Abstract
RATIONALE:
Acute respiratory failure (ARF) may complicate the course of hematologic malignancies (HMs). Our objective was to study the characteristics, outcomes and predictors of mortality of patients with HMs who required intubation for ARF.
METHODS:
This retrospective cohort study evaluated all patients with HMs who were admitted to the Intensive Care Unit (ICU) of King Abdul-Aziz Medical City-Riyadh between 2008 and 2013 and required invasive mechanical ventilation. We noted their baseline characteristics, treatments and different outcomes. Multivariable logistic regression analysis was performed to evaluate predictors of hospital mortality.
RESULTS:
During the 6-year period, 190 patients with HMs were admitted to the ICU and 122 (64.2%) required intubation for ARF. These patients had mean age of 57.2 ± 19.3 years and Acute Physiology and Chronic Health Evaluation II score of 28.0 ± 7.8 and were predominantly males (63.4%). Lymphoma (44.3%) and acute leukemia (38.5%) were the most common hematologic malignancy. Noninvasive ventilation (NIV) was tried in 22 patients (18.0%) but failed. The code status was changed to “Do-Not-Resuscitate” for 39 patients (32.0%) during ICU stay. Hospital mortality was 70.5% and most deaths (81.4%) occurred in the ICU. The mortality of patients with “Do-Not-Resuscitate” status was 97.4%. On multivariable logistic regression analysis, male gender (odds ratio (OR), 6.74; 95% confidence interval (CI), 2.24–20.30), septic shock (OR, 6.61; 95% CI, 1.93–22.66) were independent mortality predictors. Remission status, non-NIV failure and chemotherapy during ICU stay were not associated with mortality.
CONCLUSIONS:
Patients with HMs requiring intubation had high mortality (70.5%). Male gender and presence of septic shock were independent predictors of mortality.
Keywords: Hematologic malignancy, Intensive Care Unit, leukaemia, lymphoma, mechanical ventilation, multiple myeloma, respiratory failure
Hematologic malignancies (HMs) are neoplastic diseases arising from the hematopoietic system involving either myeloid or lymphoid series. They are usually treated with intensive chemotherapy and sometimes require radiotherapy and/or hematopoietic stem cell transplantation. The prognosis of HMs has improved over time, which is mostly related to improvement in supportive care and the institution of targeted therapy.[1,2] Nevertheless, many complications can occur during the illness course as a result of organ involvement by the disease itself, disease-related or treatment-induced immunosuppression or other treatment side effects. One of these complications is acute respiratory failure (ARF), which can be due to different conditions, such as pulmonary and extra-pulmonary infections, pulmonary edema, alveolar hemorrhage, leukemic or lymphomatous pulmonary involvement and drug and transfusion reactions.[3,4] ARF management may include mechanical ventilation, either via noninvasive ventilation (NIV), or invasive mechanical ventilation.
The studies that evaluated ARF in HM patients varied in methodology and showed changing outcomes over time. In one of the earliest studies in the United States, Peters et al. retrospectively reviewed 119 critically ill patients with HMs who required mechanical ventilation between 1976 and 1985 and found that the most common ARF causes were pulmonary infiltrates and hypoxemia (63.0%) and prolonged postoperative ventilation (16.0%).[5] The mortality rate was very high (82%).[5] In a secondary analysis of Intensive Care National Audit and Research Centre Case Mix Programme Database which included 7689 admissions to 178 Intensive Care Units (ICUs) in the United Kingdom (1995–2007), the mortality of ventilated patients was 70.2% compared with 45.3% for those who did not require mechanical ventilation (P < 0.0001).[6] In a prospective, cohort study of patients with HMs admitted to multiple ICUs in France and Belgium (2010–2011), ARF was the most common reason (62.5%) for ICU admission with mechanical ventilation associated with 60.5% mortality.[7] Studies on this topic from Saudi Arabia are scarce. Bahammam et al. conducted a cohort study over 9-year period (1993–2004), assessed 44 patients with HMs admitted to the ICU with life threatening complications, one of which was RF (31% of ICU admissions), and found an overall hospital mortality of 72.7%.[8]
As the prognosis of HM patients admitted to the ICU with ARF is commonly poor, identifying the factors affecting their outcomes may be important to improve medical care. Additionally, the data from Saudi Arabia about the characteristics and outcomes of HM patients with ARF are limited. Hence, this study determined the characteristics of patients with HMs who developed ARF at a tertiary-care hospital in Riyadh and evaluated their outcomes and the predictors of mortality.
