Abstract
Purpose
Trastuzumab emtansine (T-DM1), an antibody–drug conjugate comprising the cytotoxic agent DM1, a stable linker, and trastuzumab, has demonstrated substantial activity in human epidermal growth factor receptor 2 (HER2) –positive metastatic breast cancer, raising interest in evaluating the feasibility and cardiac safety of T-DM1 in early-stage breast cancer (EBC).
Patients and Methods
Patients (N = 153) with HER2-positive EBC and prechemotherapy left ventricular ejection fraction (LVEF) ≥ 55% received (neo)adjuvant doxorubicin plus cyclophosphamide or fluorouracil plus epirubicin plus cyclophosphamide followed by T-DM1 for four cycles. Patients could then receive three to four cycles of optional docetaxel with or without trastuzumab. T-DM1 was then resumed with optional radiotherapy (sequential or concurrent) for 1 year (planned) of HER2-directed therapy. The coprimary end points were rate of prespecified cardiac events and safety.
Results
Median follow-up was 24.6 months. No prespecified cardiac events or symptomatic congestive heart failures were reported. Four patients (2.7%) had asymptomatic LVEF declines (≥ 10 percentage points from baseline to LVEF < 50%), leading to T-DM1 discontinuation in one patient. Of 148 patients who received ≥ one cycle of T-DM1, 82.4% completed the planned 1-year duration of HER2-directed therapy. During T-DM1 treatment, 38.5% and 2.7% of patients experienced grade 3 and 4 adverse events, respectively. Approximately 95% of patients receiving T-DM1 plus radiotherapy completed ≥ 95% of the planned radiation dose with delay ≤ 5 days.
Conclusion
Use of T-DM1 for approximately 1 year after anthracycline-based chemotherapy was feasible and generally well tolerated by patients with HER2-positive EBC, providing support for phase III trials of T-DM1 in this setting.
INTRODUCTION
Human epidermal growth factor receptor 2 (HER2) is overexpressed in 15% to 20% of breast cancers.1–3 HER2-positive tumors have an aggressive phenotype and, before the advent of HER2-directed therapy, were associated with increased mortality and shorter time to relapse than HER2-normal tumors.1,4–6 The HER2-targeted humanized monoclonal antibody trastuzumab plus chemotherapy became standard care for the adjuvant treatment of HER2-positive early-stage breast cancer (EBC) after phase III studies showed significantly improved disease-free and overall survival (OS) rates versus chemotherapy alone.7–9 In EBC, trastuzumab is often administered with concurrent radiotherapy postsurgery10 and/or hormonal therapy (when indicated). Although trastuzumab-based chemotherapy regimens improve clinical outcome in EBC,7–9,11 they can be associated with significant toxicity resulting from the cytotoxic effects of chemotherapy on normal cells. Furthermore, some patients develop disease recurrence. Thus, more effective and less toxic treatments are desired.
Trastuzumab emtansine (T-DM1) is an antibody–drug conjugate composed of the cytotoxic agent DM1 and trastuzumab joined by a stable thioether linker.12 T-DM1 delivers DM1 directly to HER2-overexpressing tumor cells, inhibiting microtubule function and leading to cell death.12,13 Like trastuzumab, T-DM1 inhibits HER2 signaling, prevents HER2 shedding, and induces antibody-dependent cellular cytotoxicity.14 In studies of HER2-positive metastatic breast cancer (MBC), T-DM1 was well tolerated, with fewer of the toxicities typically associated with conventional chemotherapy (eg, alopecia, neutropenia).15–18 In randomized studies, T-DM1 was associated with longer median progression-free survival (PFS)15,17,18 and OS17 versus chemotherapy plus HER2-directed therapy. Overall rates of grade ≥ 3 adverse events (AEs) and treatment discontinuation because of AEs were lower with T-DM1 than control regimens. However, rates of grade ≥ 3 thrombocytopenia and increased serum aminotransferases were greater with T-DM1.15,17,18 Single-agent T-DM1 is approved in the United States19 and European Union for previously treated HER2-positive MBC.
Given the significant efficacy and favorable safety of T-DM1 in MBC, there is interest in exploring T-DM1 in EBC. In the neoadjuvant or adjuvant setting, T-DM1 could potentially replace trastuzumab plus taxane. Because DM1 is targeted directly to HER2-overexpressing cells, T-DM1 may allow exposure to cytotoxic therapy for longer (eg, 1 year) than is possible with conventional systemic chemotherapy. Trastuzumab, the targeting antibody of T-DM1, can be associated with cardiotoxicity (particularly when part of anthracycline-based regimens).20–22 Thus, the frequency and severity of cardiac dysfunction with T-DM1 should be assessed in EBC, where long-term survival is expected.9,23,24
TDM4874g (BO22857) was a single-arm, open-label, phase II study that examined the cardiac safety, efficacy, and overall feasibility of T-DM1 treatment for approximately 1 year after administration of anthracycline-based chemotherapy in the (neo)adjuvant setting in HER2-positive EBC. T-DM1 administered concurrently with adjuvant radiotherapy and/or hormonal therapy was also investigated.
PATIENTS AND METHODS
Study Design and Patients
Eligible patients had EBC, with centrally confirmed HER2-positive status (in situ hybridization positive or immunohistochemistry 3+), Eastern Cooperative Oncology Group performance status of 0 or 1, and prechemotherapy left ventricular ejection fraction (LVEF) ≥ 55%. Key exclusion criteria were stage IV or bilateral breast cancer; prior radiotherapy, immunotherapy, or biotherapy < 5 years before enrollment; history of cardiotoxic chemotherapy; or active cardiac history (eg, unstable angina, congestive heart failure [CHF], or myocardial infarction within previous 12 months; high-risk uncontrolled arrhythmias; clinically significant valvular disease; or uncontrolled hypertension). All patients provided written informed consent.
