Abstract
Background and Purpose
The visual analogue scale (VAS) is a self-reported, validated tool to measure quality of life (QoL). Our purpose was to determine whether baseline QoL predicted strokes in the ALLHAT study and evaluate determinants of post-stroke change in QoL. In the ALLHAT study, among the 33,357 patients randomized to treatment arms, 1,525 suffered strokes; 1,202 (79%) strokes were non-fatal. This study cohort includes 32,318 (97%) subjects who completed the baseline VAS QoL estimate.
Methods
QoL was measured on a VAS and adjusted using a Torrance transformation (TQoL). Kaplan-Meier curves and adjusted proportional hazards analyses were used to estimate the effect of TQoL on the risk of stroke, on a continuous scale (0–1) and by quartiles (≤0.81, >0.81 ≤0.89, >0.89≤0.95, >0.95). We analyzed the change from baseline to first post-stroke TQoL using adjusted linear regression.
Results
After adjusting for multiple stroke risk factors, the hazard ratio for stroke events for baseline TQoL was 0.93 (95% CI 0.89–0.98) per 0.1 unit increase. The lowest baseline TQoL quartile had a 20% increased stroke risk (HR =1.20 [CI 1.00–1.44]) compared to the reference highest quartile TQoL. Post-stroke TQoL change was significant within all treatment groups (p≤.001). Multivariate regression analysis revealed that baseline TQoL was the strongest predictor of post-stroke TQoL with similar results for the untransformed QoL.
Conclusions
The lowest baseline TQoL quartile had a 20% higher stroke risk than the highest quartile. Baseline TQoL was the only factor that predicted post-stroke change in TQoL.
Clinical Trials Registration
https://clinicaltrials.gov/ct2/show/NCT00000542?term=allhat&rank=3 Unique Identifier: NCT00000542
Keywords: Quality of Life, Stroke, Cerebrovascular disease, Risk Factors, Cardiovascular disease, Neurobehavior
Subject Codes: Lifestyle, Risk Factors, Cardiovascular disease, Quality and Outcomes, Cerebrovascular Disease/Stroke, Cognitive impairment
Introduction
In the United States, approximately 795,000 people experience new or recurrent stroke annually1,2. Quality of life (QoL) measures are frequently utilized tools for outcomes analysis post-stroke, but may also serve as an important independent predictor of major diseases. Health-related QoL has been reported to independently predict mortality following Coronary Artery Bypass Grafting (CABG)3, Myocardial Infarction (MI)4,5, heart failure6, Diabetes Mellitus7,8, and Hemodialysis 9. Egido et al found that psychosocial stress predicted stroke in a small case-control study, implying that QoL is a significant causative role10,11. Socioeconomic factors influencing QoL such as unemployment or multiple job losses, may be significant risk factors for acute MI and stroke12,13,14.
Since ample evidence suggests that strokes impact post-stroke QoL 15,16, we seek to explore how QoL prior to stroke-events may impact stroke risk and outcomes. Despite major QoL burdens of stroke 17, there is no consensus stroke-specific QoL measurement tool in wide usage. The National Institute of Neurological Disorders and Stroke Common Data Elements (NINDS CDE)18 and European Stroke Organization Outcomes Working Group19 recommend standardized indices to monitor outcomes within clinical trials. The Barthel Index (BI) and the modified Rankin scale (mRS), commonly utilized stroke outcome scales, quantify activities of daily living (ADL) or the extent of functional disabilities, respectively. However, they each fail to assess the broad multi-dimensional factors contributing to QoL. Ali et al demonstrated correlation between mRS, BI, and QoL (generic and disease specific); self-reported QoL had a significantly stronger association with mRS while proxy QoL had stronger association with BI20,21,22.
The simple visual analogue scale (VAS), an easily used instrument to measure self-reported QoL, has been validated with excellent reliability in assessing global QoL, when compared to multi-item questionnaire tool 23, and serves as an appropriate tool to measure QoL in the setting of stroke 24,25,26. To date, there has been no large-scale prospective studies analyzing the relationship between QoL and stroke27. We report relationship of baseline VAS QoL in Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) with subsequent stroke risk and changes in QoL after stroke.
Methods
All clinical sites had local ethics board approvals. Written informed consent was obtained from all subjects or their legally authorized representatives.
ALLHAT Design
Rationale and design of ALLHAT, sponsored by the National Heart, Lung, and Blood Institute have been previously published28. ALLHAT was a randomized, double-blinded trial in 42,418 high-risk hypertensive patients. ALLHAT’s main eligibility criteria were: 1) age ≥55 years 2) hypertension and 3) one or more additional risk factors for heart attack. Mean duration of treatment and follow-up was 4.9 years. Patients were assigned to 4 treatment groups: amlodipine, lisinopril, chlorthalidone, and doxazosin. Doxazosin arm was terminated early due to a significantly higher rate of cardiovascular disease and was not included in this analysis.
Outcomes
Primary endpoint of ALLHAT was the composite of fatal CHD and nonfatal MI. Four major protocol-defined pre-hypothesized secondary outcomes were: 1) all-cause mortality 2) combined CHD 3) strokes and 4) combined cardiovascular disease CVD. QoL was a prespecified secondary outcome28.
Quality of Life
Of ALLHAT participants, 28,534 (86%) completed at least one bi-annual VAS estimate of QoL. The VAS scale ranged from 0–100. This value was transformed using the statistical Torrance transformation29, 30. We analyzed mean baseline QoL and transformed QoL (TQoL) before and after stroke for each participant.
Statistical Analysis
Baseline characteristics are described by stroke status: participants with no stroke versus fatal or nonfatal stroke. Characteristics are also described for participants with no stroke versus those with nonfatal stroke. Groups are compared by T-tests for continuous variables and chi-squared tests for categorical variables.
