Abstract
Objective To study the strength and validity of associations between adiposity and risk of any type of obstetric or gynaecological conditions.
Design An umbrella review of meta-analyses.
Data sources PubMed, Cochrane database of systematic reviews, manual screening of references for systematic reviews or meta-analyses of observational and interventional studies evaluating the association between adiposity and risk of any obstetrical or gynaecological outcome.
Main outcomes Meta-analyses of cohort studies on associations between indices of adiposity and obstetric and gynaecological outcomes.
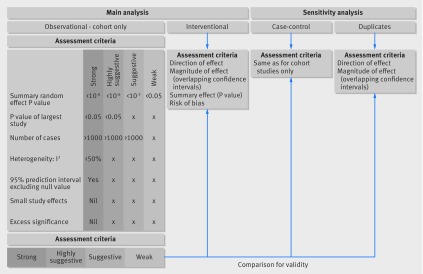
Data synthesis Evidence from observational studies was graded into strong, highly suggestive, suggestive, or weak based on the significance of the random effects summary estimate and the largest study in the included meta-analysis, the number of cases, heterogeneity between studies, 95% prediction intervals, small study effects, excess significance bias, and sensitivity analysis with credibility ceilings. Interventional meta-analyses were assessed separately.
Results 156 meta-analyses of observational studies were included, investigating associations between adiposity and risk of 84 obstetric or gynaecological outcomes. Of the 144 meta-analyses that included cohort studies, only 11 (8%) had strong evidence for eight outcomes: adiposity was associated with a higher risk of endometrial cancer, ovarian cancer, antenatal depression, total and emergency caesarean section, pre-eclampsia, fetal macrosomia, and low Apgar score. The summary effect estimates ranged from 1.21 (95% confidence interval 1.13 to 1.29) for an association between a 0.1 unit increase in waist to hip ratio and risk endometrial cancer up to 4.14 (3.61 to 4.75) for risk of pre-eclampsia for BMI >35 compared with <25. Only three out of these eight outcomes were also assessed in meta-analyses of trials evaluating weight loss interventions. These interventions significantly reduced the risk of caesarean section and pre-eclampsia, whereas there was no evidence of association with fetal macrosomia.
Conclusions Although the associations between adiposity and obstetric and gynaecological outcomes have been extensively studied, only a minority were considered strong and without hints of bias.
Introduction
The number of overweight and obese adults and children has risen globally from about 857 million in 1980 to 2.1 billion in 2013, and the prevalence of obesity in women has more than doubled within the past four decades.1 In most European countries more than 10% of pregnant women are obese, varying from 7.1% in Poland to 20.7% in Scotland.2
Obesity has been associated with increased incidences of gynaecological cancers,3 4 5 poor survival from gynaecological cancer,6 7 increased incidence of polycystic ovary syndrome,8 and infertility.9 Obesity before and during pregnancy is associated with multiple health conditions for both the mother and the fetus, including a higher rate of gestational diabetes, large for gestational age babies, hypertensive disorders, instrumental delivery and caesarean section, preterm birth, and congenital anomalies, a lower initiation rate and shorter duration of breast feeding, and obesity and cardiometabolic morbidity in adult life.10 11 12 13
Although many of the suggested associations could be causal, some might be biased. Residual confounding and selective reporting of strong positive results could overinflate the observed magnitudes of effect,14 15 16 and recent umbrella reviews have shown that, despite the strong claims of significant associations in several scientific specialties, only a fraction were considered to have robust support without hints of bias.17 18 19 20 21 22
We performed an umbrella review of systematic reviews and meta-analyses of the association between adiposity indices with risk of any type of obstetric or gynaecological morbidity to assess the breadth, strength, and validity of the reported estimates.
Methods
Search strategy and selection criteria
We searched PubMed and the Cochrane database of systematic reviews from inception to May 2016 for systematic reviews (with or without meta-analyses) of observational studies published in English that investigated the association between adiposity and risk of any disease, outcome, or health state in the specialty of obstetrics and gynaecology. Adiposity was defined as excess accumulation of fat and was used to capture all obesity indices assessed: body mass index (BMI), weight, weight gain, gestational weight gain, waist to hip ratio, waist circumference, hip circumference, or bariatric surgery. We also searched for meta-analyses of randomised or non-randomised controlled trials that investigated dietary, exercise, or mixed interventions for weight loss and obstetric and gynaecological outcomes. The search algorithms used can be found in appendix 1. We additionally hand searched the references of the articles reaching full text review to identify any articles possibly missed by the initial search or any unpublished data. Appendix 1 also provides detailed explanation of data extraction and analysis.
Inclusion and exclusion criteria
We excluded meta-analyses in which the exposure or outcome was not relevant to clinical obstetrics and gynaecology (such as congenital malformations or childhood obesity and age at menarche), single arm meta-analyses that did not contain a comparison group, prognostic studies associating adiposity with cancer survival or mortality among patients who already had a diagnosis of gynaecological cancer or another gynaecological condition, and meta-analyses assessing obesity as a predictive variable or screening test.
Meta-analyses that did not present comprehensive study specific data (number of incident events, number of study population or person years, relative risks and 95% confidence intervals) and in which the missing data were not retrievable from the authors of meta-analyses or from the original studies (considered not possible when more than one category of study specific data was missing) were also excluded.
If more than one meta-analysis existed for the same exposure-outcome association, we selected the one with the largest number of studies. Meta-analyses were evaluated as they were originally presented—expansion of one meta-analysis with studies detected by another on the same topic was beyond the scope of this review. In a sensitivity analysis we further assessed whether there were any differences in the summary findings when the same exposure-outcome association was assessed in more than one meta-analyses.
For interventional meta-analyses, we included all meta-analyses of controlled trials investigating dietary, exercise, or mixed interventions aimed at weight loss on outcomes included in the main analysis.
Evaluation of the strength of evidence
We graded the strength of the evidence in the reported associations for observational studies using several criteria (see appendix 1 and previous publications20 21). An association was deemed as strong when the P value of the random effects model was <10−6, the meta-analysis included >1000 cases/exposed women, the P value of the largest included study was <0.05, heterogeneity between studies as measured with I2 statistic was <50%, the meta-analysis did not show evidence of small study effects, the 95% prediction interval excluded the null value, there was no evidence of excess significance bias, and the meta-analysis survived the 10% credibility ceiling. An association was considered as highly suggestive if it presented a P value of <10−6 in random effects model, included >1000 cases/exposed women, and the P value of the largest study in the meta-analysis was <0.05. Suggestive associations presented a P value of <10−3 in random effects model and included >1000 cases/exposed women. P<0.05 in random effects models indicated weak associations.
Evaluation of quality of included meta-analyses
We assessed the quality of all included observational meta-analyses using the AMSTAR tool, which uses 11 items to measure the methodological quality of systematic reviews.23
To assess the quality of evidence and risk of bias in the interventional meta-analyses used in sensitivity analyses, we extracted and present here the method of quality assessment performed in each original meta-analysis.
Our primary analysis focused only on results from cohort studies, which are considered the ideal approach in observational research; sensitivity analyses included case-control studies. Evidence from interventional meta-analyses was analysed separately. Figure 1 summarises the applied methods. We used STATA version 13 for statistical analyses.24 P values were two tailed.
Fig 1 Graphical presentation of main and sensitivity analyses of meta-analyses investigating obesity and gynaecological and obstetric conditions
Patient involvement
No patients were involved in setting the research question or the outcome measures, nor were they involved in developing plans for design or implementation of the study. No patients were asked to advise on interpretation or writing up of results. It was not evaluated whether the studies included in the review had any patient involvement. The results will be disseminated to the general public through public presentations and authors’ involvement in different charities.
Results
Meta-analyses of observational studies
Characteristics of meta-analyses
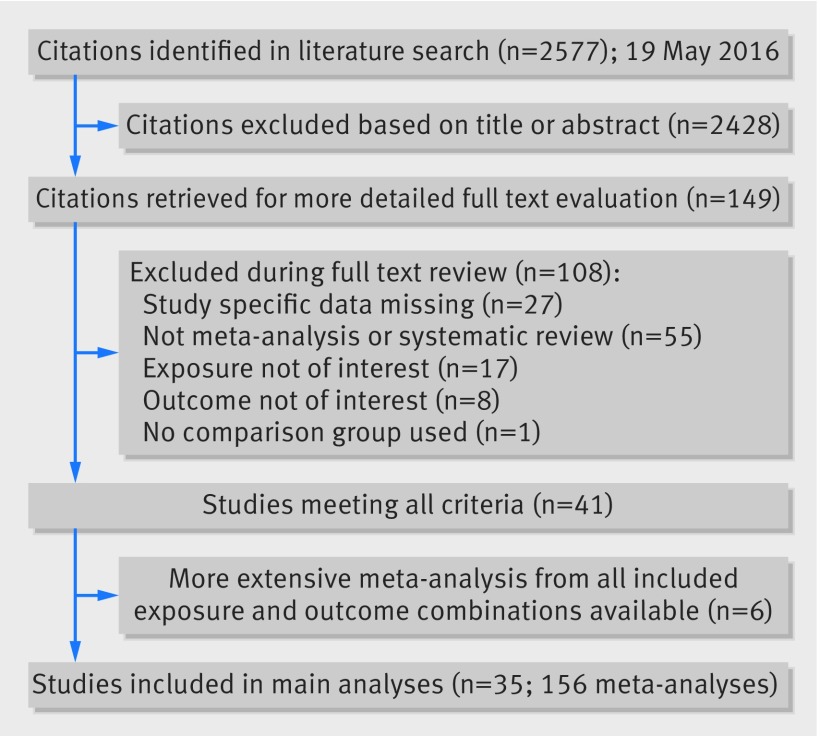
We extracted data from 35 manuscripts, which included 156 meta-analyses with 1308 individual study estimates (fig 2).3 4 5 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 Each meta-analysis combined two to 40 study estimates, with a median of six. The median number of cases and population/controls in each meta-analysis was 1733 and 151 309, respectively. The lowest number of cases in a meta-analysis was 14 and highest was 137 567. A total of 99 individual meta-analyses had at least 1000 cases of the studied outcome. Most of the 502 unique individual studies included in this umbrella review (many appearing in more than one meta-analysis) were cohort studies (427, 85.1%), 39 (7.8%) were case-control studies, 22 were cross-sectional studies (4.4%), and 14 (2.7%) were case series.
