Abstract
Context:
Intravesical Bacillus Calmette-Guérin (BCG) is a cause of bladder and systemic toxicity that is difficult to prevent and is responsible for treatment drop out in bladder cancer patients. More recently, BCG shortage has become the main cause of incomplete treatment.
Aims:
The aim of this study was to examine the impact on long-term prognosis of bladder cancer patients following discontinuation of BCG instillations.
Settings and Design:
In this retrospective study, data were examined from 333 consecutive nonmuscle invasive bladder cancer patients treated from 2005 to 2015 by transurethral resection (TUR) and had undergone adjuvant BCG therapy after TUR.
Subjects and Methods:
Rate of complete cure, the reason for the interruption, toxicity, and the associations between discontinuance of BCG therapy, tumor characteristics, association with carcinoma in situ and tumor recurrence or progression were analyzed.
Statistical Analysis Used:
Recurrence and progression-free survival rate curves were estimated using the Kaplan-Meier method and were compared using the log-rank test. Univariate and multivariate analyses were performed using the Cox proportional hazards model. Differences among groups were considered as statistically significant when P < 0.05.
Results:
Overall, 303 patients were eligible for analysis. Median follow up was 36 (confidence interval: 7–120) months. A total of 55 (18.1%) had <6 installations (Group I); 87 (28.7%) completed induction and 1-year maintenance (Group III); and 161 (53.1%) completed the induction course, but not the 1-year maintenance (Group II). Grade III–IV toxicity rates were significantly higher in Group I than Group II and III. Interruption for BCG shortage was the main cause of interrupting BCG in Group II. Multivariate analysis showed that discontinuation of BCG induction therapy was an independent predictor for tumor recurrence (P < 0.001) and 1-year BCG maintenance therapy for tumor progression (P = 0.005).
Conclusions:
Discontinuation of BCG therapy has a significantly deleterious effect on tumor recurrence and progression rates. Although BCG toxicity is a major cause of drop out, BCG shortage became a major cause of discontinuation. All effort must be done today to restore normal production of BCG worldwide.
Keywords: Bacillus Calmette-Guérin instillation, drug shortage, prognosis, transitional cell carcinoma, treatment discontinuance
INTRODUCTION
It is widely accepted that intravesical Bacillus Calmette-Guérin (BCG) instillation is the treatment of choice for high-risk stage Ta and T1 papillary carcinoma and carcinoma in situ (CIS). BCG significantly decreases recurrence rates[1] and reduces the odds of progression by 27%.[2] Guidelines from urological societies recommend maintenance BCG therapy for up to 3 years.[3] However, intravesical BCG is a cause of bladder and systemic toxicity that is difficult to prevent and is responsible for treatment discontinuation.[4] The consequences, as shown in previous retrospective studies, are that patients have an increased risk of recurrence.[5] In the experience at our center, not only BCG interruption is partly due to toxicity but also patient's and/or physician's fear of complications. More recently, since 2013 a shortage of production of BCG has dramatically impaired our capacity to follow the current guidelines of maintenance therapy. The hypothesis suggested is that BCG discontinuance at any time during induction or consolidation impacts on prognosis independently of the cause (toxicity, personal choices). This study represents a retrospective analysis of the impact of discontinuation of BCG instillations during the induction phase and maintenance phase on the long-term prognosis of bladder cancer patients.
SUBJECTS AND METHODS
Patient population and inclusion criteria
A retrospective analysis was conducted on the medical records of patients who had undergone transurethral resection (TUR) of bladder tumor and an initial course of adjuvant BCG therapy (Connaught Strain, Sanofi Pasteur® BCG-Medac, BCG seed RIVM derived from seed 1173-P2) for non-muscle-invasive bladder cancer from 2007 to 2016. Patients were included for analysis if the following criteria were met: primary bladder papillary carcinoma totally removed by TUR and at risk for recurrence or progression (pTaG3; pTa multifocal, pT1, CIS); BCG treatment commenced at 6-week post-TUR; follow-up within 24 months of surgery by urine cytology and cystoscopy (every 3 monthly during the first 2 years and every 6 monthly thereafter). Patients with either incomplete follow-up or missing information regarding the BCG course or with disease progression during BCG treatment were excluded from the study.
