Abstract
Purpose
The aim of this study is to evaluate the effects of Wnt signaling through LRP6 and Frizzled6 on the endothelial differentiation of dental pulp stem cells (DPSC).
Methods
DPSC were stably transduced with EGFP-tagged lentiviral vectors (shRNA-LRP6, shRNA-Frizzled6, or empty vector controls). We evaluated the effects of LRP6 and Frizzled6 on expression of endothelial markers and on capillary tube formation mediated by DPSC induced with rhWnt1 and/or rhVEGF165. In vivo, tooth slices/scaffolds were seeded with LRP6-silenced, Frizzled6-silenced or vector control DPSC cells and transplanted into immunodeficient mice. The density of blood vessels generated by DPSC cells differentiated into vascular endothelial cells was analyzed by immunohistochemistry for EGFP.
Results
rhWnt1 and rhVEGF165 induced expression of active-β-catenin in control DPSC cells and in Frizzled6-silenced DPSC, but not in LRP6-silenced DPSC. Further, VEGF and IL-8 were downregulated in LRP6-silenced DPSC, but not in control DPSC cells or in Frizzled6-silenced DPSC (p<0.05). Likewise, rhWnt1 and rhVEGF165 induced expression of the endothelial marker VEGFR2 in control DPSC cells and in Frizzled6-silenced DPSC, but not in LRP6-silenced DPSC. These data correlated with a trend for lower density of capillary sprouts generated by LRP6-silenced DPSC cells when compared to control DPSC in Matrigel. In vivo, tooth slice/scaffolds seeded with DPSC-shRNA-LRP6 cells showed lower density of human blood vessels (i.e. EGFP-positive blood vessels), when compared to tooth slice/scaffolds seeded with vector control cells (p<0.05).
Conclusion
Collectively, these data demonstrated that LRP6 signaling is necessary for the vasculogenic differentiation of human dental pulp stem cells.
Keywords: Regenerative Endodontics, Wnt, Angiogenesis, Cell fate, Tissue Engineering
Introduction
Dental pulp tissue engineering can be achieved with the use of a scaffold that provides a physiological three-dimensional microenvironment that is conducive to stem cell adhesion, survival and differentiation in presence of adequate morphogenic signaling molecules (1, 2). Successful dental pulp tissue regeneration also requires the fast establishment of a functional vascular network, able to supply the tissue with oxygen, nutrients and immune cells, while removing by products and waste (3). Dental pulp stem cells (DPSC) have the ability to differentiate into odontoblasts, osteoblasts, fibroblasts, adipocytes, neural-like cells, and vascular endothelial cells (4–6). Although DPSC have potential to differentiate into endothelial cells, the molecular events regulating this process are not fully understood.
Wnt signaling plays a major role in the regulation of cell fate decisions during development (7, 8). In absence of Wnt, a multi-protein complex including glycogen synthase kinase 3 beta (GSK-3β) constantly phosphorylates β-catenin, signaling its degradation (9). Activation of Wnt signaling results in the translocation of β-catenin to the nucleus where it interacts with T-cell factor/lymphoid enhancer factor (TCF/LEF) family proteins to modulate expression of several downstream genes, including several pro-angiogenic factors vascular endothelial growth factor (VEGF), Interleukin-8 (IL-8), and vascular endothelial growth factor receptor 2 (VEGFR2) (10–12). Wnt signaling involves a complex set of receptors that include the Frizzled family and low-density lipoprotein receptor-related protein (e.g. LRP5/6). Frizzled functions as a G protein coupled receptor (GPCR) that preferentially couples to Gαs heterotrimeric G proteins (13). More direct evidence for a role of G proteins in Wnt pathway activation comes from reconstitution studies showing that several of them have the capacity to inhibit β-catenin phosphorylation by GSK-3β. For example, it has been proposed that Gβγ promotes the recruitment of GSK-3β to the plasma membrane to enhance LRP6 phosphorylation and activation (14).
Studies have found that Wnt/β-catenin signaling plays role in vasculature in vivo and in the differentiation of stem cells into odontoblasts, endothelial cells, osteoblast and neural cells (5, 15–19). Here, we evaluated the role of Wnt receptors on the differentiation of DPSC into endothelial cells. We hypothesize that LRP6 and Frizzled6 regulate Wnt-induced differentiation of dental pulp stem cells into endothelial cells.