Fig 2 Identification and inclusion of observational meta-analyses investigating obesity and gynaecological and obstetric conditions
These 156 meta-analyses included associations between eight exposures (body mass index (BMI), hip circumference, waist circumference, waist to hip ratio, weight, weight gain, gestational weight gain, or bariatric surgery) and the incidence of 84 different outcomes in obstetrics and gynaecology, as reported by the authors of the original meta-analyses (emergency and elective caesarean sections, for example, are hence considered to be two separate outcomes). For each outcome more than one exposure could be included (tables A-C, appendix 2).
A total of 144 meta-analyses included at least two cohort studies with 1163 individual study estimates. The median number of cases and population/controls in each meta-analysis of cohort studies was 1990 and 243 267, respectively, and 98 individual meta-analyses had at least 1000 cases. There were two to 40 study estimates combined per meta-analysis with a median of six. Most meta-analyses (n=110, 76%) used a categorical contrast to measure the exposure, and in only 34 instances was a continuous contrast used. For our main analysis we concentrated only on these meta-analyses of cohort studies as they constitute the best available evidence among observational studies.
Summary effect size
At a threshold of P<0.05 as level of significance, the summary fixed effects estimates were significant in 115 out of the 144 meta-analyses of cohort studies (80%), whereas the summary random effects were significant in 105 (73%). At a threshold of significance at P<0.001, 97 (67%) and 78 meta-analyses (54%) produced significant summary results using the fixed and random effects model, respectively, while at a threshold of P<10−6, 83 (58%) and 51 (35%) associations were significant (table A in appendix 2).
Of the 51 associations with P<10−6 in the random effects model, 50 yielded an increased risk of adiposity with obstetric or gynaecological conditions (table A in appendix 2).
Table A in appendix 2 also shows the effect of the largest study included in each meta-analysis. Most (n=107, 74%) of these effects were nominally significant at P<0.05, and 76 showed an increased risk.
The reported odds ratios (n=64) ranged from 0.32 to 5.52, the reported relative risks (n=68) ranged from 0.39 to 4.69, and the three hazard ratios ranged from 1.09 to 1.44 (fig 3). The magnitude of these odds ratios was small (the summary estimate being between 0.8 and 1.2) in 19 of the 64 meta-analyses (30%), while 12 of the 34 meta-analyses (35%) reported a relative risk of small magnitude. In 31 (48%) and 30 (44%) the summary effect estimate was considered moderate (the summary effect estimate between 1.2 and 2.0 or 0.5 and 0.8), respectively (fig 3). The summary effect estimates approached 1.00 with decreasing variances.
Fig 3 Association between summary random effects estimates and inverse of variance in meta-analyses, stratified by type of exposure-outcome pair
Heterogeneity between studies
Τhe Q test for heterogeneity was significant at P<0.10 for 75 out of 144 meta-analyses (52%) (table B in appendix 2). There was moderate to high heterogeneity (I2=50-75%) in 29 meta-analyses (20%) and substantial heterogeneity (I2>75%) in 37 (26%). We further accounted for heterogeneity between studies by calculating 95% prediction intervals and found 41 associations in which the null value was excluded (table A in appendix 2).
Small study effects
The presence of small study effects was suggested in 12 meta-analyses (Egger’s test at P<0.10, with more conservative effects in the largest study of a meta-analysis compared with the summary random effects estimate) (table B in appendix 2). Only four of these 12 meta-analyses (associations between BMI and increased risk of ovarian cancer, miscarriage, low birth weight, and stillbirth), however, included an adequate number of studies (10 or more) for Egger’s test to have adequate statistical power to identify small study effects.
Excess significance bias
We further assessed the presence of excess significance bias and explored whether the observed number of studies with nominally significant results (“positive” studies, P<0.10) was different from the expected number of studies based on the largest study in each meta-analysis, and observed 11 (8%) meta-analyses in which the excess significance test was significant (assisted reproduction: pregnancy rate among women undergoing IVF (n=1); gynaecological oncology: complication rate in ovarian cancer surgery (n=1), incidence of ovarian cancer (n=2), incidence of endometrial cancer (n=2); obstetric fetal: stillbirth (n=1), admission to neonatal intensive care unit (n=1), neonatal death (n=1), fetal death (n=1), and low birth weight <2500 g (n=1)). When we used the random or fixed effect estimate as the plausible effect size, seven meta-analyses presented evidence of excess significance on both occasions (table B in appendix 2).
Credibility ceilings
From all 144 meta-analyses, 100 (69%) met nominal significance (P<0.05) with a credibility ceiling of 5%. With ceilings of 10%, 15%, and 20%, 70 (49%), 47 (33%), and 31 (22%) meta-analyses remained significant, respectively (table C in appendix 2).
Quality assessment
We used the 11 item AMSTAR tool to assess the methodological quality of all 35 included meta-analyses of observational studies (table D in appendix 2). Most reviews selected the studies and extracted the data in duplicate (25/35, 71%), performed a comprehensive literature search (27/35, 77%), used appropriate methods to combine the findings (29/35, 83%), assessed likelihood of publication bias (22/35, 63%), and provided the characteristics of included studies (33/35, 94%). Twenty one studies (60%) assessed the scientific quality of included studies, but only two (6%) used this assessment to appropriately formulate the conclusions. None of the included meta-analyses disclosed funding or other potential conflicts of interest of either the original studies or the meta-analysis, and only eight (23%) provided “a priori” published protocol or statement of ethical approval. Overall, most studies (32/35, 91%) scored 4 to 7 points and were considered of moderate quality, two studies (6%) were considered to be of low quality, and only one (3%) was high quality.
Grading of evidence
We further explored which of the reported associations had strong, highly suggestive, suggestive, or weak evidence (table 1). Only 8% of meta-analyses (11/144) met the criteria for strong evidence (table 2): they examined associations between obesity and increased risk of endometrial cancer (n=2), ovarian cancer (n=1), fetal macrosomia (n=1), low Apgar score at one minute (n=2), antenatal depression (n=1), total (elective and emergency) and emergency caesarean section (n=1 for both outcomes), and pre-eclampsia (n=2). Most examined the effect of BMI (nine studies), whereas one study examined gestational weight gain and one waist to hip ratio. The summary effect estimates ranged from 1.21 (95% confidence interval 1.13 to 1.29) for an association between a 0.1 unit increase in waist to hip ratio and risk of endometrial cancer up to 4.14 (3.61 to 4.75) for risk of pre-eclampsia in women with BMI >35 compared with <25.
Table 1.
Summary of evidence grading for meta-analyses associating obesity and risk of obstetric and gynaecological morbidity from cohort studies. Risk are for incidences unless stated otherwise
| Evidence* | Criteria used | Decreased risk | Increased risk | |||
|---|---|---|---|---|---|---|
| Cancer | Fetal outcomes | Maternal outcomes | Other | |||
| Strong | P<10−6 †; >1000 cases; P<0.05 of largest study in meta-analysis; I2 <50%; no small study effect‡; prediction interval excludes null value; no excess significance bias§; survives 10% credibility ceiling, n=11 | None | Endometrial total and PrMP (WHR per 0.1 units and BMI per 5 kg/m2, respectively); ovarian (>30 v <25) | Apgar score <7 at 1 minute (BMI 30-40 v <25 & >40 v <25); macrosomia (GWG, high v normal) | Antenatal depression (BMI >30 v <25); caesarean section, total (>30 v <25), emergency (30 v <25); pre-eclampsia, adj (>35 v <25 & 25-30 v <25) | — |
| Highly suggestive | P<10−6 †; >1000 cases; P<0.05 of largest study in meta-analysis, n=32 | Fetal outcome: SGA <10th centile (BMI 25-30 v <25) | Endometrial total (BMI: per 5 kg/m2, iya per 5 kg/m2, >30 v <25, WC per 10 cm, weight per 5 kg, weight gain per 5 kg); endometrial PoMP, type I & type II (per 5 kg/m2) | Apgar score <7 at 1 minute (BMI 25-29.99 v <25); Apgar score <7 at 5 minutes (30-40 v <25 & >40 v <25); LGA >90th centile, (>30 v <25; 25-30 v <25; >40 v 30-35); macrosomia >4000 or 4500 g (>30 v <25 & 25-30 v <25); stillbirth risk, after h20/28 (per 5 kg/m2) | GDM (BMI >30 v <25 & 25-30 v <25); instrumental delivery (>30 v <25); PPH (>30 v <25); PPWR 0-1 years PP (GWG, high v normal); pre-eclampsia, unadj and adj (>30 v <25 & 25-30 v <25); PTB total and induced (>25 v <25; >40 v 30-35; >40 v 30-40) | Assisted reproduction: miscarriage rate (BMI >25 v <25) |
| Suggestive | P<10−3 †; >1000 cases, n=22 | Assisted reproduction: live birth rate (BMI >25 v <25 & >30 v <25). Pregnancy rate (25 v <25 & 25-30 v <25). Fetal outcome: LBW <2500 g (GWG high v normal); SGA <10th centile (>30 v <25 & >40 v 30-35) | Ovarian (BMI per 5 kg/m2 & BMI iya per 5 kg/m2) | Antepartum stillbirth (BMI per 5 kg/m2); fetal death (per 5 kg/m2); infant death (per 5 kg/m2); macrosomia >4500 g (25-30 v <25); neonatal death risk (per 5 kg/m2); NICU admission (>30 v <25); post neonatal death (per 5 kg/m2) | PP depression (BMI >30 v <25); miscarriage (per 5 kg/m2 & >25 v <25); PPWR, 0-21 years pp (GWG, high v normal) | Assisted reproduction: miscarriage rate (BMI >30 v <25; 25-30 v <25; >30 v <30) |
| Weak | P<0.05†, n=34 | Assisted reproduction: live birth rate (BMI 25-30 v <25); ovulation rate (obese v not, varied cut offs); pregnancy rate (>30 v <25). Cancer: endometrial (bariatric surgery). Fetal outcome: LBW <2500 g (BMI 25-30 v <25 & >25 v <25); macrosomia >4000 g (bariatric surgery); GDM (bariatric surgery); gestational hypertensive disorders (bariatric surgery); PTB <37 pregnancy weeks (GWG, high v normal) | Cervical (BMI 25-30 v <25); endometrial (>25 v <25, HC per 10 cm); endometrial ever & never HRT (per 5 kg/m2 & WG per 5 kg); endometrial mortality (per 5 kg/m2); ovarian (weight per 5 kg); ovarian PrMP & PoMP (>30 v <25); ovarian C in, PoMP, never HRT (WG per 5 kg); wound complication in ovarian surgery (>30 v <30) | Apgar score <7 at 5 minutes (25-29.99 v <25); score <3 at 5 minutes (30-40 v <25); early neonatal death (per 5 kg/m2); SGA <5th/10th centile (bariatric surgery) | Antenatal anxiety (BMI >30 v < 25); GDM (30-35 <25 & >35 v <25; GWG excess v not); antepartum haemorrhage (obese v not, varied cut offs); maternal infection (>30 v <25); PPWR, 1-9 & >15 years pp (GWG, high v normal) | Assisted reproduction: No of oocytes retrieved (BMI >25 v <25 & >30 v <30). Reproductive health: pregnancy rate among COC users (>30 v <25). Other: age at natural menopause (BMI 25-29.99 v <25) |
Adj=effect estimate based only on studies with adjusted risk estimates; BMI=body mass index; COC=combined oral contraceptive; GDM=gestational diabetes mellitus; GWG=gestational weight gain; HC=hip circumference; HRT=hormone replacement therapy; iya=in young adulthood; LBW=low birth weight; LGA=large for gestational age; NICU=neonatal intensive care unit; PoMP=postmenopausal; PrMP=premenopausal; PP=postpartum; PPH=postpartum haemorrhage; PPWR=postpartum weight retention; PTB=preterm birth; SGA=small for gestational age; unadj=effect estimate based only on unadjusted raw numbers of included studies; WHR=waist to hip ratio; WC=waist circumference;
*For following outcomes no meta-analysis, regardless of exposure, met criteria even for weak evidence: assisted reproduction: cycle cancellation rate, ectopic pregnancy rate, multiple pregnancy rate, and OHSS rate; gynaecological oncology: cytoreduction rate, febrile complication rate, ileus rate, postoperative pneumonia rate, total complication rate, venous thromboembolism rate, estimated blood loss, and total operation time in ovarian cancer surgery; obstetric, fetal: asphyxia inc, intrapartum stillbirth risk, perinatal death risk; obstetric, maternal: shoulder dystocia incidence.