Mean baseline QoL and TQoL were analyzed by stroke status and by treatment groups. T-tests were used to compare between QoL and TQoL by stroke status. Cox regressions were used to evaluate the impact of baseline QoL and TQoL on stroke status, looking at the QoL variables both continuously and by quartile, both unadjusted and adjusted for selected baseline variables (antihypertensive treatment group, age, gender, race, history MI or stroke, diabetes, history of CHD, smoking status, and baseline SBP, DBP, atrial fibrillation, total cholesterol, LDL, HDL, triglycerides, and aspirin use). Five-year cumulative stroke rates were estimated using the Kaplan-Meier method.
For participants with nonfatal stroke, QoL and TQol were compared pre-stroke and post-stroke. Two analyses were performed: (1) using baseline QoL or TQol and the first post-stroke QoL or TQoL, and (2) using average of all pre-stroke Qol or TQol measurements and the average of all post-stroke Qol or TQol measurements. The difference between pre-stroke and post-stroke values was calculated and T-tests were used to evaluate if changes differed from 0. Analyses were stratified by treatment group, age, race, and gender. Lastly, predictors of pre-stroke to post-stroke changes in QoL and TQol were evaluated using linear regression, both univariate and multivariate, by age subgroups and for the total group. These were also done in the manners described above, using both single and average pre- and post-stroke values. Results using QoL and TQoL were similar; therefore, only results for TQoL are presented. Similarly, pre-stroke vs post-stroke change results were similar using single and average values; therefore, only results using single pre- and post-stroke VAS are presented.
All analyses were done in STATA by the ALLHAT Coordinating Center.
Results
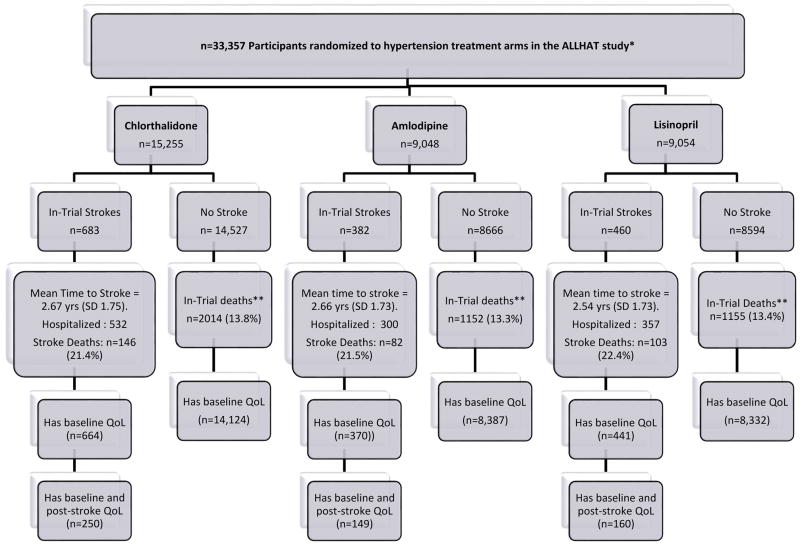
The consort diagram (Figure 1) outlines participants in ALLHAT randomized to each treatment arm (chlorthalidone, amlodipine, and lisinopril) and numbers of subjects no stroke versus with fatal and non-fatal in-trial strokes. Over mean 4.9 years of follow-up, 683 (4.5%), 382 (4.2%), 460 (5.1%) of 33,357 patients randomized to chlorthalidone (n=15,255), amlodipine (n=9,048), and lisinopril (n=9,054) groups experienced strokes, respectively. Mean times to stroke were similar across treatment arms at 2.67± 1.75 years for Chlorthalidone, 2.66±1.73 years for Amlodipine, and 2.54±1.73 years for Lisinopril arms. Stroke-related mortality across randomized groups were also similar between groups: Chlorthalidone (n=146;21%), Amlodipine (n=82;21%), and Lisinopril 22% (n=103;22%). Of all participants with strokes, 1475 (97%) had baseline VAS evaluation, and 559 (47%) with nonfatal stroke had at least one post-stroke VAS evaluation. Baseline characteristics of ALLHAT participants with nonfatal strokes with and without pre/post stroke QOL measurements (Supp. Table III) demonstrate no significant differences between each treatment groups (p=0.43).
Figure 1.
Consort diagram for stroke outcomes among participants randomized to chlorthalidone, amlodipine, or Lisinopril treatment arms in the Antihypertensive and Lipid-Lowering treatment to prevent Heart Attack Trial (ALLHAT)28. **In-trial deaths separate from/does not include incident stroke deaths.
Table 1 shows baseline characteristics of subjects with stroke compared to those without There are significant differences between groups, reflecting established risk factors such as age, race (Blacks>Non-Black), gender (male>female), educational attainment, aspirin use (higher in the stroke group), higher SBP, ASCVD, history of stroke or MI, baseline atrial fibrillation, type 2 diabetes, HDL (all p<.001).
Table 1.