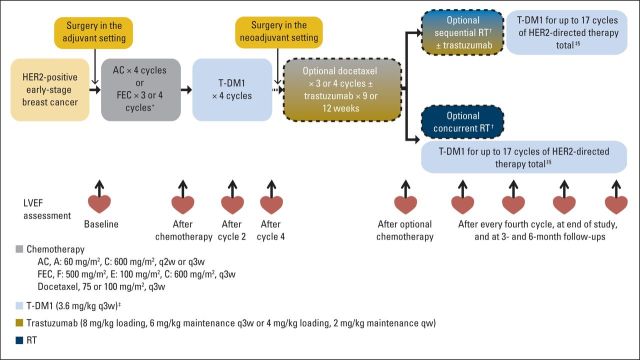
Patients received four cycles of (neo)adjuvant doxorubicin plus cyclophosphamide (AC) or three to four cycles of fluorouracil plus epirubicin plus cyclophosphamide (FEC) followed by four cycles of T-DM1 3.6 mg/kg. After the first four T-DM1 cycles, patients could receive an optional three to four cycles of docetaxel with or without trastuzumab at the treating physician's discretion (Fig 1). Use of trastuzumab and docetaxel was permitted in this curative patient population, because the efficacy of T-DM1 compared with the standard of care (ie, trastuzumab plus chemotherapy) was not established. Sequential or concurrent radiotherapy could be provided after the first four cycles of T-DM1 and optional docetaxel with or without trastuzumab; sequential radiotherapy could be administered with or without trastuzumab. The safety of concurrent T-DM1 and radiotherapy was evaluated in the first 20 patients before others were treated. Patients continued T-DM1 for a planned 17 cycles of HER2-directed therapy (total number of T-DM1 and trastuzumab cycles). Hormonal therapy was permitted in patients with hormone receptor–positive disease after having completed four T-DM1 cycles and optional docetaxel with or without trastuzumab. Patients were enrolled before or after completion of AC/FEC (Fig 1). The trial adhered to the Declaration of Helsinki, Good Clinical Practices, and applicable local laws.
Fig 1.
TDM4874g study design. AC, doxorubicin plus cyclophosphamide; FEC, fluorouracil plus epirubicin plus cyclophosphamide; HER2, human epidermal growth factor receptor 2; LVEF, left ventricular ejection fraction; qw, once every week; q2w, once every 2 weeks; q3w, once every 3 weeks; RT, radiotherapy; T-DM1, trastuzumab emtansine. (*) Enrollment in adjuvant or neoadjuvant setting and choice of AC or FEC was at discretion of investigator; patients were allowed to enroll either before or after completion of AC or FEC. (†) Radiotherapy was per investigator discretion; safety was evaluated after first 20 patients had been treated with concurrent T-DM1 and RT, and additional patients were administered sequential RT with or without trastuzumab until confirmation of concurrent T-DM1 and RT safety. (‡) Dose reductions to 3.0 and 2.4 mg/kg were permitted for toxicity. (§) Maximum number of T-DM1 cycles was determined as number of cycles to complete approximately 1 year of HER2-directed therapy, including any periods of optional trastuzumab.
Study Assessments
LVEF was evaluated by echocardiogram or multiple-gated acquisition scans at baseline, at the end of anthracycline-based treatment, after cycles two and four of T-DM1 and every four cycles of T-DM1 thereafter, before and after the start of new treatment, at the end of T-DM1 treatment (if > 6 weeks since last scan), and at 3- and 6-month follow-up visits (Fig 1). The same method of LVEF evaluation was to be used throughout. Laboratory assessments and physical examinations were conducted at baseline and at subsequent visits until the study-termination or end-of-treatment visit.
AEs were mapped to terms from the Medical Dictionary for Regulatory Activities and graded per National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0). Laboratory abnormalities were defined per established normal ranges and National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0). Serious AEs were life threatening or fatal, resulted in or prolonged hospitalization, resulted in significant disability, or were considered by the investigator to be medically significant.
T-DM1 Dose Modifications
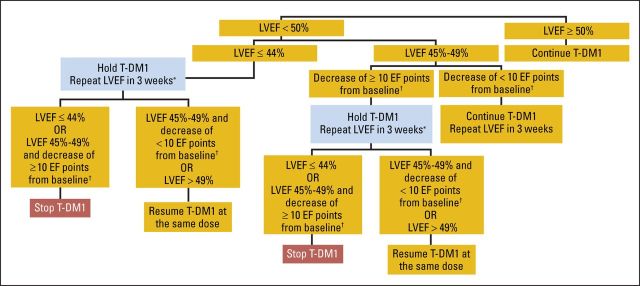
Toxicity was assessed before each T-DM1 dose; if the results from clinical assessment and laboratory testing were acceptable, T-DM1 was administered. If dose reductions were required, T-DM1 was reduced to 3.0 or 2.4 mg/kg; dose re-escalation was not allowed. T-DM1 was adjusted per a prespecified algorithm in patients experiencing cardiotoxicity (Appendix Fig A1, online only) and according to protocol guidelines in patients experiencing hematotoxicity, hepatotoxicity, neurotoxicity, or infusion-related reactions. Patients were withdrawn if they experienced two consecutive or three intermittent T-DM1 dose delays for asymptomatic LVEF decrease, symptomatic CHF, an inability to receive T-DM1 after two dose reductions for any reason, a dose delay > 42 days from last dose, disease recurrence, or unacceptable toxicity.
Statistical Methods
The coprimary end points were safety and rate of prespecified cardiac events within the first 12 weeks of T-DM1 treatment. A cardiac event was defined as death resulting from a cardiac cause or severe CHF (New York Heart Association [NYHA] class III or IV) accompanied by a decrease in LVEF of ≥ 10 percentage points from baseline to LVEF < 50%. Secondary end points included long-term (≥ 1 year) cardiac and overall safety, feasibility of administering T-DM1 concurrent with radiotherapy, feasibility of the planned duration of T-DM1 (17 cycles of HER2-directed therapy), and pathologic complete response (pCR) rate for patients administered preoperative T-DM1. pCR was defined as the absence of invasive neoplastic cells on microscopic examination of the original breast tumor area and axillary lymph nodes (ypT0/is, ypN0) after primary systemic therapy and surgery.
