Abstract
Purpose
Interleukin-10 (IL-10) stimulates the expansion and cytotoxicity of tumor-infiltrating CD8+ T cells and inhibits inflammatory CD4+ T cells. Pegylation prolongs the serum concentration of IL-10 without changing the immunologic profile. This phase I study sought to determine the safety and antitumor activity of AM0010.
Patients and Methods
Patients with selected advanced solid tumors were treated with AM0010 in a dose-escalation study, which was followed by a renal cell cancer (RCC) dose-expansion cohort. AM0010 was self-administered subcutaneously at doses of 1 to 40 μg/kg once per day. Primary end points were safety and tolerability; clinical activity and immune activation were secondary end points.
Results
In the dose-escalation and -expansion cohorts, 33 and 18 patients, respectively, were treated with daily subcutaneous injection of AM0010. AM0010 was tolerated in a heavily pretreated patient population. Treatment-related adverse events (AEs) included anemia, fatigue, thrombocytopenia, fever, and injection site reactions. Grade 3 to 4 nonhematopoietic treatment-related AEs, including rash (n = 2) and transaminitis (n = 1), were observed in five of 33 patients. Grade 3 to 4 anemia or thrombocytopenia was observed in five patients. Most treatment-related AEs were transient or reversible. AM0010 led to systemic immune activation with elevated immune-stimulatory cytokines and reduced transforming growth factor beta in the serum. Partial responses were observed in one patient with uveal melanoma and four of 15 evaluable patients with RCC treated at 20 μg/kg (overall response rate, 27%). Prolonged stable disease of at least 4 months was observed in four patients, including one with colorectal cancer with disease stabilization for 20 months.
Conclusion
AM0010 has an acceptable toxicity profile with early evidence of antitumor activity, particularly in RCC. These data support the further evaluation of AM0010 both alone and in combination with other immune therapies and chemotherapies.
INTRODUCTION
Immune therapy using checkpoint inhibitors, cytokines, or T-cell transfer therapies has shown unprecedented antitumor activity, including durable clinical responses, in patients with advanced malignancies.1,2 Many patients with melanoma, non–small-cell lung cancer, or renal cell cancer (RCC) have experienced dramatic and durable responses to checkpoint inhibition.3,4 Unfortunately, there remain a large number of patients who derive no durable benefit from these agents. For instance, only one in four patients with RCC had an objective response when treated with an anti–programmed death-1 antibody.5 Furthermore, only limited responses have been observed in immune-insensitive cancers, including microsatellite-stable colorectal cancer (CRC), pancreatic, and prostate cancers.3
Immune resistance that either presents de novo or develops over time may be driven by a low rate of somatic mutations,6 with limited T-cell recognition. Alternatively, the tumor microenvironment may lack survival factors for T cells or contain noncytotoxic (inflammatory) CD8+ T cells, deficient in granzyme and interferon gamma (IFN-γ), which will not induce major histocompatibility complex expression in the tumor.7 Interleukin-10 (IL-10) is a cytokine that has the potential to reverse some or all of these immunosuppressive mechanisms. IL-10 possesses anti-inflammatory properties at lower doses but also yields activation and proliferation of intratumoral CD8+ T cells, particularly at higher concentrations.8,9 The IL-10 receptor (IL-10R) is present on myeloid and lymphoid cells, including CD8+ T cells. On recognition of an antigen or stimulation of the T-cell receptor, CD8+ T cells upregulate IL10R.8 IL-10 leads to increased activation10 and survival of the antigen-stimulated CD8+ T cell. IL-10 is therefore thought to be a confirmatory signal for antigen-stimulated CD8+ T cells.11 Accordingly, tumor-infiltrating CD8+ T cells have high expression of IL-10R, because they recognize tumor antigens.8
Nonpegylated recombinant IL-10 has been studied in clinical trials as an anti-inflammatory molecule in immune-mediated inflammatory diseases and liver fibrosis.12 Despite its short half-life, recombinant IL-10 showed antifibrotic activity in liver fibrosis and moderate anti-inflammatory activity in psoriasis and inflammatory bowel disease. Signs of immune activation were detected at higher doses of IL-10, including elevated granzymes and IFN-γ in the serum of treated individuals.13
AM0010 is a pegylated recombinant IL-10 that allows sustained systemic exposure, leading to the expansion, activation, and cytotoxicity of tumor-infiltrating CD8+ T cells. In preclinical tumor models, pegylated recombinant murine IL-10 induces rejection of large tumors and metastases and increases intratumoral CD8+ T cells and the expression of granzymes and IFN-γ in intratumoral CD8+ T cells. In animals treated with pegylated recombinant murine IL-10, immune-stimulatory cytokines, including IFN-γ, IL-4, and IL-18, are induced in T cells and systemically. Tumor rejection is mediated by tumor-specific CD8+ T cells and is dependent on expression of IFN-γ by CD8+ T cells.14
On the basis of these considerations, we initiated a phase I clinical trial with AM0010 in patients with advanced, treatment-refractory solid tumors. We report here on the safety, pharmacodynamics, and antitumor activity in the dose-escalation cohort and one expansion cohort in RCC of this trial.
PATIENTS AND METHODS
AM0010
AM0010 is a recombinant human IL-10, produced in Escherichia coli and pegylated at the N terminus. The half maximal effective concentration (EC50) of AM0010 in cell-based in vitro assays is approximately 8 ng/mL.