Methods
Patients and setting
This was a retrospective cohort study of patients with different types of HMs admitted to the ICU with ARF. It was conducted at the Adult ICU of King Abdul-Aziz Medical City-Riyadh. The hospital was a 900-bed tertiary-care hospital which was accredited by the Joint Commission International. The Hematology Oncology Department was a referral center for patients with leukemia, lymphoma and other HMs. Hematopoietic stem cell transplant program started in January 2010, with 70–80 transplants being done annually between autologous and allogeneic cases. The ICU was a medical-surgical closed unit, which admitted approximately 900 patients with various diagnoses per year.[9] It was covered by on-site board-certified intensivists 24 h/day, 7 days/week.[9]
This study included all adolescent and adult patients (≥14-year old) with HMs admitted to the ICU between January 1, 2008 and December 31, 2013 and had ARF, defined as the need for invasive mechanical ventilation. We excluded patients who required mechanical ventilation for postsurgical procedure care.
Data collection
The HM patients were identified from the ICU database in which diagnoses were coded. Collected data included demographic information, HM type, diagnosis date and remission status, presence of chronic health illnesses as per the Acute Physiology and Chronic Health Evaluation (APACHE) II definitions, source of admission to ICU, the main reason for ICU admission, presence of sepsis on admission, presence of febrile neutropenia, admission APACHE II score,[10] admission Glasgow Coma Scale score, and baseline levels of the partial pressure of oxygen to the fraction of inspired oxygen ratio (PaO2:FiO2), platelet count, creatinine, lactate, bilirubin and International Normalized Ratio (INR).
We also noted the following ICU management aspects: Use of vasopressors within the first 24 h after ICU admission, use of noninvasive mechanical ventilation, renal replacement therapy and tracheostomy during ICU stay. We also described the change in code status during ICU stay. Our hospital had a policy in which a Do-Not-Resuscitate order can be written if three qualified physicians agreed that a patient would not benefit from cardiopulmonary resuscitation. Patients' preferences were taken into consideration. The “no resuscitation” decision was not based on predefined criteria, nevertheless, the commonest reason was the presence of a terminal illness. This medical decision would be explained to the patient or next of kin before its implementation. Limitation of life-sustaining measures may follow a Do-Not-Resuscitate order, but care withdrawal was generally not practiced. Additionally, at the time of study, advanced directives were not practiced in Saudi Arabia. We also noted the following outcomes: Hospital mortality, ICU mortality, length of stay in the ICU and hospital and duration of mechanical ventilation.
Statistical analysis
The data were analyzed using SPSS version 15.0 (SPSS Inc., Chicago, IL, USA). We presented information for all eligible patients, hospital survivors and nonsurvivors. To describe categorical variables, frequencies and percentages were presented. For continuous variables, means and standard deviations were presented. The association between categorical variables and hospital mortality was evaluated by the Chi-square or Fisher's exact test. The Student's t-test method was used to assess the association between continuous variables and hospital mortality.
Multivariable logistic regression analysis was performed to determine the predictors of hospital mortality. Independent variables that were entered in the model were clinically significant variables and those that were different between survivors and nonsurvivors (P < 0.1). These variables were gender, remission status, APACHE II score, presence of septic shock, use of NIV before intubation, admission PaO2:FiO2 ratio, INR and lactate level. There was no collinearity between the continuous independent variables. The analysis results were presented as an odds ratio (OR) with 95% confidence interval (CI). With 5% α error, the study sample size (n = 122) had 80% power to detect an effect size (OR) on hospital mortality of approximately 3.4. P < 0.05 were considered significant.
Results
Characteristics of patients
In the 6-year period (2008–2013), 190 patients with HMs were admitted to the ICU and 122 (64.2%) required intubation for ARF. These patients had a mean age of 57.2 ± 19.3 years and APACHE II score of 28.0 ± 7.8 and were predominantly males (63.4%). Before ICU admission, the majority of patients were already hospitalized in the ward (n = 82, 66.7%). They stayed in the ward before ICU admission for an average of 15.2 ± 19.0 days. Additional patient characteristics are shown in Table 1.
Table 1.