Baseline characteristics of ALLHAT participants without in-trial stroke vs. in-trial nonfatal stroke
| Baseline Characteristic | No in-trial stroke N=31,832 |
In-trial stroke N=1,525 |
P-value |
|---|---|---|---|
| Age (years), mean (SD) | 66.7 (7.7) | 69.8 (7.8) | <0.0001 |
|
| |||
| Age group, n (%) | <0.001 | ||
|
| |||
| 55–64 | 13,767 (43.3) | 417 (27.3) | |
|
| |||
| >65 | 18,065 (56.8) | 1,108 (72.7) | |
|
| |||
| Race, n (%) | <0.001 | ||
|
| |||
| Black | 11,172 (35.1) | 620 (40.7) | |
|
| |||
| Non-Black | 20,660 (64.9) | 905 (59.3) | |
|
| |||
| Men, n (%) | 16,828 (52.9) | 891 (58.4) | <0.001 |
|
| |||
| Education in years, mean (SD) | 11.0 (4.0) | 10.6 (3.9) | 0.0001 |
|
| |||
| Medication use, n (%) | |||
|
| |||
| Antihypertensive treatment | 28,691 (90.1) | 1,398 (91.7) | 0.049 |
|
| |||
| Aspirin | 11,333 (35.6) | 619 (40.6) | <0.001 |
|
| |||
| Lipid medications | 7,447 (23.4) | 326 (21.4) | 0.07 |
|
| |||
| Blood pressure (mm Hg), mean (SD) | |||
|
| |||
| SBP | 146.2 (15.6) | 148.9 (15.4) | <0.001 |
|
| |||
| DBP | 84.1 (10.0) | 83.5 (10.5) | 0.048 |
|
| |||
| Eligibility risk factors, n (%)* | |||
|
| |||
| Current smoker | 6,977 (21.9) | 326 (21.4) | 0.62 |
|
| |||
| ASCVD† | 16,291 (51.2) | 907 (59.5) | <0.001 |
|
| |||
| History Myocardial infarction or stroke | 7,189 (22.6) | 548 (35.9) | <0.001 |
|
| |||
| History coronary revascularization | 4,073 (12.8) | 237 (15.5) | 0.002 |
|
| |||
| Other ASCVD | 7,514 (23.6) | 387 (25.4) | 0.11 |
|
| |||
| Major ST depression or T-wave inversion | 3,259 (10.3) | 161 (10.7) | 0.64 |
|
| |||
| Baseline Atrial fibrillation | 287 (1.0) | 47 (3.3) | <0.001 |
|
| |||
| Type 2 diabetes | 12,436 (39.1) | 732 (48.0) | <0.001 |
|
| |||
| Left ventricular Hypertrophy | 6,273 (19.7) | 343 (22.5) | 0.008 |
|
| |||
| History CHD, n (%) | 7,962 (25.2) | 453 (30.1) | <0.001 |
|
| |||
| Body mass index, mean (SD) | 29.8 (6.2) | 29.2 (6.0) | 0.0005 |
|
| |||
| Antihypertensive treatment group, n (%) | 0.02 | ||
| Chlorthalidone | 14,572 (45.8) | 683 (44.8) | |
| Amlodipine | 8,666 (27.2) | 382 (25.1) | |
| Lisinopril | 8,594 (27.0) | 460 (30.2) | |
| Baseline QOL, mean (SD) | 0.74 (0.16) | 0.72 (0.17) | <0.001 |
| **Transformed baselineQOL, mean (SD) | 0.86 (0.13) | 0.84 (0.14) | <0.001 |
| **Transformed baselineQOL quantiles, n (%) | <0.001 | ||
| 1 (<=0.81) | 8,109 (26.3) | 463 (31.4) | |
| 2 (>0.81 & <=0.89) | 7,470 (24.2) | 373 (25.3) | |
| 3 (>0.89 & <=0.95) | 8,370 (27.1) | 340 (23.1) | |
| 4 (>0.95) | 6,894 (22.4) | 299 (20.3) | |
|
| |||
| Biochemical measures, mean (SD) | |||
|
| |||
| Total cholesterol (mg/dL) | 216.0 (43.2) | 218.6 (48.3) | 0.03 |
|
| |||
| LDL cholesterol (mg/dL) | 135.7 (37.0) | 139.2 (39.7) | 0.0006 |
|
| |||
| HDL cholesterol (mg/dL) | 46.9 (14.8) | 45.4 (14.1) | 0.0001 |
|
| |||
| Triglycerides | 172.8 (133.6) | 171.1 (132.0) | 0.68 |
SBP/DBP=systolic/diastolic blood pressure; ASCVD=atherosclerotic cardiovascular disease; CHD=coronary heart disease; QOL = Quality of Life.
For trial eligibility, participants had to have at least 1 other risk factor in addition to hypertension.
History of MI or stroke; history of coronary revascularization; major ST segment depression on T wave inversion on any ECG in the past 2 years; other ASCVD (please consult original ALLHAT trial design for detailed inclusions).
Mean baseline TQoLs (Supp. Table I) were compared by occurrence of fatal and non-fatal stroke events vs no strokes and found to be significantly lower for strokes compared with non-strokes in Chlorthalidone (0.84 vs 0.86, (p=<0.0001)) and amlodipine groups (0.84 vs.0.86,(P=0.006) but not for lisinopril group (0.85 vs. 0.86; NS).
Table 2 and Figure 2 show the association between baseline TQoL and fatal/nonfatal stroke outcomes. Cox proportional hazards regression analyses were done with and without adjustments for treatment group, age, gender, race, history of MI or stroke, diabetes, history of CHD, smoking, and baseline BP values, atrial fibrillation, cholesterol, LDL, HDL, triglycerides, and aspirin use. In unadjusted model, hazard ratio (HR) of baseline TQoL per 0.1 unit increase, was significantly associated with in-trial strokes with HR 0.90 (95% CI 0.87 – 0.93). After adjustment, HR for stroke events for baseline TQoL per 0.1 unit increase was 0.93 (95% CI 0.89 – 0.98).
Table 2.
Baseline TQOL as Predictors of In-Trial Stroke
| All TQOL | TQoL Quartile 1 (Lowest) | TQoL Quartile 2 | TQoL Quartile 3 | TQoL Quartile 4 (Highest) | |
|---|---|---|---|---|---|
| HR for stroke per 0.1 unit (95% CI) | |||||
| Unadjusted | 0.90 (0.87 – 0.93) | --- | --- | --- | --- |
| Adjusted* | 0.93 (0.89 – 0.98) | --- | --- | --- | --- |
| HR by quartile*, unadjusted | --- | 1.36 (1.18 – 1.58) | 1.16 (1.00 – 1.35) | 0.96 (0.82 – 1.12) | 1.00 (ref) |
| HR by quartile, adjusted** | --- | 1.20 (1.00 – 1.44) | 1.02 (0.85 – 1.24) | 0.97 (0.80 – 1.17) | 1.00 (ref) |
| 5-year cumulative stroke rates by quartile | 0.048 (0.045 – 0.050) | 0.060 (0.053 – 0.064) | 0.050 (0.044 – 0.055) | 0.041 (0.037 – 0.046) | 0.042 (0.038 – 0.048) |
| Number of events by quartile (% of events) | 1312 | 418 (0.31) | 333 (0.25) | 297 (0.23) | 264 (0.20) |
Abbreviations: TQOL=transformed quality of life; HR=hazard ratio
Range of VAS within each quartile of Qol - Highest Quartile >−.85, 3rd Quartile 0.75–0.85, 2nd Quartile 0.65–0.75, Lowest Quartile <0.65.
