Abstract
Interleukin (IL)-2, IL-4, IL-7, IL-9, IL-15, and IL-21 form a family of cytokines based on the sharing of a receptor component, the common cytokine receptor γ chain, γ c, which is encoded by the gene mutated in humans with X-linked severe combined immunodeficiency (XSCID). Together, these cytokines play critical roles in lymphoid development, differentiation, growth, and survival as well as mediating effector function. Here, we provide an overview of the main actions of members of this cytokine family but then primarily focus on IL-2 and IL-21, discussing their dynamic interplay and contributions to a fine-tuned immune response. Moreover, we discuss the therapeutic utility of modulating their actions, particularly for autoimmunity and cancer.
Keywords: gamma chain cytokines, interleukins, IL-2, IL-21, XSCID
Introduction: the γ c system and its association with severe combined immunodeficiency
Interleukin-2 (IL-2), IL-4, IL-7, IL-9, IL-15, and IL-21 form a family of four α-helical bundle type I cytokines that share the common cytokine receptor γ chain, γ c, as a key receptor component ( Figure 1) 1. γ c is mutated in humans with X-linked severe combined immunodeficiency (XSCID), a disease in which T and natural killer (NK) cells are greatly diminished and B cells are non-functional 2. Finding the basis for XSCID immediately allowed more precise prenatal and postnatal diagnosis and carrier female identification as well as paving the way to gene therapy for this disease 3, 4. In addition, the implications of this finding extended far beyond the management of a single disease and had major basic scientific implications as well. Although γ c was initially discovered as the IL-2 receptor γ chain (IL-2Rγ) and identifying the genetic basis for XSCID resulted from studies of the IL-2R, the fact that the phenotype in XSCID is more severe than in IL-2 deficiency led to the prediction and then discovery that IL-2Rγ was in fact a shared receptor component 5– 8, and the term γ c was proposed 5. Interestingly, the major phenotypic abnormalities do not result from defective IL-2 signaling; instead, defective signaling by IL-7 and IL-15 explain the profound decrease in T and NK cells, respectively, and defective IL-21 signaling substantially explains the non-functional B cells in this disease 9. Thus, XSCID was established to be a disease of defective cytokine signaling 10.
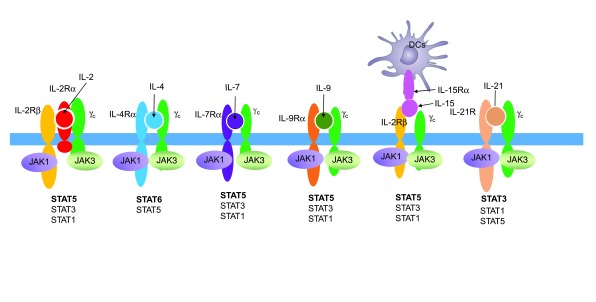
Figure 1. γ c family cytokines and their receptors.
Shown is the receptor for each γ c family cytokine as well as the interacting Janus kinase 1 (JAK1) and JAK3 kinases and the signal transducer and activator of transcription (STAT) proteins activated by each cytokine. DC, dendritic cell; IL, interleukin.
Each γ c family cytokine activates the Janus family tyrosine kinases (JAK)1 and JAK3, which then trigger signaling cascades. JAK1 associates with the more distinctive type I cytokine receptor for each cytokine and JAK3 associates with γ c 11. Because JAK3 is “downstream” of γ c, it was hypothesized 12 and then established that JAK3-deficient SCID indeed occurs, causing a T –B +NK – form of SCID that phenocopies XSCID 13, 14. Moreover, individuals with IL7R-deficient SCID were identified based on the prediction that such patients would have defective T cell development but that NK cells would develop normally given intact IL-15 signaling 15. Although IL-7 signaling is believed to be responsible for the lack of T cell development, to date human IL7-deficient SCID has still not been identified. Presumably, the frequency of inactivating mutations of IL7 is very low, the condition is unexpectedly lethal, or the phenotype is less severe than anticipated so that individuals do not come to medical attention. A less severe phenotype for IL7 than IL7R deficiency is conceivable given that signaling by thymic stromal lymphopoietin (TSLP) would also be affected in the latter but not the former, and indeed there is even more defective T cell development in mice lacking the expression of both γ c and the TSLP receptor ( Il2rg/Crlf2 double knockout mice) than in Il2rg single knockout mice 16. However, XSCID and JAK3-deficient patients have intact TSLP signaling but nevertheless have essentially absent T cell development, minimizing this as an explanation.
