Abstract
The cell’s ability to communicate with the extracellular environment, with other cells, and with itself is a crucial feature of eukaryotic organisms. In the immune system, T lymphocytes assemble a specialized structure upon contact with antigen-presenting cells bearing a peptide-major histocompatibility complex ligand, known as the immunological synapse (IS). The IS has been extensively characterized as a signaling platform essential for T-cell activation. Moreover, emerging evidence identifies the IS as a device for vesicular traffic-mediated cell-to-cell communication as well as an active release site of soluble molecules. Here, we will review recent advances in the role of vesicular trafficking in IS assembly and focused secretion of microvesicles at the synaptic area in naïve T cells and discuss the role of the IS in transcellular communication.
Keywords: immunological synapse, vesicular traffic, transcellular communication, T-cell activation
Introduction
T-cell activation crucially depends on the assembly of a complex supramolecular structure, known as the immunological synapse (IS), at the T-cell interface with the antigen-presenting cell (APC) 1. The recognition of cognate peptide ligand associated with major histocompatibility complex (pMHC) on APCs by the T-cell antigen receptor (TCR) results in the coordinate polarization of receptors, adhesion molecules, kinases, cytoskeletal elements, and organelles toward the contact area. Intracellular vesicular traffic plays a pivotal role in this polarized transport and is essential for the assembly and maintenance of the IS 2. In addition, expanding evidence indicates that microvesicles, generated and released at the synaptic area, are transferred from the T cell to the APC, where they are able to induce the activation of early signaling pathways 3, 4. Microvesicles can carry specific microRNAs (miRNAs) that modulate gene expression patterns in the recipient cell 5. Moreover, the synaptic cleft has been reported as a site for trogocytosis, a process exploited by T cells to extract pMHC from the APC during the endocytosis of engaged TCRs, which thereby can sustain signaling at endosomes 6– 12. These findings support the notion that the IS acts as a device for transcellular communication. Here, we will discuss the role of the IS in cell-to-cell communication in naïve T cells and focus on vesicular trafficking as the main regulator of this process. The polarized secretion of molecules at the IS by effector cells (that is, for example the lytic enzymes by cytotoxic T cells or immunomodulatory molecules by helper T cells) has been well characterized and described in other excellent reviews 1, 13, 14.
Vesicular trafficking at the immunological synapse
The dramatic rearrangement of molecules occurring during IS assembly leads to the formation of two concentric regions within the synaptic cleft 1, 15: the central supramolecular activation cluster (cSMAC), where the TCR and the co-stimulatory receptor CD28 accumulate, and the peripheral SMAC, where a ring of integrin bound to newly polymerized actin filaments helps stabilizing the IS. Molecules with bulky ectodomains are segregated at the outer edge of the IS, known as distal SMAC (dSMAC), by a mechanism that excludes molecules above a size threshold from the contact area 16– 18.
The orchestration of signaling by surface receptors as well as signaling molecules that accumulate at the IS area crucially depends on the polarization of the microtubule-organizing center (MTOC) 19, which brings the endosomal recycling compartment at the T cell:APC contact region, thus favoring directional intracellular trafficking 20. In naïve T cells, a number of molecules, including receptors (for example, the TCR) and membrane-associated regulators—for example, the lymphocyte-specific protein tyrosine kinase (Lck) and the linker for activation of T cells (LAT)—are clustered to the synaptic area not only from plasma membrane-associated pools but also from intracellular endosomal pools 2. A complex machinery is involved in the selective targeting of specific endosomal proteins to membrane domains. Beyond the common basic recycling pathways, which are involved in the traffic of internalized receptors to the plasma membrane through early endosomes (marked by Rab5) and recycling endosomes (marked by Rab4 and Rab11), more specific members of the Rab GTPase family have been identified as important regulators of endosomal traffic at the IS 21, 22. The emerging scenario indicates that T-cell activation relies on the synaptic delivery of receptors and signaling mediators through specific subpopulations of recycling endosomes. This notion is remarkably exemplified by the findings of Soares et al., who identified at the IS a mosaic of endosomes characterized by the presence of specific synaptic cargo associated with a unique set of traffic regulators and effectors 23. For example, LAT is associated with Rab27a + and Rab37 + vesicles, and Lck is associated with Rab11b + vesicles. It was recently reported that the endosomal localization of Lck depends on Rab11 family interacting protein-3 and alterations in its traffic impair TCR signaling, underscoring the importance of the endosomal pool of Lck for T-cell activation 24. The TCR is localized in Rab3d + and Rab8b + endosomes 23 and its traffic to the IS is regulated by Rab29 25, Rab35 26, and Rab8a 27. Surprisingly, the components of the intraflagellar transport (IFT) system, which have a well-known function in ciliogenesis, have been reported as essential for the delivery of the TCR and LAT to the IS and T-cell activation, even though lymphocytes are devoid of a primary cilium 28– 31. The complexity of the mechanism underlying the endosomal trafficking to the IS is further increased by a variety of regulators of both the microtubule and the actin cytoskeleton as well as components of the machinery involved in vesicle fusion with the synaptic membrane 2, 32. Importantly, several vesicle-soluble N-ethylmaleimide-sensitive factor attachment protein receptors (v-SNAREs) and target-SNAREs (t-SNAREs) are recruited to the IS and are implicated in TCR (VAMP3 27, 33, syntaxin4 33, and SNAP23 33) and LAT (VAMP7 34) targeting to the synaptic membrane.
