Abstract
Objectives
Clinical trials of spinal cord stimulation (SCS) have largely focused on conversion from trial to permanent SCS and the first years after implant. This study evaluates the association of type of SCS and patient characteristics with longer‐term therapy‐related explants.
Materials and Methods
Implanting centers in three European countries conducted a retrospective chart review of SCS systems implanted from 2010 to 2013. Ethics approval or waiver was obtained, and informed consent was not required. The chart review recorded implants, follow‐up visits, and date and reasons for any explants through mid‐2016. Results are presented using Cox regression to determine factors associated with explant for inadequate pain relief.
Results
Four implanting centers in three countries evaluated 955 implants, with 8720 visits over 2259 years of follow‐up. Median age was 53 years; 558 (58%) were female. Explant rate was 7.9% per year. Over half (94 of 180) of explants were for inadequate pain relief, including 32/462 (6.9%) of implants with conventional nonrechargeable SCS, 37/329 (11.2%) with conventional rechargeable and 22/155 (14.2%) with high‐frequency (10 kHz) rechargeable SCS. A higher explant rate was found in univariate regression for conventional rechargeable (HR 1.98, p = 0.005) and high‐frequency stimulation (HR 1.79, p = 0.035) than nonrechargeable SCS. After covariate adjustment, the elevated explant rate persisted for conventional rechargeable SCS (HR 1.95, p = 0.011), but was not significant for high‐frequency stimulation (HR 1.71, p = 0.069).
Conclusions
This international, real‐world study found higher explant rates for conventional rechargeable and high‐frequency SCS than nonrechargeable systems. The increased rate for conventional rechargeable stimulation persisted after covariate adjustment.
Keywords: Chronic pain, efficacy, explants, spinal cord stimulation, outcomes
Introduction
Attempts to provide safe and cost‐effective relief of chronic pain have unwittingly contributed to an epidemic of opioid overuse and abuse in the United States, with 2 million abusing or dependent on opioids and 15,000 deaths involving prescription opioids in 2015 1, 2, 3. Limited data from Europe suggest the problem of opioid abuse is less pronounced but still worthy of vigilance 4, 5. Long‐term opioid usage has little ability to improve function and is associated with work loss, physical dependence, addiction, and excess mortality 6, 7, 8, 9. In contrast, accumulating clinical evidence over decades of use has helped to establish spinal cord stimulation (SCS) as a safe, effective, programmable, and reversible therapy for many types of chronic pain 10, 11, 12, 13, 14.
The number of technologies for SCS has expanded to include rechargeable systems and paddle leads 15, 16, 17. Waveforms such as high‐frequency and burst stimulation may deliver pain relief with little or no paresthesia 18, 19, 20, 21. Additional emerging therapies include high‐density stimulation and direct stimulation of the dorsal root ganglion (DRG), and accumulating evidence can be used to help target therapy precisely to needs of the individual subject 22, 23, 24. The increasing number of therapy modalities highlights a need to validate clinical trial results with real‐world data. Registries are in development, but the only publications to date on long‐term outcomes of SCS are based on single‐center experience 25, 26, 27.
The implanting centers participating in this European chart review manage hundreds of SCS subjects every year. We undertook a multinational chart review to determine if there are any differences in rate of explant for the different types of SCS systems in use today. The chart review makes it possible to study explants as a function of type of SCS system as well as patient characteristics.
Materials and Methods
Study Design
A multinational retrospective chart review study was undertaken, including SCS systems implanted at four centers in three European countries from 2010 to 2013. Participating centers were AZ Nikolaas Hospital, Sint‐Niklaas, Belgium; Heinrich‐Heine University Hospital, Düsseldorf, Germany; Academic Medical Center, Amsterdam, and Diakonessenhuis Utrecht in the Netherlands. The period for implant dates was deliberately selected so that each implant could be followed for at least two years, exceeding the length of time of major clinical studies of SCS 10, 11, 19, 20. The chart review was performed in mid‐2016, implants at end of 2013 would have approximately two and one half years of follow‐up. In accordance with the Declaration of Helsinki, the study protocol was approved by a Medical Ethics Committee or a waiver was obtained, along with a confirmation that individual informed consent was not required for collection and analysis of the deidentified data.
The index event was implantation of any SCS implantable pulse generator (IPG) for the purpose of dorsal column stimulation from January 2010 to December 2013. Exclusions were for implants in subjects less than 18 years old; if the only therapy was Peripheral Nerve Stimulation (PNS), Peripheral Nerve Field Stimulation (PNfS), or Dorsal Root Ganglion (DRG) stimulation; or if precluded by another clinical study. A small number of implants were also excluded due to incomplete digital records. Events reviewed included implants and follow‐up visits with date and reason for any explants, revisions, lead additions, deactivations, and documented non‐use of SCS up to the point the chart review was conducted in mid‐2016.
