Abstract
We review recent findings related to the neurobiology of infant attachment, emphasizing the role of parenting quality in attachment formation and emotional development. Current findings suggest that the development of brain structures important for emotional expression and regulation (amygdala, prefrontal cortex, hippocampus) is deeply associated with the quality of care received in infancy, with sensitive caregiving providing regulation vital for programming these structures, ultimately shaping the development of emotion into adulthood. Evidence indicates that without sensitive caregiving, infants fail to develop mechanisms needed for later-life emotion and emotion regulation. Research suggests that a sensitive period exists in early life for parental shaping of emotional development, although further cross-species research is needed to discern its age limits, and thus inform interventions.
Introduction
Attachment, which is defined as the selective and enduring bond between individuals, occurs throughout the lifespan [1], encompassing both infant-caregiver attachment and adult romantic attachment. While substantial research has begun documenting the neurobiology of attachment, it has primarily focused on adult romantic attachment and adult attachment to their offspring [2,3,4]. However, more recent research is exploring the neurobiology of infant attachment to the caregiver. Within the context of infant-caregiver attachment, the term “attachment” has traditionally been used to describe a complex and highly specific bond an infant forms to their caregiver by 1 year of age [1]. However, newborns display highly specialized behaviors which can be characterized as bonding behaviors important for attachment formation [5], which we will discuss here.
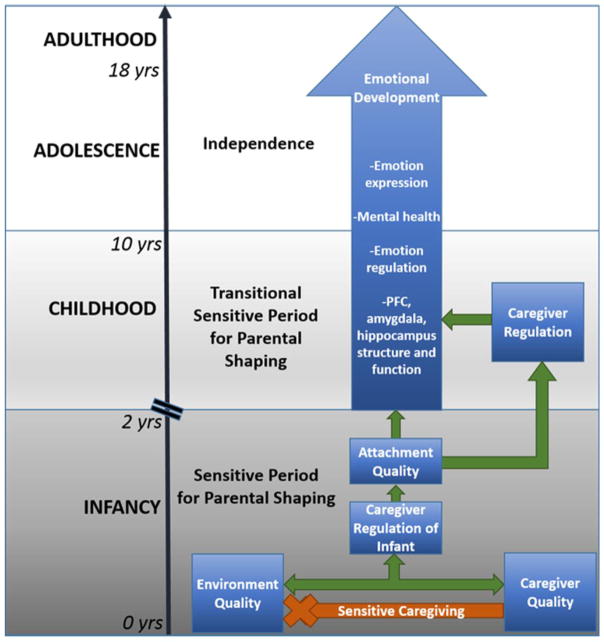
In this state of the art review, we review human and animal model research published within the past 5 years that advances our understanding of the neurobiology of infant attachment formation and the unique role of the primary caregiver in guiding attachment and infant emotional development. Recent evidence supports a working model of early-life parental shaping of lifelong emotional development, with quality of care greatly affecting emotionality and emotion regulation throughout the life course (Figure 1). Continued cross-species research will further our understanding of the mechanisms by which parenting quality in early life programs brain structures underlying lifelong emotionality.
Figure 1.
Caregiver regulation of infant behavior and physiology in early life programs later life emotionality.
Body
The Neurobiology of Infant Attachment Formation
Altricial species are not quickly mobile after birth, and rely on adults for care and nourishment. Thus, infants of altricial species, such as humans, rely on attaching to a caregiver for survival. Historically, scientists have questioned whether infant attachment is formed via biologically innate mechanisms or experience-dependent processes. To date, very little is known about the neurobiology of attachment in human infants, due to technical and ethical limitations that researchers face when working with babies. However, recent research in animal models supports a theoretical model in which the mammalian infant brain is innately biologically-predisposed to form attachments, but depends on necessary experiential input and infant learning to guide attachment formation, similar to “imprinting” which occurs in avian species [6].