Collectively, these data indicate that intracellular vesicular trafficking is essential for modulating both the intensity and duration of signaling at the IS through the polarized transport of specific molecules to the synaptic cleft.
Extracellular traffic at the immunological synapse
Emerging evidence indicates that the IS is not only a site of intense intracellular trafficking but also an area of extracellular vesicle release. Based on these observations, it has been proposed that the cSMAC can be divided into two components: the endo-cSMAC, a membrane domain where TCR signaling occurs, and the exo-cSMAC, an extracellular region between the T cell and the APC, which is characterized by the presence of TCR-enriched extracellular vesicles 35. Recently, Choudhuri et al. reported that internalized ubiquitinated TCRs can be targeted to microvesicles and budded from the plasma membrane, rather than undergo degradation in lysosomes, through the cooperation of the endosomal sorting complexes required for transport I (ESCRT-I) protein Tsg101 and the vacuolar protein sorting 4 3. Remarkably, these ectosomes carrying TCRs act as a useful device for cell-to-cell communication. Indeed, the TCRs carried by extracellular vesicles are able to engage cognate pMHC on the APC surface and this event has a dual effect: on the one hand, it triggers early intracellular signals in APCs, the intensity of which is proportional to the density of pMHC; on the other hand, the rapid endocytosis of TCR:pMHC complexes results in the signaling of the internalized T-cell ectosomes inside the APC 3.
In addition to TCR-enriched ectosomes, T cells release a different type of TCR-containing microvesicle upon antigen receptor triggering 4. Blanchard et al. showed that these microvesicles contain up to 1% of the total CD3ζ, part of which is phosphorylated on tyrosine residues, as well as Src-related tyrosine kinases (Fyn and Lck), the adaptor protein c-Cbl, the C-X-C motif chemokine receptor (CXCR)-4, and adhesion molecules (CD2 and CD18). The microvesicle content suggests that these can be delivered to cells bearing cognate pMHC, thereby becoming a means of cell-to-cell communication 4. Although the membrane compartment from which these microvesicles originate remains to be defined, it has been proposed that they may be exosomes, based on their morphology and the expression of the exosomal markers CD63 and CD18 4.
While the ectosomes containing high levels of TCR are generated at the cell surface, the exosomes appear to derive from the multivesicular bodies (MVBs) 5. During IS assembly, MTOC polarization allows MVB translocation just beneath the contact region between the T cell and the APC, which appear essential not only for polarized protein recycling but also for the synaptic release of exosomes 5, 36. MVB maturation and exosome secretion depend on the activity of diacylglycerol kinase α, which, in turn, regulates protein kinase D 1/2 36, 37. Moreover, even though traffic regulators such as the ESCRT complex, several members of Rab GTPase family, and SNARE proteins are required both for the biogenesis and for the release of exosomes, many aspects of exosome generation are still to be elucidated 38, 39. It has been reported that, in Jurkat T cells, similar to cytotoxic lymphocytes, FasL and APO2L/TRAIL localize at MVBs and are secreted in exosomes upon cell stimulation 40, 41. The authors have proposed that the release of death ligands may play an important role in the modulation of immune responses under both physiological and pathological conditions, but further studies are required to clarify this issue 41. Mittelbrun et al. demonstrated that, upon TCR stimulation, microRNA-containing exosomes, generated through membrane budding and scission from MVBs, polarize and fuse with the synaptic membrane, releasing their content into the cognate APC 5. Although a controversial issue is whether the low miRNA copy number (1–10) in exosomes is sufficient to elicit a biological response 42, 43, it has been reported that the uptake of exosomes by the APC results in the modulation of the expression of specific genes, such as the Sry-box transcription factor 4 ( Sox-4), in the recipient cell 5. Finally, although the precise mechanism involved in exosome delivery to acceptor cells remains to be clarified, these pieces of evidence highlight the exchange of genetic material mediated by extracellular vesicles at the IS as a strategy for transcellular communication and immune modulation 44.