Patients and Study Design
Eligible patients had treatment-refractory metastatic solid tumors and had received no cancer therapy for at least 4 weeks before enrollment. This was a multi-institutional, first-in-human, open-label phase I dose-escalation study conducted in centers in the United States. The study was approved by institutional review boards, and all participants signed approved informed consent forms. The primary objectives were to characterize safety and tolerability and determine the maximum tolerated dose (MTD) and pharmacokinetic properties of AM0010. Secondary objectives included the determination of antitumor activity of, characterization of immune stimulation of, and formation of antibodies to AM0010. AM0010 was subcutaneously (SC) self-administered once per day in sequential cohorts of three to six patients at 1, 2.5, 5, 10, 20, and 40 μg/kg. To avoid dosing errors and standardize the dose, doses for patients with a starting body weight up to 80 kg were based on 80 kg, and those for patients with a body weight above 80 kg were based on 100 kg.
Adverse events (AEs), serious AEs, and laboratory abnormalities were graded according to National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.03) and monitored throughout the study until 30 days after the last dose of study drug. Treatment-related AEs were reported throughout the observation period and recorded once per patient at the highest grade. The MTD was determined as the highest dose at which less than 33% of patients experienced dose-limiting toxicities (DLTs) in the first 28 days of dosing. A DLT was defined as any nontransient grade 3 or higher toxicity considered related to AM0010 (not reverting to grade 1 or baseline within 1 week). After completion of the dose escalation, expansion cohorts of 10 to 15 patients were enrolled at dose levels up to the MTD based on emerging efficacy and tolerability results.
Tumor responses were evaluated every 8 weeks following immune-related response criteria.15 Patients with continued clinical benefit could continue dosing for up to 2 years at the discretion of the investigator with the approval of the medical monitor. Responses are reported as of March 24, 2016.
Pharmacokinetics and Pharmacodynamics
Concentration of AM0010 was analyzed in serum samples collected at six time points on days 1 and 29 and once per week at predose throughout the study with an enzyme-linked immunosorbent assay against human IL-10. Immune stimulation or inhibition by AM0010 was evaluated by measuring 96 cytokines, growth factors, and soluble proteins in serum (Myriad RBM, Austin, TX). Serum was collected before treatment, after 4 weeks, and every 8 weeks thereafter.
Statistical Analysis
We based our assessment of tolerability and toxicity on the safety population, comprising all patients in each dosing cohort who received at least one dose of study drug. Numbers and percentages of patients with treatment-related AEs are presented by MedDRA system organ class, preferred term, and grade. Patients are counted once within a system organ class and grade. The MTD was based on the safety population of patients who could be assessed for DLTs. The assessment of antitumor activity was based on the response-evaluable population, defined as patients who had initiated treatment and had at least one baseline and one scheduled postbaseline tumor assessment. The calculation of response rates was determined as percentages of the evaluable patient population.
RESULTS
From November 25, 2013, to May 20, 2015, 51 patients were enrolled. Thirty-three patients with CRC, RCC, pancreatic ductal adenocarcinoma, ovarian cancer, prostate cancer, non–small-cell lung cancer, or melanoma were enrolled in six dose-escalation cohorts from 1 to 40 μg/kg. An additional 18 patients with RCC were enrolled in one expansion cohort at 20 μg/kg. Their median age was 61 years, and a majority of patients were heavily pretreated, with a median of four prior therapies for metastatic or refractory disease. Three patients had received prior treatment with anti–programmed death-1, and four patients had received prior anti–cytotoxic T-cell lymphocyte-4 treatment. Demographic and baseline patient characteristics are summarized in Table 1 and Appendix Table A1 (online only).
Table 1.
Patient Demographic and Clinical Characteristics
| Characteristic | No. of Patients | % |
|---|---|---|
| Sex* | ||
| Male | 31 | 60.8 |
| Female | 20 | 39.2 |
| Age, years* | ||
| Median | 61 | |
| Range | 22-82 | |
| Tumor histology (dose escalation) | 33 | |
| CRC | 16 | 48.5 |
| RCC | 6 | 18.2 |
| Pancreatic cancer | 4 | 12.1 |
| Melanoma | 4 | 12.1 |
| NSCLC | 1 | 3 |
| Ovarian cancer | 1 | 3 |
| Prostate cancer | 1 | 3 |
| RCC (dose expansion; 20 μg/kg) | 18 | 100 |
| ECOG PS* | ||
| 0 | 26 | 51 |
| 1 | 25 | 49 |
| Prior therapy (dose escalation) | ||
| Median | 4 | |
| Range | 1-11 | |
| Chemotherapy | 30 | |
| Radiation therapy | 3 | |
| Immunotherapy | 7 | |
| Biologics | 19 | |
| Targeted therapy | 15 | |
| Prior therapy (RCC) | ||
| Median | 3 | |
| Range | 0-10 | |
| Immunotherapy | 6 | |
| Anti–PD-1 | 2 | |
| Antiangiogenic | 15 |
Abbreviations: CRC, colorectal cancer; ECOG PS, Eastern Cooperative Oncology Group performance status; NSCLC, non–small-cell lung cancer; PD-1, programmed death-1; RCC, renal cell cancer.
Includes dose-escalation and -expansion cohorts.
