoskeletal and connective tissue disorders | 8 (9.6) | 12 (9.4) | 12 (14.8) | 6 (9.1) | 23 (10.3)/12.0 |
| Nervous system disorders | 6 (7.2) | 7 (5.5) | 1 (1.2) | 1 (1.5) | 15 (6.7)/14.1 |
| Renal and urinary disorders | 5 (6.0) | 2 (1.6) | 0 | 1 (1.5) | 8 (3.6)/3.9 |
| Respiratory, thoracic, and mediastinal disorders | 5 (6.0) | 8 (6.3) | 3 (3.7) | 1 (1.5) | 13 (5.8)/6.9 |
| Skin and subcutaneous tissue disorders | 8 (9.6) | 9 (7.1) | 3 (3.7) | 2 (3.0) | 17 (7.6)/8.6 |
| Any malignant tumor | 0 | 3 (2.4) | 2 (2.5) | 0 | 4 (1.8)/1.7 |
| Serious treatment‐emergent AEs | 4 (4.8) | 9 (7.1) | 6 (7.4) | 4 (6.1) | 16 (7.2)/6.9 |
| Serious infections and infestations | 1 (1.2) | 1 (0.8) | 2 (2.5) | 1 (1.5) | 2 (0.9)/0.9 |
| Discontinuation due to treatment‐emergent AEs | 2 (2.4) | 7 (5.5) | 4 (4.9) | 1 (1.5) | 10 (4.5)/NA |
| Treatment‐emergent AEs requiring MTX reduction | 0 | 5 (3.9) | 1 (1.2) | 1 (1.5) | NA |
| Drug‐related treatment‐emergent AEs | 25 (30.1) | 30 (23.6) | 14 (17.3) | 10 (15.2) | 60 (26.9)/NA |
| Severe treatment‐emergent AEs | 1 (1.2) | 4 (3.1) | 2 (2.5) | 3 (4.5) | 5 (2.2)/NA |
| Deaths (treatment‐emergent AEs leading to death) | 0 | 0 | 0 | 1 (1.5) | 0 |
Safety set, comprising all patients who received at least 1 dose of study medication. Terms are from Medical Dictionary for Regulatory Activities, version 17.0. NA = not available.
Includes adverse events (AEs) occurring in period 2 in any group after receiving certolizumab pegol (CZP) in period 2, including treatment‐emergent AEs occurring after induction/reinduction with CZP for patients in the methotrexate (MTX) responder and CZP stopped groups. The total number of patients exposed to CZP in period 2 was used as the denominator; event rates are per 100 patient‐years.
DISCUSSION
C‐EARLY is the first reported randomized, double‐blind study with an anti‐TNF biologic DMARD that compares the following 3 treatment strategies: continuation at full dose, tapering, or withdrawal of biologic therapy, in combination with optimized MTX, in maintaining low disease activity after 1 year of anti‐TNF treatment in DMARD‐naive patients with early and progressive, active RA. Previous studies, such as the Productivity and Remission in a Randomized Controlled Trial of Etanercept vs. Standard of Care in Early Rheumatoid Arthritis (PRIZE) study 14, compared the effect of tapering to a half dose (25 mg/week) of etanercept in combination with MTX with the effects of etanercept withdrawal (with MTX) and treatment with placebo alone. The Optimal Protocol for Methotrexate and Adalimumab Combination Therapy in Early Rheumatoid Arthritis (OPTIMA) study 15 compared the effect of continuing the full dose of adalimumab with the effects of biologic therapy withdrawal (with MTX) and MTX monotherapy.
The primary end point of the present study was not achieved. Fewer patients than projected from period 1 were eligible to enter period 2 (i.e., achieved sustained low disease activity), which may have resulted in an underpowered study. The use of sustained low disease activity at 2 consecutive study visits (both week 40 and week 52) as the entry criterion for period 2 was more difficult to achieve than a single‐visit criterion. The primary end point of period 2 was stringent, as patients were required to have maintained low disease activity throughout the entirety of the second year for 5 consecutive visits rather than just at a single study visit. The expected 20% and 15% differences between the CZP standard and reduced frequency groups versus the CZP stopped group in the maintenance of low disease activity to week 104 were also not realized. It should be noted that the study protocol design was undertaken in 2010, prior to the issuance of the 2011 ACR/EULAR guidelines 16, in which the Simplified Disease Activity Index 17 and Boolean‐based definitions of low disease activity and remission superseded DAS28‐ESR–based definitions as the closest reflection of “deep” disease control.