Adjusted for antihypertensive treatment group, age, gender, race, prior MI or stroke, diabetes, history of CHD, smoking status, and baseline SBP, DBP, atrial fibrillation, total cholesterol, LDL, HDL, triglycerides, and aspirin use.
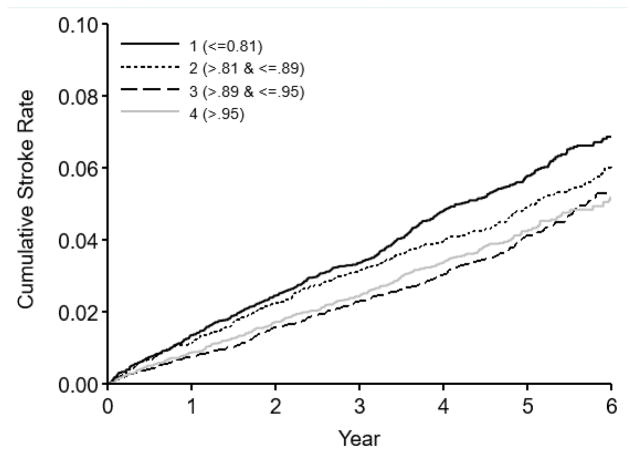
Figure 2. Stroke rates by quartile of baseline TQOL.
Kaplan-Meier event curves for first stroke up to 6 years after randomization, by baseline TQoL quartiles. In the adjusted model, the lowest quartile showed a HR of 1.20 (CI 1.00–1.44), equivalent to 20% higher stroke risk compared to highest quartile TQoL.
Figure 2 shows Kaplan-Meier event curves for first stroke up to 6 years after randomization, by baseline TQoL quartiles. The lowest quartile demonstrated a 36% higher stroke risk (HR=1.36 95% CI 1.18–1.58). In adjusted model, lowest quartile showed a HR of 1.20 (CI 1.00–1.44), equivalent to 20% higher stroke risk compared to highest quartile TQoL. The 2nd lowest quartile had significantly higher stroke risk in unadjusted models (HR=1.16 95% CI 1.00–1.35) but not after risk factor adjustment (HR=1.02, 95% CI 0.85–1.24).
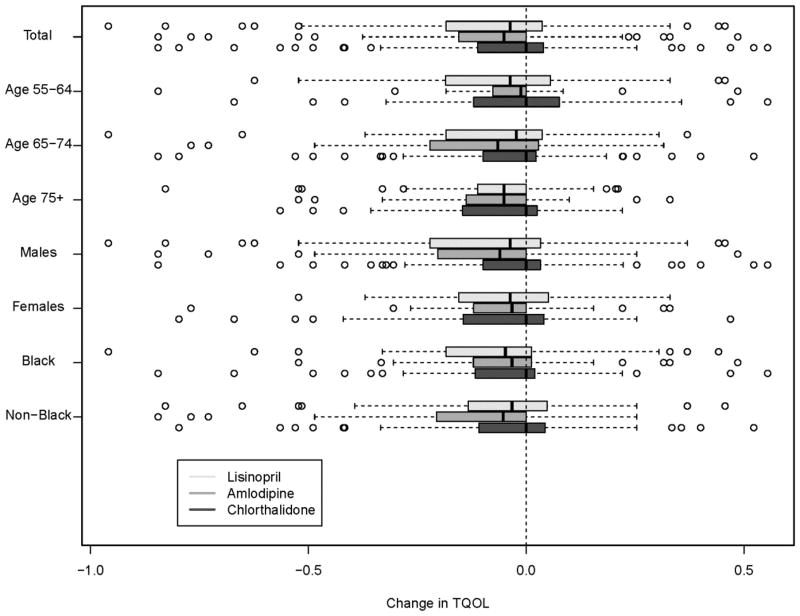
Table 3 and Figure 3 show Pre-stroke and post-stroke TQoL in patients with nonfatal strokes, stratified by treatment arm, age, gender, and race were compared. 559 (47.1%) subjects with non-fatal stroke had pre- and post-stroke QoL scores (Chlorthalidone N=250; Amlodipine N=149; Lisinopril N=160). Post-stroke TQoLs were significantly lower than pre-stroke TQoLs cumulatively (−0.06 SD 0.19, p<0.0001) and when stratified according to treatment groups (−.04; SD .19 in Chlorthalidone, −0.08 SD 0.19 in Amlodipine, and −0.07 SD 0.21 in lisinopril), (p=0.12 for comparison across treatment groups). Mean changes in TQoL increased with age, −0.04, (p=0.08) in subjects 55–64 yrs, −0.06,( p=<0.0001) in 65–75 yrs, and −0.01 (p= <0.0001) in 75+ yrs, although these differences are not significantly different across age groups (p=.18). There were no consistent patterns when TQoL was stratified by gender and race.
Table 3.