Treatment-Related AEs
Daily SC injections of AM0010 were tolerated well, with manageable AEs. The highest administered dose was 40μg/kg, and a formal MTD was not defined in this study. In the 40-μg/kg cohort, patients received the full dose for a median of 49 days (n = 5). Grade 1 to 4 treatment-related AEs in the escalation cohorts are summarized in Table 2. Most frequently observed AEs were anemia (17 patients; 51%), fatigue (15 patients; 45%), thrombocytopenia (14 patients; 42%), injection site reactions (12 patients; 36%), and fever (10 patients; 30%). Grade 3 to 4 nonhematopoietic treatment-related AEs were observed in five (15%) of 33 patients. Treatment-related grade 3 to 4 anemia or thrombocytopenia was observed in six patients (18%). One patient with pancreatic cancer with liver metastases had asymptomatic elevation of liver enzymes. Most treatment-related AEs were reversible with continued dosing. All grade 3 to 4 treatment-related AEs were reversed on temporary dose interruption or reduction. Only one patient discontinued treatment because of a DLT (recurring anemia). When observed, injection site reactions were mild, presenting as an erythematous macular rash at the injection site. A grade 2 maculopapular rash was observed in three patients, and a grade 3 maculopapular rash was observed in one patient. Rashes resolved rapidly with dose interruption and did not reoccur with redosing. The type and frequency of AEs observed in the expansion cohort in patients with RCC were comparable in number and severity to those observed at the 20-μg/kg dose in the escalation cohort. Immune-related AEs typically seen with immune checkpoint inhibitors were specifically monitored but were not observed in this study.
Table 2.
Treatment-Related AEs
| AE | Cohort (µg/kg), No. (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade 1 to 2 | Grade 3 to 4 | |||||||||||
| 1 (n = 4) | 2.5 (n = 6) | 5 (n = 6) | 10 (n = 6) | 20 (n = 6) | 40 (n = 5) | 1 (n = 4) | 2.5 (n = 6) | 5 (n = 6) | 10 (n = 6) | 20 (n = 6) | 40 (n = 5) | |
| Hematologic | ||||||||||||
| Anemia | 1 (17) | 4 (67) | 2 (33) | 2 (33) | 3 (60) | 1 (25) | 1 (17) | 2 (33) | 1 (17) | |||
| Leukopenia | 1 (17) | 1 (17) | ||||||||||
| Thrombocytopenia | 1 (17) | 3 (50) | 2 (33) | 3 (50) | 4 (80) | 1 (17) | ||||||
| Nonhematologic | ||||||||||||
| ALT increased | 2 (40) | |||||||||||
| Anorexia | 1 (25) | 1 (17) | 1 (17) | 3 (50) | 2 (33) | 2 (40) | ||||||
| Arthralgia | 1 (17) | 1 (20) | ||||||||||
| Back pain | 1 (17) | 1 (20) | ||||||||||
| Chills | 1 (17) | 3 (50) | 1 (20) | |||||||||
| Diarrhea | 1 (17) | 1 (17) | 1 (20) | |||||||||
| Dyslipidemia | 1 (25) | 1 (17) | 1 (20) | |||||||||
| Fatigue | 1 (17) | 4 (67) | 2 (33) | 4 (67) | 4 (80) | |||||||
| Fever | 1 (25) | 1 (17) | 2 (33) | 1 (17) | 3 (50) | 2 (40) | ||||||
| Flu-like symptoms | 1 (17) | 1 (17) | 1 (17) | 1 (17) | 1 (20) | |||||||
| Hypoalbuminemia | 1 (17) | 1 (20) | ||||||||||
| Increased lipase | 1 (20) | |||||||||||
| Injection site reaction | 2 (50) | 3 (50) | 3 (50) | 2 (33) | 2 (40) | |||||||
| Myalgia | 1 (17) | 1 (17) | ||||||||||
| Nausea | 2 (50) | 1 (17) | 1 (17) | 2 (33) | 1 (17) | |||||||
| Night sweats | 1 (17) | 1 (17) | ||||||||||
| Pruritis | 1 (17) | 1 (17) | 2 (33) | |||||||||
| Rash/rash maculopapular | 1 (17) | 4 (67) | 2 (40) | |||||||||
| Transaminitis | 1 (20) | |||||||||||
| Vomiting | 1 (25) | 1 (17) | 1 (17) | |||||||||
| Weakness | 2 (33) | 1 (17) | 1 (20) | |||||||||
NOTE. Does not include events that occurred in < two patients at grade 1 to 2 severity.
Abbreviation: AE, adverse event.
The recommended phase II dose (RP2D) as single agent was identified as 20 μg/kg. Patients in the dose-escalation cohort continued at this dose for a median of 90 days without interruption or dose reduction (n = 5). Patients with RCC in the expansion cohort continued dosing at the RP2D for a median of 48 days. After a median dosing time of 48 days at the RP2D, seven patients had a dose reduction by 50% because of tolerability issues, and the median duration of treatment at 50% RP2D was 65 days. No patient required additional dose reductions.