Nevertheless, results from period 2 of C‐EARLY may still be of value to rheumatologists and patients, and could inform care options, since the data suggest that after 1 year of CZP treatment at the standard dose (200 mg every 2 weeks plus MTX), continuing treatment for an additional year at a reduced frequency dosage (200 mg every 4 weeks plus MTX) provided control of disease activity comparable to continuing treatment at the standard dose. A numerically higher proportion of patients continuing CZP at both dosages maintained clinical response and improvements in physical function in comparison with patients who stopped CZP treatment after 1 year.
Unexpectedly, a slightly higher proportion of patients continuing treatment at the reduced frequency dose achieved low disease activity, remission, and normative physical function compared with patients continuing a standard dose of CZP. This could have been due to the smaller‐than‐expected sample size, which led to more variability in the results. However, the numerical differences between the CZP standard dose and reduced frequency groups are small and not considered clinically meaningful.
Treatment of patients very early in the course of RA is associated with inhibition of the progression of joint damage as well as with better clinical outcomes. The 52‐week data from C‐EARLY period 1 revealed that early initiation of CZP plus MTX significantly inhibited joint destruction compared with no CZP treatment 9. The results of exploratory analyses from period 2 suggest that early initiation of CZP treatment can provide better and continued protection against progression of structural damage over a 2‐year period, regardless of whether CZP was continued after 1 year. This difference was larger when compared with the descriptive arm of the MTX responders, even though those patients had a good clinical response with 2 years of therapy with optimized MTX alone. We note that our study was not powered to detect differences in radiographic progression rates; however, our findings are consistent with previous data from Japanese MTX‐naive patients with early RA in the Certolizumab‐Optimal Prevention of joint damage for Early RA (C‐OPERA) trial, in which patients who had been treated with CZP plus MTX for 1 year exhibited lower rates of radiographic progression for 1 additional year after stopping CZP, in comparison with patients who had been receiving 2 years of MTX monotherapy 7, 18. Similar results were also reported in DMARD‐naive patients started on adalimumab plus MTX in the High Induction Therapy with Anti‐Rheumatic Drugs (HIT HARD) study 19, although with a shorter (24‐week) treatment duration.
In addition to the clinical efficacy described above, early initiation of CZP plus MTX has demonstrated benefits over optimized MTX alone in patient‐reported measures such as workplace and household productivity 20. More patients who continued the standard or reduced frequency doses were able to maintain the initial 52‐week improvements up to week 104 in comparison with patients who stopped CZP 21.
MTX therapy in this study was optimized by week 8 (i.e., patients were given the maximum tolerated dose, between 15 mg and 25 mg) 9. To our knowledge, the Dutch U‐Act‐Early study 22 and our international C‐EARLY study are the only trials to mandate per‐protocol optimization of MTX to achieve high levels of MTX dosage in DMARD‐ and MTX‐naive patients with early RA. This optimization may in part explain the good clinical responses achieved by the MTX responders over the course of the 2 years.
There were low numbers of flares reported in this study, a limitation which was likely due to the requirement that flares be self‐reported by the patient. The physicians involved in this study did not have a systematic evaluation method and were not mandated to formally assess disease flares. Although DAS components were measured at each study visit, the investigator remained blinded with regard to the DAS score. The low number of flares is not reflected by the relatively high proportion of patients (∼50%) who did not maintain low disease activity. Of those who had disease flares, most (80%) achieved low disease activity again, and no safety issues were identified in those patients. However, because too few patients were identified as having a disease flare, no formal conclusions can be drawn about the safety and efficacy of reintroduction of the CZP standard dose in restoring low disease activity. Further evaluation of flares among the patients in this study is warranted. No new safety signals were identified for an additional 52 weeks of CZP plus MTX treatment, and the safety profile was consistent between all groups, including the MTX responder patients, who had been treated with placebo plus optimized MTX for 2 years and had never received CZP.
Overall, the data from the first 52 weeks of the C‐EARLY study demonstrated a favorable benefit‐to‐risk ratio for early initiation of CZP plus MTX therapy in DMARD‐naive patients within 1 year of receiving their diagnosis of active RA with poor prognostic factors for disease progression 9. Clinical improvements achieved by the early initiation of CZP therapy during period 1 of the study were maintained in numerically more patients who continued at the standard or reduced frequency doses, thus providing support for the “induction‐maintenance” approach for this specific population of patients with early RA who had never been exposed to either synthetic or biologic DMARDs. Exploratory analyses of radiographic data reported from period 2 suggested that early CZP initiation could be a better option for avoiding structural damage in this patient population compared with treatment with MTX alone, supporting the results reported from other studies 18. Further research would be needed to identify the characteristics of patients who would be able to stop CZP therapy successfully. By investigating the effect of continuing or tapering biologic DMARD therapy after reaching a disease target, our study contributes to the body of knowledge supporting the induction‐maintenance treatment approach, and our results inform the evolving paradigms of disease management in RA while also highlighting the need for further clinical trial evidence.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Weinblatt had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Weinblatt, Bingham, Burmester, Bykerk, Furst, Mariette, van der Heijde, van Vollenhoven, VanLunen, Ecoffet, Cioffi, Emery.