Materials and Methods
Cell culture
Dental pulp stem cells (DPSC) (20) were cultured in minimum essential medium Eagle - Alpha modification (Alpha MEM) (Invitrogen, Carlsbad, CA, USA) supplemented with 15% fetal bovine serum (FBS; Invitrogen) and 1% penicillin/streptomycin (Invitrogen) at 37°C and 5% CO2. Endothelial differentiation was induced by culturing DPSC with endothelial cell growth medium (EGM2-MV; Lonza, Walkersville, MD, USA) supplemented with 50 ng/mL recombinant human VEGF165 (rhVEGF165) (R&D Systems, Minneapolis, MN, USA) and/or 50 ng/mL rhWnt-1 (Cell Sciences, Canton, MA, USA). Endothelial differentiation was assessed in vitro by determining the expression of endothelial markers by Western blot. Human dermal microvascular endothelial cells (HDMEC; Lonza, Walkersville, MD, USA) were used as positive control and cultured in EGM2-MV. Culture medium was changed every 2 days in all experiments included here.
LRP6 and Frizzled6 gene silencing
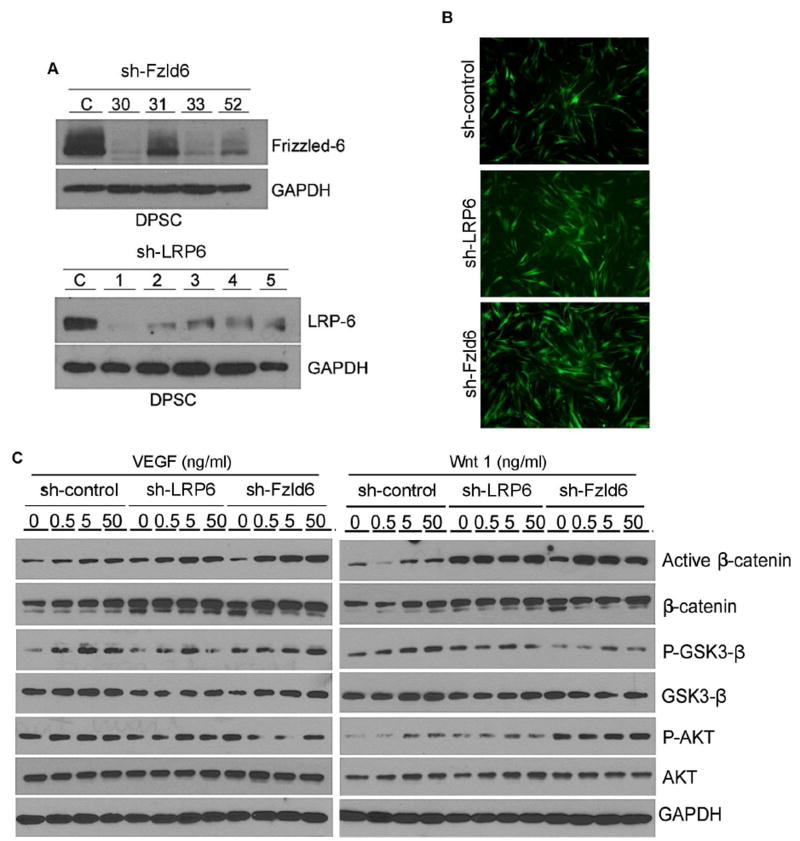
Gene silencing was performed with EGFP (green fluorescent protein)-tagged lentiviral vectors encoding shRNA (short hairpin RNA) constructs, as described previously (5, 6). 293T cells were transiently co-transfected with lentivirus packaging vector psPAX2, pMD2.G, and shRNA-C (control), shRNA-LRP6 or shRNA-Frizzled6 (Vector Core, University of Michigan) with calcium phosphate. Supernatants containing lentiviruses were used to infect DPSC overnight. Transduced DPSC were selected by exposure to 1 μg/mL puromycin (InVivogen, San Diego, CA, USA) for at least 1 week. Infection efficiency was determined by fluorescence, and gene silencing by Western blot (Fig. 1A, 1B).
Figure 1.