STAT activation and major biological effects of γ c family cytokines
γ c family cytokines collectively have broad actions ( Table 1) and activate three major signaling pathways, including the MAP kinase, PI 3-kinase, and JAK–STAT (signal transducer and activator of transcription) pathways. Of the seven STAT proteins, IL-2 mainly activates STAT5A and STAT5B, but it also signals through STAT1 and STAT3 to some degree 17 (see Figure 1 for STATs activated by each γ c family cytokine). It is a T cell growth factor that additionally augments the cytolytic activity of CD8 + T cells and NK cells and is essential for regulatory T (Treg) cell development 18. It also promotes the differentiation of T helper type 1 (Th1) 19, Th2 20, 21, and Th9 cells 22 while inhibiting Th17 cell differentiation 19, 23. Importantly, from a clinical perspective, IL-2 exhibits anti-cancer activity and is approved by the FDA for the treatment of melanoma and renal cell carcinoma 24.
Table 1. Actions of γ c family cytokines.
| IL-2 | • Promotes Th1, Th2, and Th9 differentiation and antagonizes Th17 and Tfh differentiation
• Induces T cell and NK cell proliferation • Enhances Treg cell differentiation and function • Anti-cancer role for immunotherapy |
| IL-4 | • Promotes B cell differentiation and Ig isotype switching
• Promotes Th2 and Th9 differentiation • Proliferative effects on tissue-resident macrophages • Protection from helminth infection |
| IL-7 | • Required for T cell development and homeostasis
• Promotes memory CD8 + T cell development • Essential for B cell development in mice but dispensable for B cell development in humans |
| IL-9 | • Promotes mast cell proliferation
• Augments mucus production by goblet cells • Anti-tumor activity |
| IL-15 | • Essential for NK development, expansion, and survival
• Promotes memory CD8 + T cell development • Anti-cancer role for immunotherapy via actions on CD8 + T cells and NK cells |
| IL-21 | • Promotes B cell differentiation to plasma cells and augmenting Ig production
• Has anti-cancer activity mediated in part via actions on CD8 + T cells and NK cells • Promotes Tfh differentiation and germinal center formation • Promotes Th17 differentiation • Inhibits Th9 differentiation • Promotes autoimmune disease (type I diabetes, SLE, EAE, and colitis) |
EAE, experimental autoimmune encephalomyelitis; Ig, immunoglobulin; IL, interleukin; NK, natural killer; SLE, systemic lupus erythematosus; Tfh, T follicular helper; Th, T helper; Treg, T regulatory.
IL-4 mainly activates STAT6 and plays major roles in allergic responses, including asthma and in protection against helminth infections 25– 28. IL-4 signals via two types of receptor. Type I IL-4 receptors (IL-4Rs) comprise IL-4R plus γ c 6 and are expressed mainly on lymphoid cells. In contrast, type II IL-4Rs comprise IL-4R plus IL-13Rα1 (but not γ c) and are mainly expressed on non-lymphoid cells; these IL-4Rs also represent the functional IL-13 receptor 28, 29.
IL-7 is a stromal factor which, like IL-2, predominantly activates STAT5A and STAT5B 17, 30. It drives T cell development as well as normal CD8 + T cell homeostasis, particularly of memory CD8 + T cells. IL-7 is a potent survival factor for T cells, strongly inducing the expression of BCL2 31– 34. Unlike other γ c family cytokines, IL-7 is produced within the stroma and is more constitutively expressed.
IL-9 also activates STAT5A and STAT5B 17, 30. This cytokine can promote the expansion of mast cells. Interestingly, a single nucleotide polymorphism (SNP) in the IL9R gene associates with a haplotype that is protective against wheezing in boys but not in girls 35; this sex-related difference makes sense given that IL9R is located on the X chromosome. IL-9 also promotes anti-tumor immunity 36, 37.
Like IL-2, IL-15 primarily activates STAT5A and STAT5B 17 and shares IL-2Rβ as well as γ c as receptor components. Like IL-2, IL-15 also has a specific α chain, IL-15Rα, so that these two cytokines each have three receptor components. However, whereas IL-2 signals mainly in cis by interacting with high-affinity IL-2 receptors that contain IL-2Rα, IL-2Rβ, and γ c or intermediate-affinity receptors comprising IL-2Rβ and γ c, IL-15 primarily signals via trans-presentation of IL-15Rα-bound IL-15 to cells expressing IL-2Rβ and γ c 38. IL-15 is critical for the development and expansion of NK cells as well as for memory CD8 + T cell homeostasis 39, 40.