Acquisition of data
Weinblatt, Bingham, Burmester, Bykerk, Furst, Mariette, van der Heijde, van Vollenhoven, VanLunen, Ecoffet, Cioffi, Emery.
Analysis and interpretation of data
Weinblatt, Bingham, Burmester, Bykerk, Furst, Mariette, van der Heijde, van Vollenhoven, VanLunen, Ecoffet, Cioffi, Emery.
ROLE OF THE STUDY SPONSOR
UCB Pharma sponsored the study, and all costs associated with the development of the manuscript were funded by UCB Pharma, including the funding of medical writing and editorial assistance by Costello Medical Consulting Ltd. UCB Pharma performed a full review to ensure that the data presented in the publication are scientifically, technically, and medically supportable and in compliance with the intellectual property rights of UCB Pharma. Furthermore, UCB Pharma ensured that the publication complies with applicable laws, regulations, guidelines, and good industry practice.
Supporting information
Supplementary Figure 1. Patient disposition in the C‐EARLY Period 2 study. ES: Enrolled set; SS: Safety set; FAS: Full analysis set; RAD: radiographic set. aOther included protocol violations, as well as patients withdrawing due to flares (in Period 2).
Supplementary Figure 2. DAS28(ESR) and HAQ‐DI scores. A, Mean DAS28(ESR) over 104 weeks (LOCF). B, Mean HAQ‐DI over 104 weeks (LOCF). Full analysis set. These analyses were performed post hoc. LDA: DAS28(ESR) ≤3.2; REM: DAS28(ESR) <2.6; Normative physical function: HAQ‐DI ≤0.5.
Supplementary Figure 3. Clinical response in the MTX responder group (n=66). The MTX responder group is a separate group, and included for descriptive purposes only. These analyses were performed post hoc. LOCF imputation. LDA: DAS28(ESR) ≤3.2; REM: DAS28(ESR) <2.6; Normative physical function: HAQ‐DI ≤0.5.
Supplementary Table 1. Summary of patient characteristics at study baseline and Week 52.
ACKNOWLEDGMENTS
The authors thank the patients and their caregivers in addition to the investigators and their teams who contributed to this study. The authors acknowledge Simone E. Auteri, MSc (UCB Pharma, Brussels, Belgium) for publication coordination, and Lisa Wulund, PhD (Costello Medical Consulting Ltd, Cambridge, UK) for medical writing and editorial assistance in preparing the manuscript for publication based on the authors' input and direction.
ClinicalTrials.gov identifier: NCT01521923.
Supported by UCB Pharma.
Dr. Weinblatt has received consulting fees, speaking fees, and/or honoraria from AbbVie, Amgen, Bristol‐Myers Squibb, Roche (less than $10,000 each), Lilly, Pfizer, UCB Pharma, AstraZeneca, Merck, Novartis, Crescendo Bioscience, and MedImmune (more than $10,000 each). Dr. Bingham has received consulting fees, speaking fees, and/or honoraria from UCB Pharma (less than $10,000). Dr. Burmester has received consulting fees, speaking fees, and/or honoraria from AbbVie, Bristol‐Myers Squibb, Celgene, MSD, UCB Pharma, Roche, Pfizer, and Lilly (less than $10,000 each). Dr. Bykerk has received consulting fees, speaking fees, and/or honoraria from Pfizer, AbbVie, Bristol‐Myers Squibb, Sanofi, and UCB Pharma (less than $10,000 each). Dr. Furst has received consulting fees, speaking fees, and/or honoraria from Abbott, AbbVie, Amgen, Biogen, Bristol‐Myers Squibb, Cytori, Gilead, GlaxoSmithKline, Janssen, NIH, Novartis, Pfizer, Roche/Genentech, and UCB Pharma (less than $10,000 each) and research grants from Abbott, Actelion, Amgen, Biogen, Bristol‐Myers Squibb, GlaxoSmithKline, Novartis, Pfizer, Roche/Genentech, and UCB Pharma. Dr. Mariette has received consulting fees, speaking fees, and/or honoraria from Bristol‐Myers Squibb, GlaxoSmithKline, Pfizer, and UCB Pharma (less than $10,000 each). Dr. van der Heijde has received consulting fees, speaking fees, and/or honoraria from AbbVie, Amgen, Astellas, AstraZeneca, Bristol‐Myers Squibb, Boehringer Ingelheim, Celgene, Daiichi, Eli Lilly and Company, Galapagos, Gilead, Janssen, Merck, Novartis, Pfizer, Regeneron, Roche, Sanofi, and UCB Pharma (less than $10,000 each). Dr. van Vollenhoven has received consulting fees and/or honoraria from AbbVie, Biotest, Bristol‐Myers Squibb, Celgene, Crescendo Bioscience, GlaxoSmithKline, Janssen, Lilly, Merck, Novartis, Pfizer, Roche, UCB Pharma, and Vertex (less than $10,000 each) and research grants from AbbVie, Amgen, Bristol‐Myers Squibb, GlaxoSmithKline, Pfizer, Roche, and UCB Pharma. Ms VanLunen and Drs. Ecoffet and Cioffi own stock or stock options in UCB Pharma. Dr. Emery has received consulting fees and/or honoraria from Pfizer, MSD, AbbVie, Bristol‐Myers Squibb, UCB Pharma, Roche, Novartis, Samsung, Sandoz, and Lilly (more than $10,000 each).