Clinical Response
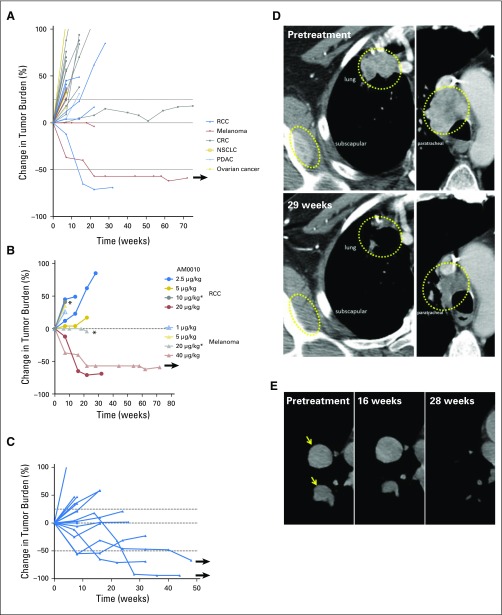
Antitumor responses were assessed according to immune-related response criteria (Table 3). Forty-one (80.4%) of 51 patients were evaluable, with at least one radiographic response assessment during treatment (8 weeks). One patient with uveal melanoma (40 μg/kg) had a partial response (PR), with histologically confirmed reduction of multiple gastric metastases (Figs 1A and 1B). Fifteen patients with RCC treated with AM0010 20 μg/kg (of 18 enrolled) had at least one scheduled assessment during treatment, and four of those patients had a PR (overall response rate, 27%; Figs 1C and 1D). Of those, one patient had a delayed response, with initial tumor increase, followed by a PR (−92%) at 28 weeks of treatment (Fig 1E). A second responding patient with RCC exhibited reductions of most lesions but the continued growth of a lung lesion. The lung lesion started to regress after 48 weeks of AM0010 treatment. Objective responses were not observed in patients treated below the RP2D. Objective responses were observed in patients who had experienced progression with prior therapy with anti–cytotoxic T-cell lymphocyte-4 or IL-2 (Appendix Table A1). Several patients in the dose-escalation and dose-expansion cohorts had mixed responses, with substantial reductions in some tumors but increases in other index lesions or lymph nodes (Table 3), including one patient with cutaneous melanoma treated with AM0010 20 μg/kg, who had a reduction in five large liver metastases but increases in lung lesions and lymph nodes. One patient with microsatellite-stable CRC experienced prolonged stable disease for more than 20 months. Responses observed were durable, with three patients having a continuing response at the time of data cutoff.
Table 3.
Clinical Response
| Variable | No. of Evaluable Patients (total) | Best Response (duration in months) |
|---|---|---|
| Dose escalation, μg/kg | ||
| 1 | 2 (4) | 1 SD (2) |
| 2.5 | 5 (6) | 1 MXR (2) |
| 5 | 5 (6) | 2 SDs (20 and 6) |
| 10 | 5 (6) | 2 MXRs (2 and 2) |
| 20 | 5 (6) | 1 PR (4), 2 MXRs (6 and 2), 1 SD (4) |
| 40 | 5 (5) | 1 PR (19+) |
| All patients (1 to 40 μg/kg) | 27 (33) | 2 PRs, 5 MXRs, 1 SD (20) |
| Tumor histology (escalation cohort) | ||
| CRC | 14 (16) | 1 SD (20), 2 MXRs (2 and 2) |
| Melanoma | 4 (4) | 1 PR (19+), 1 MXR (6) |
| Prostate cancer | 0 (1) | |
| NSCLC | 1 (1) | |
| RCC | 5 (6) | 1 PR (4), 2 MXRs (2 and 2) |
| 1 SD (6) | ||
| Pancreatic cancer | 2 (4) | 1 SD (4) |
| Ovarian cancer | 1 (1) | 1 SD (2) |
| All patients with RCC at RP2D (20 μg/kg) | 15 (19) | 4 PRs (2, 4, 4+, and 4+), 6 SDs (8, 6, 4, 4, 2, and 2) |
NOTE. SD and PR according to immune-related response criteria. MXR indicates SD with ≥ one shrinking lesion. Evaluable patients had ≥ one scheduled scan during treatment. + indicates ongoing response at data cut.
Abbreviations: CRC, colorectal cancer; MXR, mixed response; NSCLC, non–small-cell lung cancer; OR, objective response; ORR, overall response rate; PR, partial response; RCC, renal cell cancer; RP2D, recommended phase II dose; SD, stable disease.
Fig 1.
Spider plots indicating the change in tumor burden (according to immune-related response criteria) in (A) all patients in the dose-escalation cohorts, (B) patients with renal cell cancer (RCC) or melanoma in the dose-escalation cohorts, and (C) all patients with RCC treated at 20 μg/kg (arrows indicate ongoing response at data cutoff). (D) Tumor response in a patient with RCC and (E) delayed tumor response in a patient with RCC (arrows indicate tumors). CRC, colorectal cancer; NSCLC, non–small-cell lung cancer; PDAC, pancreatic ductal adenocarcinoma. (*) Mixed response (some lesions reduced).
Pharmacokinetic Assessment
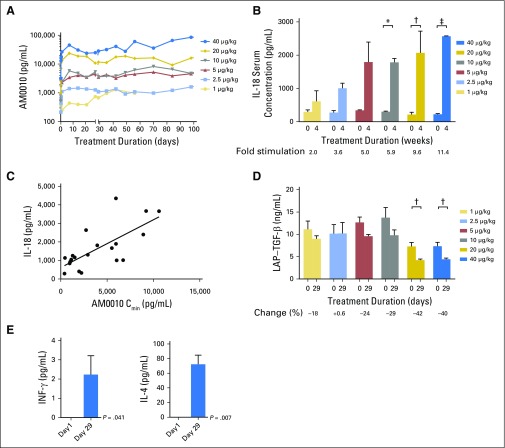
Because of its pegylation, AM0010 has a prolonged exposure compared with recombinant human IL-10 on SC delivery. The serum concentration of AM0010 was determined at six time points at days 1 and 29, and the predose concentration (Cmin) was determined once per week in patients in the dose-escalation cohorts throughout the dosing period (Fig 2A). The pretreatment serum level of IL-10 was found to be below the limit of detection (50 pg/mL) in all patients with cancer analyzed.
Fig 2.
Average serum values of cytokines for all available patients in escalation cohorts (predose serum of days 1 and 29 analyzed). (A) AM0010 serum trough (Cmin); (B) interleukin-18 (IL-18); (C) Spearman correlation between AM0010 Cmin and IL-18 (R2 = 0.5; P < .001); (D) latency-associated peptide (LAP) transforming growth factor beta (TGF-β); (E) average serum values of interferon-gamma (IFN-γ; left) and IL-4 (right) in patients in the dose-escalation cohorts treated at 20 to 40 μg/kg (predose serum analyzed on days 1 and 29). (*) P < .01. (†) P < .05. (‡) P < .001.