Characteristics of patients with hematologic malignancies requiring invasive mechanical ventilation
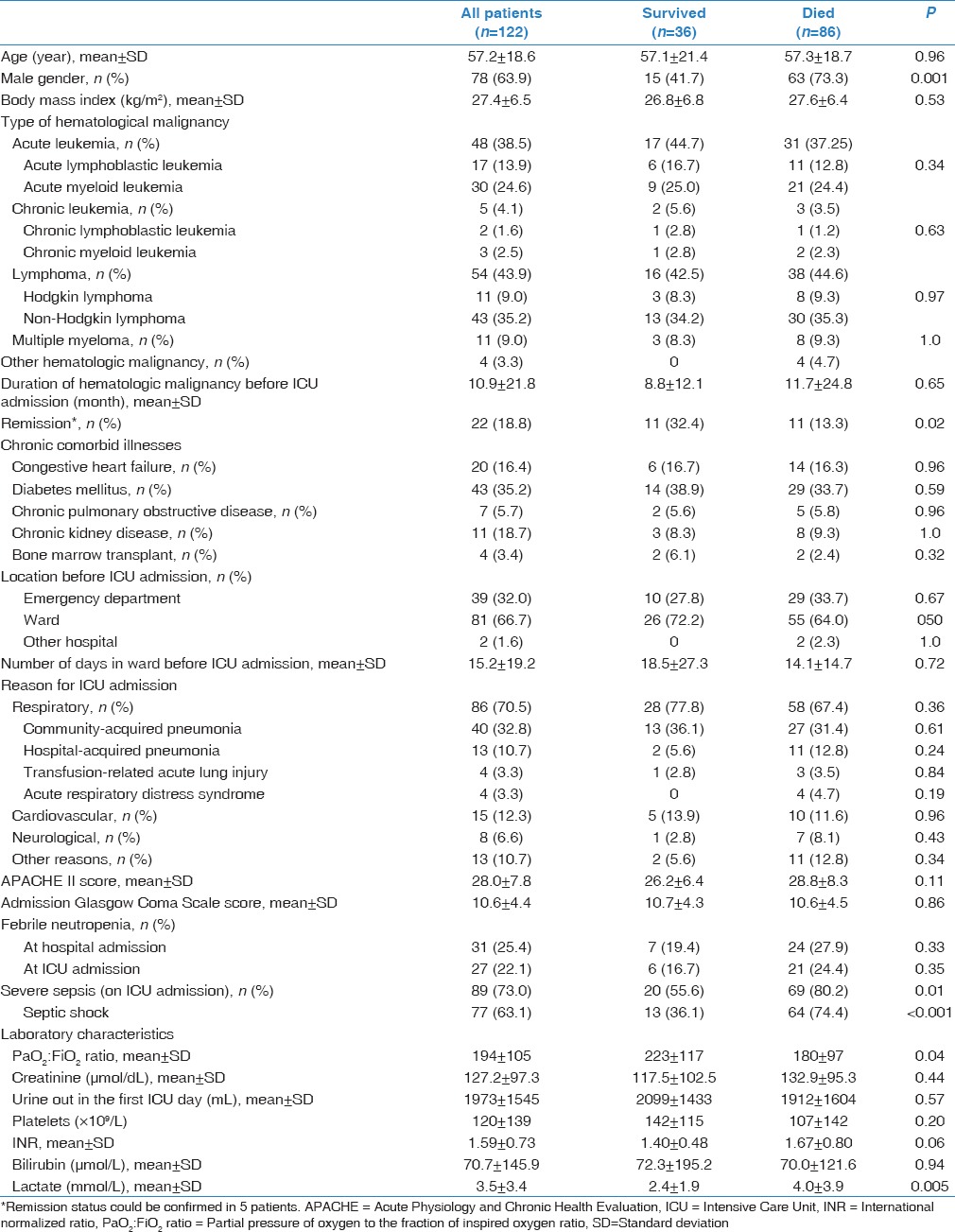
Table 1 also summarizes the types of HMs in all patients, survivors and nonsurvivors. Lymphoma (n = 54; 44.3%) was the most common HM, followed by acute leukemia (n = 48; 38.5%). There was no difference in the HM types between survivors and nonsurvivors. Only four patients (3.3%) in the study had allogeneic stem cell transplant, one with relapsed lymphoma. None of these patients had graft versus host disease. Febrile neutropenia was present in 31 patients (25.4%) at hospital admission and in 28 patients (23.0%) at ICU admission. Septic shock was present in most patients (63.1%) on ICU admission.