REFERENCES
- 1. Emamikia S, Arkema EV, Gyori N, Detert J, Chatzidionysiou K, Dougados M, et al. Induction maintenance with tumour necrosis factor‐inhibitor combination therapy with discontinuation versus methotrexate monotherapy in early rheumatoid arthritis: a systematic review and meta‐analysis of efficacy in randomised controlled trials. RMD Open 2016;2:e000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Singh JA, Saag KG, Bridges SL Jr, Akl EA, Bannuru RR, Sullivan MC, et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken) 2016;68:1–25. [DOI] [PubMed] [Google Scholar]
- 3. Van Vollenhoven RF, Nagy G, Tak PP. Early start and stop of biologics: has the time come? BMC Med 2014;12:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nagy G, van Vollenhoven RF. Sustained biologic‐free and drug‐free remission in rheumatoid arthritis, where are we now? Arthritis Res Ther 2015;17:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schett G, Emery P, Tanaka Y, Burmester G, Pisetsky DS, Naredo E, et al. Tapering biologic and conventional DMARD therapy in rheumatoid arthritis: current evidence and future directions. Ann Rheum Dis 2016;75:1428–37. [DOI] [PubMed] [Google Scholar]
- 6. Smolen JS, Landewe R, Bijlsma J, Burmester G, Chatzidionysiou K, Dougados M, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease‐modifying antirheumatic drugs: 2016 update. Ann Rheum Dis 2017;76:960–77. [DOI] [PubMed] [Google Scholar]
- 7. Atsumi T, Yamamoto K, Takeuchi T, Yamanaka H, Ishiguro N, Tanaka Y, et al. The first double‐blind, randomised, parallel‐group certolizumab pegol study in methotrexate‐naive early rheumatoid arthritis patients with poor prognostic factors, C‐OPERA, shows inhibition of radiographic progression. Ann Rheum Dis 2016;75:75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Smolen J, Landewe RB, Mease P, Brzezicki J, Mason D, Luijtens K, et al. Efficacy and safety of certolizumab pegol plus methotrexate in active rheumatoid arthritis: the RAPID 2 study. A randomised controlled trial. Ann Rheum Dis 2009;68:797–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Emery P, Bingham CO, Burmester GR, Bykerk VP, Furst DE, Mariette X, et al. Certolizumab pegol in combination with dose‐optimised methotrexate in DMARD‐naive patients with early, active rheumatoid arthritis with poor prognostic factors: 1‐year results from C‐EARLY, a randomised, double‐blind, placebo‐controlled phase III study. Ann Rheum Dis 2017;76:96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Prevoo ML, van't Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty‐eight–joint counts: development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 1995;38:44–8. [DOI] [PubMed] [Google Scholar]
- 11. Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO III, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010;62:2569–81. [DOI] [PubMed] [Google Scholar]
- 12. Fries JF, Spitz P, Kraines RG, Holman HR. Measurement of patient outcome in arthritis. Arthritis Rheum 1980;23:137–45. [DOI] [PubMed] [Google Scholar]
- 13. Van der Heijde DM, van Riel PL, Gribnau FW, Nuver‐Zwart IH, van de Putte LB. Effects of hydroxychloroquine and sulphasalazine on progression of joint damage in rheumatoid arthritis. Lancet 1989;1:1036–8. [DOI] [PubMed] [Google Scholar]
- 14. Emery P, Hammoudeh M, FitzGerald O, Combe B, Martin‐Mola E, Buch MH, et al. Sustained remission with etanercept tapering in early rheumatoid arthritis. N Engl J Med 2014;371:1781–92. [DOI] [PubMed] [Google Scholar]
