Abstract
A number of age-dependent degenerative diseases are caused by chronic endoplasmic reticulum (ER) stress in vital cells. In many cases, the afflicted cells suffer from ER stress since birth, but the death of irreplaceable cells occurs only late in life. Although our understanding of ER stress-induced cell death has advanced significantly, most of the known mechanisms involve pathways that signal within hours, and it remains unclear how these pathways regulate cell death that occurs only decades later. Here, I highlight the conceptual issues and suggest ways to make sense of the age-related effect of ER stress-induced cell death in degenerative diseases.
Keywords: Apoptosis, caspase, cell death, ER stress, Unfolded Protein Response, PERK, IRE1, degenerative disease
Introduction
Endoplasmic reticulum (ER) is a cellular organelle that stores high levels of Calcium (Ca2+) and where many membrane or secretory proteins undergo synthesis and folding before being trafficked to their final destination. Various genetic or environmental conditions that perturb the ER can cause cellular dysfunction and disease. In response, cells activate the Unfolded Protein Response (UPR) to help cells to adapt to such stress within hours [1]. Conditions of extreme or prolonged ER stress can cause cell death, and this aspect has drawn significant interest as the death of cells often correlates with the onset of degenerative diseases, and strategies to block such cell death can be developed for therapeutic purposes [2]. Examples of diseases in which ER stress induced cell death occurs includes type I diabetes caused by the death of beta-islet cells, Retinitis Pigmentosa (RP) caused by mutant rhodopsins, certain forms of Charcot-Marie-Tooth disease caused by the death of Schwann cells, Amyotrophic Lateral Sclerosis (ALS) and Parkinson’s Disease [3–8].
Much progress has been made regarding the signaling mechanisms by which cells trigger apoptosis after suffering from ER stress (see below for details). The identified mechanisms most often involve signaling pathways that are completed within hours, and it is unclear whether these models are applicable to late-onset degenerative disease, where cells die after decades of chronic ER stress. Here, I will briefly review the current models of ER stress-induced cell death, and highlight the importance of considering the timeline of events in applying those models to age-related diseases.
The role of caspases in apoptosis and non-apoptotic events
There are a number of different cell death mechanisms that includes necrosis and autophagic cell death, but many studies on ER stress response have focused on apoptosis that is executed by caspases. A number of conditions first trigger the activation of initiator caspases (caspase-2, -8, -9 and -10), which in turn, cleave and proteolytically activate effector caspases (e.g. caspase-3) for apoptosis. Once effector caspases are fully activated, they cut many other cellular proteins that collectively orchestrate a cell death program [9]. In experimental settings, the molecular cascade leading to caspase-mediated apoptosis is completed within hours.
Complicating this simple picture is the fact that caspases also have non-apoptotic roles. For example, caspase-3 is involved in the differentiation of lens epithelial cells to lens fiber cells [10], myoblasts to myotubes [11], osteoblasts to form bones [12], keratinocytes[13] and erythrocytes [14]. Caspase-8, which associates with the Death Receptors with FADD to activate caspase-3, is also involved in other branches of signaling, such as the activation of NF-kB transcription factor [15, 16]. Caspase-2 mutant mice show apoptotic defects in oocytes and B lymphoblasts [17], but also have other non-apoptotic phenotypes, which include problems with DNA damage repair and G2/M checkpoint regulation in response to ionizing irradiation [18, 19]. Drosophila genetics clearly shows the dual role of caspases in cell death as well as in non-apoptotic roles: Apoptosis-mediating caspases are also involved in sensory neuron differentiation [20], spermatid differentiation [21] and initiating signaling cascades to induce non-autonomous cell proliferation [22]. Caspases can engage in such non-apoptotic roles without killing cells, as there are feedback mechanisms that maintain their activity at sub-threshold levels [23].
A number of initiator caspases have been implicated in the mediation of ER stress induced apoptosis. These include caspase-12, caspase-9 and caspase-2 [24–26]. In ER stress response assays performed in culture dish for a short period of time, it is reasonable to interpret that these caspases directly execute cell death. However, active caspases should not take decades to kill cells in degenerative diseases. In these age-related diseases, it is possible that these UPR activated caspases may play non-apoptotic roles, indirectly affecting cell death that occurs years after UPR activation.