The most common reason (70.5%) for ICU admission was a respiratory illness, which included community-acquired pneumonia, hospital-acquired pneumonia and transfusion-related acute lung injury [Table 1]. For the forty patients with community-acquired pneumonia, at least one pathogen was recovered from respiratory secretions in 11: 4 Pseudomonas aeruginosa, 3 Klebsiella pneumoniae (2 with extended spectrum β-lactamase), 2 methicillin-sensitive Staphylococcus aureus, 2 multidrug resistant Acinetobacter baumannii and 1 Escherichia coli with extended spectrum β-lactamase. Additionally, bacteremia occurred in three patients. For the 13 patients with hospital-acquired pneumonia, multidrug resistant A. baumannii and K. pneumoniae were cultured from two patients.
Management in the Intensive Care Unit
All patients in this study were intubated and mechanically ventilated. Table 2 describes certain aspects of ICU management. NIV was tried before intubation in 22 patients (18.0%) but failed. The characteristics of patients who received NIV, including age, HM type, reason for ICU admission, presence of septic shock, were similar to those who were intubated without NIV trial. The Glasgow Coma Scale was 12.2 ± 3.9 for the NIV patients compared with 10.2 ± 4.4 (P = 0.06) for the other patients. Most patients (96.6%) had central venous catheter and required vasopressors (77.0%). New renal replacement therapy was provided to 18 patients (14.8%) for acute kidney injury. Nineteen (15.4%) patients had chemotherapy during their ICU stay.
Table 2.
Management in the Intensive Care Unit of patients with hematologic malignancies requiring invasive mechanical ventilation
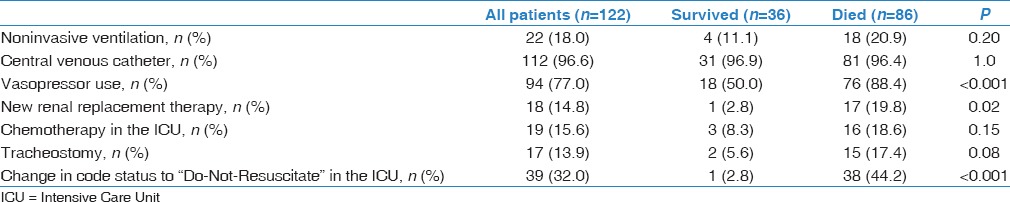
The code status was changed to “Do-Not-Resuscitate” for 39 patients (32.0%) during ICU stay. These patients had a mean age of 60.2 ± 16.7 years and APACHE II score 29.4 ± 7.9, which were not different from those who continued to be full code. “Do-Not-Resuscitate” status was instituted for 37.2% of male patients and 22.7% of female patients (P = 0.10). Patients who were in remission were less likely to have “Do-Not-Resuscitate” status (13.6% versus 36.8% for those not in remission; P = 0.04).
Outcomes
Table 3 describes the outcomes of the study patients. In the ICU, tracheostomy was performed for 17 patients (13.8%). Five patients (4.1%) had cardiac arrest in the ICU and had cardiopulmonary resuscitation. Ten patients (8.2%) had gastrointestinal bleeding. Do-Not-Resuscitate orders were associated with shorter stay in the hospital (27.4 18.7 versus 50.6 59.9 days, P = 0.002) but bot in the ICU.
Table 3.
Outcomes of patients with hematologic malignancies requiring invasive mechanical ventilation
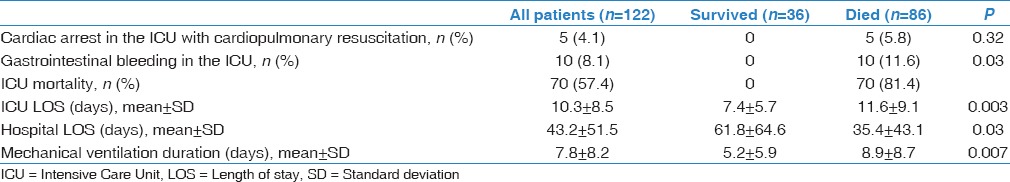
The hospital mortality was 70.5% and most deaths (81.4%) occurred in the ICU. Figure 1 shows the hospital mortality of patients according to the HM type. The hospital mortality was 70.4% for patients with lymphoma and 64.6% for those who had acute leukemia (P = 0.68). The mortality was 67.5% for patients with community-acquired pneumonia and 84.6% for hospital-acquired pneumonia. All the patients with pneumonia-associated bacteremia died. Moreover, the mortality was 81.8% for patients who failed NIV (versus 68.0% for the other patients; P = 0.30) and 84.2% for those who received chemotherapy during ICU stay (versus 68.0% for the other patients; P = 0.18). The mortality was similar in the 2008–2010 period (70.6%) compared with the 2011–2013 period (70.4%; P = 0.98). The five patients who had cardiopulmonary resuscitation in the ICU for cardiac arrest died. Only one patient who had “Do-Not-Resuscitate” status survived to hospital discharge.