Remarkably, gap junctions have been described at the IS. Of note, the GJ channel-forming protein connexin 43, a protein involved in gap junction assembly, interacts with the epithelial cell-cell junction protein zona occludens-2 (ZO-2) 45, which was recently identified as an IS component 46. This suggests that ZO-2 could participate in gap junction formation at the IS. Although this type of cell-to-cell connection has been identified as a means for the transfer of genetic information in different cell systems, a direct synaptic transfer of RNAs through gap junctions in T cells remains to be demonstrated. Nonetheless, the presence at the IS of gap junctions 47, 48, as well as of invasive T-cell pseudopodia 49 and nanotubes 50, strongly suggests that additional mechanisms besides microvesicle secretion are likely to be operational to ensure an exchange of soluble molecules between the T lymphocyte and the APC. This may be required for productive T-cell activation, as already documented for gap junctions 47, 48.
The intercellular exchange of membrane patches at the IS through phagocytosis during TCR internalization or upon T-cell dissociation from the APC has also been reported 51. The process of extraction of surface molecules, known as trogocytosis, leads to the acquisition by T cells of pMHC as well as adhesion and co-stimulatory molecules expressed on APCs 7. The acquisition of membrane patches is promoted by TCR triggering and requires R-Ras2/TC21, a member of R-Ras subfamily GTPase, and the small GTPase RhoG 6, 8– 10. T-cell uptake of TCR:pMHC complexes results in prolonged antigen presentation that, in turn, determines increased protein phosphorylation and leads to sustained TCR signaling 11, 12. Within T cells, the internalized complexes localize in MVBs 10 and can undergo either degradation or recycling to the plasma membrane. Interestingly, recycled pMHCs are exposed on the surface of T cells, allowing these to function as APCs, thus allowing them to potentiate the immune response 6, 10. The secretion by APCs of pMHC-containing exosomes able to induce T-cell activation in vitro has also been documented 52– 54.
Recently, the serine protease inhibitor neuroserpin has been reported to polarize and become secreted at the IS, where it can act as a regulator of the proteolytic balance at the synaptic cleft and affect immune cell function 55, 56, highlighting a mechanism to keep under check the contents of the synaptic cleft to which both the T cell and the APC contribute.
Immune escape mediated by targeting of the cellular vesicular machinery
Among the strategies evolved by pathogens to escape from the host immune response, transcellular communication has been shown to be exploited by the human lymphotropic virus HIV-1 to ensure its spread. Specifically, HIV-1 hijacks the polarized vesicular machinery of its host cell for both the assembly and the focused secretion of newly formed virions at the virological synapse (VS), a highly organized contact zone that forms between infected and uninfected CD4 + T cells 57. The VS and the IS share structural similarities, as well as regulators (for example, EWI-2 and α-actinin) 58 and TCR signaling components, despite their divergent kinetics in disassembly and intracellular signaling events (for example, PKCθ) 59 that lead to specific outcomes. Interestingly, TCR engagement by pMHC leads to the recruitment and the central accumulation of HIV-1 group-specific antigen (GAG) at the IS, resulting in the budding of GAG-containing microvesicles 3. Vesicles secreted by HIV-1–infected cells have been found to carry chemokine receptors, such as C-C motif chemokine receptor-5 and CXCR4, which favor their entry in non-permissive cells 60, 61. Also, HIV-1 Nef induces the secretion of extracellular vesicles that contain Nef itself 62, 63 in addition to interfering with IS assembly by impairing both the intracellular trafficking of TCR, Lck, and LAT and the organization of the actin cytoskeleton 64– 69. Although a previous study showed that Nef-containing vesicles might contribute to the depletion of CD4 + T cells by inducing the apoptosis of bystander non-infected cells 63, Nef was recently described as being required for the release of a disintegrin and metalloprotease 17-loaded exosomes, which make quiescent CD4 + T cells susceptible to HIV-1 infection 70. Hence, HIV-1 co-opts the CD4 + T-cell secretory apparatus not only to promote a direct cell-to-cell transfer of virions but also to modulate the immune response. Interestingly, extracellular vesicles released by APCs also mediate a counter-strategy to protect recipient T cells from HIV-1 by delivering apolipoprotein B editing complex 3G, a key suppressor of HIV-1 replication 71, 72.
Conclusions
The IS is a very dynamic structure that must be finely regulated in time and space to induce a productive immune response. Adaptive immunity relies on the correct assembly, maintenance, and disassembly of the IS, which is regulated by TCR and co-stimulatory receptor signaling at the cell surface, intracellular endosomal trafficking, and vesicle secretion at the synaptic cleft. The emerging scenario indicates that the IS is not only a signaling platform but also a device that allows the polarized transfer of molecules or genetic material between T cell and APC ( Figure 1). Even though these mechanisms of transcellular communication may contribute to sustained signaling, thereby potentiating the immune response, the physiological relevance of extracellular vesicle release at the IS remains to be clarified. The improvement of techniques to analyze the IS 73, 74 may help elucidate the spatiotemporal dynamics of extracellular vesicles. Moreover, pharmacological treatments as well as genetic manipulations are expected to clarify in vivo the role of donor cell–derived microvesicles released into the synaptic cleft in recipient cells as well as the possible connection to disease development.