Patients who received ≥ one dose of T-DM1 were evaluable for overall safety. Patients who received ≥ one dose of T-DM1 and underwent an echocardiogram/MUGA assessment by 12 weeks from first T-DM1 dose or discontinued the study because of per-protocol cardiac events before completing four T-DM1 cycles were evaluable for protocol-prespecified cardiac safety.
The sample size was chosen to ensure a reasonable probability of detecting cardiac events, which were expected to occur at a relatively low rate per prior trastuzumab studies.20–22 Observing a cardiac event in ≥ five patients among the first 60 cardiac safety–evaluable patients or in ≥ eight patients among 120 cardiac safety–evaluable patients would result in study termination. It was assumed that ≥ 90% of participants would complete AC/FEC without disease progression or excessive toxicity and would receive ≥ one dose of T-DM1. Under this assumption, approximately 135 patients were needed to achieve 120 cardiac safety–evaluable patients, with whom the 90% confidence limits would be within ± 8% of the observed cardiac event rate. During the actual analysis, the exact 95% CI of protocol-prespecified cardiac events, calculated using the Blyth-Still-Casella method, was provided.
RESULTS
In total, 153 patients from 35 sites were enrolled between October 2010 and June 2011 (Fig 2). As of June 12, 2013, median follow-up was 24.6 months (range, 0.2 to 29.0 months). Baseline demographic and clinical characteristics were similar between patients in the adjuvant (n = 99) and neoadjuvant settings (n = 54; Table 1).
Fig 2.
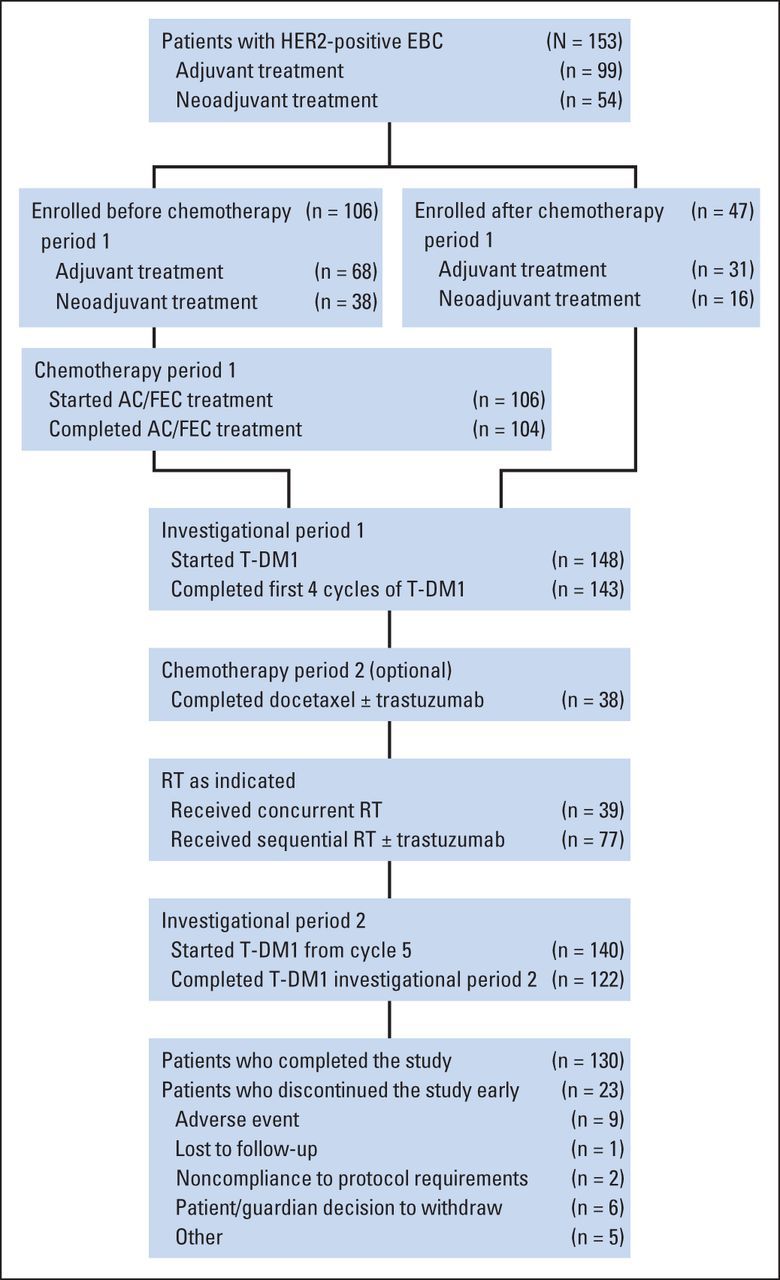
Flow diagram of patients in TDM4874g. AC, doxorubicin plus cyclophosphamide; EBC, early-stage breast cancer; FEC, fluorouracil plus epirubicin plus cyclophosphamide; HER2, human epidermal growth factor receptor 2; RT, radiotherapy; T-DM1, trastuzumab emtansine.
Table 1.