At the starting dose (1 μg/kg), patients had a serum concentration of AM0010 between 0.68 and 1 ng/mL or equivalent to 10% effective concentration. The average minimal AM0010 serum concentration at the RP2D (20 μg/kg) was 11.7 ng/mL (Fig 2A) or equivalent to the 60% effective concentration. At the RP2D, the difference between maximum serum concentration and Cmin was only 19%, indicating stable drug exposure (Appendix Table A2, online only). The area under the curve of AM0010 in patients in subsequent dose cohorts increased approximately proportional to the AM0010 dose. Serum concentrations of AM0010 remained stable throughout the duration of dosing. No significant accumulation or attenuation of AM0010 levels was observed over time. The absence of high titer antidrug antibodies was confirmed in all patients tested.
Pharmacodynamic Assessment
Pegylated recombinant murine IL-10 leads to activation, survival, and proliferation of tumor-infiltrating CD8+ T cells in mice.8,14 Through the modulation of macrophage activation, IL-10 also has anti-inflammatory activities, limiting excessive activation toward bacterial products.16 To clarify the function of AM0010 in patients with cancer, immune-activating and -suppressive cytokines were measured at days 1 and 29 in the serum. The immune-activating Th1 cytokine IL-18 was elevated on day 29 in all patients treated with AM0010 (Fig 2B). The increase was directly proportional to the AM0010 dose. IL-18 serum concentrations correlated with the Cmin of AM0010 measured in patients (Figs 2B and 2C). Increasing doses of AM0010 led to a reduction in the serum concentration of the immune-suppressive cytokine transforming growth factor beta (TGF-β) in all patients treated. At AM0010 20 μg/kg, TGF-β was reduced by 42% (Fig 2D). Most strikingly, AM0010 induced IFN-γ and IL-4, which are signature cytokines for Th1 and Th2 immune responses, respectively (Fig 2E). The changes in serum cytokines, upregulation of immune-activating cytokines, and decrease of TGF-β were durable throughout prolonged treatment periods. The absolute number and relative percentage of peripheral CD8+ or CD4+ T cells (measured bimonthly) did not change consistently (data not shown).
DISCUSSION
We report here the first-in-human trial of AM0010, showing an acceptable safety profile with early evidence of clinical activity in patients with advanced solid tumor malignancies. IL-10 has generally been perceived as an anti-inflammatory molecule, because it limits inflammatory reaction to bacterial product and cellular debris. Germline deficiency in IL-10 or its receptor in humans leads to early-onset inflammatory bowel disease17 and a high risk of large B-cell lymphomas within the first years of life. Tumors in these patients are characterized by the almost complete absence of CD8+ T cells and granzyme B.18 In mice, pegylated IL-10 induces the expansion of activated intratumoral CD8+ T cells, CD8+ T cell– and IFN-γ–dependent tumor rejection, and the formation of tumor-immune memory.9,14 Patients in this study received daily doses to continuously elevate the systemic IL-10 concentration. This led to the selective and durable increase of Th1 (IFN-γ; IL-18) and Th2 (IL-4) cytokines and a reduction of immune-suppressive TGF-β. In response to AM0010, IL-18 was upregulated in every patient tested, indicating a direct role of IL-10 in its induction. IL-18 has been shown to be induced in dendritic cells by CD8+ T cell–produced IFN-γ. As part of a positive feedback loop, IL-18 directly stimulates the proliferation of memory CD8+ T cells in the tissue.19 The combined upregulation of IFN-γ and IL-18 by AM0010 may be essential for the therapeutic activity observed. Taken together, the analyses of the systemic biomarkers confirm a predominantly stimulatory role of AM0010 in the immune system in patients with cancer.
Despite remarkable therapeutic successes, cytokine therapy has been limited by acute immune-related toxicities.20 In contrast, prolonged exposure to AM0010 did not lead to acute toxicities at the therapeutic dose. At the highest explored dose, one patient with ocular melanoma continued receiving the study drug for 17 months. However, all patients at the highest explored dose required dose reductions or interruptions after prolonged dosing. On the basis of overall tolerability and efficacy and to facilitate uninterrupted dosing in a majority of patients, 20 μg/kg was selected for the RCC expansion cohort. In the dose-escalation cohorts, several patients experienced flu-like symptoms (n = 5), fever (n = 10), or skin rashes (n = 5). Fourteen patients had thrombocytopenia and/or anemia (grade ≥ 1). Anemia and thrombocytopenia had been described in previous studies involving recombinant human IL-10.12 IL-10–induced anemia has mechanistic similarities to anemia of inflammation, wherein activated macrophages eliminate aging RBCs and platelets.21 Macrophages loaded with iron were detected in the spleen and gut of preclinical toxicology animals treated with AM0010 (data not shown). Prolonged repression of thrombocytes or erythrocytes was not observed. A reduction of thrombocytes in response to AM0010 normalized within 3 to 7 days after dose interruption or reduction. Thrombocytopenia was not associated with bleeding in this study or in previous studies of recombinant human IL-10 in inflammatory diseases.12 Treatment-related AEs were reversible, with only one patient discontinuing the study because of treatment-related anemia. Autoimmune-related toxicities such as colitis, arthropathy, pneumonitis, or endocrine AEs were not observed. This safety profile, along with tolerated exposures well above those predicted to be efficacious from the preclinical models, supports continuous daily dosing at the RP2D. However, intermittent dosing schedules are being evaluated and may be particularly important in combination studies with chemotherapy.