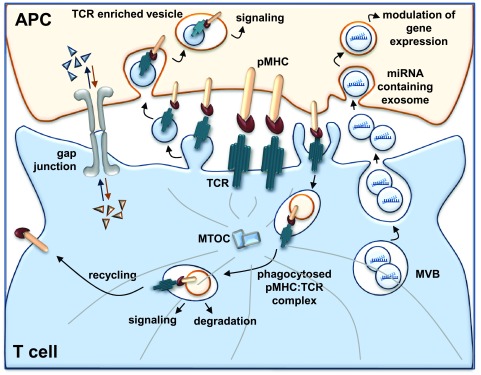
Figure 1. Cell-to-cell communication at the immunological synapse (IS).
The IS functions as a device for transcellular communication by exploiting different mechanisms: (i) polarized transfer of T-cell antigen receptor (TCR)-enriched vesicles from T cells to antigen-presenting cells (APCs), which promotes early signaling in the recipient cells; (ii) release of miRNA-loaded exosomes from T cells which modulate gene expression in APCs; (iii) trogocytosis of peptide-major histocompatibility complex:TCR (pMHC:TCR) complexes during TCR internalization, which is associated with both sustained signaling and surface expression of pMHC in T cells, the latter conferring to T cells the ability to present antigen to other T cells; and (iv) gap junction assembly between T cells and APCs that allows the exchange of soluble molecules at the IS. miRNA, microRNA; MTOC, microtubule-organizing center; MVB, multivesicular body.
The IS displays structural and functional similarities to the primary cilium 2, 75. Shared regulators of vesicular trafficking are involved in the assembly of these structures. Among these, microtubule-associated protein-4, previously identified as a regulator of cilia formation, has been implicated in MTOC polarization and in the dynamics of signaling nanovesicles during T-cell activation 76. Moreover, similar to the IS, the primary cilium has recently been identified as a site for release of active exosomes, and, interestingly, the IFT system is required for the release of extracellular vesicles in Caenorhabditis elegans 77– 79. This observation, taken together with the requirement for the IFT system in endosomal TCR trafficking and IS assembly, suggests that the IS and the primary cilium may also share regulators of the mechanisms involved in cell-to-cell communication, opening an important area for future research. Remarkably, even though the physiological role of extracellular ciliary vesicles is still unknown, it has been reported that alterations in their release may be linked to ciliary pathologies 79. Understanding of the mechanisms involved in the synaptic release and uptake of exosomes can be expected to result in the development of therapeutical applications in the context of immune disorders as well as anti-cancer immunity, as suggested by the tolerogenic effects of vesicles secreted by tumoral cells bearing immunosuppressive molecules 80, 81.
Abbreviations
APC, antigen-presenting cell; cSMAC, central supramolecular activation cluster; CXCR, C-X-C motif chemokine receptor; ESCRT-I, endosomal sorting complexes required for transport I; GAG, group-specific antigen; HIV, human immunodeficiency virus; IFT, intraflagellar transport; IS, immunological synapse; LAT, linker for activation of T cells; Lck, lymphocyte-specific protein tyrosine kinase; miRNA, microRNA; MTOC, microtubule-organizing center; MVB, multivesicular body; Nef, negative regulatory factor; pMHC, peptide-major histocompatibility complex; SNARE, soluble N-ethylmaleimide-sensitive factor attachment protein receptor; TCR, T-cell antigen receptor; VS, virological synapse; ZO-2, zona occludens-2.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
María Mittelbrunn, Hospital de 12 Octubre, Madrid, Spain
Isabel Merida, Centro Nacional de Biotecnología, Madrid, Spain
Andres Alcover, Pasteur Institute, Paris, France
Funding Statement
Part of the work discussed in this article has been supported by grants from Telethon-Italy (grant GGP16003 to CTB) and the Italian Association for Cancer Research (AIRC) (grant IG-15220 to CTB).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; referees: 3 approved]
References
- 1. Dustin ML, Choudhuri K: Signaling and Polarized Communication Across the T Cell Immunological Synapse. Annu Rev Cell Dev Biol. 2016;32:303–25. 10.1146/annurev-cellbio-100814-125330 [DOI] [PubMed] [Google Scholar]
- 2. Onnis A, Finetti F, Baldari CT: Vesicular Trafficking to the Immune Synapse: How to Assemble Receptor-Tailored Pathways from a Basic Building Set. Front Immunol. 2016;7:50. 10.3389/fimmu.2016.00050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Choudhuri K, Llodrá J, Roth EW, et al. : Polarized release of T-cell-receptor-enriched microvesicles at the immunological synapse. Nature. 2014;507(7490):118–23. 10.1038/nature12951 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 4. Blanchard N, Lankar D, Faure F, et al. : TCR activation of human T cells induces the production of exosomes bearing the TCR/CD3/zeta complex. J Immunol. 2002;168(7):3235–41. 10.4049/jimmunol.168.7.3235 [DOI] [PubMed] [Google Scholar]