†P values for meta-analysis random effects model.
‡Small study effect based on P value from Egger’s regression asymmetry test (P>0.1) where random effects summary estimate was larger compared with point estimate of largest study in meta-analysis.
§Based on P value (P>0.1) of excess significance test with largest study (smallest SE) in meta-analysis as plausible effect size.
Table 2.
Details of associations supported by strong evidence in meta-analyses of cohort studies on obesity and risk of obstetric and gynaecological morbidity. Outcomes are incidences unless stated otherwise. All statistical tests were two sided
| Exposure | Outcome | No of studies | Sample size (cases/cohort | RR (95% CI) of largest study | Random effects summary RR (95% CI)* | Random P value† | 95% prediction interval | 10% credibility RR (95% CI)* | Egger’s P‡ | I2 (%) | Excess significance§ | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| O/E | P value | |||||||||||
| Gynaecological oncology | ||||||||||||
| WHR per 0.1 units | Endometrial CA | 5 | 2447/394 340 | 1.33 (1.18 to 1.51) | 1.21 (1.13 to 1.29) | 1.0−8 | 1.09 to 1.34 | 1.17 (1.04 to 1.31) | 0.54 | 0 | 3/4.4 | NA |
| BMI per 5kg/m2 increase | Endometrial CA PrMP | 6 | 5981/2 558 935 | 1.53 (1.48 to 1.58) | 1.49 (1.39 to 1.61) | 3.1−27 | 1.27 to 1.76 | 1.36 (1.11 to 1.67) | 0.56 | 20 | 5/4.2 | 0.67 |
| BMI >30 v <25 | Ovarian CA | 13 | 6947/20 560 388¶ | 1.27 (1.19 to 1.36) | 1.27 (1.17 to 1.38) | 2.6−8 | 1.09 to 1.47 | 1.16 (1.02 to 1.30) | 0.88 | 12 | 3/5.3 | NA |
| Obstetric, fetal | ||||||||||||
| BMI 30-40 v <25 | Apgar <7 at 1 minute | 4 | 16 187/230 884 | 1.27 (1.22 to 1.32) | 1.29 (1.23 to 1.36) | <1−100 | 1.10 to 1.52 | 1.28 (1.06 to 1.56) | 0.51 | 20 | 4/3.9 | 1.00 |
| BMI >40 v < 5 | Apgar <7 at 1 minute | 3 | 9958/153 104 | 1.63 (1.52 to 1.74) | 1.63 (1.53 to 1.74) | <1−100 | 1.08 to 2.47 | 1.64 (1.06 to 2.55) | 0.62 | 0 | 3/3.0 | 1.00 |
| GWG high v normal | Macrosomia risk: >4000 g | 11 | 25 985/401 803 | 2.00 (1.90 to 2.10) | 2.08 (1.92 to 2.26) | <1−100 | 1.72 to 2.52 | 2.06 (1.46 to 2.92) | 0.14 | 41 | 9/11.0 | NA |
| Obstetric, maternal | ||||||||||||
| BMI >30 v <25 | Antenatal depression risk | 23 | 6370/46 182 | 1.48 (1.32 to 1.67) | 1.48 (1.32 to 1.66 | 1.3−11 | 1.07 to 2.05 | 1.30 (1.13 to 1.49) | 0.97 | 39 | 7/12.0 | NA |
| BMI >30 v <25 | Caesarean section-emergency | 6 | 2301/18 749 | 1.51 (1.21 to 1.89) | 1.63 (1.40 to 1.89) | 4.0−10 | 1.31 to 2.02 | 1.63 (1.15 to 2.31) | 0.29 | 0 | 5/3.9 | 0.67 |
| BMI >30 v <25 | Caesarean section-total | 16 | 8413/62 277 | 2.02 (1.79 to 2.28) | 2.00 (1.87 to 2.15) | <1−100 | 1.86 to 2.16 | 1.86 (1.45 to 2.39) | 0.66 | 0 | 12/14.5 | NA |
| BMI 25-30 v <25 | Pre-eclampsia (adjusted)** | 12 | 30 001/1 091 624 | 1.74 (1.69 to 1.80) | 1.70 (1.60 to 1.81) | <1−100 | 1.49 to 1.95 | 1.59 (1.26 to 2.01) | 0.38 | 29 | 10/10.8 | NA |
| BMI >35 v <25 | Pre-eclampsia (adjusted)** | 5 | 12 614/901 409 | 4.28 (3.48 to 5.26) | 4.14 (3.61 to 4.75) | <1−100 | 3.32 to 5.17 | 3.96 (1.54 to 10.2) | 0.84 | 0 | 5/5.0 | 1.00 |
BMI=body mass index; WHR=waist to hip ratio; CA=cancer; NA=not applicable, because estimated number is larger than observed, and there is no evidence of excess significance based on assumption made for plausible effect size; PrMP=premenopausal; GWG=gestational weight gain.
*From random effects model. RR for categorical outcome measure.
†P value of summary random effects estimate.
‡From Egger’s regression asymmetry test.
§Expected number of significant studies with point estimate of largest study (smallest SE) as plausible effect size. O/E=observed/expected number of studies with significant results. P value for excess significance test.
¶Person years.
**Estimate based on studies that used adjusted risk estimates
A higher proportion of meta-analyses were supported by highly suggestive evidence (32/144, 22%) and evaluated associations between obesity and increase in risk of miscarriage among women undergoing IVF, endometrial cancer (n=9), large for gestational age (n=3), macrosomia (n=3), low Apgar score at one and five minutes (n=3), stillbirth (n=1), gestational diabetes mellitus (n=2), instrumental delivery (n=1), postpartum haemorrhage (n=1), postpartum weight retention (n=1), pre-eclampsia (n=3), and preterm birth (n=3) and decrease in risk of babies small for gestational age (table 3).
Table 3.
Details of associations supported by highly suggestive evidence in meta-analyses of cohort studies on obesity and risk of obstetric and gynaecological morbidity. Outcomes are incidences unless stated otherwise. All statistical tests were two sided.