Antitumor activity was a secondary end point of the trial, and we observed reduction in tumor burden in two of 10 patients in the two highest dose-escalation cohorts (20%). One patient with ocular melanoma and a total of four patients with RCC had PRs (of 15 evaluable). The patient with ocular melanoma had a continuing PR at 72 weeks after AM0010 treatment initiation. The patient had multiple gastric metastases before treatment. Biopsies during treatment revealed the complete elimination of tumor cells from the gastric lesions. Two of the four patients with RCC with a PR had a delayed response in all or some of the lesions. Although consistent with other immunotherapies, delayed antitumor responses provided a challenge for treating physicians, because tumors may radiologically progress before sufficient amplification of the immune response occurs to mediate tumor reductions. Analysis of immune infiltrates and systemic immune responses before and during treatment may prospectively identify AM0010-responding patients early during treatment. In addition, patient characteristics such as International Metastatic Renal Cell Carcinoma Database Consortium criteria (Appendix Table A1)22 as well molecular and genetic profiling of the tumor are currently being evaluated to identify responding subpopulations. Mutations such as a genetic loss of BAP1 observed in uveal melanomas and a subset of RCCs may identify cancers susceptible to AM0010 monotherapy.23
In conclusion, AM0010 has an acceptable tolerability profile and encouraging clinical activity in RCC. Pegylated IL-10 (AM0010) offers a potent novel and nonredundant mechanism, adding to the arsenal of clinically active immuno-oncology drugs. The direct activation of CD8+ T cells by AM0010 in the absence of immune-related AEs should enable combination with other immune therapies that block inhibitory mechanisms.
Appendix
Table A1.
Characteristics of Patients With RCC and Melanoma
| Patient | Response | Histology | Stage | Anemia (g/dL) | Thrombocytosis (109/L) | Neutrophilia (109/L) | ECOG PS of 2 (Karnofsky score < 80) | Hypercalcemia (mg/dL) | Time From Original Diagnosis to Start of Systemic Therapy (years) | IMDC | Prior Immune Therapy | Prior Therapies (No.) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Favorable | Intermediate | Poor | IL-2 | Anti–CTLA-4 | Anti–PD-1 | |||||||||||
| RCC (AM0010 20 μg/kg) | ||||||||||||||||
| 1 | SD | Clear cell | IV | 8.6 | 332 | 7.76 | 1 | 10.56 | 0.18 | X | X | 3 | ||||
| 2 | PR | Clear cell | IV | 12.6 | 160 | 2.1 | 0 | 10.22 | 10.12 | X | X | 6 | ||||
| 3 | PR | Clear cell | IV | 12.9 | 286 | 3.3 | 0 | 9.64 | 3.74 | X | 2 | |||||
| 4 | ND | Clear cell | IV | 10.2 | 447 | 4.1 | 1 | 11.34 | 0.71 | X | 1 | |||||
| 5 | SD | Clear cell | IV | 18.5 | 221 | 3.2 | 0 | 9.32 | 0.12 | X | 4 | |||||
| 6 | PD | Translocation | IV | 13.3 | 224 | 3.73 | 0 | 9.96 | 3.07 | X | 4 | |||||
| 7 | PD | Clear cell | IV | 10.0 | 356 | 6.70 | 1 | 10.72 | 0.19 | X | 4 | |||||
| 8 | PD | Clear cell | IV | 13.1 | 231 | 3.55 | 0 | 9.98 | 0.11 | X | 3 | |||||
| 9 | SD | RCC | IV | 11.5 | 726 | 8.21 | 1 | 9.16 | 0.22 | X | X | 3 | ||||
| 10 | PD | Clear cell | IV | 12.5 | 291 | 4.94 | 1 | 9.88 | 1.63 | X | 1 | |||||
| 11 | SD | Chromophobe | IV | 11.2 | 304 | 3.48 | 0 | 9.7 | 4.18 | X | 2 | |||||
| 12 | ND | Medullary carcinoma | IV | 10.9 | 272 | 3.52 | 1 | 9.38 | 0.36 | X | 2 | |||||
| 13 | PR | Clear cell | IV | 11.2 | 241 | 4.24 | 1 | 9.36 | 3.66 | X | X | 7 | ||||
| 14 | ND | Clear cell | IV | 9.5 | 410 | 5.22 | 0 | 11.44 | 9.55 | X | X | 3 | ||||
| 15 | SD | Clear cell | IV | 10.3 | 474 | 5.45 | 0 | 10.42 | 0.65 | X | X | 3 | ||||
| 16 | PR | Clear cell | IV | 13.1 | 281 | 5.4 | 1 | 9.18 | 0.09 | X | 2 | |||||
| 17 | PD | Clear cell | IV | 9.0 | 142 | 3.3 | 1 | 8.92 | 0.06 | X | 3 | |||||
| 18 | SD | NA | IV | 13.7 | 297 | 5.9 | 1 | 10.42 | NA | X | 0 | |||||
| 19 | ND | Clear cell | IV | 12.0 | 237 | 6.33 | 0 | 10.78 | 2.19 | X | 7 | |||||
| Melanoma (AM0010 20 or 40 μg/kg) | ||||||||||||||||
| 1 | PR | MM, choroidal | IV | LDH normal | 1 | X | 3 | |||||||||
| 2 | SD (MXR) | MM, cutaneous | IV | LDH elevated | 1 | X | 1 | |||||||||
Abbreviations: CTLA-4, cytotoxic T-cell lymphocyte-4; ECOG PS, Eastern Cooperative Oncology Group performance status; IMDC, International Metastatic Renal Cell Carcinoma Database Consortium; LDH, lactate dehydrogenase; MM, metastatic melanoma; MXR, mixed response; NA, not available; ND, not determined; PD, progressive disease; PD-1, programmed death-1; PR, partial response; RCC, renal cell cancer; SD, stable disease
Table A2.