- 15. Smolen JS, Emery P, Fleischmann R, van Vollenhoven RF, Pavelka K, Durez P, et al. Adjustment of therapy in rheumatoid arthritis on the basis of achievement of stable low disease activity with adalimumab plus methotrexate or methotrexate alone: the randomised controlled OPTIMA trial. Lancet 2014;383:321–32. [DOI] [PubMed] [Google Scholar]
- 16. Felson DT, Smolen JS, Wells G, Zhang B, van Tuyl LH, Funovits J, et al. American College of Rheumatology/European League Against Rheumatism provisional definition of remission in rheumatoid arthritis for clinical trials. Arthritis Rheum 2011;63:573–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Smolen JS, Breedveld FC, Schiff MH, Kalden JR, Emery P, Eberl G, et al. A Simplified Disease Activity Index for rheumatoid arthritis for use in clinical practice. Rheumatology (Oxford) 2003;42:244–57. [DOI] [PubMed] [Google Scholar]
- 18. Atsumi T, Tanaka Y, Yamamoto K, Takeuchi T, Yamanaka H, Ishiguro N, et al. Clinical benefit of 1‐year certolizumab pegol (CZP) add‐on therapy to methotrexate treatment in patients with early rheumatoid arthritis was observed following CZP discontinuation: 2‐year results of the C‐OPERA study, a phase III randomised trial. Ann Rheum Dis 2017;76:1348–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Detert J, Bastian H, Listing J, Weiss A, Wassenberg S, Liebhaber A, et al. Induction therapy with adalimumab plus methotrexate for 24 weeks followed by methotrexate monotherapy up to week 48 versus methotrexate therapy alone for DMARD‐naive patients with early rheumatoid arthritis: HIT HARD, an investigator‐initiated study. Ann Rheum Dis 2013;72:844–50. [DOI] [PubMed] [Google Scholar]
- 20. Emery P, Bingham CO, Bykerk VP, Furst DE, Mariette X, Purcaru O, et al. Improvements in patient‐reported outcomes and workplace and household productivity following 52 weeks of treatment with certolizumab pegol in combination with methotrexate in DMARD‐naïve early rheumatoid arthritis patients: results from the C‐EARLY randomized, double‐blind, controlled phase 3 study. Ann Rheum Dis 2015;74 Suppl 2:712. [Google Scholar]
- 21. Bingham CO, Emery P, Weinblatt M, Burmester GR, Furst DE, Mariette X, et al. Maintenance of improvements in workplace and household productivity and physical function at 2 years in early RA patients with severe progressive disease who achieved sustained low disease activity following 1 year of initial therapy, with two dosing frequencies of certolizumab pegol. Ann Rheum Dis 2016;75 Suppl 2:226. 25180292 [Google Scholar]
- 22. Bijlsma JW, Welsing PM, Woodworth TG, Middelink LM, Petho‐Schramm A, Bernasconi C, et al. Early rheumatoid arthritis treated with tocilizumab, methotrexate, or their combination (U‐Act‐Early): a multicentre, randomised, double‐blind, double‐dummy, strategy trial. Lancet 2016;388:343–55. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Patient disposition in the C‐EARLY Period 2 study. ES: Enrolled set; SS: Safety set; FAS: Full analysis set; RAD: radiographic set. aOther included protocol violations, as well as patients withdrawing due to flares (in Period 2).
Supplementary Figure 2. DAS28(ESR) and HAQ‐DI scores. A, Mean DAS28(ESR) over 104 weeks (LOCF). B, Mean HAQ‐DI over 104 weeks (LOCF). Full analysis set. These analyses were performed post hoc. LDA: DAS28(ESR) ≤3.2; REM: DAS28(ESR) <2.6; Normative physical function: HAQ‐DI ≤0.5.
Supplementary Figure 3. Clinical response in the MTX responder group (n=66). The MTX responder group is a separate group, and included for descriptive purposes only. These analyses were performed post hoc. LOCF imputation. LDA: DAS28(ESR) ≤3.2; REM: DAS28(ESR) <2.6; Normative physical function: HAQ‐DI ≤0.5.
Supplementary Table 1. Summary of patient characteristics at study baseline and Week 52.