Effect of LRP6 and Frizzled6 on VEGF or Wnt1 signaling in DPSC cells. (A) Western blots to evaluate the effectiveness of LRP6 or Frizzled6 silencing in DPSC cells. We tested several shRNA sequences for Frizzled6 (#30,31,33,52) and for LRP6 (#1–5). (B) Fluorescence image to evaluate the effectiveness of lentivirus-mediated transduction of shRNA-LRP6 or shRNA-Frizzled6 into DPSC cells. All lentiviral vectors used here (including controls) contained EGFP. Photomicrographs were taken at 200× magnification. (C) Western blots depicting effect of increasing concentrations of rhVEGF165 or rhWnt1 on phosphorylated and total β-catenin, GSK-3β, and AKT.
Western Blot
Cells were lysed in NP40 (nonyl phenoxypolyethoxylethanol) buffer, proteins were electrophoresed in SDS-polyacrylamide gel (sodium dodecyl sulphate-polyacrylamide gel) and transferred to nitrocellulose membranes (Protran; Whatman, Dassel, Germany). Membranes were incubated at 4°C overnight with prim ary antibodies, as follows: mouse anti-active or anti-total B-catenin; mouse anti-phospho and anti-total GSK-3β; mouse anti-phospho and anti-total AKT; rabbit anti-VEGFR1; rabbit anti-VEGFR2; rabbit anti-LRP6; rabbit anti-Frizzled6; mouse anti-GAPDH; anti-B-actin. SuperSignal West Pico chemiluminescent substrate (Thermo Scientific, Rockford, IL, USA) was used to visualize immunoreactive proteins.
ELISA
Conditioned medium generated by DPSC cells for 24 hours was collected and used for ELISA (enzyme-linked immunosorbent assay) by Quantikine® Colorimetric Sandwich ELISAs (R&D Systems, Minneapolis, MN, USA), according to manufactory instructions. Supernatant was added to each well of a 96-well plate containing anti-VEGF or anti-CXCL8 antibody. Optical density was analyzed in spectrophotometer (450 nm).
Capillary sprouting assay
We cultured 5 ×104/well DPSC cells with endothelial cell growth medium (EGM2-MV; Lonza) supplemented with 50 ng/mL rhVEGF165 (R&D Systems) in 24-well plates pre-coated with 0.2 mL growth factor-reduced Matrigel (BD Biosciences, Bedford, MA, USA). The number of capillary sprouts was counted in 10 fields per well. Data were obtained from triplicate wells per condition and are representative of at least 3 independent experiments. Photographs were taken at 10× magnification each day for 7 days.
Tooth slice/scaffold assay
Tooth slices (1.3 mm thick) were obtained from sound human 3rd molars extracted at the Department of Oral Surgery (University of Michigan). The dental pulp was carefully removed and scaffolds were prepared using a poly-L-lactic (PLLA)/chloroform solution in the pulp chamber filled with sodium chloride (NaCl) (250 μm to 425 μm), as described previously (6). Specimens were treated with 10% EDTA (Ethylenediamine tetraacetic acid) for 1 min. 6×105 transduced DPSC were seeded in each tooth slice/scaffold (n=6 per experimental condition) and transplanted subcutaneously into the dorsum of severe combined immunodeficient mice (CB.17 SCID; Charles River, Wilmington, MA, USA) immediately after seeding. After 28 days, tooth slice/scaffolds were retrieved and fixed with 10% formaldehyde for 24 hours at 4°C and demineralized with Decalcifier II (Surgipat h; Richmond, IL, USA) for 24 hours at room temperature. Hematoxilin-eosin staining and immunohistochemistry with rabbit turbo EGFP (Bethyl Laboratories, Montgomery, TX, USA) were used to evaluate the morphology and density of DPSC-derived microvessels. Vessels were counted in 10 fields per tooth slice/scaffold by a calibrated evaluator (ICC=0.95) blinded for experimental conditions. Institutional review boards approved the protocols regulating these studies.
Results
Signaling through LRP-6 is necessary for activation of B-Catenin in DPSC
To begin to define the relative contribution of different receptors on Wnt/β-catenin signaling in DPSC, we silenced LRP6 or Frizzled6 and exposed them to rhWnt1 or rhVEGF165 (Fig. 1A,B). We used several different shRNA sequences for gene silencing, and used DPSC transduced with the sequences that were most efficient in the experiments included here, i.e. sequence #1 for LRP6 and #30 for Frizzled6 (Fig. 1A). As expected, the expression of active-β-catenin was induced by Wnt1 or VEGF in a dose-dependent manner in DPSC (Fig. 1C). In contrast, in LRP6-silenced DPSC, active-β-catenin was not upregulated by VEGF or Wnt1. Silencing Frizzled6 did not have a significant impact on VEGF- or Wnt1-induced β-catenin activation (Fig. 1C).