| Exposure | Outcome | No of studies | Sample size (cases/cohort) | RR (95% CI) of largest study | Random effects summary RR (95% CI)* | Random P value† | 95% prediction interval | 10% credibility RR (95% CI)* | Egger’s P‡ | I2 (%) | Excess significance§ | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| O/E | P value | |||||||||||
| Assisted reproduction | ||||||||||||
| BMI >25 v <25 | Miscarriage rate | 20 | 3651/17 797 | 1.35 (1.20 to 1.53) | 1.31 (1.18 to 1.45) | 1.6−7 | 0.97 to 1.77 | 1.18 (1.05 to 1.33) | 1.00 | 46 | 8/8.0 | 1.00 |
| Gynaecological oncology | ||||||||||||
| WG per 5 kg increase | Endometrial cancer | 7 | 2806/460 901 | 1.17 (1.12 to 1.22) | 1.16 (1.12 to 1.20) | 3.7−18 | 1.06 to 1.27 | 1.13 (1.05 to 1.22) | 0.95 | 47 | 6/2.8 | 0.02 |
| Weight per 5 kg | Endometrial cancer | 7 | 1778/342 382 | 1.11 (1.08 to 1.15) | 1.17 (1.13 to 1.22) | 7.7−15 | 1.04 to 1.31 | 1.15 (1.06 to 1.25) | 0.29 | 62 | 6/1.0 | <0.01 |
| BMI iya per 5 kg/m2 | Endometrial cancer | 9 | 4345/631 915 | 1.23 (1.11 to 1.35) | 1.45 (1.28 to 1.64) | 1.9−9 | 0.98 to 2.15 | 1.33 (1.14 to 1.55) | 0.41 | 75 | 8/5.2 | 0.09 |
| BMI per 5 kg/m2 | Endometrial cancer | 28 | 22 320/6 445 255 | 1.65 (1.60 to 1.71) | 1.54 (1.47 to 1.61) | <1−100 | 1.26 to 1.89 | 1.41 (1.26 to 1.57) | 0.35 | 81 | 26/25.5 | 1.00 |
| BMI >30 v <25 | Endometrial cancer | 6 | 4327/1 485 506 | 2.73 (2.48 to 2.99) | 3.10 (2.63 to 3.65) | <1−100 | 1.92 to 5.00 | 2.99 (1.49 to 6.02) | 0.24 | 66 | 6/6.0 | 1.00 |
| WC per 10 cm | Endometrial cancer | 4 | 1524/315,770 | 1.28 (1.19 to 1.37) | 1.27 (1.17 to 1.39) | 7.3−8 | 0.88 to 1.85 | 1.20 (1.03 to 1.38) | 0.59 | 70 | 3/2.9 | 1.00 |
| BMI per 5 kg/m2 | Endometrial cancer, PoMP | 6 | 10 075/2 558 935 | 1.51 (1.45 to 1.58) | 1.60 (1.40 to 1.83) | 1.4−11 | 1.01 to 2.53 | 1.52 (1.16 to 1.98) | 0.88 | 89 | 6/5.0 | 0.60 |
| BMI per 5 kg/m2 | Endometrial cancer, type I | 3 | 7125/1 102 927 | 1.58 (1.53 to 1.62) | 1.75 (1.51 to 2.03) | 1.8−13 | 0.30 to 10.24 | 1.71 (1.06 to 2.77) | 0.26 | 82 | 3/2.9 | 1.00 |
| BMI per 5 kg/m2 | Endometrial cancer, type II | 3 | 1059/1 102 927 | 1.35 (1.25 to 1.46) | 1.59 (1.29 to 1.78) | 5.7−7 | 0.24 to 9.67 | 1.47 (1.03 to 2.08) | 0.52 | 76 | 3/1.5 | 0.25 |
| Obstetric, fetal | ||||||||||||
| BMI 25-30 v <25 | Apgar score <7 at 1 minute | 3 | 14 953/215 524 | 1.13 (1.09 to 1.17) | 1.14 (1.09 to 1.18) | 1.0−10 | 0.84 to 1.54 | 1.12 (1.01 to 1.24) | 0.79 | 8 | 2/2.5 | NA |
| BMI 30-40 v <25 | Apgar score <7 at 5 minutes | 8 | 4050/340 894 | 1.26 (1.16 to 1.36) | 1.40 (1.27 to 1.54) | 2.6−11 | 1.12 to 1.75 | 1.34 (1.12 to 1.61) | 0.04 | 34 | 6/5.6 | 1.00 |
| BMI >40 v <25 | Apgar score <7 at 5 minutes | 6 | 3015/290 134 | 1.70 (1.46 to 1.98) | 1.66 (1.36 to 2.02) | 7.5−7 | 0.91 to 3.02 | 1.22 (0.96 to 1.56) | 0.67 | 67 | 4/5.9 | NA |
| BMI 25-30 v <25 | LGA >90th centile | 18 | 92 234/1 041 119 | 1.54 (1.50 to 1.57) | 1.57 (1.47 to 1.67) | <1−100 | 1.25 to 1.97 | 1.34 (1.19 to 1.52) | 0.73 | 89 | 16/17.4 | NA |
| BMI >30 v <25 | LGA >90th centile | 19 | 88 791/969 294 | 1.95 (1.90 to 1.99) | 2.11 (1.97 to 2.27) | <1−100 | 1.62 to 2.75 | 1.68 (1.35 to 2.08) | 0.34 | 90 | 17/9.0 | NA |
| BMI >40 v 30-35 | LGA >90th centile | 7 | 32 377/229 817 | 1.30 (1.26 to 1.35) | 1.36 (1.29 to 1.45) | 6.1−26 | 1.17 to 1.59 | 1.22 (1.03 to 1.45) | 0.93 | 68 | 4/5.0 | NA |
| BMI 25-30 <25 | Macrosomia >4000 g | 19 | 93 168/1 049 501 | 1.54 (1.50 to 1.57) | 1.54 (1.45 to 1.64) | <1−100 | 1.22 to 1.95 | 1.32 (1.18 to 1.47) | 0.91 | 89 | 17/18.4 | NA |
| BMI >30 v <25 | Macrosomia >4000 g | 20 | 89 849/977 613 | 1.95 (1.90 to 1.99) | 2.08 (1.94 to 2.23) | <1−100 | 1.61 to 2.70 | 1.68 (1.36 to 2.07) | 0.40 | 90 | 18/20.0 | NA |
| BMI >30 v <25 | Macrosomia >4500 g | 7 | 2405/154 197 | 1.94 (1.72 to 2.18) | 3.59 (2.53 to 5.09) | 7.9−13 | 1.23 to 10.52 | 2.89 (1.48 to 5.62) | 0.18 | 89 | 7/4.9 | 0.11 |
| BMI 25-30 v <25 | SGA <10th centile | 14 | 71 232/774 816 | 0.79 (0.77 to 0.81) | 0.80 (0.73 to 0.87) | 2.5−7 | 0.61 to 1.05 | 0.87 (0.78 to 0.96) | 0.50 | 88 | 8/9.1 | NA |
| BMI per 5kg/m2 | Stillbirth >20/28 weeks’ gestation | 18 | 16 274/3 288 688 | 1.14 (1.11 to 1.16) | 1.24 (1.18 to 1.30) | 1.4−18 | 1.03 to 1.48 | 1.12 (1.06 to 1.18) | 0.02 | 79 | 15/7.4 | <0.01 |
| Obstetric, maternal | ||||||||||||
| BMI >30 v <25 | GDM | 30 | 7941/361 340 | 4.80 (4.43 to 5.21) | 3.78 (3.31 to 4.32) | <1−100 | 2.22 to 6.43 | 3.29 (2.31 to 4.70) | 0.70 | 74 | 25/28.0 | NA |
| BMI 25-30 v <25 | GDM | 17 | 6746/394 338 | 2.29 (2.12 to 2.47) | 1.97 (1.76 to 2.19) | 7.9−35 | 1.44 to 2.67 | 1.66 (1.29 to 2.13) | 0.52 | 56 | 11/13.7 | NA |
| BMI >30 v <25 | Instrumental delivery | 4 | 114 847/1 671 077 | 1.17 (1.13 to 1.21) | 1.17 (1.12 to 1.23) | 5.9−11 | 0.99 to 1.39 | 1.12 (0.98 to 1.29) | 0.96 | 51 | 3/3.7 | NA |
| BMI >30 v <25 | PPH | 7 | 95 586/1 692 216 | 1.19 (1.15 to 1.23) | 1.48 (1.27 to 1.73) | 6.0−7 | 0.94 to 2.34 | 1.31 (1.08 to 1.59) | 0.06 | 85 | 5/4.5 | 1.00 |
| GWG high v normal | PPWR (kg) at 0-1 years | 8 | 9229/17 657¶ | 2.50 (2.35 to 2.65) | 3.02 (2.31 to 3.73) | <1−100 | 0.66 to 5.38 | 2.31 (0.90 to 3.71) | 0.88 | 95 | 7/7.2 | NA |
| BMI >30 v <25 | Pre-eclampsia, unadj | 40 | 137 567/4 430 230 | 2.64 (2.59 to 2.70) | 2.82 (2.57 to 3.10) | <1−100 | 1.65 to 4.82 | 2.49 (1.98 to 3.13) | 0.78 | 98 | 38/38.6 | NA |
| BMI 25-30 v <25 | Pre-eclampsia, unadj | 38 | 106 770/3 271 222 | 1.96 (1.91 to 2.01) | 2.08 (1.98 to 2.19) | <1−100 | 1.65 to 2.63 | 1.87 (0.58 to 2.21) | 0.56 | 89 | 35/34.7 | 1.00 |
| BMI >30 v <25 | Pre-eclampsia, adj | 10 | 36 457/1 791 255 | 3.37 (3.25 to 3.49) | 2.93 (2.58 to 3.33) | <1−100 | 2.07 to 4.15 | 2.81 (1.70 to 4.66) | 0.11 | 67 | 10/10.0 | 1.00 |
| BMI >25 v <25 | PTB (induced) at <37 weeks’ gestation | 5 | 5664/133 307 | 1.28 (1.20 to 1.36) | 1.30 (1.23 to 1.37) | 2.7−22 | 1.19 to 1.41 | 1.35 (1.09 to 1.68) | 0.03 | 0 | 3/3.2 | NA |
| BMI >40 v 30-40 | PTB <37 weeks’ gestation | 19 | 43 266/516 546 | 1.17 (1.12 to 1.21) | 1.20 (1.13 to 1.27) | 9.4−10 | 1.01 to 1.42 | 1.14 (1.06 to 1.21) | 0.51 | 60 | 10/9.3 | 0.82 |
| BMI >40 v 30-35 | PTB <37 weeks’ gestation | 10 | 22 964/295 103 | 1.19 (1.14 to 1.24) | 1.31 (1.19 to 1.43) | 1.8−8 | 1.01 to 1.68 | 1.24 (1.09 to 1.41) | 0.44 | 70 | 7/5.0 | 0.34 |
BMI=body mass index; WG=weight gain; iya=in young adulthood; WC=waist circumference; LGA=large for gestational age; NA=not applicable, because estimated number is larger than observed, and there is no evidence of excess significance based on assumption made for plausible effect size; SGA=small for gestational age; GDM=gestational diabetes mellitus; PPH=postpartum haemorrhage; PPWR=postpartum weight retention; PTB=preterm birth; adj=effect estimate based on studies with adjusted risk estimates; unadj=effect estimate based on unadjusted raw numbers of included studies.
*From random effects model. RR for categorical outcome measure.
†P value of summary random effects estimate.
‡From Egger’s regression asymmetry test.
§Expected number of significant studies with point estimate of largest study (smallest SE) as plausible effect size. O/E=observed/expected number of studies with significant results. P value for excess significance test.
¶Mean difference.
About a sixth of meta-analyses (23/144, 16%) described suggestive evidence, a quarter (39/144, 27%) were supported only by weak evidence, and 27% showed no association (P>0.05) (table 1 and table E in appendix 2).
Sensitivity analysis
When we assessed both cohort and case-control studies, two further associations met the criteria for strong evidence: the effect of BMI on macrosomia and risk of postpartum depression, both considered to be suggestive when we included only cohort studies. Two associations no longer met the criteria to be ranked as “strong,” including the association between BMI and antenatal depression and the association between gestational weight gain and macrosomia (table F in appendix 2).
We identified 107 duplicate meta-analyses, 34 from studies meeting all inclusion criteria (six studies) and 73 from studies with more than one category of study specific data missing (number of cases or cohort, relative risk, 95% confidence interval) (n=22), investigating the same exposure and outcome association for 28 outcomes (table G in appendix 2). For most of these duplicate meta-analyses, there was agreement in principle on the direction, magnitude, and significance of the summary associations with the included meta-analyses.
Meta-analyses of interventional studies
We identified 102 meta-analyses of interventional studies from 18 publications,57 58 59 60 61 62 63 64 65 66 67 68 69 70 71 72 73 74 including 292 individual study estimates, of which 287 were from randomised controlled trials, four from quasi-randomised trials, and one from a non-randomised trial, describing the effect of dietary interventions (n=33), physical exercise (n=21), or mixed interventions (n=48) aimed to reduce weight or gestational weight gain on 19 outcomes (table H in appendix 2).
Nominally significant associations were observed in 27/102 (26%) meta-analyses for 16 associations on 10 separate outcomes: between physical exercise and reduced rates of large for gestational age babies, caesarean section, gestational diabetes, gestational weight gain, excessive gestational weight gain, and postnatal depression; between dietary interventions and reduction of gestational diabetes, gestational weight gain, pre-eclampsia, preterm birth, postpartum weight retention, and shoulder dystocia; and between mixed approach with reduced risk of gestational weight gain, excessive gestational weight gain, pre-eclampsia, and shoulder dystocia (table H in appendix 2).