Treatment
The Connaught strain BCG was used for weekly administration at a dose of 81 mg in 50 ml of saline, instilled into the bladder using an 8F urethral catheter and patient attempt to retain the treatment for at least 1 h. From 2007 up to now, patients are treated by an induction course of six weekly instillations, which is followed 6 weeks later by a consolidation of 3 weekly instillations which is followed by 3 weekly instillations every 3 months. BCG instillations were done by a specialized nurse who also monitored clinical events during follow-up. Patients were followed by urine cytology and cystoscopy every 3 months for the 1st year then every 6 months.
Analysis
The following factors were recorded: age, sex, previous TURs of bladder transitional cell carcinoma (TCC), multifocality, tumor grade, pathologic stage, the presence of CIS. Toxicity was graded as mild (Grade I), moderate systemic (Grade IIA), moderate local (Grade IIB), and severe (Grade III − IV). Toxicity was classified according to the score described by Saint et al.[2]
Patients were classified according to the completeness of maintenance therapy. Patients treated by <6 instillations at induction were classified as Group I, patients treated who completed induction but had not completed at least on the year of maintenance were classified as Group II, patients who had completed a full course of induction and maintenance for 1 year were classified as Group III.
The rate of bladder carcinoma recurrence and progression was analyzed regarding morphology, pathology, toxicity and number of BCG instillations. Recurrence was defined by the occurrence of a new tumor in the bladder without stage progression. Progression was defined by the occurrence of a new tumor with stage progression or the need for cystectomy in the case of local recurrence not controllable by endoscopic treatment.
Statistical evaluation
Recurrence and progression-free survival rate curves were generated using the Kaplan–Meier method and were compared using the log-rank test. Differences among groups were considered as significant when P < 0.05. Univariate and multivariate analyses of data were performed using the Cox proportional hazards model with step-wise forward selection. These analyses were performed with the Stata version 7.0 statistical software package (Stata, College Station, TX, USA).
RESULTS
A total of 333 patients treated had their clinical and administrative charts reviewed. Thirty patients were excluded from the subsequent analysis due to tumor progression during BCG course (12 patients), lost to follow up (11 patients), or nonconcordance between administrative and clinical data regarding BCG instillations (7 patients).
Patient characteristics
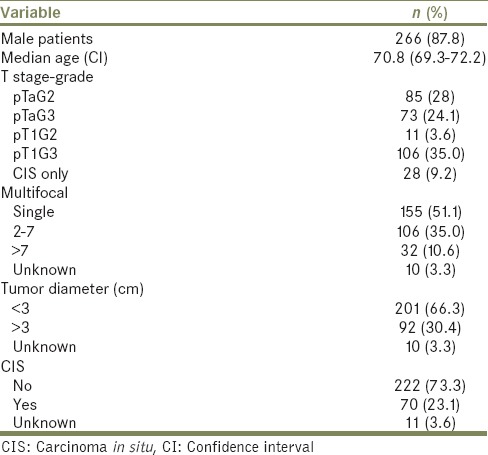
Patient characteristics are shown in Table 1. Most patients had high-grade bladder TCC (68.3%) including 28 patients (9.6%) with CIS only; 70 (23.9%) of patients had tumor surrounding CIS. Around half of patients had a single tumor and approximately 69% had a tumor of >3 cm. Median follow up was 36 (confidence interval [CI]: 7–120) months.
Table 1.
Patient characteristics (n=303)
Discontinuation rates and causes
Of the 303 patients, 55 (18.1%) had <6 instillations (Group I); 87 (28.7%) had completed induction and 1-year maintenance (Group III). The majority (161 out of 303 [53.1%]) had completed induction course but discontinued maintenance (Group II). Toxicity rates are shown in Table 2. Only Grade III–IV toxicity rates were significantly higher in Group I compared with Group II or Group III (P < 0.01).