LRP6 signaling is necessary for vasculogenic differentiation of DPSC in vitro
Here, we defined the impact of LRP6 and Frizzled6 on the expression of endothelial markers in DPSC. In shRNA-control DPSC cells, the endothelial differentiation medium (i.e. EGM2-MV supplemented with VEGF +/− Wnt1 induced expression of the endothelial marker VEGFR2 (and VEGFR1) (Fig 2A). The same was observed with Frizzled6-silenced DPSC (Fig. 2A). In contrast, LRP6-silenced DPSC did not express endothelial markers in response to the endothelial differentiation medium (Fig. 2A). Interestingly, both VEGF and IL-8 were constitutively downregulated in LRP6-silenced DPSC when compared to control DPSC cells (Fig. 2B,C).
Figure 2.
LRP6 silencing inhibits vasculogenic differentiation of DPSC cells in vitro. (A) Western blots depicting protein expression of endothelial markers (VEGFR1, VEGFR2) upon culture of DPSC in control medium (alpha-MEM), endothelial growth medium (EGM2-MV), or EGM2-MV supplemented with 50 ng/ml rhVEGF165 or rhWnt1. (B,C) Graphs depicting the results of ELISA for VEGF (B) and IL-8 (C) in DPSC cells cultured in control alpha-MEM medium. Different letters represent p<0.05.
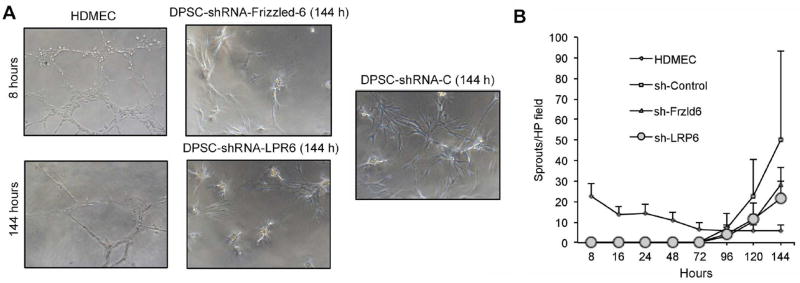
Capillary sprouting assay in Matrigel was used to analyze the impact of Wnt receptors on the vasculogenic potential of DPSC in vitro. We observed that endothelial cells (HDMEC) quickly formed capillary sprouts in Matrigel within a few hours. In contrast, DPSC took much longer to differentiate into endothelial cells and form capillary sprouts (Fig. 3A). However, DPSC-shRNA-LRP6 and DPSC-shRNA-Frizzled6 showed a trend for lower numbers of capillary sprouts compared to vector control DPSC, but the differences were not significant (Fig. 3B).
Figure 3.
Effect of LRP6 or Frizzled6 signaling on capillary sprouting of DPSC in 3-D cultures. (A) Photomicrographs of capillary tube networks generated by human dermal microvascular endothelial cells (HDMEC) or by DPSC seeded in Matrigel and exposed to EGM2-MV supplemented with 50 ng/ml rhVEGF165 for up to 144 hours (200×). (B) Graph depicting the number of sprouts/microscopic field generated by HDMEC, DPSC-silenced cells, or controls seeded on Matrigel and stimulated with EGM2-MV supplemented with 50 ng/ml rhVEGF165 for up to 144 hours.
LRP6 silencing inhibits endothelial differentiation of DPSC in vivo
To verify our in vitro results, DPSC were seeded in tooth slices/scaffolds and implanted in the subcutaneous space of immunodeficient mice. Twenty-eight days after transplantation, tooth slices/scaffolds were retrieved and pulp-like tissues were observed in the pulp chambers (Fig. 4A). As all our shRNA lentiviruses contained an EGFP cassette, we performed immunohistochemistry for this exogenous marker as a strategy to quantify the number of DPSC-derived (human) blood vessels. We observed that there were fewer EGFP-positive blood vessels (p<0.05) in the pulps engineered with LRP6-silenced DPSC, as compared to Frizzled6-silenced DPSC or empty vector control cells (Fig. 4B).
Figure 4.