Selected Baseline Demographic and Clinical Characteristics
| Characteristic | Adjuvant Setting (n = 99) |
Neoadjuvant Setting (n = 54) |
All Patients (N = 153) |
|||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| Age, years | ||||||
| 18-40 | 13 | 13.1 | 9 | 16.7 | 22 | 14.4 |
| 41-64 | 72 | 72.7 | 41 | 75.9 | 113 | 73.9 |
| ≥ 65 | 14 | 14.1 | 4 | 7.4 | 18 | 11.8 |
| Race/ethnicity | ||||||
| White | 67 | 67.7 | 34 | 63.0 | 101 | 66.0 |
| Black or African American | 1 | 1.0 | 1 | 1.9 | 2 | 1.3 |
| Asian | 6 | 6.1 | 8 | 14.8 | 14 | 9.2 |
| Multiple | 1 | 1.0 | 0 | 0 | 1 | 0.7 |
| Not available | 24 | 24.2 | 11 | 20.4 | 35 | 22.9 |
| World region | ||||||
| Europe | 76 | 76.8 | 34 | 63.0 | 110 | 71.9 |
| United States | 19 | 19.2 | 13 | 24.1 | 32 | 20.9 |
| Asia | 4 | 4.0 | 7 | 13.0 | 11 | 7.2 |
| ECOG PS | ||||||
| 0 | 89 | 89.9 | 49 | 90.7 | 138 | 90.2 |
| 1 | 10 | 10.1 | 5 | 9.3 | 15 | 9.8 |
| ER/PR status | ||||||
| ER positive and/or PR positive | 65 | 65.7 | 30 | 55.6 | 95 | 62.1 |
| ER negative and PR negative | 34 | 34.3 | 24 | 44.4 | 58 | 37.9 |
| Prechemotherapy LVEF by local assessment, % | ||||||
| Median | 69.0 | 65.0 | 67.0 | |||
| Range | 52-79 | 55-81 | 52-81 | |||
| ≥ 60 | 92 | 92.9 | 45 | 83.3 | 137 | 89.5 |
| 55 to < 60 | 6 | 6.1 | 9 | 16.7 | 15 | 9.8 |
| < 55 | 1* | 1.0 | 0 | 0 | 1 | 0.7 |
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; ER, estrogen receptor; LVEF, left ventricular ejection fraction; PR, progesterone receptor.
One patient had prechemotherapy LVEF of 52% and was enrolled onto study in postchemotherapy setting, with postchemotherapy LVEF of 64%.
Drug Exposure and Treatment Duration
In the intent-to-treat population (N = 153), 38 patients (24.8%) received optional docetaxel, 74 (48.4%) received optional trastuzumab, and 116 (75.8%) received radiotherapy (concurrent, n = 39; sequential, n = 77; Table 2). Among the 74 patients who received trastuzumab, 36 also received docetaxel, and 68 also received radiotherapy. Sixty-two patients (40.5% of intent-to-treat population) received concurrent hormonal therapy with T-DM1; others may have received hormonal therapy after T-DM1 treatment. Of the 148 patients who received ≥ one cycle of T-DM1, 82.4% (n = 122) completed the planned duration (approximately 1 year) of HER2-directed therapy (Table 2). Patients received a median of 14 cycles of T-DM1 (range, one to 17), with 75.7% (n = 112) receiving ≥ 13 cycles.
Table 2.
Drug Exposure and Duration
| Drug Exposure | Patients |
Cycles |
||
|---|---|---|---|---|
| No. | % | Median | Range | |
| All patients (N = 153) | ||||
| Anthracycline-based regimens | 152 | 99.3* | — | — |
| AC | 68 | 44.4 | 4 | 4-4 |
| FEC | 84 | 54.9 | 3 | 1-4 |
| Docetaxel | 38 | 24.8 | 4 | 1-4 |
| Trastuzumab | 74 | 48.4 | 4 | 1-10 |
| T-DM1 | 148† | 96.7 | 14 | 1-17 |
| Hormonal therapy | 62 | 40.5 | — | — |
| T-DM1–treated patients (n = 148)† | ||||
| Completed planned duration of T-DM1 | 122 | 82.4 | — | — |
| Completed planned duration without dose reduction | 98 | 66.2 | — | — |
| Dose reduced to 3.0 mg/kg | 27 | 18.2 | — | — |
| Dose reduced to 2.4 mg/kg | 5 | 3.4 | — | — |
| Early discontinuation | ||||
| AE | 20 | 13.5‡ | — | — |
| PD | 1 | 0.7 | — | — |
| Other | 5 | 3.4§ | — | — |
| RT-treated patients | 116 | 75.8 | — | — |
| Concurrent RT (n = 38)‖ | — | — | ||
| Completed ≥ 95% of planned RT dose with ≤ 5-day delay | 36 | 94.7 | — | — |
| Sequential RT (n = 77) | ||||
| Completed ≥ 95% of planned RT dose with ≤ 5-day delay | 74 | 96.1 | — | — |
Abbreviations: AC, doxorubicin plus cyclophosphamide; AE, adverse event; FEC, fluorouracil plus epirubicin plus cyclophosphamide; PD, progressive disease; RT, radiotherapy; T-DM1, trastuzumab emtansine.
One additional patient received four cycles of AC before enrollment, but detailed dosing information was incomplete.
Five patients did not receive T-DM1 because of elevated liver function tests after neoadjuvant chemotherapy (n = 1), PD during neoadjuvant chemotherapy (n = 1), patient/legal guardian decision to withdraw (n = 1), and discontinuation of FEC (n = 2).
Thrombocytopenia (n = 5); neutropenia and increased AST (n = 2 each); vertigo, reduced visual acuity, fatigue, malaise, pyrexia, hyperbilirubinemia, increased ALT, increased blood bilirubin, decreased ejection fraction, joint crepitation, muscle spasms, myalgia, and peripheral neuropathy (n = 1 each).
Patient/legal guardian decision to discontinue (n = 4); noncompliance with protocol requirements (n = 1).
One patient did not have available RT dose information and thus was not included in this section of table.
Twenty (13.5%) of the 148 patients who received ≥ one cycle of T-DM1 experienced AEs leading to T-DM1 discontinuation, most commonly thrombocytopenia (n = 5; grade 2), neutropenia (n = 2; grade 3), and increased AST (n = 2; one grade 2, one grade 3). T-DM1 dose was reduced to 3.0 mg/kg in 27 patients (18.2%) and to 2.4 mg/kg in five (3.4%). The most common reasons for T-DM1 dose reduction were grade 3 increases in ALT (n = 8) and AST (n = 6). T-DM1 dose was reduced to 3.0 mg/kg most often at cycle two (n = 7) and to 2.4 mg/kg most often at cycle 10 (n = 2).
Of those who received T-DM1 concurrently with radiotherapy and for whom radiotherapy dose information was available, 94.7% (36 of 38 patients) completed ≥ 95% of the planned radiotherapy dose with delay ≤ 5 days (Table 2). This was similar to the 96.1% (74 of 77 patients) completion rate among those who received sequential radiotherapy.