Data Collection
Site personnel with access to patient data entered findings of the chart review, including dates, into an Excel workbook that generated a de‐identified version of the data free of any names, dates or other identifying information. A quality check on a subset of implants was performed by an additional person at each site, and compared to ensure accuracy and completeness of the chart review.
Data Analysis
The focus of this analysis is unanticipated explants related to SCS therapy. Unanticipated explants are defined as any removal of an IPG with the subject still alive, except cases of battery depletion or a decision to change to a different IPG with additional waveforms, MRI compatibility, or other lead configurations. Unanticipated explants were further categorized as due to inadequate pain relief, or from infection or wound dehiscence, pocket pain, IPG problem, lead problem, need for MRI, or subject becoming free from pain. Supporting Information Table S1 contains definitions for each of the outcomes.
Statistical Analysis
Deidentified data from all sites were merged and notes from the chart review were used to identify categories for indication, predominant pain location and reasons for explant. Outcomes were classified using the notes from the chart review, and then adjudicated by investigators from each site. Timing of each explant was plotted for visualization and qualitative assessment. Continuous variables are presented using median and quartiles; categorical variables with number and percentage. Crude rate of explant and 95% confidence interval are expressed as events per year of follow‐up. Incidence of explant was evaluated using the Kaplan‐Meier estimator and compared among subgroups with log‐rank test. Plots of the Kaplan‐Meier estimator were limited to the first five years after implant. A Cox proportional‐hazards regression model was used to determine factors associated with explant for ineffective therapy. Results of the regression were presented using univariate regression, as well as an adjusted multivariable model with independent variables for type of SCS, age, gender, indication, pain location, time with chronic pain and de novo vs. replacement IPG. Age at implant was included as a continuous variable in the regression, but plotted in Kaplan‐Meier curves in subgroups of those age 65 or older, corresponding to the U.S. Medicare age group, or less than 65 years old. Lead type and location were not used in the regression because of the potential for multicollinearity. In case of indication and pain location, groups with less than 50 implants were placed in an “other” category. SAS 9.4 (Cary, NC, USA) was used for statistical analysis.
Results
The chart review included four implanting centers in three countries. There were 822 unique subjects with 955 implants performed from January 2010 to December 2013 that satisfied all inclusion and exclusion criteria (Fig. 1). Exclusions were predominantly for neurostimulation systems that did not use dorsal column SCS, with fewer than 2% excluded for any other reason.
Figure 1.
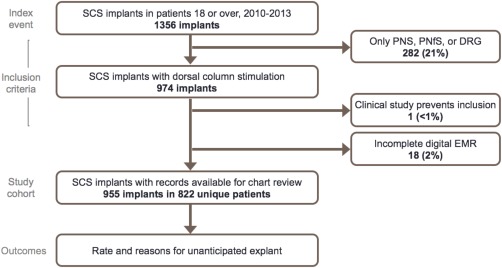
Consort diagram. [Color figure can be viewed at wileyonlinelibrary.com]
Median age at implant was 53 years; 558 (58%) were female. Table 1 contains a summary of basic characteristics at time of implant. There were 462 (48%) implants with conventional nonrechargeable SCS from any manufacturer, 329 (34%) with conventional rechargeable SCS, and 155 (16%) with high‐frequency (10 kHz) rechargeable stimulation. There were nine implants (1%) with another waveform or unknown stimulation type that were excluded from the regression analysis.
Table 1.
Demographics and Implant Characteristics. Continuous Variables Are Presented With Median and Quartiles [Q1–Q3]; Categorical Variables as Number and Percentage.