- 5. Mittelbrunn M, Gutiérrez-Vázquez C, Villarroya-Beltri C, et al. : Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat Commun. 2011;2:282. 10.1038/ncomms1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alarcón B, Martínez-Martín N: RRas2, RhoG and T-cell phagocytosis. Small GTPases. 2012;3(2):97–101. 10.4161/sgtp.19138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Huang JF, Yang Y, Sepulveda H, et al. : TCR-Mediated internalization of peptide-MHC complexes acquired by T cells. Science. 1999;286(5441):952–4. 10.1126/science.286.5441.952 [DOI] [PubMed] [Google Scholar]
- 8. Hwang I, Huang JF, Kishimoto H, et al. : T cells can use either T cell receptor or CD28 receptors to absorb and internalize cell surface molecules derived from antigen-presenting cells. J Exp Med. 2000;191(7):1137–48. 10.1084/jem.191.7.1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Singleton K, Parvaze N, Dama KR, et al. : A large T cell invagination with CD2 enrichment resets receptor engagement in the immunological synapse. J Immunol. 2006;177(7):4402–13. 10.4049/jimmunol.177.7.4402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Martínez-Martín N, Fernández-Arenas E, Cemerski S, et al. : T cell receptor internalization from the immunological synapse is mediated by TC21 and RhoG GTPase-dependent phagocytosis. Immunity. 2011;35(2):208–22. 10.1016/j.immuni.2011.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wetzel SA, McKeithan TW, Parker DC: Peptide-specific intercellular transfer of MHC class II to CD4 + T cells directly from the immunological synapse upon cellular dissociation. J Immunol. 2005;174(1):80–9. 10.4049/jimmunol.174.1.80 [DOI] [PubMed] [Google Scholar]
- 12. Osborne DG, Wetzel SA: Trogocytosis results in sustained intracellular signaling in CD4 + T cells. J Immunol. 2012;189(10):4728–39. 10.4049/jimmunol.1201507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ortega-Carrion A, Vicente-Manzanares M: Concerning immune synapses: a spatiotemporal timeline [version 1; referees: 2 approved]. F1000Res. 2016;5: pii: F1000 Faculty Rev-418. 10.12688/f1000research.7796.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dieckmann NM, Frazer GL, Asano Y, et al. : The cytotoxic T lymphocyte immune synapse at a glance. J Cell Sci. 2016;129(15):2881–6. 10.1242/jcs.186205 [DOI] [PubMed] [Google Scholar]
- 15. Monks CR, Freiberg BA, Kupfer H, et al. : Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395(6697):82–6. 10.1038/25764 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 16. Cartwright AN, Griggs J, Davis DM: The immune synapse clears and excludes molecules above a size threshold. Nat Commun. 2014;5:5479. 10.1038/ncomms6479 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 17. Springer TA: Adhesion receptors of the immune system. Nature. 1990;346(6283):425–34. 10.1038/346425a0 [DOI] [PubMed] [Google Scholar]
- 18. Davis SJ, van der Merwe PA: The kinetic-segregation model: TCR triggering and beyond. Nat Immunol. 2006;7(8):803–9. 10.1038/ni1369 [DOI] [PubMed] [Google Scholar]
- 19. Kupfer A, Swain SL, Singer SJ: The specific direct interaction of helper T cells and antigen-presenting B cells. II. Reorientation of the microtubule organizing center and reorganization of the membrane-associated cytoskeleton inside the bound helper T cells. J Exp Med. 1987;165(6):1565–80. 10.1084/jem.165.6.1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bustos-Morán E, Blas-Rus N, Martín-Cófreces NB, et al. : Orchestrating Lymphocyte Polarity in Cognate Immune Cell-Cell Interactions. Int Rev Cell Mol Biol. 2016;327:195–261. 10.1016/bs.ircmb.2016.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu H, Rhodes M, Wiest DL, et al. : On the dynamics of TCR:CD3 complex cell surface expression and downmodulation. Immunity. 2000;13(5):665–75. 10.1016/S1074-7613(00)00066-2 [DOI] [PubMed] [Google Scholar]
- 22. Kumar A, Kremer KN, Dominguez D, et al. : Gα13 and Rho mediate endosomal trafficking of CXCR4 into Rab11 + vesicles upon stromal cell-derived factor-1 stimulation. J Immunol. 2011;186(2):951–8. 10.4049/jimmunol.1002019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Soares H, Henriques R, Sachse M, et al. : Regulated vesicle fusion generates signaling nanoterritories that control T cell activation at the immunological synapse. J Exp Med. 2013;210(11):2415–33. 10.1084/jem.20130150 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 24. Bouchet J, Del Río-Iñiguez I, Vázquez-Chávez E, et al. : Rab11-FIP3 Regulation of Lck Endosomal Traffic Controls TCR Signal Transduction. J Immunol. 2017;198(7):2967–78. 