Specifically, infant rat pups possess unique neurobiological mechanisms that promote preference learning and block aversion learning in order to support attachment. This specialized attachment circuit involves a hyper-functioning locus coeruleus releasing high levels of norepinephrine, and a hypo-functioning hypothalamus-pituitary-adrenal (HPA) stress axis (for review see [7]). When this circuit is activated by external somatosensory stimuli, such as stimuli naturally provided by the mother, pups (born blind and deaf) learn to prefer any olfactory stimulus paired with this stimulation during their first 10 days of life. Such preference learning occurs regardless if the stimulation is pleasurable or aversive [7]. Although seemingly paradoxical, the system has presumably evolved to promote infant attachment to a caregiver, and thus survival, regardless of the quality of care [8]. While this animal model has identified brain circuits critical for attachment formation in rodents, it is currently unclear if the mechanisms supporting attachment formation in human infants are the same. However, norepinephrine levels are very high at the time of birth and are critical to attachment formation across numerous species [9], and human children show decreased stress reactivity in early childhood in the presence of a caregiver [10]. Furthermore, it is noteworthy that Bowlby’s original description of attachment was based on animal models, providing a strong foundation for the use of animal models to further our understanding of the neurobiology of infant attachment [1].
Attachment formation begins in the womb, where infants form preferences for maternal cues, including her odor and voice, with continued learning occurring after birth (for review see [5]). Additionally, the experience-driven neurobiological mechanisms supporting attachment formation allow the infant to bond with multiple caregivers once outside of the womb. A recent publication has highlighted the extent that attachment formation is experience-driven [11]. Perry et al. altered the smell of rat mothers’ natural odor via manipulation of their diet. Maternal odor was manipulated because it is critical for pup survival; pups require the maternal odor to orient to the mother, behave socially with the mother, and nipple attach for nursing. Two weeks of rearing with these “newly-scented” mothers produced pups that showed attachment behaviors to the new maternal odor and a loss of value of the original natural maternal odor, as indicated by pups’ failure to approach or nipple attach to the mother with the natural maternal odor. These behavioral changes were paired with drastic differences in infant brain processing of the natural maternal odor, following the dissociation of this odor from caregiving. In a second experiment, the researchers reared infant rat pups with both their mother and father to test whether or not pups also displayed attachment to their father. Pups reared in these conditions showed similar approach levels to their mothers’ and fathers’ natural odors, indicating high odor-preference learning for both. Furthermore, the father’s odor induced a neural signature similar to that of the maternal odor, suggesting that infant experiences with their fathers as a co-caregiver elicited infant attachment in a similar way to mothers. Together these experiments support a neurobiological basis for plasticity within the attachment system, as well as attachment formation to multiple caregivers.
Attachment Despite Adversity
The attachment system serves the infant the immediate benefit of promoting bonding to a caregiver, and thus survival, at this vulnerable point in development. However, since the system allows attachment to caregivers regardless of the quality of parental care received, for some infants (especially those facing adverse environments) this comes at a cost [8]. Indeed, parental care has great control over the environmental and experiential impact on the developing infant, which is due to the unique and powerful control that primary caregivers have on shaping infant development [12].
Recent research in humans suggests that the quality of parental care is critical to infant emotional development, due in part to their pronounced ability to regulate infant behavior and physiology [13,14]. For example, parental presence regulates stress hormones [15,16] and brain activity in children [17,18], but not adolescents [18,19]. While few human researchers have studied brain activity in infancy during caregiver-infant interactions, the evidence thus far further supports parental regulation of the developing infant brain [20,21]. Since it is hard to directly assess what is going on in a human infant brain, clues from animal research are helping us discover how parents regulate the infant brain. A seminal study by Sarro et al. displayed for the first time that infant rat brain activity is directly influenced by interactions with the mother in their natural nest (via, in part, to a noradrenergic neurotransmitter mechanism), with the magnitude of maternal regulation decreasing with age [22].