Altogether 16 out of 18 publications assessed the risk of bias of the included studies (table I in appendix 2). Most publications (11/18) used the Cochrane risk of bias tool75 76 or a modification of this. Other tools for assessment of methods or risk of bias included the CONSORT statement (n=2), GRADE score (n=2), Jadad score (n=3), PRISMA guidelines (n=2), and a modified Delphi list (n=1). Six publications used more than one tool to assess bias, and two publications used none. In all publications most of the primarily included studies were deemed as low risk of bias/high methodological quality, except in two57 58 in which no study scored more than 3 points on a 5 point Jadad scale (table H in appendix 2). For the two publications (30 meta-analyses) that used the GRADE method,77 21 out of the 30 meta-analyses presented moderate or high levels of evidence (table H in appendix 2). Of the 20 exposure-outcome pairs with more than one interventional meta-analysis, most presented results were essentially similar as 95% confidence intervals overlapped between duplicate meta-analyses (table H in appendix 2).
Altogether 17 outcomes were studied by both observational and interventional meta-analyses, whereas two outcomes were studied only by interventional (gestational weight gain and excessive gestational weight gain) and 67 only by observational meta-analyses. When we compared the results between interventional and observational meta-analyses, the effect was concordant (for example, adiposity increased the incidence, whereas the intervention decreased it and vice versa) for all nominally significant associations apart from one. The rate of shoulder dystocia was not associated with adiposity in the observational meta-analysis, though it decreased with interventions that reduced obesity.
Out of eight outcomes that met the strong criteria in observational studies, only three were studied in meta-analyses of interventions that could reduce obesity. These interventions were found to significantly reduce the rates of total caesarean section and pre-eclampsia, whereas the results for fetal macrosomia did not reach nominal significance. No interventional meta-analyses have been published on the five remaining outcomes with strong associations (endometrial or ovarian cancer, low Apgar score at one minute, antenatal depression, or emergency caesarean section). Out of the 13 outcomes supported by highly suggestive evidence, 11 were assessed in at least one meta-analysis of interventions. These interventions resulted in significant reduction in the rates of large for gestational age, gestational diabetes mellitus, postpartum weight retention, pre-eclampsia, and preterm birth in at least one meta-analysis. The rates of macrosomia, small for gestational age, stillbirth/intrauterine death, perinatal mortality, instrumental delivery, low Apgar score at five minutes, or risk of postpartum haemorrhage were not significantly reduced by interventions, whereas the rate of miscarriage after IVF or any type of endometrial cancer was not assessed in any interventional meta-analysis.
Discussion
Main findings and interpretation in light of evidence
We included 156 meta-analyses of observational studies and 102 meta-analyses of clinical trials that evaluated the current evidence on the association of different adiposity indices with any type of obstetric or gynaecological condition. Eleven meta-analyses of observational studies examining eight outcomes met the criteria for strong associations. These examined the associations between adiposity indices (mostly BMI) and the risk of endometrial and ovarian cancer, fetal macrosomia, pre-eclampsia, low Apgar score (at one minute), antenatal depression, and total and emergency caesarean section. Furthermore, we observed highly suggestive evidence for 32 associations that included risks of these outcomes with various other indices of obesity and, in addition, new outcomes that included the rate of miscarriage among women undergoing IVF, fetal macrosomia, low Apgar score (at five minutes), stillbirth, gestational diabetes, instrumental delivery, postpartum haemorrhage, postpartum weight retention, and preterm birth as well as inverse association with rates of small for gestational age. We identified at least one interventional meta-analysis of weight loss interventions that reported nominally significant reductions in the rates of total caesarean sections, pre-eclampsia, large for gestational age, gestational diabetes, postpartum weight retention, pre-eclampsia, and preterm birth. The results of interventional meta-analyses were not significant for other outcomes including the rate of macrosomia, small for gestational age, stillbirth/intrauterine death, perinatal mortality, Apgar score <7 at five minutes, instrumental delivery, or postpartum haemorrhage. We did not find any meta-analyses that explored the impact of interventions that reduce obesity on the risk of miscarriage after IVF, any type of endometrial or ovarian cancer, antenatal depression, or emergency caesarean section.
Two recent reviews with narrative analysis associated obesity with at least 22 pregnancy related outcomes.10 78 Nineteen out of these 22 outcomes have been assessed in observational meta-analyses and were included in our review, but only five were supported by strong evidence (macrosomia, postnatal depression, total and emergency caesarean section, and pre-eclampsia). In these former reviews the claimed associations were based on nominal significance of a meta-analysis or single studies without systematic assessment of possible biases, whereas as in the current umbrella review we applied a transparent and replicable set of criteria to evaluate the quality and categorise the level of existing observational evidence.
One of the most prominent associations identified in the current umbrella review was that between obesity and risk of endometrial cancer, for which two meta-analyses met the criteria for strong and nine for highly suggestive evidence. The association between waist to hip ratio and incidence of total endometrial cancer was supported by strong evidence. The association of BMI (per 5 unit increase) with premenopausal endometrial cancer was supported by strong evidence and with highly suggestive evidence for risk of postmenopausal endometrial cancer. It is plausible that the difference in the evidence grading is because of heterogeneity caused by possible interactions with HRT use for postmenopausal endometrial cancer.79 The evidence was highly suggestive for the association between BMI and both type I and type II endometrial cancer and the associations between total endometrial cancer and waist circumference, weight, weight gain, and BMI in young adulthood around the age of 20. These results agree with those from recent Mendelian randomisation studies, in which increasing BMI (but not waist to hip ratio), hyperinsulinemia, and genetically predicted higher BMI were found to be causally associated with risk of endometrial cancer.80 81 Furthermore, the results are consistent with the reports of the World Cancer Research Fund Continuous Update Project (WCRF CUP), which rates the association between total endometrial cancer and BMI as convincingly causal and between waist circumference and waist to hip ratio as probably causal and with a recent report from the International Agency for Research on Cancer (IARC) on body fatness and cancer that concluded a strong causal relation between adiposity measures and risk of endometrial cancer.82 None of these studies have rated the evidence separately for pre-menopausal and post-menopausal women and for different histological subtypes,83 and no meta-analyses of randomised controlled trials have studied the effects of weight loss interventions on risk of endometrial cancer.
The association between BMI as a categorical variable, evaluated as obese versus normal weight, and the risk of ovarian cancer was strong. When this was assessed for BMI as a continuous variable (per 5 unit increase), the summary effect estimate was smaller (1.27 v 1.08), and the evidence was judged as only suggestive. A recent Mendelian randomisation study suggested a causal positive association between adult BMI and risk of ovarian cancer,84 while the WCRF CUP graded the evidence as probably causal.85 Similarly, IARC recent reported that there was strong evidence for an association between adiposity and ovarian cancer.82
Several meta-analyses have investigated the impact of maternal weight or weight gain on the weight of the fetus/neonate. The evidence supporting the association between gestational weight gain and birth weight above 4000 g was strong, while the evidence supporting the association between BMI and macrosomia at birth and fetuses large for gestational age was highly suggestive because of large heterogeneity between studies. These findings are in line with a recent Mendelian randomisation study that showed maternal BMI to be causally associated with higher birth weight in offspring.86 Macrosomia at birth has important implications, with links to obesity later in life87 as well as obstetric complications (such as shoulder dystocia, caesarean delivery, perineal trauma, and postpartum haemorrhage88 89 90). The “inter-connectivity”’ of these strong or highly suggestive associations with obstetric complications was obvious, as the evidence for adiposity and increased rate of both emergency and total caesarean section was strong in observational meta-analyses. This was further corroborated by one meta-analysis of trials that reported a significant decrease in the rate of caesarean section with physical exercise when a physical exercise programme was compared with standard care. Although the results were not significant, there was a trend towards reduced rates of caesarean section in meta-analyses that assessed other interventions, including diet and the combination of diet and exercise.59 60 65 67 68 70 Furthermore, the associations between adiposity and low Apgar score at birth were supported by strong and highly suggestive evidence, probably because obesity predisposes to higher risk of gestational diabetes, pre-eclampsia, and fetal macrosomia.
The association between obesity and risk of gestational diabetes was supported by highly suggestive evidence. The summary effect estimates in the included meta-analyses were high (varying from 2.0 to 3.8), while both dietary interventions and physical exercise significantly reduced the incidence. Although this suggests that the association is likely to be true, the evidence was not graded as strong, possibly because of high heterogeneity arising from variation of the clinical definitions across countries.
Two out of five observational meta-analyses showed strong evidence to suggest an association between BMI and the risk of pre-eclampsia, with large summary point estimates, varying between 1.7 and 4.1, while the three others were considered to be highly suggestive. This was further confirmed by three interventional meta-analyses that documented significant reductions in the risk of pre-eclampsia by interventions that reduce obesity, which predominantly had a low risk of bias.
Meta-analyses of interventions that reduce obesity (such as dietary interventions or physical exercise) have been shown to reduce gestational weight gain,58 63 65 66 67 68 69 72 though this did not lead to a reduction for most obstetric complications. Only 15% of the included interventional meta-analyses on obstetric outcomes other than gestational weight gain reached nominal significance for eight studied outcomes (physical exercise and rates of large for gestational age, caesarean section, gestational diabetes, postnatal depression; dietary interventions and rates of gestational diabetes, pre-eclampsia, preterm birth, postpartum weight retention, shoulder dystocia; mixed approach and rates of pre-eclampsia and shoulder dystocia). Obesity creates an unfavourable metabolic environment from early gestation and initiation of weight loss interventions during pregnancy might be too late to prevent or reverse adverse effects,91 which underlines the importance of weight management strategies before conception.
Strengths and limitations
This umbrella review presents the most comprehensive critical appraisal of the literature of published associations between indices of adiposity and risk of any type of obstetric or gynaecological morbidity to date. The categorisation of the evidence was based on a wide range of statistical tests and sensitivity analyses aimed to assess evidence strength and validity.17 18 19 20 21 22 92 93 94 95
Possible limitations and caveats should be considered in the interpretation of our findings. Our review relied on previously published meta-analyses and literature searches performed by the authors of the meta-analyses. Some individual studies might have been missed, but this is unlikely to have influenced our findings because our assessment of duplicate meta-analyses on the same associations between exposure and outcome reported similar summary results. In addition, we have currently evaluated all associations (such as primary outcomes, outcome subtypes, different definitions of same outcome, associations by menopausal status, and use of HRT) for which the original published meta-analyses reported study specific results, but we could have missed other subanalyses that meta-analyses have not reported on with sufficient detail. Although the overall number of studies was large, for some associations the number of studies included in a meta-analysis was small. This would therefore limit the ability to assess the presence of small study effects and excess significance bias because of low power of the statistical tests. For this reason, our estimates are conservative and the problem might be more severe. Finally, the statistical tests used to explore presence of bias can offer only hints of bias but do not prove its definitive presence or its exact source. Our estimates, however, are likely to be conservative as a negative test for bias does not exclude the potential for bias.