AM0010 Pharmacokinetic Assessment (day 29)
| Dose (mg/kg) | Cmax (ng/mL) | Tmax | Cmin (24 hours) | AUC (ng/mL × 24 hours) |
|---|---|---|---|---|
| 1 | 0.85 | 7.3 | 0.68 | 18.4 |
| 2.5 | 1.45 | 9.2 | 1.08 | 30.6 |
| 5 | 5.30 | 14.4 | 3.54 | 106 |
| 10 | 5.35 | 9.2 | 3.60 | 108 |
| 20 | 13.9 | 9.2 | 11.7 | 307 |
| 40 | 31.1 | 12.5 | 28.5 | 716 |
Abbreviations: AUC, area under the curve; Cmax, maximum serum concentration; Cmin, minimum serum concentration; Tmax, time to maximum serum concentration.
Footnotes
Supported by ARMO BioSciences, which provided funding, data collection, medical writing, and editorial assistance.
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Clinical trial information: NCT02009449.
See accompanying article on page 3576
AUTHOR CONTRIBUTIONS
Conception and design: Aung Naing, Kyriakos P. Papadopoulos, Karen A. Autio, Manish R. Patel, Deborah J. Wong, Shubham Pant, Melinda Whiteside, Peter Van Vlasselaer, Nizar M. Tannir, Martin Oft, Jeffrey R. Infante
Provision of study materials or patients: Aung Naing, Gerald S. Falchook, Johanna C. Bendell
Collection and assembly of data: Aung Naing, Kyriakos P. Papadopoulos, Karen A. Autio, Patrick A. Ott, Manish R. Patel, Deborah J. Wong, Gerald S. Falchook, Shubham Pant, Melinda Whiteside, Drew R. Rasco, John B. Mumm, Ivan H. Chan, Johanna C. Bendell, Todd M. Bauer, Rivka R. Colen, David S. Hong, Nizar M. Tannir, Martin Oft, Jeffrey R. Infante
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Safety, Antitumor Activity, and Immune Activation of Pegylated Recombinant Human Interleukin-10 (AM0010) in Patients With Advanced Solid Tumors
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Aung Naing
Research Funding: National Cancer Institute, EMD Serono, MedImmune, Healios, Atterocor, Amplimmune, ARMO BioSciences, Karyopharm Therapeutics, Incyte, Novartis, Regeneron, Baxter (I)
Travel, Accommodations, Expenses: ARMO BioSciences
Kyriakos P. Papadopoulos
Research Funding: AbbVie (Inst), MedImmune (Inst), Daiichi Sankyo (Inst), GlaxoSmithKline (Inst), Onyx Pharmaceuticals (Inst), Regeneron (Inst), Sanofi (Inst), Novartis (Inst), ARMO BioSciences (Inst), ArQule (Inst)
Karen A. Autio
Research Funding: Merck (Inst), Eli Lilly (Inst), Pfizer (Inst)
Patrick A. Ott
Honoraria: Merck
Consulting or Advisory Role: Alexion Pharmaceuticals, CytomX Therapeutics, Celldex, Neon Therapeutics, Genentech, Bristol-Myers Squibb, Amgen
Research Funding: Bristol-Myers Squibb (Inst), Merck (Inst), Celldex (Inst), ARMO BioSciences (Inst), AstraZeneca/MedImmune (Inst)
Manish R. Patel
Honoraria: Medivation, Gilead Sciences, Taiho Pharmaceutical
Speakers’ Bureau: Medivation, Gilead Sciences, Taiho Pharmaceutical
Deborah J. Wong
Consulting or Advisory Role: Bristol-Myers Squibb
Research Funding: ARMO BioSciences (Inst), Merck (Inst), AstraZeneca (Inst), Kura Oncology (Inst), EMD Serono (Inst), Genentech (Inst), BioMed Valley Discoveries (Inst)
Travel, Accommodations, Expenses: Bristol-Myers Squibb, Merck
Gerald S. Falchook
Research Funding: ARMO BioSciences (Inst)
Shubham Pant
Consulting or Advisory Role: Celgene, Halozyme, Bayer HealthCare Pharmaceuticals
Melinda Whiteside
Employment: ARMO BioSciences
Stock or Other Ownership: ARMO BioSciences
Drew R. Rasco
Consulting or Advisory Role: TaiRx
Research Funding: Celgene (Inst), Millennium Pharmaceuticals (Inst), Rexahn Pharmaceuticals (Inst), Santa Maria Biotherapeutics (Inst), Five Prime Therapeutics (Inst), Bayer HealthCare Pharmaceuticals (Inst), Pharmacyclics (Inst), Asana Biosciences (Inst), Eisai (Inst), Aeglea Biotherapeutics (Inst)
John B. Mumm
Employment: ARMO BioSciences
Ivan H. Chan
Employment: ARMO BioSciences
Johanna C. Bendell
No relationship to disclose
Todd M. Bauer
No relationship to disclose
Rivka R. Colen
No relationship to disclose
David S. Hong
Research Funding: Novartis, Genentech, Eisai, AstraZeneca, Pfizer, miRNA Therapeutics, Amgen, Daiichi Sankyo, Merck, Mirati Therapeutics, Eli Lilly
Travel, Accommodations, Expenses: Loxo, miRNA Therapeutics
Peter Van Vlasselaer
Employment: ARMO BioSciences
Leadership: ARMO BioSciences
Nizar M. Tannir
Honoraria: Pfizer, Novartis, GlaxoSmithKline, Bristol-Myers Squibb, Exelixis, Nektar
Consulting or Advisory Role: GlaxoSmithKline, Novartis, Exelixis, Bristol-Myers Squibb, Nektar
Research Funding: Bristol-Myers Squibb, Novartis, Exelixis, Epizyme