Cardiac Safety
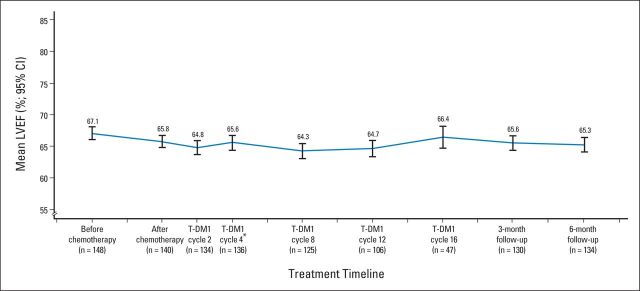
No protocol-prespecified cardiac events (95% CI, 0% to 2.45%) or symptomatic CHF events were reported after T-DM1 treatment (Table 3). Five patients (3.4%) experienced cardiac AEs that the investigator suspected were related to T-DM1. Four patients (2.7%) experienced asymptomatic LVEF declines (≥ 10 percentage points from baseline to LVEF < 50%). One of these patients, with a postanthracycline LVEF of 50%, discontinued T-DM1 with an LVEF of 45%. LVEF for this patient had recovered to 50% at last follow-up (14 months after last T-DM1 dose). The other three LVEF declines were unrelated to T-DM1 per the investigator, with two occurring during treatment with optional docetaxel plus trastuzumab. No patient had an LVEF < 40% at any time during the study. Mean LVEF was stable throughout T-DM1 treatment (Appendix Fig A2, online only).
Table 3.
Summary of Cardiac Safety
| Cardiac Event | T-DM1–Treated Patients (n = 148) |
|
|---|---|---|
| No. | % | |
| Protocol-prespecified cardiac event | 0 | 0.0 |
| Symptomatic CHF event | 0 | 0.0 |
| Asymptomatic LVEF decline* | 4 | 2.7 |
| T-DM1 discontinuation because of cardiac dysfunction | 1 | 0.7† |
| T-DM1–related cardiac disorder | 5 | 3.4 |
| Atrial fibrillation (grade 4) | 1 | 0.7 |
| Tricuspid valve incompetence (grade 2) | 1 | 0.7 |
| Palpitations (all grade 1) | 4 | 2.7 |
| Supraventricular extrasystoles (grade 2) | 1 | 0.7 |
Abbreviations: CHF, congestive heart failure; LVEF, left ventricular ejection fraction; T-DM1, trastuzumab emtansine.
LVEF decline of ≥ 10 percentage points from baseline to < 50%. Three were considered by investigators to be unrelated to T-DM1; two of these events occurred during treatment with trastuzumab.
With LVEF of 45%, last LVEF after discontinuation was 50%.
Overall Safety
During T-DM1 therapy (n = 148), the most common any-grade AEs were nausea (37.8%; n = 56) and headache (37.2%; n = 55; Table 4). Fifty-seven patients (38.5%) experienced grade 3 AEs, and four (2.7%) experienced grade 4 AEs; there were no grade 5 AEs. The most common grade ≥ 3 AEs were thrombocytopenia (8.1%; n = 12), increased ALT (7.4%; n = 11), and increased AST (7.4%; n = 11; Table 4). Fifteen patients (10.1%) experienced serious AEs during T-DM1 therapy, with atrial fibrillation (n = 2), pyrexia (n = 2), and device-related infection (n = 2) occurring in > one patient. No patient met Hy's law laboratory criteria for hepatotoxicity (> three-fold increase in ALT or AST above upper limit of normal and > two-fold increase in bilirubin above upper limit of normal)25; nodular regenerative hyperplasia and portal hypertension were not reported.
Table 4.
Summary of AEs Among T-DM1–Treated Patients During Treatment With T-DM1 (n = 148)
| AE | Any-Grade AEs Occurring in > 15% of Patients |
|||
|---|---|---|---|---|
| Any Grade |
Grade 3* |
|||
| No. | % | No. | % | |
| Nausea | 56 | 37.8 | 0 | 0.0 |
| Headache | 55 | 37.2 | 1 | 0.7 |
| Epistaxis | 47 | 31.8 | 2 | 1.4 |
| Asthenia | 45 | 30.4 | 2 | 1.4 |
| Pyrexia | 39 | 26.4 | 1 | 0.7 |
| Fatigue | 34 | 23.0 | 3 | 2.0 |
| Arthralgia | 33 | 22.3 | 0 | 0.0 |
| Thrombocytopenia | 32 | 21.6 | 12 | 8.1 |
| Myalgia | 31 | 20.9 | 1 | 0.7 |
| Vomiting | 25 | 16.9 | 0 | 0.0 |
| Rash | 24 | 16.2 | 0 | 0.0 |
Abbreviations: AE, adverse event; T-DM1, trastuzumab emtansine.
Total of five grade 4 AEs were reported in four T-DM1–treated patients while receiving treatment with T-DM1: febrile neutropenia (n = 1) and pancytopenia (n = 1; same patient), atrial fibrillation (n = 1), decreased platelet count (n = 1), and hypokalemia (n = 1).
During concurrent T-DM1 and hormonal therapy (n = 62), the rate of any-grade AEs was 69.4% (n = 43); the rate of grade 3 AEs was 12.9% (n = 8); no grade 4 AEs were reported. Neutropenia (n = 2) was the only grade 3 AE reported in ≥ two patients administered concurrent hormonal therapy.
During concurrent T-DM1 and radiotherapy (n = 39), three patients (7.7%) had grade 3 AEs (one each: neutropenia, asthenia, erythema), and one patient (2.6%) experienced radiotherapy-associated pneumonitis (grade 2). During sequential radiotherapy (n = 77), two patients (2.6%) had grade 3 AEs (neutropenia, radiotherapy pneumonitis), and one additional patient had grade 2 radiotherapy pneumonitis; thus, in total, 2.6% of patients experienced radiotherapy-associated pneumonitis. No grade 4 AEs were reported during concurrent or sequential radiotherapy.