LRP6 silencing inhibits endothelial differentiation of DPSC in vivo. (A) Tooth slices/scaffolds seeded with LRP6-silenced DPSC, Frizzled6-silenced DPSC, or vector control DPSC were transplanted into the subcutaneous space of immunodeficient mice. After 28 days, tooth slice/scaffolds were retrieved, fixed, and analyzed by hematoxilin-eosin staining (200×), or immunohistochemistry with EGFP antibody (400×). (B) Graph depicting the density of EGFP-positive blood vessels (i.e. generated by DPSC cells) in 6 tooth slice/scaffolds per experimental condition. Blood vessels were counted in 10 microscopic fields/specimen at 200× magnification. Different letters represent p<0.05.
Discussion
It is known that dental pulp stem cells have ability to differentiate into multiple cells lineage (4–6) being a viable source for dental pulp tissue engineering (4). We have demonstrated that human dental pulp stem cells are capable to differentiate into endothelial cells that organize themselves into functional blood vessels that connect with the host vasculature (5, 6). We have also show that VEGF and Wnt1 trigger signaling events that result in the differentiation of DPSC into endothelial cells (5). However, we did not know which Wnt receptor was responsible for the vasculogenic differentiation of endothelial cells.
We have previously shown that dental pulp stem cells of exfoliated teeth (SHED) do not express endothelial markers (i.e. CD31, VEGFR2) when cultured in control a-MEM medium (23). However, when SHED cells are exposed to EGM2-MV medium supplemented with rhVEGF165 they differentiated into endothelial cells (23). Here, we report similar trends for dental pulp stem cells retrieved from permanent teeth. It is known that Wnt/β-catenin signaling plays an important role in vasculogenesis (21). As VEGF expression can be upregulated by activation of the Wnt pathway (10), it is plausible that autologous VEGF can induce angiogenesis (6, 22, 23). Also, Wnt1 was found to directly promote proliferation, tube formation and induction of the pro-angiogenic IL-8 transcription in human endothelial cells (11, 21). β-catenin is the key-mediator of the canonical Wnt signaling. This signaling pathway is activated when Wnt ligands bind to a member of the Frizzled family and a member of LRP family of co-receptors. We found that Frizzled3, 4, 5 and 6, and LRP5 and 6 are expressed in dental pulp stem cells (data not shown). Here, we decided to focus on Frizzled6 and LRP6 as these receptors were consistently highly expressed in DPSC.
Interestingly, we noticed that active β-catenin expression was higher in LRP6-silenced DPSC and in Frizzled6-silenced DPSC treated with rhVEGF165 or rhWnt1. This result is in line with the fact that one of the functions of LRP6 is the inhibition of the β-catenin destruction complex through direct inhibition of GSK3-β activity. However, it is important to notice that β-catenin and GSK3-β participate in other signaling pathways (e.g. AKT signaling) or as a structural component of cellular adhesion complex (24–26). In addition, LRP6 and Frizzled6 also play a role as negative regulators of Wnt/β-catenin signaling through TCF/LEF (25, 27). Our data suggest that LRP6 and Frizzled6 also regulate the activity of β-catenin amount in postnatal stem cells (e.g. DPSC).
We observed that the angiogenic potential of Frizzled6-silenced DPSC is similar to control cells in most of the experiments performed here. Silencing of Frizzled6 does not interfere with the transcription of VEGFR1 and VEGFR2 that play critical roles in the endothelial differentiation of dental pulp stem cells (6). In contrast, LRP6-silenced DPSC did not show upregulation of VEGFR2 in response to the endothelial differentiation medium. These data suggest that LRP6 plays a critical role in the regulation of the angiogenic potential in dental pulp stem cells. This might be related to the observation that LRP6 is required for the activation of LEF/TCF activity (28, 29).
It has been reported that undifferentiated DPSC expressed soluble pro-angiogenic factors such as VEGF and IL-8 (30). Notably, LRP6-silenced DPSC cells showed lower expression levels of VEGF and IL-8 than vector control cells. This observation might provide additional mechanistic explanation for the lower vasculogenic activity that was found in LRP6-silenced cells in vitro and in vivo. We observed that LRP6-silenced DPSC transplanted into SCID mice generated fewer blood vessels, when compared to vector control DPSC cells. In contrast, the pulps generated with Frizzled6-silenced DPSC showed similar vascularization as compared to pulps generated with control DPSC cells. These data confirm our in vitro observations, and demonstrate a critical role for Wnt signaling in the determination of the vasculogenic fate of DPSC. These data also suggest the possibility of directing the fate of DPSC towards non-vasculogenic phenotypes upon targeted inhibition of LRP6.