Travel, Accommodations, Expenses: Pfizer, Novartis, GlaxoSmithKline, Exelixis, Nektar, Bristol-Myers Squibb
Martin Oft
Employment: ARMO BioSciences
Jeffrey R. Infante
No relationship to disclose
REFERENCES
- 1.Page DB Postow MA Callahan MK, etal: Immune modulation in cancer with antibodies Annu Rev Med 65:185–202,2014 [DOI] [PubMed] [Google Scholar]
- 2.Gill S, June CH: Going viral: Chimeric antigen receptor T-cell therapy for hematological malignancies Immunol Rev 263:68–89,2015 [DOI] [PubMed] [Google Scholar]
- 3.Topalian SL Hodi FS Brahmer JR, etal: Safety, activity, and immune correlates of anti-PD-1 antibody in cancer N Engl J Med 366:2443–2454,2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larkin J Chiarion-Sileni V Gonzalez R, etal: Combined nivolumab and ipilimumab or monotherapy in untreated melanoma N Engl J Med 373:23–34,2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Motzer RJ Escudier B McDermott DF, etal: Nivolumab versus everolimus in advanced renal-cell carcinoma N Engl J Med 373:1803–1813,2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rizvi NA Hellmann MD Snyder A, etal: Cancer immunology: Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer Science 348:124–128,2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gartlan KH Markey KA Varelias A, etal: Tc17 cells are a proinflammatory, plastic lineage of pathogenic CD8+ T cells that induce GVHD without antileukemic effects Blood 126:1609–1620,2015 [DOI] [PubMed] [Google Scholar]
- 8.Emmerich J Mumm JB Chan IH, etal: IL-10 directly activates and expands tumor-resident CD8(+) T cells without de novo infiltration from secondary lymphoid organs Cancer Res 72:3570–3581,2012 [DOI] [PubMed] [Google Scholar]
- 9.Fujii S Shimizu K Shimizu T, etal: Interleukin-10 promotes the maintenance of antitumor CD8(+) T-cell effector function in situ Blood 98:2143–2151,2001 [DOI] [PubMed] [Google Scholar]
- 10.Chan IH Wu V Bilardello M, etal: The potentiation of IFN-γ and induction of cytotoxic proteins by pegylated IL-10 in human CD8 T cells J Interferon Cytokine Res 35:948–955,2015 [DOI] [PubMed] [Google Scholar]
- 11.Oft M: IL-10: Master switch from tumor-promoting inflammation to antitumor immunity Cancer Immunol Res 2:194–199,2014 [DOI] [PubMed] [Google Scholar]
- 12.Asadullah K, Sterry W, Volk HD: Interleukin-10 therapy: Review of a new approach Pharmacol Rev 55:241–269,2003 [DOI] [PubMed] [Google Scholar]
- 13.Lauw FN Pajkrt D Hack CE, etal: Proinflammatory effects of IL-10 during human endotoxemia J Immunol 165:2783–2789,2000 [DOI] [PubMed] [Google Scholar]
- 14.Mumm JB Emmerich J Zhang X, etal: IL-10 elicits IFNγ-dependent tumor immune surveillance Cancer Cell 20:781–796,2011 [DOI] [PubMed] [Google Scholar]
- 15.Wolchok JD Kluger H Callahan MK, etal: Nivolumab plus ipilimumab in advanced melanoma N Engl J Med 369:122–133,2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li MO, Flavell RA: Contextual regulation of inflammation: a duet by transforming growth factor-beta and interleukin-10 Immunity 28:468–476,2008 [DOI] [PubMed] [Google Scholar]
- 17.Glocker EO Kotlarz D Boztug K, etal: Inflammatory bowel disease and mutations affecting the interleukin-10 receptor N Engl J Med 361:2033–2045,2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neven B Mamessier E Bruneau J, etal: A Mendelian predisposition to B-cell lymphoma caused by IL-10R deficiency Blood 122:3713–3722,2013 [DOI] [PubMed] [Google Scholar]
- 19.Iwai Y Hemmi H Mizenina O, etal: An IFN-gamma-IL-18 signaling loop accelerates memory CD8+ T cell proliferation PLoS One 3:e2404,2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Atkins MB: Cytokine-based therapy and biochemotherapy for advanced melanoma Clin Cancer Res 12:2353s–2358s,2006 [DOI] [PubMed] [Google Scholar]
- 21.Tilg H Ulmer H Kaser A, etal: Role of IL-10 for induction of anemia during inflammation J Immunol 169:2204–2209,2002 [DOI] [PubMed] [Google Scholar]
- 22.Heng DY Xie W Regan MM, etal: Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor–targeted agents: Results from a large, multicenter study J Clin Oncol 27:5794–5799,2009 [DOI] [PubMed] [Google Scholar]
- 23.Peña-Llopis S Vega-Rubín-de-Celis S Liao A, etal: BAP1 loss defines a new class of renal cell carcinoma Nat Genet 44:751–759,2012 [DOI] [PMC free article] [PubMed] [Google Scholar]