Efficacy
The pCR (ypT0/isN0) rate was 56.0% (28 of 50 patients; 95% CI, 41.3% to 69.6%) in those who were administered neoadjuvant therapy (anthracycline followed by four T-DM1 cycles) and underwent surgery. The pCR rate was 51.7% (15 of 29 patients; 95% CI, 33.9 to 70.1) in those with hormone receptor–positive disease and 61.9% (13 of 21 patients; 95% CI, 39.8 to 80.3) in those with hormone receptor–negative disease. One patient who was treated in the neoadjuvant setting discontinued T-DM1 because of disease progression before surgery.
DISCUSSION
In this study, the first to our knowledge to evaluate T-DM1 in patients with EBC, approximately 1 year of (neo)adjuvant T-DM1 after anthracycline-based chemotherapy was feasible and generally well tolerated. Only 17.6% of patients who initiated treatment with T-DM1 did not complete the planned therapy duration. In comparison, 31.4% discontinued trastuzumab before completing 1 year of therapy in the joint analysis of the NSABP B-31 (National Surgical Adjuvant Breast and Bowel Project) trial and NCCTG N9831 (North Central Cancer Treatment Group) trial.8 However, in the HERA (Herceptin Adjuvant) study of patients with HER2-positive EBC who completed locoregional therapy and received ≥ four cycles of (neo) adjuvant chemotherapy, only 8.5% did not complete 1 year of treatment with single-agent trastuzumab.7
The sample size of our study was chosen to ensure a reasonable probability of detecting cardiac events; however, no patient experienced a prespecified cardiac event or symptomatic CHF. Although the sample size of our study was relatively small (n = 148), and the patient population was heterogeneous, with patients having different treatment exposures, the rate of NYHA class III or IV CHF was numerically lower than that reported in pivotal trials of adjuvant trastuzumab, where an incidence of 2.3% to 3.8% was observed in patients administered adjuvant trastuzumab plus taxane after anthracycline-based chemotherapy.23 The rate of NYHA class III or IV CHF in the HERA trial, in which patients were administered adjuvant trastuzumab after chemotherapy, was 0.60%.26
In our study, 2.7% of patients experienced asymptomatic declines in LVEF (≥ 10 percentage points from baseline to LVEF < 50%). Cross-trial comparison of the incidence of asymptomatic LVEF decline is difficult because of variations in how this event is defined and differences in prior treatment exposure and population characteristics. However, HERA used the same definition of asymptomatic LVEF decline as our study. In HERA, in which patients received single-agent trastuzumab after locoregional therapy and ≥ four cycles of (neo)adjuvant chemotherapy, the rate of asymptomatic LVEF decline was 7.1% (although baseline was recorded after anthracycline treatment).7 In NSABP B-31, the incidence of LVEF declines ≥ 10% to < 55% was 34% in the AC plus paclitaxel plus trastuzumab arm.27 In the BCIRG (Breast Cancer International Research Group) 006 trial, the subclinical loss of mean LVEF (> 10% relative loss) was 18.6% in the AC plus paclitaxel plus trastuzumab arm versus 9.4% in the docetaxel plus carboplatin plus trastuzumab arm.9 Most cardiac events in these and other studies occurred during active anti-HER2 therapy. With ≤ 8 years of follow-up, cardiac event rates did not increase significantly.9,22,28,29 Thus, we do not expect the cardiac event rate in our study would increase with longer follow-up.
The cardiac safety profile of T-DM1 in our study was similar to that reported in other studies of T-DM1 in patients with HER2-positive MBC. Only one patient, who had a postanthracycline LVEF of 50%, experienced an asymptomatic LVEF decline that was considered related to T-DM1; although LVEF in this patient declined to 45%, it had recovered to 50% when the patient was assessed approximately 14 months after the last T-DM1 dose. In a pooled analysis of six phase I to III clinical trials (n = 882), four patients (0.5%) had a postbaseline LVEF < 40%, and 16 (1.8%) had an LVEF decline ≥ 15 percentage points from baseline to < 50%.16 In the phase III TH3RESA study of patients with HER2-positive MBC previously treated with ≥ two HER2-directed therapies for MBC, 1.5% of T-DM1–treated patients experienced declines in LVEF of ≥ 15 percentage points from baseline to < 50%—similar to the rate observed in the treatment of physician's choice control arm (1.1%).18 Moreover, T-DM1 had no clinically significant effect on the corrected QT interval in a dedicated phase II study of patients with previously treated HER2-positive MBC.30
The overall safety profile of T-DM1 in EBC was also similar to that seen in phase III studies of T-DM1 in MBC, with the most commonly reported grade ≥ 3 AEs being thrombocytopenia (8.1%), increased AST (7.4%), and increased ALT (7.4%). The corresponding rates in the EMILIA trial were 12.9%, 4.3%, and 2.9%, respectively.17 In TH3RESA, these incidences were 4.7%, 2.2%, and < 2%, respectively.18 In our study, 2.6% of patients administered T-DM1 concurrent with or sequential to radiotherapy experienced pneumonitis versus 1.1% in the trastuzumab-containing arms and 0.6% in the chemotherapy-only arm of the NCCTG N9831 study.10 Among patients receiving sequential treatment, no minimum period was required to have elapsed between the completion of radiotherapy and the initiation of T-DM1 in our study, and the extent of radiotherapy was per investigator discretion. Considering the relatively small study sample size and the low frequency of pneumonitis overall, larger sample sizes are needed to fully establish the overall safety profile of T-DM1 in the EBC setting, including in patients receiving T-DM1 and radiotherapy.