We propose that parental regulation of infant physiology in early life, a time of heightened and rapid brain development, is critical for the programming of circuitry underlying emotion. When the infant bonds to a caregiver that provides low quality of care, however, the lack of regulation and expected species-specific experiences in early life enduringly disrupts brain areas underlying emotion (Figure 1). Furthermore, we propose that parental regulation of the infant is tightly linked to the patterning and quality of parental care the infant receives. Indeed, parental control of infant physiology decreases as the quality of parenting decreases (i.e. intrusiveness, unpredictability, neglect) [23,24]. We draw further evidence from studies showing that stressful conditions within the home place parents at risk for becoming less sensitive caregivers [25,26], which mediates many adverse child outcomes related to emotion regulation and behavioral problems [27–29]. These enduring outcomes are associated with altered HPA-axis activity [30,31], vagal withdrawal [32], and connectivity of brain areas important for emotion and emotion regulation [33–35]. Research with animal models is providing additional mechanistic insight into how early-life parenting quality enduringly alters brain areas supporting emotion [36–39]. One such model introduces adversity to the attachment system by exposing rodent mothers and pups to a scarce resource environment, which produces an increase in negative maltreatment caregiving behaviors. This model has identified the amygdala as being particularly vulnerable to effects of caregiving quality, as indicated by an increase in depressive-like symptoms and antisocial behaviors in adult offspring who experienced negative caregiving, as well as altered fear-related behaviors, via an amygdala-dependent mechanism [8,40,41] involving decreased amygdala-prefrontal cortex functional connectivity [42]. These infant rodent results mirror altered amygdala-prefrontal cortex connectivity found in orphanage reared human children and nonhuman primates reared with maltreating caregivers [33,43].
Researchers are now exploring what specific aspects of sensitive caregiving promote optimal emotional development in humans, and have identified two main elements of sensitive caregiving that provide the greatest benefits, even in adverse environments: nurturance to the infant, such as sensitivity following a distressful event [44,45], and synchrony, such as caregiver responsiveness to a child’s bid [15,46]. These aspects of sensitive caregiving are associated with many behavioral and physiological outcomes, with parental regulation of infant physiology hypothesized as a mediator [47]. Animal models of caregiver nurturance and caregiver-infant synchrony are needed to better understand the mechanism by which these caregiver styles in early life promote optimal emotional development throughout the lifespan. However, insight can be drawn from existing rodent and primate studies related to mother-infant social buffering [23,48], and social learning [49], which provide evidence of strong maternal regulation of emotional states, emotional learning, and the associated underlying physiology in early life.
Infant attachment to the caregiver occurs regardless of the quality of care received. However, both caregiver quality and the quality of the rearing environment impact the caregiver’s ability to regulate their infant’s brain and physiology, ultimately determining the quality of the parent-infant attachment, and the infant’s emotional development throughout the lifespan. Importantly, sensitive caregiving, such as nurturing and synchronous interactions with the infant, can buffer the effects of adverse environments on infant outcome, making sensitive caregiving an important target for interventions for at-risk families. Lastly, a sensitive period for parental shaping of emotional development occurs in early life and is thought to coincide with strong maternal regulation of infant behavior and physiology for the programming of emotion circuitry. Caregiver regulation of offspring persists into childhood (as a function of attachment quality), although it wanes as offspring approach adolescence, and transition to independence.
Sensitive Periods of Emotional Development
Sensitive periods are conceptualized as developmental windows during which a system displays high plasticity and vulnerability to shaping and attunement by environmental input [50]. A growing body of literature suggests that there is a sensitive period for when parenting can influence systems underlying emotional development of their offspring, although the exact time points of this sensitive period remain to be determined (for review see [14,51]).
We argue that this sensitive period may be intimately linked to the caregiver’s ability to profoundly regulate infant physiology during typical caregiver-infant interactions [22], and in the presence of stressors by buffering infant reactivity [52] (Figure 1). In rodents, powerful maternal regulation of stress hormone reactivity and amygdala fear learning occurs throughout the first two weeks of life [7,49], and maternal regulation of infant brain state during caregiving interactions wanes as pups approach weaning [22], supporting the notion that this sensitive period is confined to infancy and childhood.