IL-21 is the most recently identified γ c family cytokine. IL-21 has pleiotropic actions, driving the terminal differentiation of B cells to plasma cells 41, 42 and cooperating with IL-7 and IL-15 to expand CD8 + T cells 43. Moreover, IL-21 serves a key role in promoting T follicular helper (Tfh) cell differentiation 44 and can augment Th17 differentiation in vitro 45– 47. Furthermore, IL-21 has anti-cancer activity in animal models and is being evaluated in human clinical trials. In addition, a broad range of animal models indicate that IL-21 plays a key role in the development of autoimmune disease, including for type 1 diabetes, systemic lupus erythematosus, and experimental autoimmune uveitis 48. Collectively, γ c family cytokines therefore play broad and important biological roles, many of which are associated with diseases and may represent targets for therapeutic application. Below, we will focus on IL-2 and IL-21, which exhibit both overlapping and opposing actions.
IL-2 and IL-21
IL-2 and IL-21 are encoded by adjacent genes on human chromosome 4q27 and mouse chromosome 3, and their structural homology suggests that these two genes may have arisen from a gene duplication event during evolution. Despite their proximity, the IL2 and IL21 genes are differentially regulated, and analysis of the chromatin region between these genes has revealed the presence of insulator regions that ensure their independent regulation 49, 50. IL-2 and IL-21 exert distinctive actions on immune cell populations, sometimes with opposite outcomes, which results, at least in part, from their differential activation of STAT proteins. Although both cytokines can activate STAT1, STAT3, STAT5A, and STAT5B, IL-2 predominantly activates STAT5A and STAT5B, whereas IL-21 mostly signals via STAT3. Consistent with these signaling differences, analysis of the effects of IL-2 and IL-21 on the anti-tumor activity of CD8 + T cells revealed that these cytokines induced distinctive transcriptional profiles, with associated differences in disease outcome 51. Whereas IL-2 enhanced CD8 + T cell proliferation and effector function, IL-21-treated cells exhibited a central memory phenotype, with greater persistence of the cells and higher anti-tumor activity in vivo. IL-2 and IL-21 also have markedly different effects on the in vivo function of Tfh cells and the in vitro differentiation of Th9 cells, as we discuss below. As continued investigation yields more insights into the mechanistic underpinnings of these two cytokines, investigators may learn how to specifically enhance the “good” features and inhibit the “bad” features of each. Here, we review recent advances in our understanding of IL-2 and IL-21, how they regulate multiple lymphoid populations, and potential strategies for utilizing the strengths of each cytokine in the treatment of disease.
IL-2 signaling
As noted above, each γ c family cytokine can activate the JAK–STAT pathway, but these cytokines collectively also activate phosphoinositide (PI) 3-kinase and extracellular signal-regulated kinase (ERK)-dependent pathways as well 18. The relative kinetics and potency of activation of JAK–STAT, PI 3-kinase, and ERK pathways and the kinetics of their activation are critical for determining the specificity of signaling. Each of these three major signaling pathways involves kinases, and IL-2, like other cytokines and growth factors, influences the exchange of phosphate groups. Understanding the mechanisms that these intracellular systems use to initiate specific differentiation programs, often by increasing the expression of key transcriptional regulators, represents a major step towards creating future interventions to alter cell fate 18.
Using mass spectrometry, a phosphoproteomic signature was recently identified in pre-activated CD8 + T cells that were cultured with IL-12 to maintain viability and then stimulated with IL-2 52. IL-2 was shown to induce the phosphorylation and dephosphorylation of a large number of proteins that carry out vital functions, including transcription, RNA stabilization, nuclear translocation, protein translation, cell trafficking, metabolism, and cell cycle, thus potentially identifying new targets for manipulating IL-2 signaling. Surprisingly, inhibition of JAK3 and JAK1 signaling with tofacitinib affected only 4% of the phosphoproteome, suggesting that many of these phosphorylation events were independent of JAK signaling 52. Interestingly, Treg cells have been shown to express high levels of phosphatase and tensin homolog (PTEN) 53, which inhibits IL-2-induced PI 3-kinase signaling but does not affect STAT5 activation. PTEN thus may be differentially important in the control of IL-2-mediated Treg cell versus effector T cell function. Consistent with this possibility, the absence of PTEN can reduce IL-2Rα and FoxP3 expression by Treg cells, leading to autoimmune disease 53.