Figure 1.
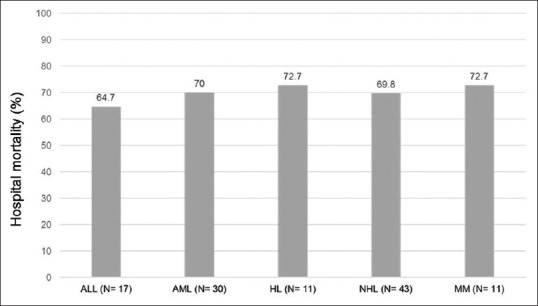
Hospital mortality of patients according to the types of hematologic malignancy. ALL: Acute lymphoblastic leukemia, AML: Acute myeloblastic leukemia, HL: Hodgkin's lymphoma, NHL: Non-Hodgkin's lymphoma, MM: Multiple myeloma
Predictors of hospital mortality
On multivariable logistic regression analysis, male gender (OR, 6.74; 95% CI, 2.24–20.30) and septic shock (OR, 6.61; 95% CI, 1.93–22.66) were independent predictors of hospital mortality. Remission status was not associated with increased mortality risk.
Discussion
The main findings of this study were the following: Patients with HMs requiring intubation had a high hospital mortality rate; the code status was changed to “Do-Not-Resuscitate” for almost one third of patients with the vast majority of these patients dying in the hospital and male gender and the presence of septic shock were independent predictors of in-hospital death.
In our study and in the 6-year period (2008–2013), approximately two-thirds of patients with HM admitted to the ICU required intubation for ARF. This is consistent with the findings of other studies where ARF was the most common reason for ICU admission.[7,11] Pneumonia was the cause of ARF in more than one-third of patients. Acute respiratory distress syndrome (four patients) and transfusion-related acute lung injury (four patients) were uncommon. Febrile neutropenia, sepsis and septic shock requiring vasopressor therapy were frequent in our cohort. Only four patients had bone marrow transplantation, as this therapy was started in 2010 in our center.
In the current study, NIV was tried in 22 patients (18.0%) before intubation. NIV is frequently used for ARF treatment in HM patients[12] as it had been associated with improved survival.[13] An multicenter observational study found that 21% of 1302 HM patients with ARF were managed initially with NIV.[14] However, NIV may fail in approximately half of patients,[13,14] and a larger randomized trial in 374 critically ill immunocompromised patients (238 with HMs) found no difference in mortality between NIV and standard oxygen therapy.[15] Additionally, a recent meta-analysis of five randomized controlled trials which included 592 immunocompromised patients with ARF found that early NIV was associated with lower short-term (risk ratio, 0.62; 95% CI 0.40–0.97) but not long-term mortality.[16] High-flow oxygen by nasal cannula might be a better therapeutic option than NIV for immunocompromised patients.[17]
In this study, the code status was changed during ICU stay from full support to “Do-Not-Resuscitate” for 39 patients (32.0%) as they deteriorated despite full support and/or short-term prognosis was considered poor (“Do-Not-Resuscitate” orders were more likely for patients with HM relapse). The hospital mortality of these patients was very high at 97.4%. This suggests that “Do-Not-Resuscitate” orders may have been implemented before imminent death or were associated with limitations of life-sustaining measures. In this study, five patients (4.1%) had cardiac arrest in the ICU and had cardiopulmonary resuscitation and eventually died in the ICU. The prognosis of cancer patients who develop cardiac arrest in the ICU is poor. In one study, the hospital mortality rate of such patients was 94.2% with acute kidney injury (OR, 1.7; 95% CI, 1.1–2.6), mechanical ventilation (OR, 3.8; 95% CI, 1.3–11), refractory shock (OR, 4.7; 95% CI, 1.8–12) and cardiopulmonary duration (OR, 1.1; 95% CI, 1.1–1.2) predicting resuscitation failure.[18] It should be noted that the outcome of patients with HMs may not be accurately predicted at ICU admission.[19] Lecuyer et al. found that HM characteristics were not significantly different between survivors and nonsurvivors and that organ failure scores on the sixth ICU day were more accurate for predicting survival than earlier scores.[19] Hence, ICU admission with full support followed by reevaluation on day 6 may be advocated for HM patients without severe disability.[19] In general, HM patients and/or their surrogate decision makers should be provided with realistic information to make an informed decision about code status and cardiopulmonary resuscitation,[20] especially in the presence of deteriorating organ function.