Signaling pathways associated with ER stress
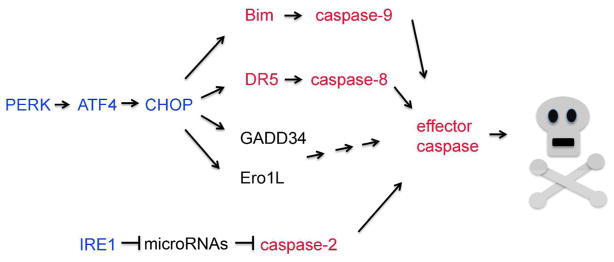
Three signaling pathways of the Unfolded Protein Response (UPR) attract most attention when considering cellular ER stress response. These are the pathways initiated by ER stress sensors, PERK, IRE1 and ATF6 [1]. Among these, the PERK branch is most frequently studied in terms of cell death induction (Figure 1). PERK phosphorylates eIF2alpha to reduce the overall rate of translational initiation [27], and also activates downstream transcriptional factors, ATF4 and CHOP [28, 29]. CHOP mediates cell death in a number of ER stress models, and knockout of this gene suppresses a few disease phenotypes associated with excessive ER stress [3, 30]. A number of CHOP’s transcriptional targets are also associated with mediating cell death. These include Ero1L, GADD34 and Bim [25, 31, 32]. Bim is a BH3 only domain gene that initiates the mitochondrial pathway of apoptosis, ultimately resulting in caspase-9 activation. Most recently, a study has found that a death receptor gene, DR5, is induced downstream of CHOP, thereby activating caspase-8 for apoptosis induction [33].
Figure 1. A diagram of reported cell death pathways regulated by UPR.
The signaling pathways by PERK, ATF4, CHOP and IRE1 (blue) have effectors that trigger caspase-mediated cell death. These include caspases -9, -8, -2, and effector caspases, as well as their upstream activators (red).
Aside from the PERK-mediated UPR, studies have found evidence that IRE1 contributes to ER stress-induced cell death (Figure 1). IRE1 has an RNase domain that is activated upon ER stress, and Scott Oakes’ group has found that IRE1 cleaves microRNAs that normally inhibit caspase-2 synthesis. Thus, in response to stress, caspase-2 levels build up and contribute to cell death in cultured cells suffering from ER stress [34]. Consistently, inhibition of IRE1 RNase suppresses ER stress-associated loss of beta islet cells and retinal degeneration in animal models [35].
Sequence of events during age-related degeneration
Can these signaling pathways explain how ER-stress kills cells in age-related degenerative disorders? A careful observer may notice that timeframe of events for UPR activation and cell death induction may not coincide in many diseases.
In response to ER stress conditions in a culture dish, IRE1 signaling is activated within hours, but their signaling activity returns to basal levels through negative feedback loops within a day. Similarly, PERK activation induces ATF4 within hours, and ATF4’s transcriptional targets are induced not long thereafter. PERK/ATF4 signaling also has negative feedback loops that inhibit its activity within a day or two [1]. However, ER stressed cells in degenerative diseases often trigger cell death years after exposure to chronic stress. There is a clear difference in the timing of UPR activity and cell death induction. Thus, it is likely that cell death is not triggered directly by the UPR signaling cascades, at least in these age-related diseases.
Alternative ways to think about ER stress induced cell death in age-related diseases
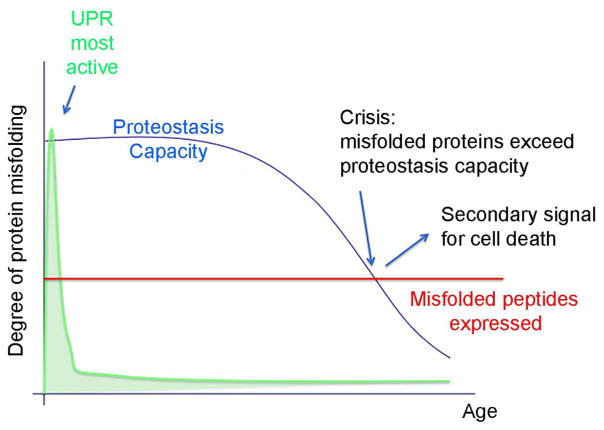
What is the trigger of cell death in age-related diseases caused by chronic ER stress? Worthy of consideration is an alternative possibility that cell death is not directly triggered by the UPR. Rather, there may be secondary signals that initiate cell death only later in life (Figure 2). What changes in later stages of life? It is helpful to note that a cell’s ability to handle misfolded proteins decline with age [36]. According to this view, young cells are able to cope with the misfolded proteins that underlie degenerative disease due to their robust capacity of the UPR and the proteostasis network. As that capacity declines, a point of crisis emerges, when the ER can no longer handle the amount of misfolded peptides generated in cells (Figure 2). Those conditions may activate a distinct secondary cell death signal. Based on the literature, one may speculate that Ca2+ or Reactive Oxygen Species (ROS) serve that role. Such speculation is supported in part by studies that have examined how cholesterol overload in macrophages trigger ER stress-induced cell death. The Tabas laboratory found that kinases activated by Ca2+ and ROS are required for the death of those macrophages [37, 38]. Independently, my own group had also screened for kinases involved in ER stress-induced cell death, using a Drosophila model for Retinitis Pigmentosa. We uncovered two kinases, Mekk1 and CDK5, which can be activated by ROS in cells [39]. Thus, it is possible that old cells under secondary crisis may have Ca2+ leaking into the cytoplasm or ROS being generated, that activates these cell death-triggering kinases.