| Basic characteristic | All implants (N = 955) |
|---|---|
| Age in years at implant | 53 [45–63] range 18–87 |
| Female | 558 (58%) |
| Male | 397 (42%) |
| Indications for SCS | |
| Prior spine surgery (FBSS/FNSS) | 700 (73%) |
| CRPS type I or II | 60 (6%) |
| Peripheral neuropathy | 130 (14%) |
| Neuritis/Radiculitis | 17 (2%) |
| Lumbosacral neuropathy with no prior surgery | 20 (2%) |
| Radiculopathies | 42 (4%) |
| Angina pectoris | 25 (3%) |
| Abdominal, pelvic or cancer pain | 6 (1%) |
| Peripheral vascular disease | 51 (5%) |
| Predominant pain location | |
| Low back and lower extremity | 599 (63%) |
| Low back | 66 (7%) |
| Lower extremity | 185 (19%) |
| Upper extremity | 47 (5%) |
| Thoracic | 33 (3%) |
| Cervical | 42 (4%) |
| Other | 23 (2%) |
| Time from chronic pain diagnosis to implant (years) | 3.8 [1.0–8.4] |
| First (de novo) SCS implant | 715 (75%) |
| Trial performed | 568 of 715 (79%) |
| Days from start of trial to permanent implant | 14 [10–31] |
| One previous SCS pulse generator implant | 156 (16%) |
| More than one previous SCS implant | 84 (9%) |
| Type of SCS system | |
| Conventional nonrechargeable | 462 (48%) |
| Conventional rechargeable | 329 (34%) |
| High‐frequency (10 kHz) rechargeable | 155 (16%) |
| Other or not known | 9 (1%) |
| One percutaneous lead | 615 (64%) |
| Two percutaneous leads | 256 (27%) |
| More than two percutaneous leads | 23 (3%) |
| Paddle lead | 61 (6%) |
| Lead 1 location | |
| Cervical | 82 (9%) |
| Upper thoracic (T1‐T5) | 15 (2%) |
| Mid‐thoracic (T6‐T9) | 622 (67%) |
| Lower thoracic (T10‐T12) | 171 (19%) |
| Lumbar or sacral | 31 (3%) |
Note: Summary statistics are calculated on each implant. An individual subject may have more than one implant. Additionally, there may be more than one indication per implant.
Postimplant management of the 955 implants included 8720 follow‐up visits over 2259 years of follow‐up. An event timeline, shown in Figure 2, revealed median follow‐up for all implants of 2.24 years. There were 180 unanticipated explants (19%). In addition, there were 13 deaths, 173 battery depletions, and 38 IPG upgrades. Of the 173 battery depletions, a replacement IPG was implanted in 167 (97%).
Figure 2.
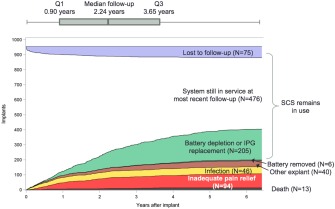
Event timeline. The figure shows a timeline of the 955 SCS systems in the chart review, including unanticipated explants due to inadequate pain relief, unanticipated explants for any other reason, deaths, battery EOL occurrences, upgrades, loss to follow‐up, and SCS systems still in use.
Table 2 details the unanticipated explants by reason for explant. Overall explant rate was 8.0% per year of follow‐up [95% confidence interval 6.9–9.2%]. Over half (94 of 180) of explants were for inadequate pain relief. As shown in Table 3, the breakdown of explants for inadequate pain relief was 32/462 (6.9%) with nonrechargeable SCS, 37/329 (11.2%) with conventional rechargeable, and 22/155 (14.2%) with high‐frequency SCS. The explant rate for inadequate pain relief was 2.8% per year of follow‐up for nonrechargeable SCS, 5.5% for conventional rechargeable SCS, and 5.0% for high‐frequency stimulation.
Table 2.
Follow‐Ups and Unanticipated IPG Explants.
| Number of implants | 955 |
| Total follow‐ups | 8720 |
| Follow‐ups per implant | 7 [3–12] |
| Total follow‐up duration | 2259 years |
| All unanticipated IPG explants | 180 (19% of implants) |
| Explant rate per year of follow‐up | 8.0% per year |
| [95% confidence interval] | [6.9–9.2%] |
| Explants for inadequate pain relief | 94 (10% of implants) |
| Rate per year of follow‐up | 4.2% per year |
| [95% confidence interval] | [3.4–5.1%] |
| Other reasons for unanticipated explant | |
| Infection or wound dehiscence | 46 (5%) |
| IPG problem | 22 (2%) |
| Lead problem | 6 (<1%) |
| Pain at pocket | 4 (<1%) |
| MRI required | 3 (<1%) |
| Free of pain | 3 (<1%) |
| No specific reason identified | 2 (<1%) |
*Additional IPGs removed and censored at event but not counted as unanticipated explant: 173 with battery depletion (167 replaced).
Thirty‐eight IPG replacements to obtain access to additional features, including burst, high‐frequency, high‐density, MRI conditional system, or additional leads.
Thirteen deaths.
Table 3.