Conclusion
The association between obesity and the risk of any obstetric or gynaecological morbidity has been extensively studied. Observational associations between obesity and risk of eight outcomes are supported by strong evidence, but for only two of them (total caesarean section and pre-eclampsia) is there supporting evidence from meta-analyses of clinical trials. Other associations could also be valid, but there is still uncertainty about them.
There is still paucity of data in many of the explored associations and the number of interventional meta-analyses is limited. With obesity becoming a global epidemic, the assessment of the strength of the evidence supporting the impact of adiposity in gynaecological and obstetric conditions could allow the identification of women at high risk for adverse outcomes and increased morbidity and allow better prevention. Observed large heterogeneity in many of the explored associations might reflect biases, such as residual confounding or selective reporting bias. To draw firmer conclusions, we need more prospective studies and large collaborations with better assessment of the changing nature of body fatness and with comprehensive standardised reporting of analyses.
What is already known on this topic
An increasing number of women are obese, including while pregnant
Several meta-analyses have studied the association between obesity and any gynaecological or obstetric morbidity and the effect of weight loss interventions on these outcomes
The proposed associations in previous meta-analyses could be causal but could also be affected by different inherent biases
What this study adds
This umbrella review provides a comprehensive critical appraisal of the literature exploring the strength and validity of the published associations between obesity, interventions to reduce it, and the risk of obstetric and gynaecological morbidity
In 258 observational or interventional meta-analyses that assessed the association between obesity and the incidence of 84 different obstetric or gynaecological outcome there was strong evidence to support the association between obesity and increased risk of only eight outcomes (risk of endometrial and ovarian cancer, fetal macrosomia, pre-eclampsia, low Apgar score (at one minute), antenatal depression, and total and emergency caesarean section)
Web Extra.
Extra material supplied by the author
Appendix 1: Supplementary methods
Appendix 2: Supplementary tables A-I and figure
We thank Nicola Heslehurst for her assistance in providing missing study specific data.
Contributions: MK (joint last author), IK, PMH, EP, and KT (joint last author) conceived and designed the study. IK, MK, and KT acquired and collated the data, which were analysed by IK, MK, GM, and KT. All authors drafted and critically revised the manuscript for important intellectual content. All authors gave final approval of the version to be published and contributed to the manuscript. MK is guarantor.
Funding: This work was supported by the Imperial Healthcare NHS Trust NIHR Biomedical Research Centre (P45272 to MK and PB); Genesis Research Trust (Garfield Weston Foundation, P63522 to MK); Ovarian Cancer Action (MK, MG, HG); Sigrid Jusélius Fellowship (P52483 to IK and MK); World Cancer Research Fund International Regular Grant Programme (2014/1180 to KT); and Imperial College Healthcare Charity (P47907 to AM, MK). None of the funders had any influence on the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: Not required.
Transparency: The lead authors had full access to all the data in the study and the final responsibility for the decision to submit for publication. The lead author affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Data sharing: No additional data available.
References
- 1. NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet 2016;387:1377-96. 10.1016/S0140-6736(16)30054-X pmid:27115820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Project E-P. European perinatal health report. Health and care of pregnant women and babies in Europe in 2010. 2010; http://www.europeristat.com
- 3.Aune D, Navarro Rosenblatt DA, Chan DS, et al. Anthropometric factors and endometrial cancer risk: a systematic review and dose-response meta-analysis of prospective studies. Ann Oncol 2015;26:1635-48. 10.1093/annonc/mdv142 pmid:25791635. [DOI] [PubMed] [Google Scholar]
- 4.Aune D, Navarro Rosenblatt DA, Chan DS, et al. Anthropometric factors and ovarian cancer risk: a systematic review and nonlinear dose-response meta-analysis of prospective studies. Int J Cancer 2015;136:1888-98. 10.1002/ijc.29207 pmid:25250505. [DOI] [PubMed] [Google Scholar]
- 5.Poorolajal J, Jenabi E. The association between BMI and cervical cancer risk: a meta-analysis. Eur J Cancer Prev 2016;25:232-8. 10.1097/CEJ.0000000000000164 pmid:25932869. [DOI] [PubMed] [Google Scholar]
- 6.Protani MM, Nagle CM, Webb PM. Obesity and ovarian cancer survival: a systematic review and meta-analysis. Cancer Prev Res (Phila) 2012;5:901-10. 10.1158/1940-6207.CAPR-12-0048 pmid:22609763. [DOI] [PubMed] [Google Scholar]
- 7.Yang HS, Yoon C, Myung SK, Park SM. Effect of obesity on survival of women with epithelial ovarian cancer: a systematic review and meta-analysis of observational studies. Int J Gynecol Cancer 2011;21:1525-32. 10.1097/IGC.0b013e31822eb5f8 pmid:22080892. [DOI] [PubMed] [Google Scholar]
- 8.Lim SS, Norman RJ, Davies MJ, Moran LJ. The effect of obesity on polycystic ovary syndrome: a systematic review and meta-analysis. Obes Rev 2013;14:95-109. 10.1111/j.1467-789X.2012.01053.x pmid:23114091. [DOI] [PubMed] [Google Scholar]
- 9.Rich-Edwards JW, Goldman MB, Willett WC, et al. Adolescent body mass index and infertility caused by ovulatory disorder. Am J Obstet Gynecol 1994;171:171-7. 10.1016/0002-9378(94)90465-0 pmid:8030695. [DOI] [PubMed] [Google Scholar]
- 10.Marchi J, Berg M, Dencker A, Olander EK, Begley C. Risks associated with obesity in pregnancy, for the mother and baby: a systematic review of reviews. Obes Rev 2015;16:621-38. 10.1111/obr.12288 pmid:26016557. [DOI] [PubMed] [Google Scholar]
- 11. ESHRE Capri Workshop Group. Nutrition and reproduction in women. Hum Reprod Update 2006;12:193-207. 10.1093/humupd/dmk003 pmid:16449360. [DOI] [PubMed] [Google Scholar]
- 12.Berti C, Cetin I, Agostoni C, et al. Pregnancy and Infants’ Outcome: Nutritional and Metabolic Implications. Crit Rev Food Sci Nutr 2016;56:82-91. 10.1080/10408398.2012.745477 pmid:24628089. [DOI] [PubMed] [Google Scholar]
- 13.Amir LH, Donath S. A systematic review of maternal obesity and breastfeeding intention, initiation and duration. BMC Pregnancy Childbirth 2007;7:9 10.1186/1471-2393-7-9 pmid:17608952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ioannidis JP. Why most discovered true associations are inflated. Epidemiology 2008;19:640-8. 10.1097/EDE.0b013e31818131e7 pmid:18633328. [DOI] [PubMed] [Google Scholar]
- 15.Ioannidis JP. Why most published research findings are false. PLoS Med 2005;2:e124 10.1371/journal.pmed.0020124 pmid:16060722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dwan K, Gamble C, Williamson PR, Kirkham JJ, Reporting Bias G. Reporting Bias Group. Systematic review of the empirical evidence of study publication bias and outcome reporting bias - an updated review. PLoS One 2013;8:e66844 10.1371/journal.pone.0066844 pmid:23861749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsilidis KK, Kasimis JC, Lopez DS, Ntzani EE, Ioannidis JP. Type 2 diabetes and cancer: umbrella review of meta-analyses of observational studies. BMJ 2015;350:g7607 10.1136/bmj.g7607 pmid:25555821. [DOI] [PubMed] [Google Scholar]
- 18.Theodoratou E, Tzoulaki I, Zgaga L, Ioannidis JP. Vitamin D and multiple health outcomes: umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials. BMJ 2014;348:g2035 10.1136/bmj.g2035 pmid:24690624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bellou V, Belbasis L, Tzoulaki I, Evangelou E, Ioannidis JP. Environmental risk factors and Parkinson’s disease: An umbrella review of meta-analyses. Parkinsonism Relat Disord 2016;23:1-9. 10.1016/j.parkreldis.2015.12.008 pmid:26739246. [DOI] [PubMed] [Google Scholar]
- 20.Belbasis L, Bellou V, Evangelou E, Ioannidis JP, Tzoulaki I. Environmental risk factors and multiple sclerosis: an umbrella review of systematic reviews and meta-analyses. Lancet Neurol 2015;14:263-73. 10.1016/S1474-4422(14)70267-4 pmid:25662901. [DOI] [PubMed] [Google Scholar]
- 21.Markozannes G, Tzoulaki I, Karli D, et al. Diet, body size, physical activity and risk of prostate cancer: An umbrella review of the evidence. Eur J Cancer 2016;69:61-9. 10.1016/j.ejca.2016.09.026 pmid:27816833. [DOI] [PubMed] [Google Scholar]
- 22.Kyrgiou M, Kalliala I, Markozannes G, et al. Adiposity and cancer at major anatomical sites: umbrella review of the literature. BMJ 2017;356:j477 10.1136/bmj.j477 pmid:28246088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shea BJ, Grimshaw JM, Wells GA, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol 2007;7:10 10.1186/1471-2288-7-10 pmid:17302989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. StataCorp. Stata Statistical Software: Release 13.StataCorp LP, 2013. [Google Scholar]
- 25.Aune D, Saugstad OD, Henriksen T, Tonstad S. Maternal body mass index and the risk of fetal death, stillbirth, and infant death: a systematic review and meta-analysis. JAMA 2014;311:1536-46. 10.1001/jama.2014.2269 pmid:24737366. [DOI] [PubMed] [Google Scholar]