In humans, caregivers provide strong regulation of behavior and physiology in childhood (by decreasing amygdala and stress reactivity through prefrontal cortex engagement) but not in adolescence, similarly supporting the idea that a sensitive period exists prior to adolescence [18]. Studies following the outcome of children reared in orphanages with low-quality caregiver interactions found that foster care interventions effectively restored HPA-axis and parasympathetic nervous system reactivity only among children placed in foster care prior to two years of age, suggesting that the first two years of life are particularly open to parental shaping of stress reactivity [53]. Additionally, a recent study provided evidence that a sensitive period for maternal support influencing hippocampal development occurs in preschool [54]. In this longitudinal study, hippocampal development from school-aged children to adolescents increased faster for children with higher levels of preschool maternal support.
Finally, a common theme in recent literature is emerging in which it is proposed that caregiver adversity promotes accelerated infant development via premature closure of sensitive periods [13,33]. While this may be true in some instances, we argue that this conceptualization is too simplistic and has the potential to dissuade researchers from exploring alternative hypotheses, thus limiting our understanding of neurobiological pathways to pathology. For example, there is evidence that early-life adversity precociously activates amygdala activity in humans and rodents [33,55]. However, using rodent models, deeper levels of analyses of gene expression, cell-type specific development, and the development of receptors used for cell-to-cell communication indicate that adversity can accelerate or delay different aspects of brain development [56,57]. Furthermore, rodent research exploring ways to repair the impact of early-life caregiver adversity on later-life emotionality discovered that in adulthood the presence of maternal odor can paradoxically normalize depressive-like and fear behaviors that arise as a result of early-life adversity [41]. Since maternal odor typically loses its regulatory power by weaning [11], this indicates that maternal cues become regulatory at a delayed, developmentally-inappropriate time point following infant experience with negative caregiving. These findings provide exciting advances for research efforts attempting to re-open sensitive periods of maternal regulation of emotional development, in order to enduringly repair early-life effects of negative caregiving in adulthood.
Conclusion
Research on the neurobiology of infant attachment is revealing that the infant brain is uniquely primed for learning about the world in a way that promotes attachment to a caregiver. This attachment bias has immediate benefits, but enduring consequences, due to the caregiver’s powerful ability to program the rapidly developing infant brain. Sensitive caregivers, particularly those who are in synchrony with their infant and provide nurturance during distress, provide the most optimal early-life programming of brain structures important for lifelong emotionality, seemingly via regulation of the infant brain and physiology. Adversity within the attachment system via negative caregiving has an enduring impact on brain areas underlying emotion and emotional regulation. Recent research suggests that there is a sensitive period for parental shaping of emotional development in early life, although further cross-species research is necessary for understanding the age limits of this period and how to re-open this sensitive period for later-life intervention efforts.
Highlights.
Infants form attachment to their caregiver despite the quality of parenting.
Parents program emotionality via regulation of infant physiology.
Parental nurturance and caregiver-infant synchrony are key to infant regulation.
Neglectful, intrusive, and/or unpredictable caregiving disrupts regulation.
Sensitive caregiving protects infant development in adverse environments.
Acknowledgments
We thank Stephen H. Braren for his thoughtful comments during revisions of this article. Preparation of this manuscript was supported by National Institutes of Health Grants R01HD881252 and UG3OD023332 to Clancy Blair, and R37HD083217 and R01MH091451 to Regina M. Sullivan.