Table 2.
Toxicity rates in patients completing Bacillus Calmette-Guérin induction and consolidation courses (Group III), induction only (Group II) or <6 installations (Group I)
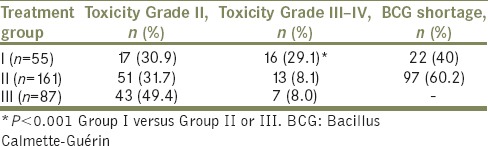
The main reason for induction discontinuation (Group I) was local toxicity (Grade II) for 17 patients (30.9%) and systemic toxicity (Grade III − IV) for 16 patients (29.1%). BCG was discontinued for factory shortage for 22 patients in Group I (40.0%). The main reason for maintenance discontinuation was BCG shortage (60.2%) In this Group II, maintenance was also discontinued for grade II and II toxicity (31% and 8.1% respectively) [Table 2].
Recurrence and progression rates after Bacillus Calmette-Guérin
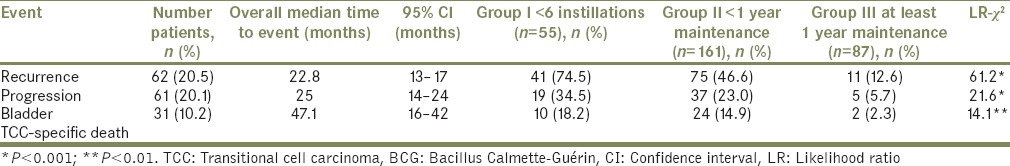
Recurrence, progression, and specific death rates are shown in Table 3. At the time of analysis, the recurrence rate was 27% (restricted to patients without progression, median time to recurrence: 22.8 months), progression rate was 17.8% (CI: 14–24; median time to progression: 25 months) and specific mortality rate was 10.2% (CI: 6–47.1; median time to cancer-related death) months. Comparison between groups by Mantel–Haenszel analysis showed that events of recurrence (P < 0.001), progression (P < 0.001), and specific mortality (P < 0.01) were significantly more frequent in Groups I and II as compared to Group III [Table 3]. Median time to cancer recurrence and progression and specific death was equivalent between groups. For Groups I, II, and II, recurrence occurred at a median time of 30.5, 24.5, and 28.5 months, respectively; progression occurred at a median time of 36, 30, and 30 months, respectively; and cancer-specific death occurred at a median time of 46, 36, and 30 months, respectively.
Table 3.
Rates of recurrence, progression, and bladder transitional cell carcinoma-specific death according to the number of Bacillus Calmette-Guérin instillations
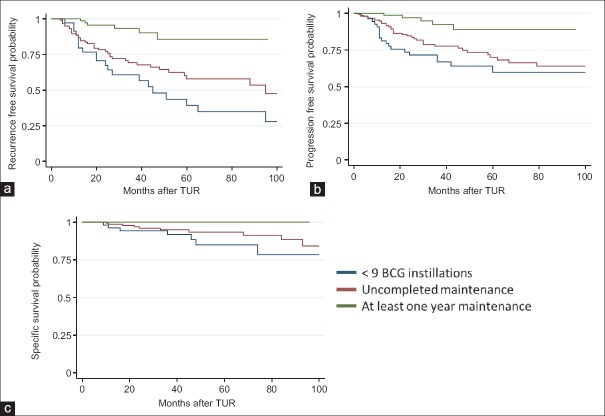
Survival time analysis according to Kaplan-Meier method showed that there was a statistically significant difference between groups in terms of recurrence, progression, and survival. At 36 months (median follow up time), the recurrence rate was 59% (CI: 46–73) for Group I, 48% (CI: 39–57) for Group II and 13% (CI: 6–24) for Group III. According to the log rank test, the difference between groups was significant (log rank: 39, P < 0.0001). At 36 months, progression rate was 33% (CI: 22–48) for Group I, 22% (CI: 15–31) for Group II and 7.5% (CI: 2.8–19.5) for Group III; the difference between groups was also significant (log rank: 14.2, P = 0.008). At 36 months, the rate of death from bladder cancer was 8.6%, 2.9%, and 2.1% for Groups I, II, and III, respectively; the difference between groups was not significant (log rank: 5.55, P = 0.06). Actuarial survival curves are shown in Figure 1.