Explants for Inadequate Pain Relief and Type of SCS System.
| Type of SCS | Number of implants | Explants for inadequate pain relief (%) | Total years of follow‐up | Explants (% per year) [95% CI] |
|---|---|---|---|---|
| Conventional nonrechargeable | 462 | 32 (6.9%) | 1125.2 | 2.8% [2.0–4.0%] |
| Conventional rechargeable | 329 | 37 (11.2%) | 671.5 | 5.5% [4.0–7.6%] |
| High‐frequency (10 kHz) rechargeable | 155 | 22 (14.2%) | 439.4 | 5.0% [3.3–7.6%] |
Figure 3 illustrates timing of explants for each case of an unanticipated explant. This qualitative analysis demonstrates that some reasons for explant, such as infection or wound dehiscence, occur most frequently in the first year after implant, while other less common reasons such as the need for MRI could occur at any time. The Kaplan‐Meier estimators for all implants in Figure 4 show the incidence of any unanticipated explant, as well as incidence of explant for inadequate pain relief. Figure 5 compares Kaplan‐Meier estimates of the incidence of explant for inadequate pain relief in different subgroups. Type of SCS (panel a) was a significant predictor of explant (p = 0.011). Explants for inadequate pain relief were also more common in women than men (p = 0.015). There was no significant association between age in subgroups above or below 65 years old, indication or predominant pain location and incidence of explant.
Figure 3.
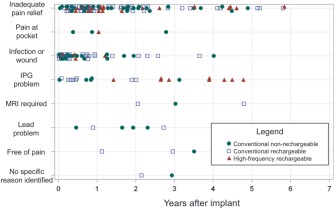
Timing of explants. Each marker indicates an unanticipated explant. As shown in the legend, the color and shape of the marker indicate the type of SCS system.
Figure 4.
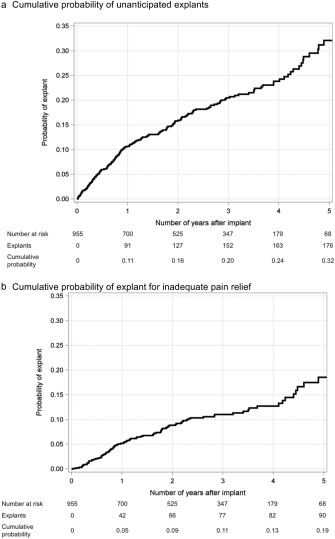
Cumulative probability of explant using the Kaplan‐Meier estimator. Panel a shows estimated probability of unanticipated explant for any reason, while panel b is probability of explant for inadequate pain relief.
Figure 5.
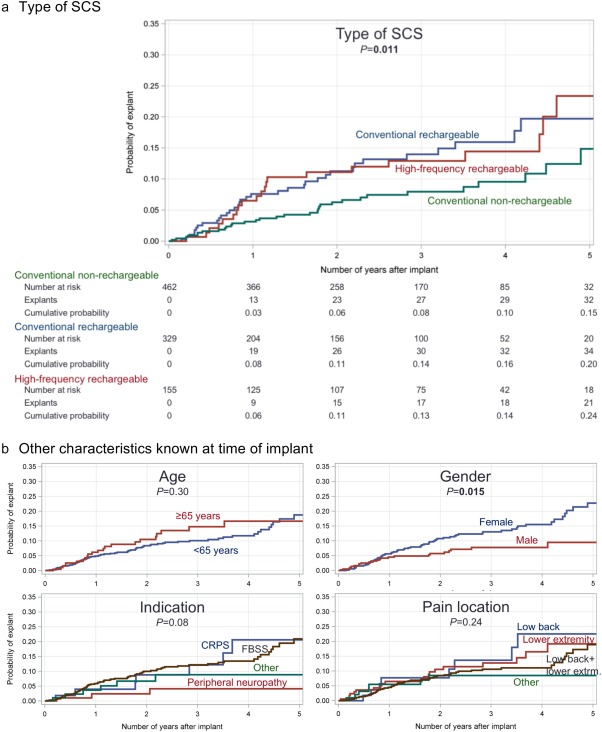
Explants by subgroup. Kaplan‐Meier curves show probability of explant for inadequate pain relief as a function of type of SCS (panel a), age, gender, SCS indication, and predominant pain location (panel b).
The results of Cox proportional‐hazards regression are shown in Figure 6. In the univariate regression shown on the left side, conventional rechargeable, high‐frequency rechargeable stimulation, and female gender were associated with greater risk of explant. Among the different indications for SCS, peripheral neuropathy was least associated with risk of explant. Significant differences persisting in the multivariable regression were for conventional rechargeable (HR 1.95, p = 0.011), female gender (HR 1.87, p = 0.011), and peripheral neuropathy (HR 0.28, p = 0.039). For high‐frequency stimulation, the HR was 1.71 and p value 0.069 after covariate adjustment.