Comparison of Prestroke and Poststroke TQoL in Patients With Nonfatal Stroke
| Chlorthalidone | Amlodipine | Lisinopril | Total | |
|---|---|---|---|---|
| Total | ||||
| Total participants with stroke and TQoL | 250 | 149 | 160 | 559 |
| BL TQoL, mean (SD) | 0.84 (0.14) | 0.85 (0.13) | 0.86 (0.13) | 0.85 (0.14) |
| First poststroke TQoL, mean (SD) | 0.81 (0.17) | 0.78 (0.18) | 0.79 (0.19) | 0.79 (0.18) |
| Change in TQoL, mean (SD) | −0.04 (0.19) | −0.08 (0.19) | −0.07 (0.21) | −0.06 (0.19) |
| P value | 0.0011 | <0.0001 | 0.0001 | <0.0001 |
| Age 55–64 y | ||||
| Total participants with stroke and TQoL | 50 | 27 | 44 | 121 |
| BL TQoL, mean (SD) | 0.82 (0.16) | 0.81 (0.16) | 0.85 (0.14) | 0.83 (0.15) |
| First poststroke TQoL, mean (SD) | 0.81 (0.17) | 0.77 (0.19) | 0.80 (0.18) | 0.80 (0.18) |
| Change in TQoL, mean (SD) | −0.02 (0.22) | −0.04 (0.21) | −0.05 (0.22) | −0.04 (0.22) |
| P value | 0.5763 | 0.3103 | 0.1270 | 0.0754 |
| Age 65–74 y | ||||
| Total participants with stroke and TQoL | 116 | 74 | 75 | 265 |
| BL TQoL, mean (SD) | 0.84 (0.14) | 0.85 (0.14) | 0.85 (0.13) | 0.85 (0.14) |
| First poststroke TQoL, mean (SD) | 0.81 (0.18) | 0.77 (0.18) | 0.79 (0.18) | 0.80 (0.18) |
| Change in TQoL, mean (SD) | −0.04 (0.19) | −0.09 (0.20) | −0.07 (0.21) | −0.06 (0.20) |
| P value | 0.0411 | 0.0005 | 0.0079 | <0.0001 |
| Age 75+ y | ||||
| Total participants with stroke and TQoL | 84 | 48 | 41 | 173 |
| BL TQoL, mean (SD) | 0.86 (0.13) | 0.88 (0.11) | 0.88 (0.12) | 0.87 (0.12) |
| First poststroke TQoL, mean (SD) | 0.81 (0.17) | 0.80 (0.16) | 0.79 (0.21) | 0.80 (0.17) |
| Change in TQoL, mean (SD) | −0.05 (0.15) | −0.08 (0.16) | −0.09 (0.20) | −0.07 (0.17) |
| P value | 0.0015 | 0.0014 | 0.0089 | <0.0001 |
| Men | ||||
| Total participants with stroke and TQoL | 154 | 92 | 102 | 348 |
| BL TQoL, mean (SD) | 0.85 (0.15) | 0.85 (0.14) | 0.86 (0.14) | 0.85 (0.15) |
| First poststroke TQoL, mean (SD) | 0.82 (0.15) | 0.76 (0.18) | 0.78 (0.20) | 0.79 (0.18) |
| Change in TQoL, mean (SD) | −0.03 (0.17) | −0.09 (0.21) | −0.08 (0.23) | −0.06 (0.20) |
| P value | 0.0686 | 0.0001 | 0.0006 | <0.0001 |
| women | ||||
| Total participants with stroke and TQoL | 96 | 57 | 58 | 211 |
| BL TQoL, mean (SD) | 0.84 (0.13) | 0.86 (0.12) | 0.85 (0.12) | 0.85 (0.12) |
| First poststroke TQoL, mean (SD) | 0.78 (0.20) | 0.81 (0.16) | 0.81 (0.16) | 0.79 (0.18) |
| Change in TQoL, mean (SD) | −0.06 (0.20) | −0.05 (0.16) | −0.05 (0.17) | −0.05 (0.18) |
| P value | 0.0046 | 0.0145 | 0.0490 | <0.0001 |
| Blacks | ||||
| Total participants with stroke and TQoL | 95 | 51 | 68 | 214 |
| BL TQoL, mean (SD) | 0.84 (0.16) | 0.85 (0.14) | 0.86 (0.14) | 0.85 (0.15) |
| First poststroke TQoL, mean (SD) | 0.79 (0.19) | 0.81 (0.13) | 0.79 (0.19) | 0.80 (0.18) |
| Change in TQoL, mean (SD) | −0.05 (0.20) | −0.04 (0.17) | −0.07 (0.22) | −0.07 (0.23) |
| P value | 0.0162 | 0.0925 | 0.0085 | 0.0001 |
| Non-blacks | ||||
| Total participants with stroke and TQoL | 155 | 98 | 92 | 345 |
| BL TQoL, mean (SD) | 0.85 (0.13) | 0.86 (0.13) | 0.86 (0.12) | 0.85 (0.13) |
| First poststroke TQoL, mean (SD) | 0.81 (0.16) | 0.76 (0.19) | 0.79 (0.19) | 0.79 (0.18) |
| Change in TQoL, mean (SD) | −0.03 (0.18) | −0.09 (0.20) | −0.06 (0.20) | −0.06 (0.19) |
| P value | 0.0259 | <0.0001 | 0.0033 | <0.0001 |
BL indicates baseline; and TQoL, transformed quality of life.
Figure 3.
Box-and-whisker plot of changes in TQOL from pre-stroke to post-stroke (line in shaded boxes indicates median)
The goal of our multivariate analyses, (Table 3 and Supp. Table II), was to determine predictors of change in TQoL in participants with nonfatal stroke, unadjusted (Table 3) and after adjusting for clinical risk variables (Supp. Table II). Table 3 presents unadjusted TQOL change by treatment group, age, sex, and race. Most of the changes were significantly different from 0. However, there were no significant differences across treatment groups, age at stroke, sex, or race. Multivariable analyses, including tests for interaction for age at stroke and baseline TQol and treatment group and baseline TQoL, are shown in Supplemental Table 2. Baseline TQOL was a significant predictor of post-stroke TQoL change. No other clinical risk factor significantly impacted TQOL change. Similarly multivariate regression analysis stratified by age revealed that baseline TQoL was strongest predictor of post-stroke change in TQol in all age groups.