Generally, the mortality of patients with HMs who develop critical illness is high.[5,6,7,21] The earlier studies reported mortality rate >80%.[5] The mortality is usually higher for patients requiring mechanical ventilation.[6,7,21,22] Kroschinsky et al. found a significantly higher ICU mortality in ventilated patients (74% vs. 12% in nonventilated patients, P < 0.001).[21] Hampshire et al. also found higher hospital mortality in ventilated patients (70.2% vs. 45.3% for nonventilated patients, P < 0.0001).[6] Survival seems to have improved over time.[7,23] A more recent prospective multicenter observational study (2010-2011) of 1100 critically ill patients with HMs (25% had hematopoietic stem cell transplantation) reported hospital, 90-day, and 1-year survival rates of 60.7, 52.5, and 43.3%, respectively.[7] However, mechanical ventilation was associated with high mortality (60.5%).[7] In the current study, the mortality of HM patients who required intubation was higher (69.1%), but comparable with other studies from different countries.[24,25] The differences in the reported mortality rates could be related to differences in patient characteristics and implemented treatments such as hematopoietic stem cell transplantation.
In our study, male gender was associated with higher mortality (OR, 6.74; 95% CI, 2.24–20.30). Depuydt et al. studied the outcomes of HM patients who required mechanical ventilation and also found had that female sex (OR, 0.36; 95% CI, 0.16–0.82) was associated with lower mortality risk in the multivariable logistic regression analysis.[26] Evidence suggests that women cope better with cancer. Sex hormones may affect outcomes.[27] Men probably had higher cardiovascular disease which may have affected the outcome. Septic shock was also associated with higher mortality risk (OR, 6.61; 95% CI, 1.93–22.66) in this study. This was observed in other studies.[11,22,25] Benoit et al. observed on multivariable logistic regression analysis that vasopressor requirement was associated with higher mortality in patients with HMs admitted to the ICU (OR, 3.74; 95% CI, 1.4–9.8).[11] In this study, HM patients in remission had lower rate of hospital mortality (50%) compared with 75.0% for those not in remission. Bahammam et al. also found lower mortality rate in patients who were in remission (33.3%) compared with those who had relapse (79.0%, P = 0.04).[8] However, remission status was not independently associated with survival in our study. Additionally, having chemotherapy in ICU was not associated with mortality, similar to another study done by Benoit et al.[28]
The results of this study should be interpreted taking into consideration its strengths and limitations. This study was the first to assess the outcomes of HM patients who required intubation in the region. However, it was retrospective and was performed in one center. The study (sample size = 122) was underpowered to detect smaller effect size. Confounding factors that may have affected mortality may have not been captured in our data collection. Sepsis management elements, such as the timing and appropriateness of antibiotics, details of chronic illnesses and various organ dysfunction are potential confounding factors. Moreover, organ failure scores on ICU admission and during stay, which were not calculated, may have additional prognostic value. We also did not have information about the discussions surrounding changes in code status and the associated care limitations.