Figure 1.
Kaplan–Meier survival analysis according to the completion of induction and consolidation courses of Bacillus Calmette-Guérin received: (a) recurrence-free survival; (b) progression-free survival (c) bladder transitional cell carcinoma-specific survival
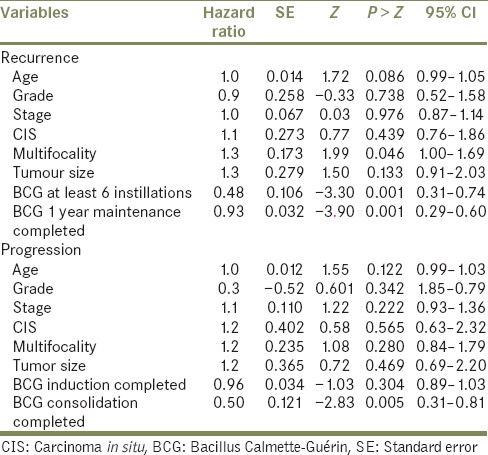
Multivariate analysis showed that discontinuation of BCG induction (Group I) and discontinuation of consolidation and tumor multifocality were independent adverse predictors of tumor recurrence, while the latter was also a risk factor for tumor progression [Table 4]. Age, tumor grade and stage, CIS and size of a tumor were not significant for predicting tumor recurrence or progression on multivariate analysis.
Table 4.
Multivariate analysis of independent variables using cox proportional hazard model to test variables in regards to risk of bladder recurrence and progression
DISCUSSION
Randomized controlled clinical trials and meta-analyses have shown that BCG bladder instillations provide superior protection against bladder tumor recurrence as well as a positive impact on disease progression.[1] Current guidelines recommend that patients with bladder cancer should be treated by an induction course of 6 weekly instillations, followed by bi-annual maintenance of 3 weekly BCG instillations for 3 years.[6] Despite these recommendations, 30% of patients with a high-risk tumor are not treated by instillations of BCG or chemotherapy.[7] In addition, data from published studies show that BCG dropout rates are between 2% and 32%, due to toxicity, the patient's fear of side-effects and the constraints associated with treatment. In addition, since 2013, factory shortage of BCG has become a major cause of BCG discontinuation. In this study, discontinuation rates were 18.1% for the induction course and 53.1% for the maintenance course. The main reason for induction course discontinuation was Grade II or III toxicity (60%); fewer patients discontinued their treatment for BCG shortage reason. Regarding those patients discontinuing the maintenance course, the majority (19.8%) did so for BCG shortage reason. It was also noted that although Grade III toxicity was the most frequent cause of early BCG drop out, Grade II toxicity was equally frequent in all groups. These results suggest that independently from BCG shortage, individual tolerance to BCG instillation is a determinant factor of drop out and should be considered.
Intravesical BCG induces a nonspecific immune reaction and consequently, bladder toxicity is inevitable. BCG therapy relies on the interaction between the bacillus, the immunocompetent cells which causes the secretion of toxic factors (mostly cytokines: interleukin 1 [IL-2], IL-6 and interferon).[8,9,10] Previous retrospective studies have found that patients developing local and/or systemic side-effects to BCG may have a better clinical outcome. In these studies, authors have described a correlation between side-effects or inflammatory urinary markers (leucocytes) and efficacy in terms of decreasing the rate of bladder recurrence.[11,12,13] However, these results were not confirmed by larger controlled EORTC randomized studies.[14] In contrast, bladder and systemic toxicity are major causes of BCG discontinuation, which may impact on the general management of high-risk bladder carcinoma. Previous reports have shown that discontinuation of the induction course of BCG is associated with an increased risk of recurrence and possible progression.[15,16] In a retrospective multivariate analysis on a cohort of 236 bladder cancer patients treated with BCG, Andius and Holmäng reported that BCG induction discontinuation was associated with progression (hazard ratio: 0.334 [0.169–0.659]).[15] More recently in a cohort of 106 patients, Takeda et al. found an increased risk of bladder recurrence for patients who had <6 BCG instillations.[5] Similar results were noted in the current study, with a higher rate of recurrence and progression in patients who did not complete the BCG induction and maintenance courses. We also noticed that treatment discontinuation during the maintenance course had an impact on recurrence. Patients who had completed the induction and maintenance course had better recurrence and progression free survival rates.