10.4049/jimmunol.1600671 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 25. Onnis A, Finetti F, Patrussi L, et al. : The small GTPase Rab29 is a common regulator of immune synapse assembly and ciliogenesis. Cell Death Differ. 2015;22(10):1687–99. 10.1038/cdd.2015.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Patino-Lopez G, Dong X, Ben-Aissa K, et al. : Rab35 and its GAP EPI64C in T cells regulate receptor recycling and immunological synapse formation. J Biol Chem. 2008;283(26):18323–30. 10.1074/jbc.M800056200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Finetti F, Patrussi L, Galgano D, et al. : The small GTPase Rab8 interacts with VAMP-3 to regulate the delivery of recycling T-cell receptors to the immune synapse. J Cell Sci. 2015;128(14):2541–52. 10.1242/jcs.171652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Finetti F, Paccani SR, Riparbelli MG, et al. : Intraflagellar transport is required for polarized recycling of the TCR/CD3 complex to the immune synapse. Nat Cell Biol. 2009;11(11):1332–9. 10.1038/ncb1977 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 29. Finetti F, Patrussi L, Masi G, et al. : Specific recycling receptors are targeted to the immune synapse by the intraflagellar transport system. J Cell Sci. 2014;127(Pt 9):1924–37. 10.1242/jcs.139337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vivar OI, Masi G, Carpier JM, et al. : IFT20 controls LAT recruitment to the immune synapse and T-cell activation in vivo. Proc Natl Acad Sci U S A. 2016;113(2):386–91. 10.1073/pnas.1513601113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Galgano D, Onnis A, Pappalardo E, et al. : The T cell IFT20 interactome reveals new players in immune synapse assembly. J Cell Sci. 2017;130(6):1110–21. 10.1242/jcs.200006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ueda H, Zhou J, Xie J, et al. : Distinct Roles of Cytoskeletal Components in Immunological Synapse Formation and Directed Secretion. J Immunol. 2015;195(9):4117–25. 10.4049/jimmunol.1402175 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 33. Das V, Nal B, Dujeancourt A, et al. : Activation-induced polarized recycling targets T cell antigen receptors to the immunological synapse; involvement of SNARE complexes. Immunity. 2004;20(5):577–88. 10.1016/S1074-7613(04)00106-2 [DOI] [PubMed] [Google Scholar]
- 34. Larghi P, Williamson DJ, Carpier J, et al. : VAMP7 controls T cell activation by regulating the recruitment and phosphorylation of vesicular Lat at TCR-activation sites. Nat Immunol. 2013;14(7):723–31. 10.1038/ni.2609 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 35. Dustin ML: The immunological synapse. Cancer Immunol Res. 2014;2(11):1023–33. 10.1158/2326-6066.CIR-14-0161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Alonso R, Mazzeo C, Rodriguez MC, et al. : Diacylglycerol kinase α regulates the formation and polarisation of mature multivesicular bodies involved in the secretion of Fas ligand-containing exosomes in T lymphocytes. Cell Death Differ. 2011;18(7):1161–73. 10.1038/cdd.2010.184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mazzeo C, Calvo V, Alonso R, et al. : Protein kinase D1/2 is involved in the maturation of multivesicular bodies and secretion of exosomes in T and B lymphocytes. Cell Death Differ. 2016;23(1):99–109. 10.1038/cdd.2015.72 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 38. Colombo M, Raposo G, Théry C: Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–89. 10.1146/annurev-cellbio-101512-122326 [DOI] [PubMed] [Google Scholar]
- 39. Edgar JR: Q&A: What are exosomes, exactly? BMC Biol. 2016;14:46. 10.1186/s12915-016-0268-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Martínez-Lorenzo MJ, Anel A, Gamen S, et al. : Activated human T cells release bioactive Fas ligand and APO2 ligand in microvesicles. J Immunol. 1999;163(3):1274–81. [PubMed] [Google Scholar]
- 41. Monleón I, Martínez-Lorenzo MJ, Monteagudo L, et al. : Differential secretion of Fas ligand- or APO2 ligand/TNF-related apoptosis-inducing ligand-carrying microvesicles during activation-induced death of human T cells. J Immunol. 2001;167(12):6736–44. 10.4049/jimmunol.167.12.6736 [DOI] [PubMed] [Google Scholar]