Footnotes
Conflict of interest: The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Papers of particular interest, published within the period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Bowlby J. Attachment and loss: retrospect and prospect. Am J Orthopsychiatry. 1982;52:664–678. doi: 10.1111/j.1939-0025.1982.tb01456.x. [DOI] [PubMed] [Google Scholar]
- 2.Swain JE. Baby stimuli and the parent brain: functional neuroimaging of the neural substrates of parent-infant attachment. Psychiatry (Edgmont) 2008;5:28–36. [PMC free article] [PubMed] [Google Scholar]
- 3.Zeki S. The neurobiology of love. FEBS Lett. 2007;581:2575–2579. doi: 10.1016/j.febslet.2007.03.094. [DOI] [PubMed] [Google Scholar]
- 4.Atzil S, Touroutoglou A, Rudy T, Salcedo S, Feldman R, Hooker JM, Dickerson BC, Catana C, Barrett LF. Dopamine in the medial amygdala network mediates human bonding. Proc Natl Acad Sci U S A. 2017;114:2361–2366. doi: 10.1073/pnas.1612233114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sullivan R, Perry R, Sloan A, Kleinhaus K, Burtchen N. Infant bonding and attachment to the caregiver: insights from basic and clinical science. Clin Perinatol. 2011;38:643–655. doi: 10.1016/j.clp.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gottlieb G. Synthesizing nature-nurture: Prenatal roots of instinctive behavior. Mahwah, NJ: Erlbaum; 1997. [Google Scholar]
- 7.Landers MS, Sullivan RM. The development and neurobiology of infant attachment and fear. Dev Neurosci. 2012;34:101–114. doi: 10.1159/000336732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perry R, Sullivan RM. Neurobiology of attachment to an abusive caregiver: short-term benefits and long-term costs. Dev Psychobiol. 2014;56:1626–1634. doi: 10.1002/dev.21219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Numan M, Young LJ. Neural mechanisms of mother-infant bonding and pair bonding: Similarities, differences, and broader implications. Horm Behav. 2016;77:98–112. doi: 10.1016/j.yhbeh.2015.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doom JR, Gunnar MR. Stress physiology and developmental psychopathology: past, present, and future. Dev Psychopathol. 2013;25:1359–1373. doi: 10.1017/S0954579413000667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11**.Perry RE, Al Ain S, Raineki C, Sullivan RM, Wilson DA. Development of Odor Hedonics: Experience-Dependent Ontogeny of Circuits Supporting Maternal and Predator Odor Responses in Rats. J Neurosci. 2016;36:6634–6650. doi: 10.1523/JNEUROSCI.0632-16.2016. Here researchers provided novel evidence that 1) infant rat pups are capable of forming attachments to multiple caregivers (i.e. mother and father), via seemingly similar neurobiological mechanisms, and 2) attachment learning is highly plastic and based on infant experiences with the caregiver. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang AC, Reeb-Sutherland BC, Romeo RD, McEwen BS. On the causes of early life experience effects: evaluating the role of mom. Front Neuroendocrinol. 2014;35:245–251. doi: 10.1016/j.yfrne.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Tottenham N. Social scaffolding of human amygdala-mPFCcircuit development. Soc Neurosci. 2015;10:489–499. doi: 10.1080/17470919.2015.1087424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gee DG. Sensitive Periods of Emotion Regulation: Influences of Parental Care on Frontoamygdala Circuitry and Plasticity. New Dir Child Adolesc Dev. 2016;2016:87–110. doi: 10.1002/cad.20166. [DOI] [PubMed] [Google Scholar]
- 15*.Hibel LC, Trumbell JM, Mercado E. Work/non-workday differences in mother, child, and mother-child morning cortisol in a sample of working mothers and their children. Early Hum Dev. 2014;90:1–7. doi: 10.1016/j.earlhumdev.2013.11.007. This paper explores physiological synchrony in mothers and infants, by sampling cortisol levels simultaneously from both mothers and infants. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seltzer LJ, Prososki AR, Ziegler TE, Pollak SD. Instant messages vs. speech: hormones and why we still need to hear each other. Evol Hum Behav. 2012;33:42–45. doi: 10.1016/j.evolhumbehav.