Figure 6.
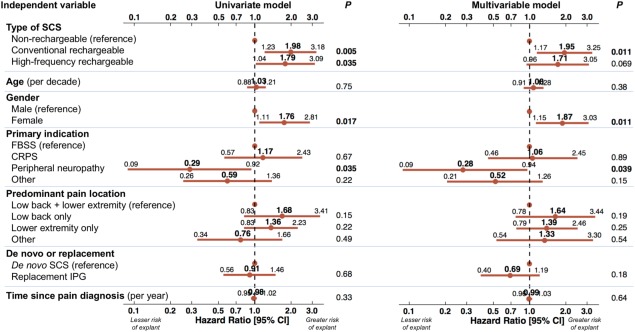
Factors associated with explant for inadequate pain relief. Univariate Cox proportional‐hazards regression (left) shows relationship of each individual variable with explants for inadequate pain relief. Multivariable Cox regression (right) includes all variables in a single model. Each point is the Hazard Ratio; lines indicate 95% confidence interval. Significant associations have p value shown in bold.
Discussion
This multinational chart review study of 955 SCS implants, performed from 2010 to 2013 and followed until mid‐2016, found that the rate of unanticipated explants was 8.0% per year of follow‐up in a cohort of subjects known to suffer from complex chronic pain. The most frequent reason, inadequate pain relief, occurred in 4.2% of implants per year of follow‐up. The survival curve showed a total rate of explant for inadequate pain relief of 19% at five years after implant. Stated another way, pain relief was achieved up to five years after implant in 81% of implants. Furthermore, the vast majority of subjects who used SCS until battery depletion (167 of 173, 97%) chose to continue on with a replacement SCS system.
A higher explant rate was found for devices with conventional rechargeable SCS compared to conventional nonrechargeable SCS, both in unadjusted regression and after adjusting for covariates. There was also an elevated risk of explant for high‐frequency rechargeable SCS in the unadjusted regression, but the difference did not reach significance after covariate adjustment.
Possible reasons for higher explant rate for rechargeable SCS systems are varied and may include patient compliance, as well as physiology and technical aspects of the SCS system design. Habituation may be a possible physiological reason inadequate pain relief, but it is not possible from the available data to determine if there were any differences in habituation. Also, compliance may be reduced as a result of the burden of recharging. Manufacturers of SCS systems should be encouraged to develop technologies for rapid recharging, wireless communication, and hardware design with smaller batteries and ergonomic shape. Updateable software may also help to reduce risk of explantation.
The rate of explant for inadequate pain relief was also higher in females than males. The covariates collected in this retrospective chart review did not clarify the difference, which persisted in the multivariable model. There may be additional covariates that might help to clarify this finding, such as prescription medications, prior treatments, pain scores, and work and disability status. However, the reasons may also involve complex physiological and psychosocial factors, expectations, and individual variations in perception of pain.
Comparison With Prior Work
In a single‐center chart review of 234 subjects who received permanent SCS implants, Hayek et al. reported an explant rate of 23.9% (56 of 234) over a mean follow‐up of 44.5 months.26 The most common reason was loss of therapeutic effect, which was followed by infection. These findings are largely concordant with results of this multinational chart review. Also notable in the present multicenter chart review was the observation that explants for MRI were relatively infrequent (amounting to <1% of implants), even though the implant period was before availability of MRI‐conditional SCS systems. While there have been few reports on long‐term outcomes of SCS, it is hoped that this systematic investigation of outcomes will encourage further exploration of the longer‐term rate and reasons for explants. There is a particular need for such analysis given the proliferation of technologies available in SCS. Likewise, there is a need for further exploration of the appropriate clinical response when challenges occur with the therapy. Strategies such as lead addition and new stimulation waveforms have the potential to avoid some explants.
The recently published SENZA‐RCT19 and ongoing SUNBURST studies demonstrate promise of new waveforms. The former study found that 84.5% of patients with high‐frequency (10 kHz) stimulation were responders for back pain and 83.1% for leg pain, while the responder rate was 43.8% for back pain and 55.5% for leg pain with conventional stimulation.19 Recent work with burst stimulation found that a 62.5% responder rate was achieved with burst SCS in the fraction of patients who did not respond to tonic SCS 28. The findings of the present chart review study, particularly with regard to high‐frequency stimulation, demonstrate the need for further real‐world experiences to complement the findings of carefully conducted clinical trials.