In the present study, we show the effect of BCG factory shortage on discontinuation. BCG shortage occurred in 2013 because of the shutting down of the production site of the Connaught strain in Toronto, Canada. For 3 years, BCG Connaught was not available causing a restriction in dependent countries. Despite the availability of other strains of BCG, the overall production was not sufficient to cover patient's needs.[17,18,19,20] The study results clearly show that BCG shortage has become a major cause of discontinuation and therefore a cause of patient's undertreatment causing recurrence and progression. Further studies will be focused on cost efficacy studies to have an estimate on the financial impact as well.
CONCLUSIONS
BCG has proven efficacy in preventing bladder cancer recurrence and has enabled conservative treatment of most high-risk, nonmuscle-invasive bladder cancer.[21,22,23] Nevertheless, BCG treatment causes toxicity for most patients, is a time-consuming treatment that interrupts the patient's usual activities and is also a source of fear and depression in patients. We believe that any patient undergoing BCG therapy must be informed not only of its side-effects but also its goals.[24] Measures should be taken to decrease such side-effects, as well as provide rapid treatment of local and general toxicity.[25,26] On the other hand, health authorities should be aware of the impact of BCG shortage in terms of disease recurrence, progression, and over costs.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We thank Nurse Alfreda Pages in charge of our BCG unit for assistance in the care and follow up of bladder cancer patients.
REFERENCES
- 1.Shelley MD, Court JB, Kynaston H, Wilt TJ, Fish RG, Mason M, et al. Intravesical Bacillus Calmette-Guerin in ta and T1 bladder cancer. Cochrane Database Syst Rev. 2000;4:CD001986. doi: 10.1002/14651858.CD001986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sylvester RJ, van der Meijden AP, Lamm DL. Intravesical Bacillus Calmette-Guerin reduces the risk of progression in patients with superficial bladder cancer: A meta-analysis of the published results of randomized clinical trials. J Urol. 2002;168:1964–70. doi: 10.1016/S0022-5347(05)64273-5. [DOI] [PubMed] [Google Scholar]
- 3.Persad R, Lamm D, Brausi M, Soloway M, Palou J, Böhle A, et al. Current approaches to the management of non-muscle invasive bladder cancer: Comparison of current guidelines and recommendations. Eur Urol Suppl. 2008;7:637–50. [Google Scholar]
- 4.Witjes JA, Palou J, Soloway M, Lamm D, Brausi M, Spermon JR, et al. Clinical practice recommendations for the prevention and management of intravesical therapy-associated adverse events. Eur Urol Suppl. 2008;7:615–74. [Google Scholar]
- 5.Takeda T, Kikuchi E, Yuge K, Matsumoto K, Miyajima A, Nakagawa K, et al. Discontinuance of bacille Calmette-Guérin instillation therapy for nonmuscle-invasive bladder cancer has negative effect on tumor recurrence. Urology. 2009;73:1318–22. doi: 10.1016/j.urology.2008.12.039. [DOI] [PubMed] [Google Scholar]
- 6.Lamm D, Colombel M, Persad R, Soloway M, Böhle A, Palou J, et al. Clinical practice recommendations for the management of non-muscle invasive bladder cancer. Eur Urol Suppl. 2008;7:651–66. [Google Scholar]