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conner OL, Siegle GJ, McFarland AM, Silk JS, Ladouceur CD, Dahl RE, Coan JA, Ryan ND. Mom-it helps when you’re right here! Attenuation of neural stress markers in anxious youths whose caregivers are present during fMRI. PLoS One. 2012;7:e50680. doi: 10.1371/journal.pone.0050680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18**.Gee DG, Gabard-Durnam L, Telzer EH, Humphreys KL, Goff B, Shapiro M, Flannery J, Lumian DS, Fareri DS, Caldera C, et al. Maternal buffering of human amygdala-prefrontal circuitry during childhood but not during adolescence. Psychol Sci. 2014;25:2067–2078. doi: 10.1177/0956797614550878. This paper eloquently demonstrated evidence of maternal regulation of amygdala reactivity and affect in human children, but not adolescents, translationally extending previous animal findings of a senstive period for maternal regulation of offspring prior to adolescence. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hostinar CE, Johnson AE, Gunnar MR. Parent support is less effective in buffering cortisol stress reactivity for adolescents compared to children. Dev Sci. 2015;18:281–297. doi: 10.1111/desc.12195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Graham AM, Fisher PA, Pfeifer JH. What sleeping babies hear: a functional MRI study of interparental conflict and infants’ emotion processing. Psychol Sci. 2013;24:782–789. doi: 10.1177/0956797612458803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mash C, Bornstein MH, Arterberry ME. Brain dynamics in young infants’ recognition of faces: EEG oscillatory activity in response to mother and stranger. Neuroreport. 2013;24:359–363. doi: 10.1097/WNR.0b013e32835f6828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22**.Sarro EC, Wilson DA, Sullivan RM. Maternal regulation of infant brain state. Curr Biol. 2014;24:1664–1669. doi: 10.1016/j.cub.2014.06.017. This paper demonstrated for the first time, in awake behaving rat pups, that infant pup brain physiology is directly regulated by interactions with the caregiver in the nest. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hostinar CE, Sullivan RM, Gunnar MR. Psychobiological mechanisms underlying the social buffering of the hypothalamic-pituitary-adrenocortical axis: a review of animal models and human studies across development. Psychol Bull. 2014;140:256–282. doi: 10.1037/a0032671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perry NB, Nelson JA, Swingler MM, Leerkes EM, Calkins SD, Marcovitch S, O’Brien M. The relation between maternal emotional support and child physiological regulation across the preschool years. Dev Psychobiol. 2013;55:382–394. doi: 10.1002/dev.21042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Finegood ED, Blair C, Granger DA, Hibel LC, Mills-Koonce R Family Life Project Key I. Psychobiological influences on maternal sensitivity in the context of adversity. Dev Psychol. 2016;52:1073–1087. doi: 10.1037/dev0000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Asok A, Bernard K, Roth TL, Rosen JB, Dozier M. Parental responsiveness moderates the association between early-life stress and reduced telomere length. Dev Psychopathol. 2013;25:577–585. doi: 10.1017/S0954579413000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mills-Koonce WR, Willoughby MT, Garrett-Peters P, Wagner N, Vernon-Feagans L Family Life Project Key I. The interplay among socioeconomic status, household chaos, and parenting in the prediction of child conduct problems and callous-unemotional behaviors. Dev Psychopathol. 2016;28:757–771. doi: 10.1017/S0954579416000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sulik MJ, Blair C, Mills-Koonce R, Berry D, Greenberg M Family Life Project I. Early Parenting and the Development of Externalizing Behavior Problems: Longitudinal Mediation Through Children’s Executive Function. Child Dev. 2015;86:1588–1603. doi: 10.1111/cdev.12386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raver CC, Roy AL, Pressler E, Ursache AM, Charles McCoy D. Poverty-Related Adversity and Emotion Regulation Predict Internalizing Behavior Problems among Low-Income Children Ages 8–11. Behav Sci (Basel) 2016;7 doi: 10.3390/bs7010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berry D, Blair C, Willoughby M, Granger DA, Mills-Koonce WR. Family Life Project Key I. Maternal sensitivity and adrenocortical functioning across infancy and toddlerhood: Physiological adaptation to context? Dev Psychopathol. 