The overall rate of cardiac events in this heterogeneous patient population was low, suggesting that T-DM1 may provide a potentially less toxic alternative to treatment with conventional chemotherapy plus trastuzumab in patients with EBC and that phase III trials of T-DM1 in this setting are warranted. If validated in phase III studies, the favorable tolerability of T-DM1 may allow use of targeted cytotoxic therapy for a longer duration (eg, 1 year) than is feasible with conventional cytotoxic agents. Three randomized phase III studies of T-DM1 in HER2-positive EBC have begun: KATHERINE (BO27938; ClinicalTrials.gov No. NCT01772472) compares adjuvant T-DM1 with trastuzumab in patients with residual invasive tumors after preoperative therapy and surgery; KAITLIN (BO28407; ClinicalTrials.gov No. NCT01966471) compares T-DM1 plus pertuzumab with trastuzumab plus pertuzumab plus taxane after an adjuvant anthracycline regimen; and KRISTINE (BO28408; ClinicalTrials.gov No. NCT02131064) examines neoadjuvant T-DM1 plus pertuzumab versus neoadjuvant trastuzumab plus pertuzumab plus docetaxel plus carboplatin. These studies will provide further understanding of the safety and efficacy of (neo)adjuvant T-DM1 in HER2-positive EBC.
Acknowledgment
We thank Laurence Lehuu, MSc, PhD, global study leader for the TDM4874g study, and Susan Yee, PhD, who provided writing assistance.
Appendix
Fig A1.
Algorithm for trastuzumab emtansine (T-DM1) discontinuation based on left ventricular ejection fraction (LVEF) assessments in asymptomatic patients. (*) Three intermittent holds of T-DM1 led to discontinuation. (†) Baseline refers to prechemotherapy LVEF measurement, regardless of whether patient was enrolled before or after completing chemotherapy. EF, ejection fraction.
Fig A2.
Mean left ventricular ejection fraction (LVEF) in trastuzumab emtansine (T-DM1) –treated patients over time. (*) Optional trastuzumab could have been administered between cycles four and eight.
Footnotes
Supported by Genentech, a member of the Roche group, which also funded third-party writing assistance.
Presented in part at the 48th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, June 1-5, 2012; Heart Failure Congress, Lisbon, Portugal, May 25-28, 2013; and San Antonio Breast Cancer Symposium, San Antonio, TX, December 10-14, 2013.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Clinical trial information: NCT01196052.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Ian E. Krop, Thomas M. Suter, Chau T. Dang, Mario Campone, Na Xu, Melanie Smitt, Luca Gianni
Provision of study materials or patients: Ian E. Krop, Gilles Romieu, Claudio Zamagni, Mario Campone
Collection and assembly of data: Luc Dirix, Gilles Romieu, Na Xu, Melanie Smitt, Luca Gianni
Data analysis and interpretation: Ian E. Krop, Thomas M. Suter, Chau T. Dang, Claudio Zamagni, Marc L. Citron, Na Xu, Melanie Smitt, Luca Gianni
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Feasibility and Cardiac Safety of Trastuzumab Emtansine After Anthracycline-Based Chemotherapy As (neo)Adjuvant Therapy for Human Epidermal Growth Factor Receptor 2–Positive Early-Stage Breast Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Ian E. Krop
Employment: Vertex Pharmaceuticals (I)
Stock or Other Ownership: Vertex Pharmaceuticals (I)
Research Funding: Genentech
Thomas M. Suter
Honoraria: Novartis, Ratiopharm
Speakers' Bureau: Novartis, Ratiopharm
Chau T. Dang
Honoraria: Pfizer
Consulting or Advisory Role: Pfizer
Research Funding: Roche, Genentech, GlaxoSmithKline
Luc Dirix
Travel, Accommodations, Expenses: Novartis, Roche
Gilles Romieu
Expert Testimony: Roche
Claudio Zamagni
Consulting or Advisory Role: Roche, Fresenius Biotech, Genomic Health, Pierre Fabre
Travel, Accommodations, Expenses: Roche, Celgene
Marc L. Citron
Honoraria: Novartis, Genentech/Roche, Kyowa Hakko Kirin
Consulting or Advisory Role: Novartis, Kyowa Hakko Kirin
Speakers' Bureau: Genentech/Roche, Novartis, Kyowa Hakko Kirin
Research Funding: Novartis, Genentech/Roche, Bayer Schering Pharma, Merck, Boehringer Ingelheim, Pfizer, Puma, Geron
Travel, Accommodations, Expenses: Kyowa Hakko Kirin
Mario Campone
Honoraria: Novartis, Roche, Servier, Menarini
Consulting or Advisory Role: Novartis, Servier
Speakers' Bureau: Novartis
Travel, Accommodations, Expenses: Novartis
Na Xu
Employment: Genentech/Roche
Stock or Other Ownership: Roche
Melanie Smitt
Employment: Roche
Stock or Other Ownership: Roche
Luca Gianni
Consulting or Advisory Role: Roche/Genentech, Pfizer, GlaxoSmithKline, Synthon, Tahio, AstraZeneca, Genomic Health, Merck Sharp & Dohme, Boehringer Ingelheim
Patents, Royalties, Other Intellectual Property: Roche
REFERENCES
- 1.Slamon DJ Clark GM Wong SG, etal: Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene Science 235:177–182,1987 [DOI] [PubMed] [Google Scholar]
- 2.Ross JS Slodkowska EA Symmans WF, etal: The HER-2 receptor and breast cancer: Ten years of targeted anti-HER-2 therapy and personalized medicine Oncologist 14:320–368,2009 [DOI] [PubMed] [Google Scholar]
- 3.Pathmanathan N Provan PJ Mahajan H, etal: Characteristics of HER2-positive breast cancer diagnosed following the introduction of universal HER2 testing Breast 21:724–729,2012 [DOI] [PubMed] [Google Scholar]
- 4.Paik S Hazan R Fisher ER, etal: Pathologic findings from the National Surgical Adjuvant Breast and Bowel Project: Prognostic significance of erbB-2 protein overexpression in primary breast cancer J Clin Oncol 8:103–112,1990 [DOI] [PubMed] [Google Scholar]
- 5.Gabos Z Sinha R Hanson J, etal: Prognostic significance of human epidermal growth factor receptor positivity for the development of brain metastases after newly diagnosed breast cancer J Clin Oncol 24:5658–5663,2006 [DOI] [PubMed] [Google Scholar]