Figure 2. A speculative model by which cell death signals are activated in late stages of life.
Blue curve indicates cellular proteostasis capacity, which starts high in early life, but declines with age. The x axis indicates age of individual. UPR can be most robustly activated at this early stage for effective cellular quality control, but declines quickly through feedback regulation (green). Disease causing mutant protein expression remains constant (red line). At a late stage of life, there is a point where the proteostasis capacity begins to decline below the misfolded protein levels (Crisis point: indicated with arrow). Such conditions may activate a secondary signal that initiates cell death.
Concluding Remarks
The issue of timing in ER stress-induced cell death has not been sufficiently resolved in the field that frequently relies on cultured cells exposed to acute stress. There is no doubt that cultured cells exposed to ER stress-causing chemicals have facilitated the elucidation of detailed UPR mechanisms. However, it appears that these models are less suited to understand age-related degenerative phenotypes, as these experimental conditions are aggressive enough to breach the proteostasis threshold early, and activate within hours a death signal that can otherwise take decades to activate. On the other hand, many age-related degenerative diseases associated with ER stress show that the afflicted cells maintain their function for many years, indicating that the degree of ER stress in those young cells, while sufficient to activate adaptive UPR, is not enough to activate cell death. Some hints regarding the nature of the late-acting cell death signaling pathways have now emerged. Future studies may provide a better understanding of their temporal dynamics.
Acknowledgments
This work was supported by R01 EY020866 of the N.I.H.
Abbreviations
- ER
endoplasmic reticulum
- UPR
Unfolded Protein Response
- RP
Retinitis Pigmentosa
- ALS
Amyotrophic Lateral Sclerosis
- ROS
Reactive Oxygen Species
References
- 1.Walter P, Ron D. The unfolded protein response: from stress pathway to hemeostatic regulation. Science. 2011;334:1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 2.Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol. 2011;13:184–190. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pennuto M, Tinelli E, Malaguti M, Del Carro U, D’Antonio M, Ron D, Quattrini A, Feltri ML, Wrabetz L. Ablation of the UPR-mediator CHOP restores motor function and reduces demyelination in Charcot-Marie-Tooth 1B mice. Neuron. 2008;57:393–405. doi: 10.1016/j.neuron.2007.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsuda H, Han SM, Yang Y, Tong C, Lin YQ, Mohan K, Haueter C, Zoghbi A, Harati Y, Kwan J, Miller MA, Bellen HJ. The amyotriphic lateral sclerosis 8 protein VAPB is cleaved, secreted, and acts as a ligand for Eph receptors. Cell. 2008;133:963–977. doi: 10.1016/j.cell.2008.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ryoo HD, Domingos PM, Kang MJ, Steller H. Unfolded protein response in a Drosophila model for retinal degeneration. Embo J. 2007;26:242–52. doi: 10.1038/sj.emboj.7601477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin JH, Walter P, Yen TS. Endoplasmic Reticulum Stress in Disease Pathogenesis. Annu Rev Pathol. 2007 doi: 10.1146/annurev.pathmechdis.3.121806.151434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mercado G, Valdes P, Hetz C. An ERcentric view of Parkinson’s disease. Trends Mol Med. 2013;19:165–175. doi: 10.1016/j.molmed.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Oakes SA, Papa FR. The role of endoplasmic reticulum stress in human pathology. Annu Rev Pathol. 2015;10:173–194. doi: 10.1146/annurev-pathol-012513-104649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ashkenazi A, Salvesen G. Regulated cell death: signaling and mechanisms. Annu Rev Cell Dev Biol. 2014;30:337–356. doi: 10.1146/annurev-cellbio-100913-013226. [DOI] [PubMed] [Google Scholar]