- 7.Snyder C, Harlan L, Knopf K, Potosky A, Kaplan R. Patterns of care for the treatment of bladder cancer. J Urol. 2003;169:1697–701. doi: 10.1097/01.ju.0000056727.30546.b7. [DOI] [PubMed] [Google Scholar]
- 8.Böhle A. BCG's mechanism of action – Increasing our understanding. For the EBIN group. Eur Urol. 2000;37(Suppl 1):1–8. doi: 10.1159/000052375. [DOI] [PubMed] [Google Scholar]
- 9.Bisiaux A, Thiounn N, Timsit MO, Eladaoui A, Chang HH, Mapes J, et al. Molecular analyte profiling of the early events and tissue conditioning following intravesical Bacillus Calmette-Guerin therapy in patients with superficial bladder cancer. J Urol. 2009;181:1571–80. doi: 10.1016/j.juro.2008.11.124. [DOI] [PubMed] [Google Scholar]
- 10.Higuchi T, Shimizu M, Owaki A, Takahashi M, Shinya E, Nishimura T, et al. A possible mechanism of intravesical BCG therapy for human bladder carcinoma: Involvement of innate effector cells for the inhibition of tumor growth. Cancer Immunol Immunother. 2009;58:1245–55. doi: 10.1007/s00262-008-0643-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orihuela E, Herr HW, Pinsky CM, Whitmore WF., Jr Toxicity of intravesical BCG and its management in patients with superficial bladder tumors. Cancer. 1987;60:326–33. doi: 10.1002/1097-0142(19870801)60:3<326::aid-cncr2820600309>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 12.Lüftenegger W, Ackermann DK, Futterlieb A, Kraft R, Minder CE, Nadelhaft P, et al. Intravesical versus intravesical plus intradermal Bacillus Calmette-Guerin: A prospective randomized study in patients with recurrent superficial bladder tumors. J Urol. 1996;155:483–7. doi: 10.1016/s0022-5347(01)66427-9. [DOI] [PubMed] [Google Scholar]
- 13.Saint F, Patard JJ, Irani J, Salomon L, Hoznek A, Legrand P, et al. Leukocyturia as a predictor of tolerance and efficacy of intravesical BCG maintenance therapy for superficial bladder cancer. Urology. 2001;57:617–21. doi: 10.1016/s0090-4295(01)00921-9. [DOI] [PubMed] [Google Scholar]
- 14.van der Meijden AP, Sylvester RJ, Oosterlinck W, Hoeltl W, Bono AV EORTC Genito-Urinary Tract Cancer Group. Maintenance Bacillus Calmette-Guerin for ta T1 bladder tumors is not associated with increased toxicity: Results from a European organisation for research and treatment of cancer genito-urinary group phase III trial. Eur Urol. 2003;44:429–34. doi: 10.1016/s0302-2838(03)00357-9. [DOI] [PubMed] [Google Scholar]
- 15.Andius P, Holmöng S. Bacillus Calmette-Guérin therapy in stage ta/T1 bladder cancer: Prognostic factors for time to recurrence and progression. BJU Int. 2004;93:980–4. doi: 10.1111/j.1464-410X.2003.04764.x. [DOI] [PubMed] [Google Scholar]
- 16.Lamm DL, van der Meijden AP, Akaza H, Brendler C, Hedlund PO, Mizutani Y, et al. Intravesical chemotherapy and immunotherapy: How do we assess their effectiveness and what are their limitations and uses? Int J Urol. 1995;2(Suppl 2):23–35. [PubMed] [Google Scholar]
- 17.Krege S, Giani G, Meyer R, Otto T, Rübben H. A randomized multicenter trial of adjuvant therapy in superficial bladder cancer: Transurethral resection only versus transurethral resection plus Mitomycin C versus transurethral resection plus Bacillus Calmette-Guerin. Participating clinics. J Urol. 1996;156:962–6. doi: 10.1016/s0022-5347(01)65673-8. [DOI] [PubMed] [Google Scholar]