Investigators have also studied IL-2 diffusion through cell “niches” as measured by STAT5 phosphorylation in target cells 54. By using IL-2/anti-IL-2 complexes to expand Treg cells in vivo and assessing STAT5 signaling in conventional T cells, it was shown that the dimensions of cytokine gradients changed rapidly, depending on the number of cells (e.g., Treg cells) that were consuming cytokine. The IL-2 gradients identified suggest that there may be functional heterogeneity in response to antigen and cytokine signals, depending on the position of responding cells in three-dimensional space within a given organ 54, with possible therapeutic implications for manipulating IL-2 concentrations in vivo.
Manipulating IL-2 signals in immunotherapy
The anti-cancer activity of IL-2 has long been known, and high-dose IL-2 can be toxic and is associated with capillary leak syndrome; thus, efforts to lower the toxicity have focused in part on lowering IL-2 24. IL-2 can also promote activation-induced cell death (AICD), an unwanted effect that can be diminished by lowering doses of IL-2. Previous studies showed that in addition to it inducing the proliferation of CD8 + T cells, IL-2 preferentially stimulates short-lived effector T cells that are detrimental to cancer immunotherapy 51. IL-15 is another important cytokine for immunotherapy that, unlike IL-2, does not bind to IL-2Rα and thus does not preferentially stimulate Treg cells. Unlike IL-2, IL-15 is not associated with capillary leak syndrome nor does it mediate AICD 55, 56. Despite these potential advantages for IL-15, IL-2–anti-IL-2 complexes were superior to IL-15–soluble IL-15Rα complexes at supporting the anti-tumor activity of transferred CD8 + T cells 57. One basis for this could be that IL-15 is quickly internalized and mediates only brief STAT5 signaling in a lymphoreplete host, whereas IL-2 remains in a surface “reservoir”, trapped by excess IL-2Rα and recycled after internalization, which results in sustained STAT5 signaling 57. Indeed, a membrane-tethered form of IL-15 on tumor-specific T cells demonstrated improved T cell survival and enhanced anti-tumor effects in vivo due to the preferential growth of T memory stem cells 58. Thus, both IL-2 and IL-15 show considerable potential that is worthy of additional investigation.
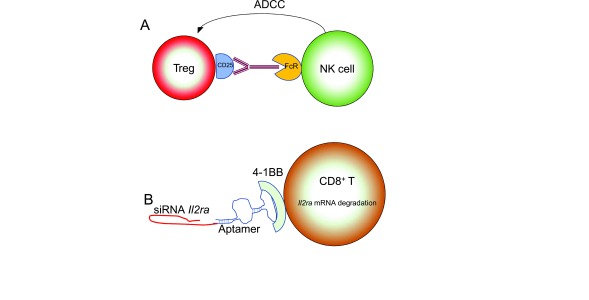
Another strategy for improving immunotherapeutic outcomes in malignancy involves disabling or eliminating tumor-associated Treg cells. Effector T cells within tumors have much lower expression of IL-2Rα (CD25) than do Treg cells, suggesting that targeting CD25 might be a therapeutically useful approach for preferentially depleting Treg cells 59. Although earlier attempts to deplete intratumoral Treg cells with antibodies to CD25 were not successful 60, 61, a new CD25-directed antibody with enhanced binding to an activating Fc region allowed Treg cell-specific antibody-dependent cell-mediated cytotoxicity (ADCC) ( Figure 2A), and when combined with a programmed death-1 (PD-1) blockade in mice, this treatment skewed the tumor-infiltrating lymphocyte (TIL) landscape towards activated, conventional T cells and improved rejection of established tumors 59.
Figure 2. Schematic for mechanisms of manipulating interleukin (IL)-2 signals.
( A) CD25-specific antibody with an activating Fc region binds to CD25 on regulatory T (Treg) cells and activates natural killer (NK)-mediated antibody-dependent cell-mediated cytotoxicity (ADCC) via stimulation of the FcR. ( B) Silencing RNAs to Il2ra are conjugated to an aptamer that allows specific binding to 4-1BB on CD8 + T cells, allowing delivery specifically to activated cells. siRNA, small interfering RNA.