- 26.Bartsch E, Medcalf KE, Park AL, Ray JG. High Risk of Pre-eclampsia Identification Group. Clinical risk factors for pre-eclampsia determined in early pregnancy: systematic review and meta-analysis of large cohort studies. BMJ 2016;353:i1753 10.1136/bmj.i1753 pmid:27094586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crosbie EJ, Zwahlen M, Kitchener HC, Egger M, Renehan AG. Body mass index, hormone replacement therapy, and endometrial cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev 2010;19:3119-30. 10.1158/1055-9965.EPI-10-0832 pmid:21030602. [DOI] [PubMed] [Google Scholar]
- 28.Heslehurst N, Simpson H, Ells LJ, et al. The impact of maternal BMI status on pregnancy outcomes with immediate short-term obstetric resource implications: a meta-analysis. Obes Rev 2008;9:635-83. 10.1111/j.1467-789X.2008.00511.x pmid:18673307. [DOI] [PubMed] [Google Scholar]
- 29.Koning AM, Mutsaerts MA, Kuchenbecker WK, et al. Complications and outcome of assisted reproduction technologies in overweight and obese women. Hum Reprod 2012;27:457-67. 10.1093/humrep/der416 pmid:22144420. [DOI] [PubMed] [Google Scholar]
- 30.Lutsiv O, Mah J, Beyene J, McDonald SD. The effects of morbid obesity on maternal and neonatal health outcomes: a systematic review and meta-analyses. Obes Rev 2015;16:531-46. 10.1111/obr.12283 pmid:25912896. [DOI] [PubMed] [Google Scholar]
- 31.Maheshwari A, Stofberg L, Bhattacharya S. Effect of overweight and obesity on assisted reproductive technology--a systematic review. Hum Reprod Update 2007;13:433-44. 10.1093/humupd/dmm017 pmid:17584821. [DOI] [PubMed] [Google Scholar]
- 32.Mannan M, Doi SA, Mamun AA. Association between weight gain during pregnancy and postpartum weight retention and obesity: a bias-adjusted meta-analysis. Nutr Rev 2013;71:343-52. 10.1111/nure.12034 pmid:23731445. [DOI] [PubMed] [Google Scholar]
- 33.McDonald SD, Han Z, Mulla S, Beyene J. Knowledge Synthesis Group. Overweight and obesity in mothers and risk of preterm birth and low birth weight infants: systematic review and meta-analyses. BMJ 2010;341:c3428 10.1136/bmj.c3428 pmid:20647282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McDonald SD, Han Z, Mulla S, et al. Knowledge Synthesis Group. High gestational weight gain and the risk of preterm birth and low birth weight: a systematic review and meta-analysis. J Obstet Gynaecol Can 2011;33:1223-33. 10.1016/S1701-2163(16)35107-6 pmid:22166276. [DOI] [PubMed] [Google Scholar]
- 35.Metwally M, Ong KJ, Ledger WL, Li TC. Does high body mass index increase the risk of miscarriage after spontaneous and assisted conception? A meta-analysis of the evidence. Fertil Steril 2008;90:714-26. 10.1016/j.fertnstert.2007.07.1290 pmid:18068166. [DOI] [PubMed] [Google Scholar]
- 36.Molyneaux E, Poston L, Ashurst-Williams S, Howard LM. Obesity and mental disorders during pregnancy and postpartum: a systematic review and meta-analysis. Obstet Gynecol 2014;123:857-67. 10.1097/AOG.0000000000000170 pmid:24785615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mulders AG, Laven JS, Eijkemans MJ, Hughes EG, Fauser BC. Patient predictors for outcome of gonadotrophin ovulation induction in women with normogonadotrophic anovulatory infertility: a meta-analysis. Hum Reprod Update 2003;9:429-49. 10.1093/humupd/dmg035 pmid:14640376. [DOI] [PubMed] [Google Scholar]
- 38.Onubi OJ, Marais D, Aucott L, Okonofua F, Poobalan AS. Maternal obesity in Africa: a systematic review and meta-analysis. J Public Health (Oxf) 2016;38:e218-31. 10.1093/pubmed/fdv138 pmid:26487702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poorolajal J, Jenabi E, Masoumi SZ. Body mass index effects on risk of ovarian cancer: a meta- analysis. Asian Pac J Cancer Prev 2014;15:7665-71. 10.7314/APJCP.2014.15.18.7665 pmid:25292044. [DOI] [PubMed] [Google Scholar]
- 40.Rittenberg V, Seshadri S, Sunkara SK, Sobaleva S, Oteng-Ntim E, El-Toukhy T. Effect of body mass index on IVF treatment outcome: an updated systematic review and meta-analysis. Reprod Biomed Online 2011;23:421-39. 10.1016/j.rbmo.2011.06.018 pmid:21885344. [DOI] [PubMed] [Google Scholar]
- 41.Skubleny D, Switzer NJ, Gill RS, et al. The Impact of Bariatric Surgery on Polycystic Ovary Syndrome: a Systematic Review and Meta-analysis. Obes Surg 2016;26:169-76. 10.1007/s11695-015-1902-5 pmid:26431698. [DOI] [PubMed] [Google Scholar]
- 42.Smits A, Lopes A, Bekkers R, Galaal K. Body mass index and the quality of life of endometrial cancer survivors--a systematic review and meta-analysis. Gynecol Oncol 2015;137:180-7. 10.1016/j.ygyno.2015.01.540 pmid:25636459. [DOI] [PubMed] [Google Scholar]
- 43.Tian C, Hu C, He X, et al. Excessive weight gain during pregnancy and risk of macrosomia: a meta-analysis. Arch Gynecol Obstet 2016;293:29-35. 10.1007/s00404-015-3825-8 pmid:26246412. [DOI] [PubMed] [Google Scholar]
- 44.Torloni MR, Betrán AP, Horta BL, et al. Prepregnancy BMI and the risk of gestational diabetes: a systematic review of the literature with meta-analysis. Obes Rev 2009;10:194-203. 10.1111/j.1467-789X.2008.00541.x pmid:19055539. [DOI] [PubMed] [Google Scholar]
- 45.Upala S, Anawin Sanguankeo . Bariatric surgery and risk of postoperative endometrial cancer: a systematic review and meta-analysis. Surg Obes Relat Dis 2015;11:949-55. 10.1016/j.soard.2014.09.024 pmid:25620433. [DOI] [PubMed] [Google Scholar]
- 46.Wang Z, Wang P, Liu H, et al. Maternal adiposity as an independent risk factor for pre-eclampsia: a meta-analysis of prospective cohort studies. Obes Rev 2013;14:508-21. 10.1111/obr.12025 pmid:23530552. [DOI] [PubMed] [Google Scholar]
- 47.Wise MR, Jordan V, Lagas A, et al. Obesity and endometrial hyperplasia and cancer in premenopausal women: A systematic review. Am J Obstet Gynecol 2016;214:689.e1-17. 10.1016/j.ajog.2016.01.175 pmid:26829507. [DOI] [PubMed] [Google Scholar]
- 48.Yamazaki M, Dwyer K, Sobhan M, et al. Effect of obesity on the effectiveness of hormonal contraceptives: an individual participant data meta-analysis. Contraception 2015;92:445-52. 10.1016/j.contraception.2015.07.016 pmid:26247330. [DOI] [PubMed] [Google Scholar]
- 49.Yi XY, Li QF, Zhang J, Wang ZH. A meta-analysis of maternal and fetal outcomes of pregnancy after bariatric surgery. Int J Gynaecol Obstet 2015;130:3-9. 10.1016/j.ijgo.2015.01.011 pmid:25863541. [DOI] [PubMed] [Google Scholar]
- 50.Yu Z, Han S, Zhu J, Sun X, Ji C, Guo X. Pre-pregnancy body mass index in relation to infant birth weight and offspring overweight/obesity: a systematic review and meta-analysis. PLoS One 2013;8:e61627 10.1371/journal.pone.0061627 pmid:23613888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang Y, Liu H, Yang S, Zhang J, Qian L, Chen X. Overweight, obesity and endometrial cancer risk: results from a systematic review and meta-analysis. Int J Biol Markers 2014;29:e21-9. 10.5301/jbm.5000047 pmid:24170556. [DOI] [PubMed] [Google Scholar]
- 52.Keum N, Greenwood DC, Lee DH, et al. Adult weight gain and adiposity-related cancers: a dose-response meta-analysis of prospective observational studies. J Natl Cancer Inst 2015;107 10.1093/jnci/djv088. [DOI] [PubMed] [Google Scholar]
- 53.Brunner S, Stecher L, Ziebarth S, et al. Excessive gestational weight gain prior to glucose screening and the risk of gestational diabetes: a meta-analysis. Diabetologia 2015;58:2229-37. 10.1007/s00125-015-3686-5 pmid:26141788. [DOI] [PubMed] [Google Scholar]
- 54.Zhu T, Tang J, Zhao F, Qu Y, Mu D. Association between maternal obesity and offspring Apgar score or cord pH: a systematic review and meta-analysis. Sci Rep 2015;5:18386 10.1038/srep18386 pmid:26692415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smits A, Lopes A, Das N, et al. Surgical morbidity and clinical outcomes in ovarian cancer - the role of obesity. BJOG 2016;123:300-8. 10.1111/1471-0528.13585 pmid:26331299. [DOI] [PubMed] [Google Scholar]
- 56.Schoenaker DA, Jackson CA, Rowlands JV, Mishra GD. Socioeconomic position, lifestyle factors and age at natural menopause: a systematic review and meta-analyses of studies across six continents. Int J Epidemiol 2014;43:1542-62. 10.1093/ije/dyu094 pmid:24771324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wiebe HW, Boulé NG, Chari R, Davenport MH. The effect of supervised prenatal exercise on fetal growth: a meta-analysis. Obstet Gynecol 2015;125:1185-94. 10.1097/AOG.0000000000000801 pmid:25932847. [DOI] [PubMed] [Google Scholar]
- 58.Sanabria-Martínez G, García-Hermoso A, Poyatos-León R, Álvarez-Bueno C, Sánchez-López M, Martínez-Vizcaíno V. Effectiveness of physical activity interventions on preventing gestational diabetes mellitus and excessive maternal weight gain: a meta-analysis. BJOG 2015;122:1167-74. 10.1111/1471-0528.13429 pmid:26036300. [DOI] [PubMed] [Google Scholar]
- 59.Rogozińska E, Chamillard M, Hitman GA, Khan KS, Thangaratinam S. Nutritional manipulation for the primary prevention of gestational diabetes mellitus: a meta-analysis of randomised studies. PLoS One 2015;10:e0115526 10.1371/journal.pone.0115526 pmid:25719363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Muktabhant B, Lawrie TA, Lumbiganon P, Laopaiboon M. Diet or exercise, or both, for preventing excessive weight gain in pregnancy. Cochrane Database Syst Rev 2015;(6):CD007145.pmid:26068707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bain E, Crane M, Tieu J, Han S, Crowther CA, Middleton P. Diet and exercise interventions for preventing gestational diabetes mellitus. Cochrane Database Syst Rev 2015;(4):CD010443.pmid:25864059. [DOI] [PubMed] [Google Scholar]