DISCUSSION
The objective of this study was to determine effect of baseline QoL on stroke risk and outcomes in the ALLHAT study, demonstrating utility of simple VAS after previous work has demonstrated VAS and its transformation useful in cost-effectiveness studies in ALLHAT study population31.
The major findings of this study indicate that baseline QoL is significantly lower in those who suffered a stroke during the average 4.9 years of ALLHAT follow-up, except in Lisinopril treatment group. Subjects in lowest quartile of baseline TQoL had a 20% higher stroke risk compared to reference group of highest quartile in a model adjusted for commonly known stroke risk factors.
Change in QoL showed a strong age effect from 50–64, 65–74 and 75+, which trended across all treatment arms. Amlodipine group had greatest change in QoL and TQoL, but biological and functional meaning of this is unclear. There was no consistent effect by gender or ethnicity.
We examined risk factors for change in TQoL in both univariate and multivariate models. Baseline TQoL was the strongest predictor of average post-stroke TQoL. A smaller effect was seen in stroke subjects less than 75 years of age. Greater TQoL change in older subjects is consistent with previous findings that stroke in older individuals is associated with less favorable outcomes32,33.
The study has several substantial limitations which affect the conclusions and generalizability. ALLHAT remains the largest anti-hypertensive treatment trial, but was not designed as a stroke study. ALLHAT study was a parallel group study. We have created new sub-cohorts in our analysis of this secondary outcome measure, whose power to detect differences may be limited, although sample sizes remain quite large due to size of the complete ALLHAT study population. Conducted between 1994 and 2002, with an average of 4.9 years of follow up (3.2 years for the Doxazosin group) this study unfortunately did not collect data on type of stroke (nor prior stroke type), traditional Neurological outcomes, nor currently used functional outcome measures(BI and mRS). No cognitive outcomes are available, limiting attribution of cognitive change due to stroke post-stroke- QoL. Further, no clinical correlation with QoL and activities of daily living (ADLs) is available and warrants future investigation.
Special effort was made to recruit minorities, and 32% of final cohort were Black non-hispanic34. This group alone recorded 620 strokes (40% of total in-study strokes), which is a significant contribution to existing literature. While this minority recruitment effort contributed to groundbreaking utility of the primary outcome in changing hypertension treatment, ALLHAT is not a community based study, and data on Hispanics and Native Americans are not available to make additional generalizations regarding the overall population. Furthermore, individuals may enroll in clinical drug studies for many reasons, further skewing the population studied from the general population.
Prior stroke was not an exclusion criteria for subjects, and inclusion of this group can skew data on baseline QOL measures which correlate with outcome QOL. For this reason, our analysis included analysis of QOL change with in-study stroke, and there is no evidence that subjects with prior strokes were preferentially represented in any treatment arm nor other subgroup. Aspirin usage was higher in subjects with stroke (which includes infarcts and cerebral hemorrhages), and this may affect clinical outcomes 35,36 and further analysis of the meaning of this effect cannot be considered. BI and mRS were not collected and comparison with other stroke clinical trials is limited 18,37 . The effect of lipid lowering was not included in our analyses, and is beyond the scope of this paper. Furthermore, generalization of our results to agents other than the treatment groups cannot be assessed.
In a study of ALLHAT size and breadth, it is inevitable that missing data will affect the analysis. There is no evidence of biased data ascertainment, and we acknowledge that missing follow up data on QoL/TQoL post-stroke might affect analytical conclusions, although robustness and face validity of findings remains. Lastly, conclusions derived from subgroup analysis (table 2, Figure 3), especially when stratified by treatment groups, may be underpowered.
QoL is a multiply determined integrative function 16,38, and seeming simplicity of VAS captures a global measure but does not distinguish which QoL component contributes to the results. The magnitude of stroke risk change between highest to lowest QoL group suggests possibility lifestyle change affecting global QoL could make a contribution to stroke risk reduction.
The VAS is a simply administered validated instrument. Its application in this study has revealed important outcomes seen in other conditions such as CABG, MI, hemodialysis, and DM. The use of a VAS tool for QoL assessment should be considered for inclusion in other stroke registries and clinical trials.
Supplementary Material
Acknowledgments
SOURCES OF FUNDING
This study was supported until June 1, 2016, by contracts N01-HC-35130 and HHSN268201100036C with National Heart, Lung, and Blood Institute (NHLBI) of National Institutes of Health (NIH). ALLHAT investigators acknowledge contributions of study medications by Pfizer, Inc. (amlodipine and doxazosin), AstraZeneca (atenolol and lisinopril) and Bristol-Myers Squibb (pravastatin) and financial support provided by Pfizer, Inc. Opinions expressed in the manuscript are those of authors and do not necessarily represent official views of NHLBI, NIH, or Department of Health and Human Services.
Footnotes
Disclosures:
Alan Lerner receives research funding from NIH, Axovant, Novartis, TauRX, Eli Lily.
Suzanne Oparil (S.O.) has received research grant support or reimbursement for travel to meetings or other, non-financial support from: Actelion Clinical Research/George Clinical; AstraZeneca AB; Bayer; Lundbeck; Novartis; Novo Nordisk; Rox Medical.” Suzanne Oparil (S.O.) has consulted for: Actelion/George Clinical, Lundbeck, Novo Nordisk and ROX Medical.