- 10.Weber GF, Menko AS. The canonical intrinsic mitochondrial death pathway has a non-apoptotic role in signaling lens cell differentiation. J Biol Chem. 2005;280:22135–22145. doi: 10.1074/jbc.M414270200. [DOI] [PubMed] [Google Scholar]
- 11.Fernando P, Kelly JF, Balazsi K, Slack RS, Megeney LA. Caspase 3 activity is required for skeletal muscle differentiation. Proc Natl Acad Sci USA. 2002;99:11025–11030. doi: 10.1073/pnas.162172899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mogi M, Togari A. Activation of caspases is required for osteoblastic differentiation. J Biol Chem. 2003;278:47477–47482. doi: 10.1074/jbc.M307055200. [DOI] [PubMed] [Google Scholar]
- 13.Okuyama R, Nguyen B-C, Talora C, Ogawa E, di Vignano AT, Lioumi M, Chiorino G, Tagami H, Woo M, Dotto GP. High commitment of embryonic keratinocytes to terminal differentiation through a Notch1-caspase 3 regulatory mechanism. Dev Cell. 2004;6:551–562. doi: 10.1016/s1534-5807(04)00098-x. [DOI] [PubMed] [Google Scholar]
- 14.Zermati Y, Garrido C, Amsellem S, Fishelson S, Bouscary D, Valensi F, Varet B, Solary E, Hermine O. Caspase activation is required for terminal erythroid differentiation. J Exp Med. 2001;193:247–254. doi: 10.1084/jem.193.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kataoka T, Tschopp J. N-terminal fragment of c-FLIPL processed by caspase 8 specifically interacts with TRAF2 and induces activation of the NF-kB signaling pathway. Mol Cell Biol. 2004;24:2627–2636. doi: 10.1128/MCB.24.7.2627-2636.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bidere N, Snow AL, Sakai K, Zheng L, Lenardo MJ. Caspase-8 regulation by direct interaction with TRAF6 in T cell receptor-induced NF-kB activation. Curr Biol. 2006;16:1666–1671. doi: 10.1016/j.cub.2006.06.062. [DOI] [PubMed] [Google Scholar]
- 17.Bergeron L, Perez GI, Macdonald G, Shi L, Sun Y, Jurisicova A, Varmuza S, Latham KE, Flaws JA, Salter JC, Hara H, Moskowitz MA, Li E, Greenberg A, Tillly JL, Yuan J. Defects in regulation of apoptosis in caspase-2 deficient mice. Genes Dev. 1998:12. doi: 10.1101/gad.12.9.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ho LH, Taylor R, Dorstyn L, Cakouros D, Bouillet P, Kumar S. A tumor suppressor function for caspase-2. Proc Natl Acad Sci USA. 2009;106:5336–5341. doi: 10.1073/pnas.0811928106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi M, Vivian CJ, Lee KJ, Ge C, Morotomi-Yano K, Manzl C, Bock F, Sato S, Tomomori-Sato C, Zhu R, Haug JS, Swanson SK, Washburn MP, Chen DJ, Chen BP, Vilunger A, Florens L, Du C. DNA-PKcs-PIDDosome: a nuclear caspase-2 activating complex with role in G2/M checkpoint maintenance. Cell. 2009;136:508–520. doi: 10.1016/j.cell.2008.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Kanuka H, Kuranaga E, Takemoto K, Hiratou T, Okano H, Miura M. Drosophila caspase transduces Shaggy/GSK-3beta kinase activity in neural precursor development. EMBO J. 2005;24:3793–3806. doi: 10.1038/sj.emboj.7600822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arama E, Agapite J, Steller H. Caspase activity and a specific cytochrome C are required for sperm differentiation in Drosophila. Dev Cell. 2003;4:687–697. doi: 10.1016/s1534-5807(03)00120-5. [DOI] [PubMed] [Google Scholar]
- 22.Ryoo HD, Bergmann A. The role of apoptosis-induced proliferation for regeneration and cancer. Cold Spring Harb Perspect Biol. 2012;4:a008797. doi: 10.1101/cshperspect.a008797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shapiro PJ, Hsu HH, Jung H, Robbins ES, Ryoo HD. Regulation of the Drosopphila apoptosome through feedback inhibition. Nat Cell Biol. 2008;10:1440–1446. doi: 10.1038/ncb1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, Yuan J. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000;403:98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- 25.Puthalakath H, O’Reilly LA, Gunn P, Lee L, Kelly PN, Huntington ND, Hughes PD, Michalak EM, McKimm-Breschkin J, Motoyama N, Gotoh T, Akira S, Bouillet P, Strasser A. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell. 2007;129:1337–49. doi: 10.1016/j.cell.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 26.Upton JP, Austgen K, Nishino M, Coakley KM, Hagen A, Han D, Papa FR, Oakes SA. Caspase-2 cleavage of BID is a critical apoptotic signal downstream of endoplasmic reticulum stress. Mol Cell Biol. 2008;28:3943–3951. doi: 10.1128/MCB.00013-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397:271–274. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- 28.Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, Ron D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000;6:1099–1108. doi: 10.1016/s1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- 29.Palam LR, Baird TD, Wek RC. Phosphorylation of eIF2 facilitates ribosomal bypass of an inhibitory upstream ORF to enhance CHOP translation. J Biol Chem. 2011;286:10939–10949. doi: 10.1074/jbc.M110.216093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zinszner H, Kuroda M, Wang X, Batchvarova N, Lightfoot RT, Remotti H, Stevens JL, Ron D. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 1998;12:982–95. doi: 10.1101/gad.12.7.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marciniak SJ, Yun CJ, Oyadomari S, Novoa I, Zhang Y, Jungreis R, Nagata K, Harding HP, Ron D. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004;18:3066–3077. doi: 10.1101/gad.1250704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Han J, Back SH, Hur J, Lin YH, Gildersleeve R, Shan J, Yuan CL, Krokowski D, Wang S, Hatzoglou M, Kilberg MS, Sartor MA, Kaufman RJ. ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat Cell Biol. 2013;15:481–490. doi: 10.1038/ncb2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu M, Lawrence DA, Marsters S, Acosta-Alvear D, Kimmig P, Mendez AS, Paton AW, Paton JC, Walter P, Ashkenazi A. Opposing unfolded-protein-response signals converge on death receptor 5 to control apoptosis. Science. 2014;345:98–101. doi: 10.1126/science.1254312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Upton JP, Wang L, Han D, Wang ES, Huskey NE, Lim L, Truitt M, McManus MT, Ruggero D, Goga A, Papa FR, Oakes SA. IRE1alpha cleaves select microRNAs during ER stress to derepress translation of proapoptotic Caspase-2. Science. 2012;338:818–822. doi: 10.1126/science.1226191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghosh R, Wang L, Wang ES, Perera BG, Igbaria A, Morita S, Prado K, Thamsen M, Caswell D, Macias H, Weiberth KF, Gliedt MJ, Alavi MV, Hari SB, Mitra AK, Bhhatarai B, Schurer SC, Snapp EL, Gould DB, German MS, Backes BJ, Maly DJ, Oakes SA, Papa FR. Allosteric inhibition of the IRE1alpha RNase preserves cell viability and function during endoplasmic reticulum stress. Cell. 2014;158:534–548. doi: 10.1016/j.cell.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science. 2008:319. doi: 10.1126/science.1141448. [DOI] [PubMed] [Google Scholar]
- 37.Li G, Scull C, Ozcan L, Tabas I. NADPH oxidase links endoplasmic reticulum stress, oxidative stress and PKR activation to induce apoptosis. J Cell Biol. 2010;191:1113–1125. doi: 10.1083/jcb.201006121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li G, Mongillo M, Chin KT, Harding H, Ron D, Marks AR, Tabas I. Role of ERO1-alpha-mediated stimulation of inositol 1,4,5-triphosphate receptor activity in endoplasmic reticulum stress-induced apoptosis. J Cell Biol. 2009;186:783–792. doi: 10.1083/jcb.200904060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kang MJ, Chung J, Ryoo HD. CDK5 and MEKK1 mediate pro-apoptotic signalling following endoplasmic reticulum stress in an autosomal dominant retinitis pigmentosa model. Nat Cell Biol. 2012;14:409–415. doi: 10.1038/ncb2447. [DOI] [PMC free article] [PubMed] [Google Scholar]