2016:1–15. doi: 10.1017/S0954579416000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blair C, Ursache A, Mills-Koonce R, Stifter C, Voegtline K, Granger DA Family Life Project I. Emotional reactivity and parenting sensitivity interact to predict cortisol output in toddlers. Dev Psychol. 2015;51:1271–1277. doi: 10.1037/dev0000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perry NB, Calkins SD, Bell MA. Indirect Effects of Maternal Sensitivity on Infant Emotion Regulation Behaviors: The Role of Vagal Withdrawal. Infancy. 2016;21:128–153. doi: 10.1111/infa.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gee DG, Gabard-Durnam LJ, Flannery J, Goff B, Humphreys KL, Telzer EH, Hare TA, Bookheimer SY, Tottenham N. Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proc Natl Acad Sci U S A. 2013;110:15638–15643. doi: 10.1073/pnas.1307893110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luby J, Belden A, Botteron K, Marrus N, Harms MP, Babb C, Nishino T, Barch D. The effects of poverty on childhood brain development: the mediating effect of caregiving and stressful life events. JAMA Pediatr. 2013;167:1135–1142. doi: 10.1001/jamapediatrics.2013.3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jedd K, Hunt RH, Cicchetti D, Hunt E, Cowell RA, Rogosch FA, Toth SL, Thomas KM. Long-term consequences of childhood maltreatment: Altered amygdala functional connectivity. Dev Psychopathol. 2015;27:1577–1589. doi: 10.1017/S0954579415000954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roth TL, Matt S, Chen K, Blaze J. Bdnf DNA methylation modifications in the hippocampus and amygdala of male and female rats exposed to different caregiving environments outside the homecage. Dev Psychobiol. 2014;56:1755–1763. doi: 10.1002/dev.21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baram TZ, Davis EP, Obenaus A, Sandman CA, Small SL, Solodkin A, Stern H. Fragmentation and unpredictability of early-life experience in mental disorders. Am J Psychiatry. 2012;169:907–915. doi: 10.1176/appi.ajp.2012.11091347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Howell BR, Grand AP, McCormack KM, Shi Y, LaPrarie JL, Maestripieri D, Styner MA, Sanchez MM. Early adverse experience increases emotional reactivity in juvenile rhesus macaques: relation to amygdala volume. Dev Psychobiol. 2014;56:1735–1746. doi: 10.1002/dev.21237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang TY, Labonte B, Wen XL, Turecki G, Meaney MJ. Epigenetic mechanisms for the early environmental regulation of hippocampal glucocorticoid receptor gene expression in rodents and humans. Neuropsychopharmacology. 2013;38:111–123. doi: 10.1038/npp.2012.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raineki C, Cortes MR, Belnoue L, Sullivan RM. Effects of early-life abuse differ across development: infant social behavior deficits are followed by adolescent depressive-like behaviors mediated by the amygdala. J Neurosci. 2012;32:7758–7765. doi: 10.1523/JNEUROSCI.5843-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raineki C, Sarro E, Rincon-Cortes M, Perry R, Boggs J, Holman CJ, Wilson DA, Sullivan RM. Paradoxical neurobehavioral rescue by memories of early-life abuse: the safety signal value of odors learned during abusive attachment. Neuropsychopharmacology. 2015;40:906–914. doi: 10.1038/npp.2014.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yan C, Rincon-Cortes M, Raineki C, Sarro E, Colcombe S, Guilfoyle DN, Yang Z, Gerum S, Biswal BB, Milham MP, et al. Aberrant development of intrinsic brain activity in a rat model of caregiver maltreatment of offspring. Translational Psychiatry. 2017 doi: 10.1038/tp.2016.276. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Howell BR, McMurray MS, Guzman DB, Nair G, Shi Y, McCormack KM, Hu X, Styner MA, Sanchez MM. Maternal buffering beyond glucocorticoids: impact of early life stress on corticolimbic circuits that control infant responses to novelty. Soc Neurosci. 2017;12:50–64. doi: 10.1080/17470919.2016.1200481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bernard K, Meade EB, Dozier M. Parental synchrony and nurturance as targets in an attachment based intervention: building upon Mary Ainsworth’s insights about mother-infant interaction. Attach Hum Dev. 2013;15:507–523. doi: 10.1080/14616734.2013.820920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dozier M, Roben CK, Caron EB, Hoye J, Bernard K. Attachment and Biobehavioral Catch-up: An evidence-based intervention for vulnerable infants and their families. Psychother Res. 2016:1–12. doi: 10.1080/10503307.2016.1229873. [DOI] [PubMed] [Google Scholar]