References
- 1.Papademetriou V, Piller LB, Ford CE, Gordon D, Harney T, Geraci T, et al. Characteristics and lipid distribution of a large, high-risk, hypertensive population: the lipid-lowering component of the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) J Clin Hypertens Greenwich Conn. 2003;5:377–384. doi: 10.1111/j.1524-6175.2003.03163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Go AS, Mozaffarian D, Roger V, Benjamin E, Berry J, Blaha M, et al. Executive summary: heart disease and stroke statistics--2014 update: a report from the american heart association. Circulation. 2014;129:399–410. doi: 10.1161/01.cir.0000442015.53336.12. [DOI] [PubMed] [Google Scholar]
- 3.Rumsfeld JS, MaWhinney S, McCarthy M, Shroyer AL, VillaNueva CB, O’Brien M, et al. Health-related quality of life as a predictor of mortality following coronary artery bypass graft surgery. Participants of the Department of Veterans Affairs Cooperative Study Group on Processes, Structures, and Outcomes of Care in Cardiac Surgery. JAMA J Am Med Assoc. 1999;281:1298–1303. doi: 10.1001/jama.281.14.1298. [DOI] [PubMed] [Google Scholar]
- 4.Venskutonyte L, Brismar K, Öhrvik J, Rydén L, Kjellström B. Self-rated health predicts outcome in patients with type 2 diabetes and myocardial infarction: a DIGAMI 2 quality of life sub-study. Diabetes Vasc Dis Res Off J Int Soc Diabetes Vasc Dis. 2013;10:361–367. doi: 10.1177/1479164113482694. [DOI] [PubMed] [Google Scholar]
- 5.Venskutonyte L, Brismar K, Ryden-Bergsten T, Ryden L, Kjellstrom B. Satisfaction with glucose-lowering treatment and well-being in patients with type 2 diabetes and myocardial infarction: A DIGAMI2 QoL sub-study. Diab Vasc Dis Res. 2013;10:263–269. doi: 10.1177/1479164112463711. [DOI] [PubMed] [Google Scholar]
- 6.Alla F, Briancon S, Guillemin F, Juilliere Y, Mertes PM, Vellemot JP, et al. Self-rating of quality of life provides additional prognostic information in heart failure. Insights into the EPICAL study Eur J Heart Fail. 2002;4:337–343. doi: 10.1016/s1388-9842(02)00006-5. [DOI] [PubMed] [Google Scholar]
- 7.Tentori F, Mapes DL. Health-related quality of life and depression among participants in the DOPPS: predictors and associations with clinical outcomes. Semin Dial. 2010;23:14–16. doi: 10.1111/j.1525-139X.2009.00677.x. [DOI] [PubMed] [Google Scholar]
- 8.Hayes AJ, Clarke PM, Glasziou PG, Simes RJ, Drury PL, Keech AC, et al. Can self-rated health scores be used for risk prediction in patients with type 2 diabetes? Diabetes Care. 2008;31:795–797. doi: 10.2337/dc07-1391. [DOI] [PubMed] [Google Scholar]
- 9.Tsai YC, Hung C, Hwang SJ, Wang SL, Hsiao SM, Lin MY, et al. Quality of life predicts risks of end-stage renal disease and mortality in patients with chronic kidney disease. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc - Eur Ren Assoc. 2010;25:1621–1626. doi: 10.1093/ndt/gfp671. [DOI] [PubMed] [Google Scholar]
- 10.Egido JA, Castillo O, Roig B, Sanz I, Herrero MR, Garay MT, et al. Is psycho-physical stress a risk factor for stroke? A case-control study. J Neurol Neurosurg Psychiatry. 2012;83:1104–1110. doi: 10.1136/jnnp-2012-302420. [DOI] [PubMed] [Google Scholar]
- 11.Henderson KM, Clark CJ, Lewis TT, Aggarwal NT, Beck T, Guo H, et al. Psychosocial distress and stroke risk in older adults. Stroke J Cereb Circ. 2013;44:367–372. doi: 10.1161/STROKEAHA.112.679159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dupre ME, George LK, Liu G, Peterson ED. The cumulative effect of unemployment on risks for acute myocardial infarction. Arch Intern Med. 2012;172:1731–1737. doi: 10.1001/2013.jamainternmed.447. [DOI] [PubMed] [Google Scholar]
- 13.Gallo WT, Teng HM, Falba TA, Kasl SV, Krumholz HM, Bradley EH. The impact of late career job loss on myocardial infarction and stroke: a 10 year follow up using the health and retirement survey. Occup Environ Med. 2006;63:683–687. doi: 10.1136/oem.2006.026823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lewis BL, Pearce LA, Field TS, White CL, Benavente OR. The relevance of living supports on antiplatelet adherence and trial participation: the SPS3 trial. Int J Stroke Off J Int Stroke Soc. 2014;9:443–448. doi: 10.1111/ijs.12267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holloway RG, Arnold RM, Cretzfeldt CJ, Lewis EF, Lutz BJ, McCann RM, et al. Palliative and End-of-Life Care in Stroke: A Statement for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2014;45:1887–916. doi: 10.1161/STR.0000000000000015. [DOI] [PubMed] [Google Scholar]
- 16.Patel MD, McKevitt C, Lawrence E, Rudd AG, Wolfe CDA. Clinical determinants of long-term quality of life after stroke. Age Ageing. 2007;36:316–322. doi: 10.1093/ageing/afm014. [DOI] [PubMed] [Google Scholar]
- 17.Sheldenkar A, Crichton S, Douiri A, Rudd AG, Wolfe CDA, Chen R. Temporal trends in health-related quality of life after stroke: analysis from the South London Stroke Register 1995–2011. Int J Stroke Off J Int Stroke Soc. 2014;9:721–727. doi: 10.1111/ijs.12257. [DOI] [PubMed] [Google Scholar]
- 18.Saver JL, Warach S, Janis S, Odenkirchen J, Becker K, Benavente O, et al. Standardizing the structure of stroke clinical and epidemiologic research data: the National Institute of Neurological Disorders and Stroke (NINDS) Stroke Common Data Element (CDE) project. Stroke J Cereb Circ. 2012;43:967–973. doi: 10.1161/STROKEAHA.111.634352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lees KR, Bath PMW, Schellinger PD, Kerr DM, Fulton R, Hacke W, et al. Contemporary outcome measures in acute stroke research: choice of primary outcome measure. Stroke J Cereb Circ. 2012;43:1163–1170. doi: 10.1161/STROKEAHA.111.641423. [DOI] [PubMed] [Google Scholar]