Conclusion
Patients with HMs requiring intubation had high mortality (69%). Male gender and the presence of septic shock were independent predictors of mortality. Selecting chemotherapeutic agents with less pulmonary toxicity is advisable. As the outcome of these patients is frequently poor, physicians should discuss with them and/or their surrogate decision makers the realistic treatment goals and address code status especially when persistent multiorgan failure occurs.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Brenner H, Gondos A, Arndt V. Recent major progress in long-term cancer patient survival disclosed by modeled period analysis. J Clin Oncol. 2007;25:3274–80. doi: 10.1200/JCO.2007.11.3431. [DOI] [PubMed] [Google Scholar]
- 2.Gooley TA, Chien JW, Pergam SA, Hingorani S, Sorror ML, Boeckh M, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010;363:2091–101. doi: 10.1056/NEJMoa1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaoui D, Legrand O, Roche N, Cornet M, Lefebvre A, Peffault de Latour R, et al. Incidence and prognostic value of respiratory events in acute leukemia. Leukemia. 2004;18:670–5. doi: 10.1038/sj.leu.2403270. [DOI] [PubMed] [Google Scholar]
- 4.Azoulay E, Mokart D, Lambert J, Lemiale V, Rabbat A, Kouatchet A, et al. Diagnostic strategy for hematology and oncology patients with acute respiratory failure: Randomized controlled trial. Am J Respir Crit Care Med. 2010;182:1038–46. doi: 10.1164/rccm.201001-0018OC. [DOI] [PubMed] [Google Scholar]
- 5.Peters SG, Meadows JA, 3rd, Gracey DR. Outcome of respiratory failure in hematologic malignancy. Chest. 1988;94:99–102. doi: 10.1378/chest.94.1.99. [DOI] [PubMed] [Google Scholar]
- 6.Hampshire PA, Welch CA, McCrossan LA, Francis K, Harrison DA. Admission factors associated with hospital mortality in patients with haematological malignancy admitted to UK adult, general critical care units: A secondary analysis of the ICNARC Case Mix Programme Database. Crit Care. 2009;13:R137. doi: 10.1186/cc8016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Azoulay E, Mokart D, Pène F, Lambert J, Kouatchet A, Mayaux J, et al. Outcomes of critically ill patients with hematologic malignancies: Prospective multicenter data from France and Belgium – A groupe de recherche respiratoire en réanimation onco-hématologique study. J Clin Oncol. 2013;31:2810–8. doi: 10.1200/JCO.2012.47.2365. [DOI] [PubMed] [Google Scholar]
- 8.Bahammam AS, Basha SJ, Masood MI, Shaik SA. Outcome of patients with hematological malignancies admitted to the Intensive Care Unit with life-threatening complications. Saudi Med J. 2005;26:246–50. [PubMed] [Google Scholar]
- 9.Arabi Y, Alshimemeri A, Taher S. Weekend and weeknight admissions have the same outcome of weekday admissions to an Intensive Care Unit with onsite intensivist coverage. Crit Care Med. 2006;34:605–11. doi: 10.1097/01.ccm.0000203947.60552.dd. [DOI] [PubMed] [Google Scholar]
- 10.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: A severity of disease classification system. Crit Care Med. 1985;13:818–29. [PubMed] [Google Scholar]
- 11.Benoit DD, Vandewoude KH, Decruyenaere JM, Hoste EA, Colardyn FA. Outcome and early prognostic indicators in patients with a hematologic malignancy admitted to the Intensive Care Unit for a life-threatening complication. Crit Care Med. 2003;31:104–12. doi: 10.1097/00003246-200301000-00017. [DOI] [PubMed] [Google Scholar]
- 12.Rathi NK, Haque SA, Nates R, Kosturakis A, Wang H, Dong W, et al. Noninvasivepositive pressure ventilation vsinvasive mechanical ventilation as first-line therapy for acute hypoxemic respiratory failure in cancer patients. J Crit Care. 2017;39:56–61. doi: 10.1016/j.jcrc.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 13.Hilbert G, Gruson D, Vargas F, Valentino R, Gbikpi-Benissan G, Dupon M, et al. Noninvasive ventilation in immunosuppressed patients with pulmonary infiltrates, fever, and acute respiratory failure. N Engl J Med. 2001;344:481–7. doi: 10.1056/NEJM200102153440703. [DOI] [PubMed] [Google Scholar]
- 14.Gristina GR, Antonelli M, Conti G, Ciarlone A, Rogante S, Rossi C, et al. Noninvasive versus invasive ventilation for acute respiratory failure in patients with hematologic malignancies: A 5-year multicenter observational survey. Crit Care Med. 2011;39:2232–9. doi: 10.1097/CCM.0b013e3182227a27. [DOI] [PubMed] [Google Scholar]