- 46.Pratt M, Singer M, Kanat-Maymon Y, Feldman R. Infant negative reactivity defines the effects of parent-child synchrony on physiological and behavioral regulation of social stress. Dev Psychopathol. 2015;27:1191–1204. doi: 10.1017/S0954579415000760. [DOI] [PubMed] [Google Scholar]
- 47.Feldman R. Bio-behavioral synchrony: A model for integrating biological and microsocial behavioral processes in the study of parenting. Parenting. 2012;12:154–164. [Google Scholar]
- 48.Al Ain S, Perry RE, Nunez B, Kayser K, Hochman C, Brehman E, LaComb M, Wilson DA, Sullivan RM. Neurobehavioral assessment of maternal odor in developing rat pups: implications for social buffering. Soc Neurosci. 2016:1–18. doi: 10.1080/17470919.2016.1159605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Debiec J, Sullivan RM. Intergenerational transmission of emotional trauma through amygdala-dependent mother-to-infant transfer of specific fear. Proc Natl Acad Sci U S A. 2014;111:12222–12227. doi: 10.1073/pnas.1316740111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Werker JF, Hensch TK. Critical periods in speech perception: new directions. Annu Rev Psychol. 2015;66:173–196. doi: 10.1146/annurev-psych-010814-015104. [DOI] [PubMed] [Google Scholar]
- 51.Feldman R. Sensitive periods in human social development: New insights from research on oxytocin, synchrony, and high-risk parenting. Dev Psychopathol. 2015;27:369–395. doi: 10.1017/S0954579415000048. [DOI] [PubMed] [Google Scholar]
- 52.Gunnar MR, Hostinar CE, Sanchez MM, Tottenham N, Sullivan RM. Parental buffering of fear and stress neurobiology: Reviewing parallels across rodent, monkey, and human models. Soc Neurosci. 2015;10:474–478. doi: 10.1080/17470919.2015.1070198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53*.McLaughlin KA, Sheridan MA, Tibu F, Fox NA, Zeanah CH, Nelson CA., 3rd Causal effects of the early caregiving environment on development of stress response systems in children. Proc Natl Acad Sci U S A. 2015;112:5637–5642. doi: 10.1073/pnas.1423363112. In this study, researchers tracked the outcome of children reared in Romanian orphanages, where they lacked quality caregiver interactions. They reported evidence of a sensitive period for parental shapting of emotional development in the first two years of life, for infants placed into foster care interventions prior to two years of age showed healthier emotional outcomes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54**.Luby JL, Belden A, Harms MP, Tillman R, Barch DM. Preschool is a sensitive period for the influence of maternal support on the trajectory of hippocampal development. Proc Natl Acad Sci U S A. 2016;113:5742–5747. doi: 10.1073/pnas.1601443113. Here researchers present novel findings from a longitudinal study, indicating that maternal support in early childhood powerfully increases hippocampal growth across development, promoting better emotion regulation in early adolescence. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Raineki C, Moriceau S, Sullivan RM. Developing a neurobehavioral animal model of infant attachment to an abusive caregiver. Biol Psychiatry. 2010;67:1137–1145. doi: 10.1016/j.biopsych.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Santiago AN, KYL, Perry RE, Sullivan RM, Aoki C. Neurodevelopment of parvalbumin cells and perineuronal nets following early life trauma. 46th meeting of the Society for Neuroscience; San Diego, CA. 2016. Edited by. [Google Scholar]
- 57.Barr GA, Opendak MM, Perry RE, Kayser K, Sullivan RM. Neonatal pain experienced in the presence of the caregiver has short and long-term consequences for pain and emotion. 46th meeting of the Society for Neuroscience; San Diego, CA. 2016. Edited by. [Google Scholar]