Because IL-2 drives CD8 + T cells toward an “effector” phenotype, which confers poor anti-tumor performance in some models, reducing IL-2 signaling favors the production of memory CD8 + T cells 62, 63. A new strategy for reducing IL-2 signaling is to conjugate Il2ra siRNAs to a 4-1BB-binding oligonucleotide aptamer. Since 4-1BB is expressed on activated CD8 + T cells, this approach was designed to decrease IL-2 signaling specifically on these cells ( Figure 2B). Indeed, this aptamer increased “central memory” phenotype cells in vitro and enhanced tumor rejection in mice, demonstrating its potential efficacy 64.
Above, we discussed ways of modulating IL-2 signaling by efforts to diminish the expression of CD25 in conventional T cells, deplete CD25 high Treg cells, or to increase the availability of IL-2, either locally or systemically. The effects of complexes of IL-2 and IL-2-specific antibodies have also been studied. One monoclonal antibody binds with high affinity to human IL-2 at the CD25-binding epitope, thus preventing interaction with high-affinity receptors. Instead, the stabilized IL-2–anti-IL-2 complex preferentially acts on cells with a high level of IL-2Rβ-γ c intermediate-affinity receptors, such as NK and CD8 + T cells, inducing their proliferation and STAT phosphorylation, decreasing TIL markers of “exhaustion”, and improving anti-tumor responses 65. Other IL-2–anti-IL-2 complexes can selectively stimulate either Treg or effector T cells, and protein interaction modeling coupled with biological experiments allows the engineering of therapeutic antibodies directed at specific immune subpopulations 66.
Besides the anti-cancer actions of IL-2, augmenting in vivo T cell exposure to IL-2 might be beneficial in vaccine strategies or in efforts to treat autoimmune disease. In one approach, IL-2 plasmid was co-administered with human papillomavirus (HPV) vaccination of mice, which increased the proliferation and effector differentiation of HPV E7-specific CD8 + T cells and their production of interferon (IFN)γ. Importantly, this augmented the ratio of effector cells to Treg cells, with an enhanced anti-tumor response 67.
Another approach to enhance the activity of IL-2 was to develop an IL-2 superkine with augmented affinity for IL-2Rβ 68. Normally, IL-2 first binds IL-2Rα, resulting in a conformational change in IL-2 that allows it to then efficiently bind IL-2Rβ; the IL-2–IL-2Rβ complex then efficiently recruits γ c. The IL-2 superkine "locks in" the altered conformation that normally results after binding IL-2Rα so that it efficiently binds to IL-2Rβ even in the absence of IL-2Rα 68. This superkine had increased activity, even at a low concentration, with decreased capillary leak syndrome 68. Derivatives of the IL-2 superkine have also been generated in order to fine-tune IL-2 signaling (discussed below) 69.
Using IL-2 to preferentially expand Treg cells constitutes an area of intense focus related to autoimmune disease. For example, providing low doses of IL-2 to patients with systemic lupus erythematosus (SLE) can increase the percentage of circulating Treg cells and may prove clinically beneficial 70. An initial study in which patients with SLE were given low-dose IL-2 reported an increase in the number of Treg cells, which was associated with an apparent decrease in a clinical SLE disease index 71, suggesting that such an approach may warrant evaluation in future clinical trials. A similar IL-2 regimen in mice resulted in augmented PD-1 expression in an “activated-memory” Treg cell subset, and PD-1 blockade resulted in apoptosis of these cells. Low-dose IL-2 treatment of humans with graft-versus-host disease was originally found to lead to expansion of Treg cells and reduced symptoms in a group of patients 72. Moreover, humans with graft-versus-host disease receiving low-dose IL-2 treatment could be retrospectively divided into likely responders and non-responders based on PD-1 expression on their peripheral Treg cells 73.
Effects of IL-21 on germinal center T cells
IL-21 is a key regulator of T follicular helper (Tfh) cell development in germinal centers and represents a major cytokine secreted by Tfh cells that critically regulates the differentiation of memory B cells and plasma cells 44. Tfh cells appear to be heterogeneous with regard to their cytokine profiles and their anatomical location 74, 75. For example, when IL-4–IL-21 double reporter mice were infected with Nippostrongylus brasiliensis, few Tfh cells produced both IL-4 and IL-21, and those producing either IL-4 or IL-21 were localized to different regions of the germinal center. Tfh cells expressing IL-4, IL-21, or both cytokines also had distinct transcriptional profiles 76. Interestingly, in the germinal center, IL-21-producing Tfh cells, which are localized at a region involved in immunoglobulin (Ig) hypermutation, can differentiate into IL-4-producing Tfh cells, which are localized in an area more involved in the differentiation of plasma cells. When these individual Tfh populations were transferred into mice and immunized, Tfh cells producing IL-21 or IL-21 plus IL-4 induced higher expression of B-cell lymphoma 6 protein (BCL6) in the germinal center, with the cells producing both cytokines inducing a greater increase in germinal center size and more plasma cells 76, consistent with the synergistic effects of IL-4 and IL-21, as was first shown for immunoglobulin (Ig) production 9.