- 62.Ruifrok AE, van Poppel MN, van Wely M, et al. Association between weight gain during pregnancy and pregnancy outcomes after dietary and lifestyle interventions: a meta-analysis. Am J Perinatol 2014;31:353-64.pmid:23918523. [DOI] [PubMed] [Google Scholar]
- 63.Domenjoz I, Kayser B, Boulvain M. Effect of physical activity during pregnancy on mode of delivery. Am J Obstet Gynecol 2014;211:401.e1-11. 10.1016/j.ajog.2014.03.030 pmid:24631706. [DOI] [PubMed] [Google Scholar]
- 64.Allen R, Rogozinska E, Sivarajasingam P, Khan KS, Thangaratinam S. Effect of diet- and lifestyle-based metabolic risk-modifying interventions on preeclampsia: a meta-analysis. Acta Obstet Gynecol Scand 2014;93:973-85. 10.1111/aogs.12467 pmid:25138651. [DOI] [PubMed] [Google Scholar]
- 65.Thangaratinam S, Rogozinska E, Jolly K, et al. Effects of interventions in pregnancy on maternal weight and obstetric outcomes: meta-analysis of randomised evidence. BMJ 2012;344:e2088 10.1136/bmj.e2088 pmid:22596383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sui Z, Grivell RM, Dodd JM. Antenatal exercise to improve outcomes in overweight or obese women: A systematic review. Acta Obstet Gynecol Scand 2012;91:538-45. 10.1111/j.1600-0412.2012.01357.x pmid:22229625. [DOI] [PubMed] [Google Scholar]
- 67.Oteng-Ntim E, Varma R, Croker H, Poston L, Doyle P. Lifestyle interventions for overweight and obese pregnant women to improve pregnancy outcome: systematic review and meta-analysis. BMC Med 2012;10:47 10.1186/1741-7015-10-47 pmid:22574949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tanentsapf I, Heitmann BL, Adegboye AR. Systematic review of clinical trials on dietary interventions to prevent excessive weight gain during pregnancy among normal weight, overweight and obese women. BMC Pregnancy Childbirth 2011;11:81 10.1186/1471-2393-11-81 pmid:22029725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Streuling I, Beyerlein A, Rosenfeld E, Hofmann H, Schulz T, von Kries R. Physical activity and gestational weight gain: a meta-analysis of intervention trials. BJOG 2011;118:278-84. 10.1111/j.1471-0528.2010.02801.x pmid:21134106. [DOI] [PubMed] [Google Scholar]
- 70.Dodd JM, Grivell RM, Crowther CA, Robinson JS. Antenatal interventions for overweight or obese pregnant women: a systematic review of randomised trials. BJOG 2010;117:1316-26. 10.1111/j.1471-0528.2010.02540.x pmid:20353459. [DOI] [PubMed] [Google Scholar]
- 71.Daley A, Jolly K, MacArthur C. The effectiveness of exercise in the management of post-natal depression: systematic review and meta-analysis. Fam Pract 2009;26:154-62. 10.1093/fampra/cmn101 pmid:19126829. [DOI] [PubMed] [Google Scholar]
- 72.Choi J, Fukuoka Y, Lee JH. The effects of physical activity and physical activity plus diet interventions on body weight in overweight or obese women who are pregnant or in postpartum: a systematic review and meta-analysis of randomized controlled trials. Prev Med 2013;56:351-64. 10.1016/j.ypmed.2013.02.021 pmid:23480971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gresham E, Byles JE, Bisquera A, Hure AJ. Effects of dietary interventions on neonatal and infant outcomes: a systematic review and meta-analysis. Am J Clin Nutr 2014;100:1298-321. 10.3945/ajcn.113.080655 pmid:25332328. [DOI] [PubMed] [Google Scholar]
- 74.Quinlivan JA, Julania S, Lam L. Antenatal dietary interventions in obese pregnant women to restrict gestational weight gain to Institute of Medicine recommendations: a meta-analysis. Obstet Gynecol 2011;118:1395-401. 10.1097/AOG.0b013e3182396bc6 pmid:22105270. [DOI] [PubMed] [Google Scholar]
- 75.Furlan AD, Pennick V, Bombardier C, van Tulder M. Editorial Board, Cochrane Back Review Group. 2009 updated method guidelines for systematic reviews in the Cochrane Back Review Group. Spine (Phila Pa 1976) 2009;34:1929-41. 10.1097/BRS.0b013e3181b1c99f pmid:19680101. [DOI] [PubMed] [Google Scholar]
- 76.Green S. Cochrane handbook for systematic reviews of interventions version 5.1. 0.Cochrane Collaboration, 2011. [Google Scholar]
- 77.Langendam MW, Akl EA, Dahm P, Glasziou P, Guyatt G, Schünemann HJ. Assessing and presenting summaries of evidence in Cochrane Reviews. Syst Rev 2013;2:81 10.1186/2046-4053-2-81 pmid:24059250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Poston L, Caleyachetty R, Cnattingius S, et al. Preconceptional and maternal obesity: epidemiology and health consequences. Lancet Diabetes Endocrinol 2016;4:1025-36. 10.1016/S2213-8587(16)30217-0 pmid:27743975. [DOI] [PubMed] [Google Scholar]
- 79.McCullough ML, Patel AV, Patel R, et al. Body mass and endometrial cancer risk by hormone replacement therapy and cancer subtype. Cancer Epidemiol Biomarkers Prev 2008;17:73-9. 10.1158/1055-9965.EPI-07-2567 pmid:18187388. [DOI] [PubMed] [Google Scholar]
- 80.Nead KT, Sharp SJ, Thompson DJ, et al. Australian National Endometrial Cancer Study Group (ANECS). Evidence of a Causal Association Between Insulinemia and Endometrial Cancer: A Mendelian Randomization Analysis. J Natl Cancer Inst 2015;107:djv178 10.1093/jnci/djv178 pmid:26134033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Painter JN, O’Mara TA, Marquart L, et al. AOCS Group for RENDOCAS National Study of Endometrial Cancer Genetics Group (NSECG) Australian National Endometrial Cancer Study Group (ANECS). Genetic Risk Score Mendelian Randomization Shows that Obesity Measured as Body Mass Index, but not Waist:Hip Ratio, Is Causal for Endometrial Cancer. Cancer Epidemiol Biomarkers Prev 2016;25:1503-10. 10.1158/1055-9965.EPI-16-0147 pmid:27550749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K. International Agency for Research on Cancer Handbook Working Group. Body Fatness and Cancer--Viewpoint of the IARC Working Group. N Engl J Med 2016;375:794-8. 10.1056/NEJMsr1606602 pmid:27557308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.World Cancer Research Fund International/American Institute for Cancer Research Continuous Update Project Report. Diet, Nutrition, Physical Activity, and Endometrial Cancer. 2013; Available at: http://www.wcrf.org/sites/default/files/Endometrial-Cancer-2013-Report.pdf.
- 84.Gao C, Patel CJ, Michailidou K, et al. on behalf of: the Colorectal Transdisciplinary Study (CORECT); Discovery, Biology and Risk of Inherited Variants in Breast Cancer (DRIVE); Elucidating Loci Involved in Prostate Cancer Susceptibility (ELLIPSE); Follow-up of Ovarian Cancer Genetic Association and Interaction Studies (FOCI); and Transdisciplinary Research in Cancer of the Lung (TRICL). Mendelian randomization study of adiposity-related traits and risk of breast, ovarian, prostate, lung and colorectal cancer. Int J Epidemiol 2016;45:896-908. 10.1093/ije/dyw129 pmid:27427428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.World Cancer Research Fund International/American Institute for Cancer Research CUPR. Diet, Nutrition, Physical Activity, and Ovarian Cancer. 2014; Available at: http://www.wcrf.org/sites/default/files/Ovarian-Cancer-2014-Report.pdf.
- 86.Tyrrell J, Richmond RC, Palmer TM, et al. Early Growth Genetics (EGG) Consortium. Genetic Evidence for Causal Relationships Between Maternal Obesity-Related Traits and Birth Weight. JAMA 2016;315:1129-40. 10.1001/jama.2016.1975 pmid:26978208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Muhlhausler BS, Ong ZY. The fetal origins of obesity: early origins of altered food intake. Endocr Metab Immune Disord Drug Targets 2011;11:189-97. 10.2174/187153011796429835 pmid:21831032. [DOI] [PubMed] [Google Scholar]
- 88.Wispelwey BP, Sheiner E. Cesarean delivery in obese women: a comprehensive review. J Matern Fetal Neonatal Med 2013;26:547-51. 10.3109/14767058.2012.745506 pmid:23130683. [DOI] [PubMed] [Google Scholar]
- 89.Tsur A, Sergienko R, Wiznitzer A, Zlotnik A, Sheiner E. Critical analysis of risk factors for shoulder dystocia. Arch Gynecol Obstet 2012;285:1225-9. 10.1007/s00404-011-2139-8 pmid:22083313. [DOI] [PubMed] [Google Scholar]
- 90.Temtanakitpaisan T, Bunyacejchevin S, Koyama M. Obstetrics anal sphincter injury and repair technique: a review. J Obstet Gynaecol Res 2015;41:329-33. 10.1111/jog.12630 pmid:25545893. [DOI] [PubMed] [Google Scholar]
- 91.Catalano P, deMouzon SH. Maternal obesity and metabolic risk to the offspring: why lifestyle interventions may have not achieved the desired outcomes. Int J Obes (Lond) 2015;39:642-9. 10.1038/ijo.2015.15 pmid:25777180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bellou V, Belbasis L, Tzoulaki I, Middleton LT, Ioannidis JP, Evangelou E. Systematic evaluation of the associations between environmental risk factors and dementia: An umbrella review of systematic reviews and meta-analyses. Alzheimers Dement 2017;13:406-18. 10.1016/j.jalz.2016.07.152 pmid:27599208. [DOI] [PubMed] [Google Scholar]
- 93.Belbasis L, Savvidou MD, Kanu C, Evangelou E, Tzoulaki I. Birth weight in relation to health and disease in later life: an umbrella review of systematic reviews and meta-analyses. BMC Med 2016;14:147 10.1186/s12916-016-0692-5 pmid:27677312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ioannidis JP, Zhou Y, Chang CQ, Schully SD, Khoury MJ, Freedman AN. Potential increased risk of cancer from commonly used medications: an umbrella review of meta-analyses. Ann Oncol 2014;25:16-23. 10.1093/annonc/mdt372 pmid:24310915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ioannidis JP. Integration of evidence from multiple meta-analyses: a primer on umbrella reviews, treatment networks and multiple treatments meta-analyses. CMAJ 2009;181:488-93. 10.1503/cmaj.081086 pmid:19654195. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 1: Supplementary methods
Appendix 2: Supplementary tables A-I and figure