- 42. Chevillet JR, Kang Q, Ruf IK, et al. : Quantitative and stoichiometric analysis of the microRNA content of exosomes. Proc Natl Acad Sci U S A. 2014;111(41):14888–93. 10.1073/pnas.1408301111 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 43. Stevanato L, Thanabalasundaram L, Vysokov N, et al. : Investigation of Content, Stoichiometry and Transfer of miRNA from Human Neural Stem Cell Line Derived Exosomes. PLoS One. 2016;11(1):e0146353. 10.1371/journal.pone.0146353 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 44. Mittelbrunn M, Sánchez-Madrid F: Intercellular communication: diverse structures for exchange of genetic information. Nat Rev Mol Cell Biol. 2012;13(5):328–35. 10.1038/nrm3335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dbouk HA, Mroue RM, El-Sabban ME, et al. : Connexins: a myriad of functions extending beyond assembly of gap junction channels. Cell Commun Signal. 2009;7(4):4. 10.1186/1478-811X-7-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tello-Lafoz M, Martínez-Martínez G, Rodríguez-Rodríguez C, et al. : Sorting nexin 27 interactome in T-lymphocytes identifies zona occludens-2 dynamic redistribution at the immune synapse. Traffic. 2017;18(8):491–504. 10.1111/tra.12492 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 47. Elgueta R, Tobar JA, Shoji KF, et al. : Gap junctions at the dendritic cell-T cell interface are key elements for antigen-dependent T cell activation. J Immunol. 2009;183(1):277–84. 10.4049/jimmunol.0801854 [DOI] [PubMed] [Google Scholar]
- 48. Mendoza-Naranjo A, Bouma G, Pereda C, et al. : Functional gap junctions accumulate at the immunological synapse and contribute to T cell activation. J Immunol. 2011;187(6):3121–32. 10.4049/jimmunol.1100378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ueda H, Morphew MK, McIntosh JR, et al. : CD4 + T-cell synapses involve multiple distinct stages. Proc Natl Acad Sci U S A. 2011;108(41):17099–104. 10.1073/pnas.1113703108 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 50. Chauveau A, Aucher A, Eissmann P, et al. : Membrane nanotubes facilitate long-distance interactions between natural killer cells and target cells. Proc Natl Acad Sci U S A. 2010;107(12):5545–50. 10.1073/pnas.0910074107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dhainaut M, Moser M: Regulation of immune reactivity by intercellular transfer. Front Immunol. 2014;5:112. 10.3389/fimmu.2014.00112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Raposo G, Nijman HW, Stoorvogel W, et al. : B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183(3):1161–72. 10.1084/jem.183.3.1161 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 53. Zitvogel L, Regnault A, Lozier A, et al. : Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med. 1998;4(5):594–600. 10.1038/nm0598-594 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 54. Denzer K, Kleijmeer MJ, Heijnen HF, et al. : Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J Cell Sci. 2000;113Pt 19:3365–74. [DOI] [PubMed] [Google Scholar]
- 55. Lorenz N, Loef EJ, Verdon DJ, et al. : Human T cell activation induces synaptic translocation and alters expression of the serine protease inhibitor neuroserpin and its target protease. J Leukoc Biol. 2015;97(4):699–710. 10.1189/jlb.1A0814-392R [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 56. Munuswamy-Ramanujam G, Dai E, Liu L, et al. : Neuroserpin, a thrombolytic serine protease inhibitor (serpin), blocks transplant vasculopathy with associated modification of T-helper cell subsets. Thromb Haemost. 2010;103(3):545–55. 10.1160/TH09-07-0441 [DOI] [PubMed] [Google Scholar]
- 57. Soares H: HIV-1 Intersection with CD4 T Cell Vesicle Exocytosis: Intercellular Communication Goes Viral. Front Immunol. 2014;5:454. 10.3389/fimmu.2014.00454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gordón-Alonso M, Sala-Valdés M, Rocha-Perugini V, et al. : EWI-2 association with α-actinin regulates T cell immune synapses and HIV viral infection. J Immunol. 2012;189(2):689–700. 10.4049/jimmunol.1103708 [DOI] [PubMed] [Google Scholar]
- 59. Vasiliver-Shamis G, Cho MW, Hioe CE, et al. : Human immunodeficiency virus type 1 envelope gp120-induced partial T-cell receptor signaling creates an F-actin-depleted zone in the virological synapse. J Virol. 2009;83(21):11341–55. 10.1128/JVI.01440-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mack M, Kleinschmidt A, Brühl H, et al. : Transfer of the chemokine receptor CCR5 between cells by membrane-derived microparticles: a mechanism for cellular human immunodeficiency virus 1 infection. Nat Med. 2000;6(7):769–75. 10.1038/77498 [DOI] [PubMed] [Google Scholar]
- 61. Rozmyslowicz T, Majka M, Kijowski J, et al. : Platelet- and megakaryocyte-derived microparticles transfer CXCR4 receptor to CXCR4-null cells and make them susceptible to infection by X4-HIV. AIDS. 2003;17(1):33–42. [DOI] [PubMed] [Google Scholar]
- 62. Muratori C, Cavallin LE, Krätzel K, et al. : Massive secretion by T cells is caused by HIV Nef in infected cells and by Nef transfer to bystander cells. Cell Host Microbe. 2009;6(3):218–30. 10.1016/j.chom.2009.06.009 [DOI] [PubMed] [Google Scholar]