Limitations
Several additional limitations of this retrospective chart review should be considered. Data in the medical charts were not recorded systematically in a standardized manner across all centers. The charts only provide general reasons for explant; a prospectively designed study would be necessary to assess the specific factors that lead to loss of efficacy in some implants. The results of this chart review study draw on data from four experienced, high‐volume centers, and may be different from long‐term outcomes at other sites. However, the centers draw on a patient population from three different countries, have slightly different implant indications due to national reimbursement policies, and make use of a variety of SCS devices and technologies from various manufacturers. The implant period selected for this chart review from 2010 to 2013 allowed for a comparison of several technologies, but are not recent enough to make a comparison with newer technologies such as burst and high‐density stimulation. As the chart review did not collect information on pain scores, medications, SCS usage patterns, and patient satisfaction, we are not able to draw further conclusions on the exact causes of inadequate pain relief. There were 75 implants (8%) lost to follow‐up, in whom it was unable to determine if SCS continued in use or if an explant may have occurred elsewhere. Finally, a competing risk analysis was not performed. Competing risks that could have affected the rate of explants for inadequate pain relief included death (1% of the study cohort), explant for another reason, battery depletion, and device upgrade. It would not be possible to use battery depletion as a competing risk, since it disproportionately affects the nonrechargeable SCS systems in the first few years after implant. However, the replacement rate of 97% indicates that is was not a widespread practice to discontinue SCS upon battery depletion.
Conclusion
This multinational European real‐world outcomes study found a higher rate of explants for inadequate pain relief for conventional rechargeable and high frequency rechargeable SCS than for nonrechargeable systems. The increased rate for conventional rechargeable stimulation persisted after covariate adjustment. Prospective investigation is warranted to understand the precise reasons for loss of efficacy over time.
Authorship Statement
Prof. Vesper, Drs. Van Buyten, Wille, and Smet, Ms. Wensing, Mrs. Breel, and Mr. Karst designed the study. Drs. Van Buyten, Wille, Smet, and Vesper, Ms. Wensing, Mrs. Breel, and Mr. Karst contributed to data analysis and interpretation and participated in writing and reviewing the manuscript. Ms. Devos and Mrs. Pöggel‐Krämer contributed to the data collection and analysis. All authors approved the final version of the manuscript.
COMMENTS
This multicentre, multinational, chart review is a laudable addition to the clinical literature on spinal cord stimulation. It represents a cumulative total of 2259 years of follow‐up for 995 implants. More importantly, it focuses on why implanted devices are removed and for what reason. The authors report an explant rate of 8% per year, half of which are due to inadequate pain relief. Female gender and implantation with a conventional rechargeable device were associated with a higher rate of explantation. This knowledge has significant implications for patients giving informed consent and benchmarking of clinical outcomes for clinicians and institutions.
Kevin McCarthy, PhD
Edinburgh, Scotland, UK
***
Spinal cord stimulation is an established treatment for certain types of chronic pain. This international retrospective chart review analyzes associations between patient demographics and type of SCS with explantation. The authors evaluated patients that had long‐term follow‐up data, which allows changes in the explant rate to be examined over several years. Associations found in this review may be helpful in lowering explant rates for future SCS procedures.
Ryan Holland, MD
Piscataway, NJ, USA
***
This multi‐national chart review study of 955 SCS implants is focusing on the type of SCS and the overall explant rate with a follow‐up period of 3 to 6 years. Interestingly, it has been shown that the newer systems (conventional rechargeable and high frequency rechargeable SCS) have an elevated risk of explant compared to the conventional non‐rechargeable SCS (11.2% and 14.2% vs. 6.9%, respectively). It was stated by the authors that the possible reasons for the higher explant rate are patient compliance, as well as physiology and technical aspects of the SCS system design.
But in my experience, factors contributing to a poor therapy outcome are suboptimal lead placement, insufficient or no intraoperative testing, insufficient paresthesia coverage to the area of pain, multifocal pain syndromes or just a loss of pain‐relieving effect of the SCS in the long run.
Unfortunately, in this study the precise reason for explant remains unclear. It would be of interest to obtain information about the frequency of reprogramming sessions to compare explanted and still running SCS systems. Also, the kind of indication and patient selection for SCS should be addressed. The new multichannel and rechargeable SCS systems are capable of producing high frequency, high density, whisper or burst stimulation patterns. These new stimulation patterns have been invented to improve stimulation outcome and also to expand the indication for SCS. This is especially true for patients having predominant back pain. As we know, there are several influencing factors causing back pain. Usually, the physician is obliged to take a careful physical, psychological and radiological examination of the patient to determine the best treatment. As is generally known, undifferentiated surgical approaches do not have a promising and long lasting effect on chronic back pain. Therefore, a more sophisticated approach to “low‐back” patients is mandatory. SCS might be one option for treating back pain in selected patients, but SCS is not the universal solution for all back pain patients.