Tfh cells do not appear to be restricted to classical secondary lymphoid organs, as a population of these cells has also been identified in Peyer’s patches of the intestine 77. These Tfh cells produce high levels of IL-21, which is essential for the production of IgG1 by germinal center B cells in the intestine. Treatment of mice with antibiotics led to a dramatic decrease in the number of Tfh cells within the Peyer’s patches, indicating that an intact gut microbiome is required for the maintenance of these cells. Not only are Tfh cells capable of differentiating or migrating outside of the spleen and lymph nodes but they can also can exhibit functional plasticity. For example, when animals were exposed to house dust mite allergen and IL-21-expressing Tfh cells from these mice were then transferred into other primed mice, they migrated to the lung where they lost expression of IL-21 and differentiated into effector Th2 cells that expressed both IL-4 and IL-13 78.
Interestingly, high production of IL-21 has been detected in populations of Tfh and Tfh-like cells that are external to germinal centers, and these cells can also regulate B cell activation and Ig production. For example, high levels of peripheral Tfh cells that secrete high levels of IL-21 have been found in a subset of HIV-infected patients, and the presence of this population correlated with an effective response to influenza vaccine 79. In addition, a population known as T peripheral helper (Tph) cells, comprising 30% of synovial fluid CD4 + T cells in rheumatoid arthritis patients, expresses chemokine receptors (CCR2, CX3CR1, CCR5) that allow the cells to migrate to sites of inflammation 80. In contrast to Tfh cells localized in germinal centers, Tph cells are PD-1 hi but are not exhausted, and they express high levels of B lymphocyte-induced maturation protein 1 (BLIMP1) but low levels of BCL6. Moreover, Tph and Tfh cells have not been interconverted in vitro, suggesting that Tph cells develop in vivo to induce B cell responses in pathological situations, such as within inflamed synovium 80.
IL-21 can also influence another small population of cells in the germinal center, known as T follicular regulatory (Tfr) cells, which negatively regulate Tfh-directed germinal center responses 81– 83. These Tfr cells share some phenotypic markers (CXCR5 +BCL6 +ICOS +PD1 +) with Tfh cells, but they also express the transcription factor FoxP3. Tfr cells can interact directly with Tfh cells to suppress their production and secretion of IL-21 and IL-4, thereby decreasing B cell Ig production 84. Tfr cells can also interact directly with B cells in the germinal center, inhibiting several metabolic pathways and diminishing their effector function 84. IL-21 can overcome the effects of Tfr, both by inhibiting their proliferation and by upregulating glycolysis in B cells, making them resistant to suppression by Tfr. Although IL-4 plays an important role in germinal center B cell responses, unlike IL-21, it cannot overcome Tfr-mediated suppression 84.
IL-21 versus IL-2 effects on regulating CD4 + T cell responses within the germinal center
As noted above, IL-2 and IL-21 are differentially regulated and have distinct effects on immune responses. Indeed, these cytokines may even have opposing effects on the formation of the cell populations within the germinal center that contribute to the generation of a humoral response to pathogens. Within the germinal center, Tfh and B cells interact with each other via IL-21 and ICOS:ICOS ligand interactions, leading to high expression of BCL6 by Tfh cells and the specification of the Tfh cell transcriptional profile ( Figure 3). Although IL-2 can expand effector T cell populations 18, interestingly, it can suppress the generation of Tfh cells within the germinal center in an influenza infection model 85. This inhibitory effect of IL-2 on Tfh cell generation was not due to the accumulation of Treg cells but rather was attributed to the ability of IL-2 to induce BLIMP1, which suppresses BCL6. Thus, whereas IL-21 induces BCL6 and promotes the accumulation of Tfh cells, IL-2 suppresses BCL6 and inhibits the germinal center response.
Figure 3. Roles of interleukin (IL)-2 and IL-21 in the regulation of germinal center development and function.