- 6.Dawood S Broglio K Buzdar AU, etal: Prognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: An institutional-based review J Clin Oncol 28:92–98,2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piccart-Gebhart MJ Procter M Leyland-Jones B, etal: Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer N Engl J Med 353:1659–1672,2005 [DOI] [PubMed] [Google Scholar]
- 8.Romond EH Perez EA Bryant J, etal: Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer N Engl J Med 353:1673–1684,2005 [DOI] [PubMed] [Google Scholar]
- 9.Slamon D Eiermann W Robert N, etal: Adjuvant trastuzumab in HER2-positive breast cancer N Engl J Med 365:1273–1283,2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halyard MY Pisansky TM Dueck AC, etal: Radiotherapy and adjuvant trastuzumab in operable breast cancer: Tolerability and adverse event data from the NCCTG phase III trial N9831 J Clin Oncol 27:2638–2644,2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gianni L Pienkowski T Im YH, etal: Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): A randomised multicentre, open-label, phase 2 trial Lancet Oncol 13:25–32,2012 [DOI] [PubMed] [Google Scholar]
- 12.Lewis Phillips GD Li G Dugger DL, etal: Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate Cancer Res 68:9280–9290,2008 [DOI] [PubMed] [Google Scholar]
- 13.Erickson HK Park PU Widdison WC, etal: Antibody-maytansinoid conjugates are activated in targeted cancer cells by lysosomal degradation and linker-dependent intracellular processing Cancer Res 66:4426–4433,2006 [DOI] [PubMed] [Google Scholar]
- 14.Junttila TT Li G Parsons K, etal: Trastuzumab-DM1 (T-DM1) retains all the mechanisms of action of trastuzumab and efficiently inhibits growth of lapatinib insensitive breast cancer Breast Cancer Res Treat 128:347–356,2011 [DOI] [PubMed] [Google Scholar]
- 15.Hurvitz SA Dirix L Kocsis J, etal: Phase II randomized study of trastuzumab emtansine versus trastuzumab plus docetaxel in patients with human epidermal growth factor receptor 2–positive metastatic breast cancer J Clin Oncol 31:1157–1163,2013 [DOI] [PubMed] [Google Scholar]
- 16.Diéras V Harbeck N Budd GT, etal: Trastuzumab emtansine in human epidermal growth factor receptor 2–positive metastatic breast cancer: An integrated safety analysis J Clin Oncol 32:2750–2757,2014 [DOI] [PubMed] [Google Scholar]
- 17.Verma S Miles D Gianni L, etal: Trastuzumab emtansine for HER2-positive advanced breast cancer N Engl J Med 367:1783–1791,2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krop IE Kim SB González Martín A, etal: Trastuzumab emtansine versus treatment of physician's choice for pretreated HER2-positive advanced breast cancer (TH3RESA): a randomized, open-label, phase 3 trial Lancet Oncol 15:689–699,2014 [DOI] [PubMed] [Google Scholar]
- 19.Kadcyla prescribing information. South San Francisco, CA: Genentech; 2013. [Google Scholar]
- 20.Perez EA Suman VJ Davidson NE, etal: Cardiac safety analysis of doxorubicin and cyclophosphamide followed by paclitaxel with or without trastuzumab in the North Central Cancer Treatment Group N9831 adjuvant breast cancer trial J Clin Oncol 26:1231–1238,2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Procter M Suter TM de Azambuja E, etal: Longer-term assessment of trastuzumab-related cardiac adverse events in the Herceptin Adjuvant (HERA) trial J Clin Oncol 28:3422–3428,2010 [DOI] [PubMed] [Google Scholar]
- 22.Romond EH Jeong JH Rastogi P, etal: Seven-year follow-up assessment of cardiac function in NSABP B-31, a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel (ACP) with ACP plus trastuzumab as adjuvant therapy for patients with node-positive, human epidermal growth factor receptor 2–positive breast cancer J Clin Oncol 30:3792–3799,2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perez EA Romond EH Suman VJ, etal: Four-year follow-up of trastuzumab plus adjuvant chemotherapy for operable human epidermal growth factor receptor 2–positive breast cancer: Joint analysis of data from NCCTG N9831 and NSABP B-31 J Clin Oncol 29:3366–3373,2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gianni L Dafni U Gelber RD, etal: Treatment with trastuzumab for 1 year after adjuvant chemotherapy in patients with HER2-positive early breast cancer: A 4-year follow-up of a randomised controlled trial Lancet Oncol 12:236–244,2011 [DOI] [PubMed] [Google Scholar]
- 25.US Department of Health and Human Services. Guidance for Industry Drug-Induced Liver Injury: Premarketing Clinical Evaluation. http://www.fda.gov/downloads/Drugs/../Guidances/UCM174090.pdf.
- 26.Suter TM Procter M van Veldhuisen DJ, etal: Trastuzumab-associated cardiac adverse effects in the herceptin adjuvant trial J Clin Oncol 25:3859–3865,2007 [DOI] [PubMed] [Google Scholar]
- 27.Tan-Chiu E Yothers G Romond E, etal: Assessment of cardiac dysfunction in a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel, with or without trastuzumab as adjuvant therapy in node-positive, human epidermal growth factor receptor 2–overexpressing breast cancer: NSABP B-31 J Clin Oncol 23:7811–7819,2005 [DOI] [PubMed] [Google Scholar]
- 28.Goldhirsch A Gelber RD Piccart-Gebhart MJ, etal: 2 years versus 1 year of adjuvant trastuzumab for HER2-positive breast cancer (HERA): An open-label, randomised controlled trial Lancet 382:1021–1028,2013 [DOI] [PubMed] [Google Scholar]
- 29.Morris PG Iyengar NM Patil S, etal: Long-term cardiac safety and outcomes of dose-dense doxorubicin and cyclophosphamide followed by paclitaxel and trastuzumab with and without lapatinib in patients with early breast cancer Cancer 119:3943–3951,2013 [DOI] [PubMed] [Google Scholar]
- 30.Gupta M Wang B Carrothers TJ, etal: Effects of trastuzumab emtansine (T-DM1) on QT interval and safety of pertuzumab plus T-DM1 in patients with previously treated human epidermal growth factor receptor 2-positive metastatic breast cancer Clin Pharmacol Drug Dev 2:11–24,2013 [DOI] [PubMed] [Google Scholar]