Furthermore, a comparison of the data of each of the four implanting centers would be helpful to get characteristic results of each single center.
This multi‐national chart review study is a further contribution to analyze failure of SCS, but for receiving “real‐world‐data” a more sophisticated and detailed approach is needed.
Dr. med. Matthias Winkelmüller
Hannover, Germany
Comments not included in the Early View version of this paper.
Supporting information
Supporting Table S1
Acknowledgements
The authors thank Mr. Hernán Castro of St. Jude Medical for his contributions in study management, evaluation of data accuracy, and manuscript review. The authors also thank the additional persons who performed data input.
For more information on author guidelines, an explanation of our peer review process, and conflict of interest informed consent policies, please go to http://www.wiley.com/WileyCDA/Section/id-301854.html
Source(s) of financial support: St. Jude Medical provided financial support for the study. Mr. Edward Karst, who contributed to data analysis and writing of the manuscript, and Mr. Hernán Castro, who made contributions in study management, evaluation of data accuracy, and manuscript review, are employees of St. Jude Medical.
Conflict of Interest: Drs. Van Buyten and Smet have received consulting fees and travel sponsorship from Nevro Corporation, Medtronic, St. Jude Medical and Mainstay Medical. Prof. Vesper hNER‐2131as received consulting fees, research grants, and travel sponsorship from St. Jude Medical and Boston Scientific Corporation. Dr. Wille and Mrs. Breel have received consulting fees from Medtronic and St. Jude Medical. Ms. Wensing was formerly an employee of St. Jude Medical. Mr. Karst is employed by St. Jude Medical. Mrs. Pöggel‐Krämer received compensation from research grants provided by St. Jude Medical. Ms. Devos has received compensation from research grants provided by Nevro Corporation, Medtronic, St. Jude Medical, and Mainstay Medical.
References
- 1. Cahana A, Bateman BT, Landau R. The death of a Prince: Chronicle of a death foretold. Pain Pract 2016;16:788–790. [DOI] [PubMed] [Google Scholar]
- 2. U.S. Centers for Disease Control . www.cdc.gov/drugoverdose/data/overdose.html
- 3. Volkow ND, McLellan T. Opioid abuse in chronic pain – Misconceptions and mitigation strategies. N Engl J Med 2016;374:1253–1263. [DOI] [PubMed] [Google Scholar]
- 4. Novak SP, Håkansson A, Martinez‐Raga J, Reimer J, Krotki K, Varughese S. Nonmedical use of prescription drugs in the European Union. BMC Psychiatry 2016;16:274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Van Amsterdam J, Van den Brink W. The misuse of prescription opioids: A threat for Europe?. Curr Drug Abuse Rev 2015;8:3–14. [DOI] [PubMed] [Google Scholar]
- 6. Ballantyne JC, LaForge KS. Opioid addiction and dependence during opioid treatment of chronic pain. Pain 2007;129:235–255. [DOI] [PubMed] [Google Scholar]
- 7. Franklin GM, Sover BD, Turner JA, Fulton‐Kehoe D, Wickizer TM. Early opioid prescription and subsequent disability among workers with back injuries: The disability risk identification study cohort. Spine 2008;33:199–204. [DOI] [PubMed] [Google Scholar]
- 8. Volinn E, Fargo JD, Fine PG. Opioid therapy for nonspecific low back pain and the outcome of chronic work loss. Pain 2009;142:194–201. [DOI] [PubMed] [Google Scholar]
- 9. Cheatle MD. Prescription opioid misuse, abuse, morbidity, and mortality: Balancing effective pain management and safety. Pain Med 2015;16:S3–S8. [DOI] [PubMed] [Google Scholar]
- 10. North RB, Kidd DH, Forrokhi F, Piantadosi SA. Spinal cord stimulation versus repeated lumbosacral spine surgery for chronic pain: A randomized, controlled trial. Neurosurgery 2005;56:98–106. [DOI] [PubMed] [Google Scholar]
- 11. Kumar K, Taylor RS, Jacques L et al. Spinal cord stimulation versus conventional medical management for neuropathic pain: A multicentre randomised controlled trial in patients with failed back surgery syndrome. Pain 2007;132:179–188. [DOI] [PubMed] [Google Scholar]