In conclusion, our data confirmed the hypothesis that Wnt/β-catenin pathway induces the vasculogenic differentiation of dental pulp stem cells, and unveiled a new role for LRP6 signaling in this process. These data align nicely with the seminal observation that the Wnt/β-catenin pathway inhibits odontoblastic differentiation of dental pulp stem cells (17). We propose that the Wnt/β-catenin pathway serves as a “switch” regulating the fate of DPSC between odontoblastic/osteoblastic and vasculogenic. Such knowledge could be exploited in translational dental pulp tissue engineering. For example, one could enforce activation of the Wnt/β-catenin pathway immediately after injection of DPSC in the root canal to maximize the rapid generation of functional blood vessels (5, 6) that will bring the oxygen and nutrients required to maintain cell viability and function in the critical first days after transplantation. This could be potentially achieved by intracanal delivery of microspheres for controlled release of rhWnt1 for a period that would likely not be longer than 7–10 days. Once a vascular network is established, the exogenous Wnt-1 would no longer be necessary and DPSC cells would be primed to respond to dentin-derived factors (e.g. BMP2) that will induce their differentiation into functional odontoblasts (6, 31). We propose that a deep understanding of processes involved in the regulation of DPSC fate will enable the development of a mechanism-based therapeutic strategy for the engineering of a functional dental pulp tissue in the treatment of necrotic immature permanent teeth.
Acknowledgments
Financial support: This work was funded by grant R01DE21410 from NIH/NIDCR (JEN) and by grant 2012/13039-0 and 2012/24244-3 from FAPESP (GOS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- 1.Nakashima M, Akamine A. The application of tissue engineering to regeneration of pulp and dentin in endodontics. J Endod. 2005;31:711–8. doi: 10.1097/01.don.0000164138.49923.e5. [DOI] [PubMed] [Google Scholar]
- 2.Nör JE. Tooth regeneration in operative dentistry. Oper Dent. 2006;31:633–2. doi: 10.2341/06-000. [DOI] [PubMed] [Google Scholar]
- 3.Brey EM, Uriel S, Greisler HP, McIntire LV. Therapeutic neovascularization: contributions from bioengineering. Tissue Eng. 2005;11:567–84. doi: 10.1089/ten.2005.11.567. [DOI] [PubMed] [Google Scholar]
- 4.Gronthos S, Brahim J, Li W, et al. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002;81:531–5. doi: 10.1177/154405910208100806. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Z, Nör F, Oh M, Cucco C, Nör JE. Wnt/β-catenin signaling determines the vasculogenic fate of post-natal mesenchymal stem cells. Stem Cells. 2016;34:1576–87. doi: 10.1002/stem.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sakai VT, Zhang Z, Dong Z, et al. SHED differentiate into functional odontoblasts and endothelium. J Dent Res. 2010;89:791–6. doi: 10.1177/0022034510368647. [DOI] [PubMed] [Google Scholar]
- 7.Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303:1483–7. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cadigan KM, Nusse R. Wnt signaling: a common theme in animal development. Genes Dev. 1997;11:3286–305. doi: 10.1101/gad.11.24.3286. [DOI] [PubMed] [Google Scholar]
- 9.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–50. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 10.Zhang X, Gaspard JP, Chung DC. Regulation of vascular endothelial growth factor by the Wnt and K-ras pathways in colonic neoplasia. Cancer Res. 2001;61:6050–4. [PubMed] [Google Scholar]
- 11.Masckauchán TN, Shawber CJ, Funahashi Y, et al. Wnt/beta-catenin signaling induces proliferation, survival and interleukin-8 in human endothelial cells. Angiogenesis. 2005;8:43–51. doi: 10.1007/s10456-005-5612-9. [DOI] [PubMed] [Google Scholar]
- 12.Lindsley RC, Gill JG, Kyba M, et al. Canonical Wnt signaling is required for development of embryonic stem cell-derived mesoderm. Development. 2006;133:3787–96. doi: 10.1242/dev.02551. [DOI] [PubMed] [Google Scholar]