- 15.Lemiale V, Resche-Rigon M, Mokart D, Pène F, Rabbat A, Kouatchet A, et al. Acute respiratory failure in patients with hematological malignancies: Outcomes according to initial ventilation strategy. A groupe de recherche respiratoire en réanimation onco-hématologique (Grrr-OH) study. Ann Intensive Care. 2015;5:28. doi: 10.1186/s13613-015-0070-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang HB, Xu B, Liu GY, Lin JD, Du B. Use of noninvasive ventilation in immunocompromised patients with acute respiratory failure: A systematic review and meta-analysis. Crit Care. 2017;21:4. doi: 10.1186/s13054-016-1586-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coudroy R, Jamet A, Petua P, Robert R, Frat JP, Thille AW. High-flow nasal cannula oxygen therapy versus noninvasive ventilation in immunocompromised patients with acute respiratory failure: An observational cohort study. Ann Intensive Care. 2016;6:45. doi: 10.1186/s13613-016-0151-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khasawneh FA, Kamel MT, Abu-Zaid MI. Predictors of cardiopulmonary arrest outcome in a comprehensive cancer center Intensive Care Unit. Scand J Trauma Resusc Emerg Med. 2013;21:18. doi: 10.1186/1757-7241-21-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lecuyer L, Chevret S, Thiery G, Darmon M, Schlemmer B, Azoulay E. The ICU trial: A new admission policy for cancer patients requiring mechanical ventilation. Crit Care Med. 2007;35:808–14. doi: 10.1097/01.CCM.0000256846.27192.7A. [DOI] [PubMed] [Google Scholar]
- 20.Hill QA. Intensify, resuscitate or palliate: Decision making in the critically ill patient with haematological malignancy. Blood Rev. 2010;24:17–25. doi: 10.1016/j.blre.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 21.Kroschinsky F, Weise M, Illmer T, Haenel M, Bornhaeuser M, Hoeffken G, et al. Outcome and prognostic features of Intensive Care Unit treatment in patients with hematological malignancies. Intensive Care Med. 2002;28:1294–300. doi: 10.1007/s00134-002-1420-5. [DOI] [PubMed] [Google Scholar]
- 22.Benoit DD, Hoste EA, Depuydt PO, Offner FC, Lameire NH, Vandewoude KH, et al. Outcome in critically ill medical patients treated with renal replacement therapy for acute renal failure: Comparison between patients with and those without haematological malignancies. Nephrol Dial Transplant. 2005;20:552–8. doi: 10.1093/ndt/gfh637. [DOI] [PubMed] [Google Scholar]
- 23.Pène F, Percheron S, Lemiale V, Viallon V, Claessens YE, Marqué S, et al. Temporal changes in management and outcome of septic shock in patients with malignancies in the Intensive Care Unit. Crit Care Med. 2008;36:690–6. doi: 10.1097/CCM.0B013E318165314B. [DOI] [PubMed] [Google Scholar]
- 24.Oliver C, Peixoto A, Guillermo C, Zunino J, Stevenazzi M, Biestro A, et al. Outcomes and prognostic factors in critically ill patients with hematologic malignancies admitted in an Intensive Care Unit: A single center experience. Hospital de clínicas, montevideo, uruguay. Blood. 2014;124:6017. [Google Scholar]
- 25.Grgic Medic M, Gornik I, Gašparovic V. Hematologic malignancies in the medical Intensive Care Unit – Outcomes and prognostic factors. Hematology. 2015;20:247–53. doi: 10.1179/1607845414Y.0000000206. [DOI] [PubMed] [Google Scholar]
- 26.Depuydt PO, Benoit DD, Vandewoude KH, Decruyenaere JM, Colardyn FA. Outcome in noninvasively and invasively ventilated hematologic patients with acute respiratory failure. Chest. 2004;126:1299–306. doi: 10.1378/chest.126.4.1299. [DOI] [PubMed] [Google Scholar]
- 27.Micheli A, Ciampichini R, Oberaigner W, Ciccolallo L, de Vries E, Izarzugaza I, et al. The advantage of women in cancer survival: An analysis of EUROCARE-4 data. Eur J Cancer. 2009;45:1017–27. doi: 10.1016/j.ejca.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 28.Benoit DD, Depuydt PO, Vandewoude KH, Offner FC, Boterberg T, De Cock CA, et al. Outcome in severely ill patients with hematological malignancies who received intravenous chemotherapy in the Intensive Care Unit. Intensive Care Med. 2006;32:93–9. doi: 10.1007/s00134-005-2836-5. [DOI] [PubMed] [Google Scholar]