- 12. Taylor RS, Van Buyten JP, Buscher E. Spinal cord stimulation for chronic back pain and leg pain and failed back surgery syndrome: A systematic review and analysis of progressive factors. Spine 2005;30:152–160. [DOI] [PubMed] [Google Scholar]
- 13. Deer TR, Mekhail N, Provenzano D et al. The appropriate use of neurostimulation of the spinal cord and peripheral nervous system for the treatment of chronic pain and ischemic diseases: The Neuromodulation Appropriateness Consensus Committee. Neuromodulation 2014;17:515–550. [DOI] [PubMed] [Google Scholar]
- 14. Grider JS, Manchikanti L, Carayannopoulos A et al. Effectiveness of spinal cord stimulation in chronic spinal pain: A systematic review. Pain Physician 2016;19:E33–E54. [PubMed] [Google Scholar]
- 15. Hornberger J, Kumar K, Verhulst E, Clark MA, Hernandez J. Rechargeable spinal cord stimulation versus non‐rechargeable systems for patients with failed back surgery syndrome: A cost‐consequences analysis. Clin J Pain 2008;24:244–252. [DOI] [PubMed] [Google Scholar]
- 16. Kinfe TM, Schu S, Quack FJ, Wille C, Vesper J. Percutaneous implanted paddle lead for spinal cord stimulation: Technical considerations and long‐term follow‐up. Neuromodulation 2012;15:402–407. [DOI] [PubMed] [Google Scholar]
- 17. Babu R, Hazzard MA, Huang KT et al. Outcomes of percutaneous and paddle lead implantation for spinal cord stimulation: A comparative analysis of complications, reoperation rates, and health‐care costs. Neuromodulation 2013; 16:418–426. [DOI] [PubMed] [Google Scholar]
- 18. Al‐Kaisy A, Van Buyten JP, Smet I, Palmisani S, Pang D, Smith T. Sustained effectiveness of 10 kHz high‐frequency spinal cord simulation for patients with chronic, low back pain: 24‐month results of a prospective multicenter study. Pain Med 2014;15:347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kapural L, Yu C, Doust MW et al. Novel 10‐kHz high‐frequency therapy (HF10 therapy) is superior to traditional low‐frequency spinal cord stimulation. Anesthesiology 2015;123:851–860. [DOI] [PubMed] [Google Scholar]
- 20. De Ridder D, Plazier M, Kamerling N, Menovsky T, Vanneste S. Burst spinal cord stimulation for limb and back pain. World Neurosurg 2013;80:642–649. [DOI] [PubMed] [Google Scholar]
- 21. Schu S, Slotty PJ, Bara G, von Knop M, Edgar D, Vesper J. A prospective, randomised, double‐blind, placebo‐controlled study to examine the effectiveness of burst spinal cord stimulation patterns for the treatment of failed back surgery syndrome. Neuromodulation 2014;17:443–450. [DOI] [PubMed] [Google Scholar]
- 22. Liem L, Russo M, Huygen FJ et al. One‐year outcomes of spinal cord stimulation of the dorsal root ganglion in the treatment of chronic neuropathic pain. Neuromodulation 2015;18:41–48. [DOI] [PubMed] [Google Scholar]
- 23. Wille F, Breel JS, Bakker EW, Hollmann MW. Altering Conventional to high density spinal cord stimulation: An energy dose‐response relationship in neuropathic pain therapy. Neuromodulation 2017;20:71–80. [DOI] [PubMed] [Google Scholar]
- 24. Miller JP, Eldabe S, Buchser E, Johanek LM, Guan Y, Linderoth B. Parameters of spinal cord stimulation and their role in electrical charge delivery: A review. Neuromodulation 2016;19:373. [DOI] [PubMed] [Google Scholar]
- 25. Meier K, Nikolaisen L, Flink M et al. The Aarhus neuromodulation database. Neuromodulation 2013;16:506–513. [DOI] [PubMed] [Google Scholar]
- 26. Hayek SM, Veizi E, Hanes M. Treatment‐limiting complications of percutaneous spinal cord stimulator implants: A review of eight years of experience from an academic center database. Neuromodulation 2015;18:603–608. [DOI] [PubMed] [Google Scholar]
- 27. Fenech C, Bandikatla V, Harris S et al. Retrospective analysis of explantation of electrical neuromodulation implants. Pain in Europe 2013. (abstract). [Google Scholar]
- 28. De Ridder D, Lenders MW, De Vos CC et al. A 2‐center comparative study on tonic versus burst spinal cord stimulation: Amount of responders and amount of pain suppression. Clin J Pain 2015;31:433–437. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Table S1