- 13.Nichols AS, Floyd DH, Bruinsma SP, et al. Frizzled receptors signal through G proteins. Cell Signal. 2013;25:1468–75. doi: 10.1016/j.cellsig.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jernigan KK, Cselenyi CS, Thorne CA, et al. Gbetagamma activates GSK3 to promote LRP6-mediated beta-catenin transcriptional activity. Science Signal. 2010;3:ra37. doi: 10.1126/scisignal.2000647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maretto S, Cordenonsi M, Dupont S, et al. Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc Natl Acad Sci USA. 2003;100:3299–304. doi: 10.1073/pnas.0434590100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ye X, Wang Y, Cahill H, et al. Norrin, frizzled-4, and Lrp5 signaling in endothelial cells controls a genetic program for retinal vascularization. Cell. 2009;139:285–98. doi: 10.1016/j.cell.2009.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scheller EL, Chang J, Wang CY. Wnt/beta-catenin inhibits dental pulp stem cell differentiation. J Dent Res. 2008;87:126–30. doi: 10.1177/154405910808700206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silvério KG, Davidson KC, James RG, et al. Wnt/β-catenin pathway regulates bone morphogenetic protein (BMP2)-mediated differentiation of dental follicle cells. J Period Res. 2012;47:309–19. doi: 10.1111/j.1600-0765.2011.01433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feng X, Xing J, Feng G, et al. Age-dependent impaired neurogenic differentiation capacity of dental stem cell is associated with Wnt/β-catenin signaling. Cell Mol Neurobiol. 2013;33:1023–31. doi: 10.1007/s10571-013-9965-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gronthos S, Mankani M, Brahim J, et al. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97:13625–30. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wright M, Aikawa M, Szeto W, Papkoff J. Identification of a Wnt-responsive signal transduction pathway in primary endothelial cells. Biochem Biophys Res Commun. 1999;263:384–8. doi: 10.1006/bbrc.1999.1344. [DOI] [PubMed] [Google Scholar]
- 22.Mullane EM, Dong Z, Sedgley CM, et al. Effects of VEGF and FGF2 on the revascularization of severed human dental pulps. J Dent Res. 2005;87:1144–8. doi: 10.1177/154405910808701204. [DOI] [PubMed] [Google Scholar]
- 23.Bento LW, Zhang Z, Imai A, et al. Endothelial differentiation of SHED requires MEK1/ERK signaling. J Dent Res. 2013;92:51–7. doi: 10.1177/0022034512466263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang J, Shemezis JR, McQuinn ER, et al. AKT activation by N-cadherin regulates beta-catenin signaling and neuronal differentiation during cortical development. Neural Dev. 2013;8:7. doi: 10.1186/1749-8104-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Golan T, Yaniv A, Bafico A, et al. The human Frizzled 6 (HFz6) acts as a negative regulator of the canonical Wnt/beta-catenin signaling cascade. J Biol Chem. 2004;279:14879–88. doi: 10.1074/jbc.M306421200. [DOI] [PubMed] [Google Scholar]
- 26.McCrea PD, Turck CW, Gumbiner B. A homolog of the armadillo protein in Drosophila (plakoglobin) associated with E-cadherin. Science. 1991;254:1359–61. doi: 10.1126/science.1962194. [DOI] [PubMed] [Google Scholar]
- 27.Beagle B, Johnson GV. Differential modulation of TCF/LEF-1 activity be the soluble LRP6-ICD. PLoS One. 2010;5:e11821. doi: 10.1371/journal.pone.0011821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beagle B, Mi K, Johnson GV. Phosphorylation of PPP(S/T)P motif of the free LRP6 intracellular domain is not required to activate the Wnt/betacatenin pathway and attenuate GSK3beta activity. J Cell Biochem. 2009;108:886–95. doi: 10.1002/jcb.22318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu G, Huang H, Abreu JG, He X. Inhibition of GSK3 phosphorylation of β-Catenin via phosphorylated PPSPXS Motifs of Wnt coreceptor LRP6. PLoS One. 2009;4:e4926. doi: 10.1371/journal.pone.0004926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bronckaers A, Hilkens P, Fanton Y, et al. Angiogenic properties of human dental pulp stem cells. PLoS One. 2013;8:e71104. doi: 10.1371/journal.pone.0071104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Casagrande L, Demarco F, Zhang Z, Araujo FB, Shi S, Nör JE. Dentin-derived BMP2 and odontoblastic differentiation of SHED. J Dent Res. 2010;89:603–8. doi: 10.1177/0022034510364487. [DOI] [PubMed] [Google Scholar]