IL-21 drives T follicular helper (Tfh) differentiation and function through the upregulation of B-cell lymphoma 6 protein (BCL6); moreover, it also induces the differentiation of B cells to immunoglobulin (Ig)-producing plasma cells through the upregulation of the B lymphocyte-induced maturation protein 1 (BLIMP1). IL-2 negatively regulates this process by inhibiting Tfh cell generation through the repression of BCL6 and also by inducing the function of T follicular regulatory (Tfr) cells that can directly interact with and inhibit B cell differentiation. AID, activation-induced deaminase; CTLA4, cytotoxic T lymphocyte-associated molecule-4; CXCR5, C-X-C chemokine receptor type 5; ICOS, inducible costimulator ligand; IFN, interferon; IL, interleukin; PD1, programmed death protein 1; PDL1, programmed death ligand 1.
Interestingly, mounting evidence suggests that CD4 + T cells destined to become Tfh cells are guided to a niche where IL-2 cannot alter their course of differentiation. These cells exhibit a G-protein-coupled receptor, EBI2, that promotes migration to the “outer T zone”, and they co-localize with CD4 + dendritic cells that consume free IL-2 with soluble and transmembrane CD25 86. This is consistent with the evolving model of antagonistic actions for IL-2 and IL-21 at the germinal center and that opposing effects of IL-2 and IL-21 are physiologically relevant.
Other studies have used an acute lymphocytic choriomeningitis virus (LCMV) infection model to dissect the dynamics of signaling and metabolism in Tfh and Th1 cells. As noted above, IL-2 inhibits Tfh differentiation but promotes Th1 differentiation. Activation of AKT, PI 3-kinase, and the mechanistic target of rapamycin (mTOR) by IL-2 was necessary for the generation of optimal levels of phospho-S6 and BLIMP-1, which by repressing the transcription of BCL6 can decrease Tfh cell differentiation. Additionally, the expression of T-Bet, a master regulator of Th1 differentiation, depends on AKT and Raptor (mTORc1) 87. Interestingly, Tfh cells in the LCMV infection model relied more on mitochondrial oxidation pathways and were less proliferative and less glycolytic than Th1 cells, although the significance of these observations remains unclear.
Although IL-2 signaling inhibits the germinal center response, as noted above, IL-2 induces the survival and function of Treg cells, which suppress both humoral and cellular immune responses 88. It was therefore surprising that when FoxP3 + Treg cells were depleted from mice prior to influenza infection, the Tfh cell response to virus infection was greatly reduced 89. Without Treg cells to bind and consume IL-2, more IL-2 was available to suppress Tfh cell generation and functional germinal center responses. Thus, under steady-state conditions, Treg cells with their high CD25 expression can compete for excess IL-2 in the follicle and promote the Tfh response. However, when IL-2 levels are sufficiently high, Tfh cell generation is suppressed.
Above, we showed an interplay between IL-21 and IL-2 related to Treg cells and Tfh cells, and indeed previous studies in mice had demonstrated that IL-21 inhibited Treg expansion after viral infection 90. While IL-2 enhances the survival of FoxP3 + Treg cells, IL-21 diminishes IL-2 production by conventional T cells and thereby lowers Treg cell numbers 91. As a result, one would predict that humans depleted of IL-21 would have increased Treg cells. In fact, IL21R-deficient patients have high numbers of both Treg cells and Tfr-like cells in their peripheral blood, suggesting that these populations are normally negatively regulated by IL-21 92. Mechanistically, IL-21 induces BCL6 and lowers IL2RA gene expression, leading to a decrease in the ability of inhibitory Treg and Tfr cells to proliferate in response to IL-2, with a correspondingly more robust germinal center antibody response 92.
Opposing actions of IL-2 and IL-21 in Th9 differentiation
IL-2 and IL-21 can drive alternative and sometimes opposing differentiation programs in a range of cell types, including the stimulatory and inhibitory germinal center populations noted above. Another example of opposing actions for these cytokines is in the differentiation of IL-9-producing Th9 cells. These cells, which were initially characterized as a population generated by stimulation with transforming growth factor (TGF)β and IL-4, have been shown to play a role in multiple inflammatory disease processes as well as in anti-tumor responses 93. IL-2 is known to promote the development of IL-9-producing cells 94, and details of the mechanisms involved have been elucidated 22, 95. IL-2-mediated activation of STAT5 is required for IL-9 production, with STAT5 binding at the Il9 promoter. In contrast, IL-21 negatively regulates the initial production of IL-9, at least in part owing to the induction of transcription factor BCL6, which binds to the Il9 promoter in close proximity to STAT5, suggesting that STAT5 and BCL6 compete for access to these promoter sites 22. Consistent with direct effects of IL-2-induced STAT5 signals on IL-9 transcription, mice deficient for Itk, a Tec family kinase activated via the T cell receptor, had defective production of interferon regulatory factor 4 (IRF4) and IL-9 production, and the expression of these factors could be rescued by IL-2 or constitutively activated STAT5 96.