- 63. Lenassi M, Cagney G, Liao M, et al. : HIV Nef is secreted in exosomes and triggers apoptosis in bystander CD4 + T cells. Traffic. 2010;11(1):110–22. 10.1111/j.1600-0854.2009.01006.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Fackler OT, Alcover A, Schwartz O: Modulation of the immunological synapse: a key to HIV-1 pathogenesis? Nat Rev Immunol. 2007;7(4):310–7. 10.1038/nri2041 [DOI] [PubMed] [Google Scholar]
- 65. Silva JG, Martins NP, Henriques R, et al. : HIV-1 Nef Impairs the Formation of Calcium Membrane Territories Controlling the Signaling Nanoarchitecture at the Immunological Synapse. J Immunol. 2016;197(10):4042–52. 10.4049/jimmunol.1601132 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 66. Thoulouze MI, Sol-Foulon N, Blanchet F, et al. : Human immunodeficiency virus type-1 infection impairs the formation of the immunological synapse. Immunity. 2006;24(5):547–61. 10.1016/j.immuni.2006.02.016 [DOI] [PubMed] [Google Scholar]
- 67. Haller C, Rauch S, Michel N, et al. : The HIV-1 pathogenicity factor Nef interferes with maturation of stimulatory T-lymphocyte contacts by modulation of N-Wasp activity. J Biol Chem. 2006;281(8):19618–30. 10.1074/jbc.M513802200 [DOI] [PubMed] [Google Scholar]
- 68. Abraham L, Bankhead P, Pan X, et al. : HIV-1 Nef limits communication between linker of activated T cells and SLP-76 to reduce formation of SLP-76-signaling microclusters following TCR stimulation. J Immunol. 2012;189(4):1898–910. 10.4049/jimmunol.1200652 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 69. Pan X, Rudolph JM, Abraham L, et al. : HIV-1 Nef compensates for disorganization of the immunological synapse by inducing trans-Golgi network-associated Lck signaling. Blood. 2012;119(3):786–97. 10.1182/blood-2011-08-373209 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 70. Arenaccio C, Chiozzini C, Columba-Cabezas S, et al. : Exosomes from human immunodeficiency virus type 1 (HIV-1)-infected cells license quiescent CD4 + T lymphocytes to replicate HIV-1 through a Nef- and ADAM17-dependent mechanism. J Virol. 2014;88(19):11529–39. 10.1128/JVI.01712-14 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 71. Khatua AK, Taylor HE, Hildreth JE, et al. : Exosomes packaging APOBEC3G confer human immunodeficiency virus resistance to recipient cells. J Virol. 2009;83(2):512–21. 10.1128/JVI.01658-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Seidl T, Whittall T, Babaahmady K, et al. : B-cell agonists up-regulate AID and APOBEC3G deaminases, which induce IgA and IgG class antibodies and anti-viral function. Immunology. 2012;135(3):207–15. 10.1111/j.1365-2567.2011.03524.x [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 73. Jang JH, Huang Y, Zheng P, et al. : Imaging of Cell-Cell Communication in a Vertical Orientation Reveals High-Resolution Structure of Immunological Synapse and Novel PD-1 Dynamics. J Immunol. 2015;195(3):1320–30. 10.4049/jimmunol.1403143 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 74. Vu TQ, de Castro RM, Qin L: Bridging the gap: microfluidic devices for short and long distance cell-cell communication. Lab Chip. 2017;17(6):1009–23. 10.1039/c6lc01367h [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Finetti F, Baldari CT: Compartmentalization of signaling by vesicular trafficking: a shared building design for the immune synapse and the primary cilium. Immunol Rev. 2013;251(1):97–112. 10.1111/imr.12018 [DOI] [PubMed] [Google Scholar]
- 76. Bustos-Morán E, Blas-Rus N, Martin-Cófreces NB, et al. : Microtubule-associated protein-4 controls nanovesicle dynamics and T cell activation. J Cell Sci. 2017;130(7):1217–23. 10.1242/jcs.199042 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 77. Wood CR, Huang K, Diener DR, et al. : The cilium secretes bioactive ectosomes. Curr Biol. 2013;23(10):906–11. 10.1016/j.cub.2013.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 78. Wood CR, Rosenbaum JL: Ciliary ectosomes: transmissions from the cell's antenna. Trends Cell Biol. 2015;25(5):276–85. 10.1016/j.tcb.2014.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wang J, Barr MM: Ciliary Extracellular Vesicles: Txt Msg Organelles. Cell Mol Neurobiol. 2016;36(3):449–57. 10.1007/s10571-016-0345-4 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 80. Bobrie A, Colombo M, Raposo G, et al. : Exosome secretion: molecular mechanisms and roles in immune responses. Traffic. 2011;12(12):1659–68. 10.1111/j.1600-0854.2011.01225.x [DOI] [PubMed] [Google Scholar]
- 81. Chaput N, Théry C: Exosomes: immune properties and potential clinical implementations. Semin Immunopathol. 2011;33(5):419–40. 10.1007/s00281-010-0233-9 [DOI] [PubMed] [Google Scholar]