- 18.Lundholm C, Norlén BJ, Ekman P, Jahnson S, Lagerkvist M, Lindeborg T, et al. A randomized prospective study comparing long-term intravesical instillations of Mitomycin C and Bacillus Calmette-Guerin in patients with superficial bladder carcinoma. J Urol. 1996;156:372–6. doi: 10.1016/s0022-5347(01)65853-1. [DOI] [PubMed] [Google Scholar]
- 19.Witjes JA, Caris CT, Mungan NA, Debruyne FM, Witjes WP. Results of a randomized phase III trial of sequential intravesical therapy with Mitomycin C and Bacillus Calmette-Guerin versus Mitomycin C alone in patients with superficial bladder cancer. J Urol. 1998;160:1668–71. [PubMed] [Google Scholar]
- 20.van der Meijden AP, Brausi M, Zambon V, Kirkels W, de Balincourt C, Sylvester R, et al. Intravesical instillation of Epirubicin, Bacillus Calmette-Guerin and Bacillus Calmette-Guerin plus isoniazid for intermediate and high risk ta, T1 papillary carcinoma of the bladder: A European organization for research and treatment of cancer genito-urinary group randomized phase III trial. J Urol. 2001;166:476–81. doi: 10.1097/00005392-200108000-00016. [DOI] [PubMed] [Google Scholar]
- 21.Martínez-Piñeiro JA, Flores N, Isorna S, Solsona E, Sebastián JL, Pertusa C, et al. Long-term follow-up of a randomized prospective trial comparing a standard 81 mg dose of intravesical bacille Calmette-Guérin with a reduced dose of 27 mg in superficial bladder cancer. BJU Int. 2002;89:671–80. doi: 10.1046/j.1464-410x.2002.02722.x. [DOI] [PubMed] [Google Scholar]
- 22.Martínez-Piñeiro JA, Martínez-Piñeiro L, Solsona E, Rodríguez RH, Gómez JM, Martín MG, et al. Has a 3-fold decreased dose of Bacillus Calmette-Guerin the same efficacy against recurrences and progression of T1G3 and tis bladder tumors than the standard dose? Results of a prospective randomized trial. J Urol. 2005;174:1242–7. doi: 10.1097/01.ju.0000173919.28835.aa. [DOI] [PubMed] [Google Scholar]
- 23.de Reijke TM, Kurth KH, Sylvester RJ, Hall RR, Brausi M, van de Beek K, et al. Bacillus Calmette-Guerin versus Epirubicin for primary, secondary or concurrent carcinoma in situ of the bladder: Results of a European organization for the research and treatment of cancer – Genito-urinary group phase III trial (30906) J Urol. 2005;173:405–9. doi: 10.1097/01.ju.0000150425.09317.67. [DOI] [PubMed] [Google Scholar]
- 24.Colombel M, Saint F, Chopin D, Malavaud B, Nicolas L, Rischmann P, et al. The effect of ofloxacin on Bacillus Calmette-Guerin induced toxicity in patients with superficial bladder cancer: Results of a randomized, prospective, double-blind, placebo controlled, multicenter study. J Urol. 2006;176:935–9. doi: 10.1016/j.juro.2006.04.104. [DOI] [PubMed] [Google Scholar]
- 25.Di Stasi SM, Giannantoni A, Giurioli A, Valenti M, Zampa G, Storti L, et al. Sequential BCG and electromotive mitomycin versus BCG alone for high-risk superficial bladder cancer: A randomised controlled trial. Lancet Oncol. 2006;7:43–51. doi: 10.1016/S1470-2045(05)70472-1. [DOI] [PubMed] [Google Scholar]
- 26.Saint F, Irani J, Patard JJ, Salomon L, Hoznek A, Zammattio S, et al. Leukocyturia as a predictor of tolerance and efficacy of intravesical BCG maintenance therapy for superficial bladder cancer. Urology. 2001;57:883–8. doi: 10.1016/s0090-4295(01)00921-9. [DOI] [PubMed] [Google Scholar]