- 20.Ali M, Fulton R, Quinn T, Brady M VISTA Collaboration. How well do standard stroke outcome measures reflect quality of life? A retrospective analysis of clinical trial data. Stroke J Cereb Circ. 2013;44:3161–3165. doi: 10.1161/STROKEAHA.113.001126. [DOI] [PubMed] [Google Scholar]
- 21.Lai S-M, Perera S, Duncan PW, Bode R. Physical and social functioning after stroke: comparison of the Stroke Impact Scale and Short Form-36. Stroke J Cereb Circ. 2003;34:488–493. doi: 10.1161/01.str.0000054162.94998.c0. [DOI] [PubMed] [Google Scholar]
- 22.Pinto EB, Maso I, Vilela RNR, Santos LC, Oliveira-Filho J. Validation of the EuroQol quality of life questionnaire on stroke victims. Arq Neuropsiquiatr. 2011;69:320–323. doi: 10.1590/s0004-282x2011000300010. [DOI] [PubMed] [Google Scholar]
- 23.de Boer A, van Lanschot J, Stalmeier P, van Sandick J, Stalmeier P, van Sandick J, et al. Is a single-item visual analogue scale as valid, reliable and responsive as multi-item scales in measuring quality of life? Qual. Life Res Int J Qual Life Asp Treat Care Rehabil. 2004;13:311–320. doi: 10.1023/B:QURE.0000018499.64574.1f. [DOI] [PubMed] [Google Scholar]
- 24.Hansen H, Schfer I, Schon G, Riedel-Heller S, Gensichen J, Weyerer S, et al. Agreement between self-reported and general practitioner-reported chronic conditions among multimorbid patients in primary care - results of the MultiCare Cohort Study. BMC Fam Pract. 2014;15:39. doi: 10.1186/1471-2296-15-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hilari K, Boreham LD. Visual analogue scales in stroke: what can they tell us about health-related quality of life? BMJ Open. 2013;3:e003309. doi: 10.1136/bmjopen-2013-003309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hilari K, Needle JJ, Harrison KL. What are the important factors in health-related quality of life for people with aphasia? A systematic review. Arch Phys Med Rehabil. 2012;93:S86–95. doi: 10.1016/j.apmr.2011.05.028. [DOI] [PubMed] [Google Scholar]
- 27.Haacke C, Althaus A, Spottke A, Siebert U, Back T, Dodel R. Long-term outcome after stroke: evaluating health-related quality of life using utility measurements. Stroke J Cereb Circ. 2006;37:193–198. doi: 10.1161/01.STR.0000196990.69412.fb. [DOI] [PubMed] [Google Scholar]
- 28.Davis B, Cutler J, Gordon D, Furberg C, Wright J, Jr, Cushman W, et al. Rationale and design for the Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). ALLHAT Research Group. Am J Hypertens. 1996;9:342–360. doi: 10.1016/0895-7061(96)00037-4. [DOI] [PubMed] [Google Scholar]
- 29.Torrance GW, Boyle MH, Horwood SP. Application of multi-attribute utility theory to measure social preferences for health states. Oper Res. 1982;30:1043–1069. doi: 10.1287/opre.30.6.1043. [DOI] [PubMed] [Google Scholar]
- 30.Torrance GW, Feeny D, Furlong W. Visual analog scales: do they have a role in the measurement of preferences for health states? Med. Decis Mak Int J Soc Med Decis Mak. 2001;21:329–334. doi: 10.1177/0272989X0102100408. [DOI] [PubMed] [Google Scholar]
- 31.Heidenreich P, Davis B, Cutler J, Furberg C, Lairson D, Shlipak M, et al. Cost-effectiveness of chlorthalidone, amlodipine, and lisinopril as first-step treatment for patients with hypertension: an analysis of the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) J Gen Intern Med. 2008;23:509–516. doi: 10.1007/s11606-008-0515-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharma JC, Fletcher S, Vassallo M. Strokes in the elderly - higher acute and 3-month mortality - an explanation. Cerebrovasc Dis Basel Switz. 1999;9:2–9. doi: 10.1159/000015889. [DOI] [PubMed] [Google Scholar]
- 33.Hacke W, Donnan G, Fieschi C, Kaste M, von Kummer R, Broderick J, et al. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 2004;363:768–774. doi: 10.1016/S0140-6736(04)15692-4. [DOI] [PubMed] [Google Scholar]
- 34.Pressel S, et al. Participant recruitment in the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) Control Clin Trials. 2001;22:674–686. doi: 10.1016/s0197-2456(01)00177-5. [DOI] [PubMed] [Google Scholar]
- 35.Kolominsky-Rabas PL, Weber M, Gefeller O, Neundoerfer B, Heuschmann PU. Epidemiology of ischemic stroke subtypes according to TOAST criteria: incidence, recurrence, and long-term survival in ischemic stroke subtypes: a population-based study. Stroke J Cereb Circ. 2001;32:2735–2740. doi: 10.1161/hs1201.100209. [DOI] [PubMed] [Google Scholar]
- 36.Lovett JK, Coull AJ, Rothwell PM. Early risk of recurrence by subtype of ischemic stroke in population-based incidence studies. Neurology. 2004;62:569–573. doi: 10.1212/01.wnl.0000110311.09970.83. [DOI] [PubMed] [Google Scholar]
- 37.Lerdal A, Gay CL. Fatigue in the acute phase after first stroke predicts poorer physical health 18 months later. Neurology. 2013;81:1581–1587. doi: 10.1212/WNL.0b013e3182a9f471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carod-Artal FJ, Egido JA. Quality of life after stroke: the importance of a good recovery. Cerebrovasc Dis Basel Switz. 2009;27(Suppl 1):204–214. doi: 10.1159/000200461. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.