Unlike STAT5, STAT3 activation has been shown to negatively regulate both the initiation and the maintenance of IL-9 expression. Th9 cells subjected to multiple rounds of in vitro differentiation produced increasing amounts of IL-21 and IL-10, which then led to the extinction of IL-9 production 97. It remains to be determined whether this effect is relevant in vivo, but in this regard, IL-1β could induce the production of high levels of IL-21 by Th9 cells, but these cells nevertheless continued to produce IL-9 and acquired potent IL-21-mediated anti-tumor activity 98.
IL-2 was also reported to contribute to the pathogenic role of IL-9 in lung disease and inflammation in cystic fibrosis through a self-amplifying circuit involving IL-2 99. In this circuit, lung epithelial damage resulted in the release of IL-33, which induced the expansion of innate lymphoid cells (ILCs) and their production of IL-9, which triggered mast cells to secrete IL-2. The mast cell-produced IL-2 then further expanded both CD25 + ILC2s as well as Th9 cells in the lung, promoting the ongoing inflammatory process.
Fine-tuning of cytokine signals
Above we have discussed a range of physiological effects and potential therapeutic approaches using IL-2 and IL-21 and provided examples where they can oppose each other’s actions. These are illustrative, and many of the lessons learned in these studies can be extended to other systems. It is important to underscore that differential actions for single cytokines have been noted and fine actions of the cytokines can be attributed to the utilization of different STAT proteins—for example, where one cytokine exhibits the induction of different genes depending on which STAT is activated. For instance, IL-21 mainly acts via the activation of STAT3 43, yet it induces some genes, such as Tbx21 encoding T-Bet and Ifng, via STAT1, with STAT3 opposing their induction 100. Differential STAT utilization thus represents a mechanism for fine-tuning the signaling induced by a single cytokine. In addition, IL-2 and IL-21 differentially regulate Th9, Tfh, and Th17 differentiation, providing an example of fine-tuning based on which cytokine is dominant in a given context. Another mechanism of fine-tuning can occur at the level of STAT tetramerization. For example, IL-2 activates STAT5A and STAT5B to form dimers (homodimers and potentially heterodimers) as well as tetramers (potentially both homotetramers and a range of heterotetramers) 101. Tetramerization occurs via dimerization of STAT dimers, mediated by an N-terminal region known as the N-domain. Some genes require STAT5 tetramers for their normal expression, whereas STAT5 dimers are sufficient for viability, red cell production, and normal thymic development. Interestingly, STAT5 tetramer-deficient mice have diminished numbers of CD8 and NK cells, defective proliferation, including in response to infection with LCMV, and diminished (albeit not absent) Treg cell function 101.
In addition to these physiological mechanisms of fine-tuning signals, IL-2 partial agonists have now been generated. The IL-2 superkine 68, which exhibits enhanced binding to IL-2Rβ, was used as a background for selecting mutants that exhibited diminished interaction with γ c 69. In contrast to the super-IL-2 full agonist, such molecules could either abrogate or attenuate IL-2 signaling based on the level of recruitment of γ c, thereby altering the E max and signaling threshold. Because these molecules have enhanced binding to IL-2Rβ, they outcompete endogenous IL-2 as well as IL-15 (which also shares IL-2Rβ) and confer a new level of signaling. One such molecule, denoted as H9-RETR, has four amino acid mutations and disrupts the γ c binding interface completely. Not only can this molecule inhibit cytokine-induced STAT5 phosphorylation on T and NK cells but it also inhibits IL-2 or IL-15-induced cytolytic activity of NK cells in vitro, prolongs survival in a mouse model of graft-versus-host disease, and inhibits the proliferation of cells from patients with the chronic/smoldering form of human T-cell lymphotropic virus-I (HTLV-I)-induced adult T cell leukemia 69. The approach for generating these partial agonists may be able to provide a range of interesting new IL-2 variants and should be broadly applicable to other cytokines as well.
Concluding remarks
The γ c family of cytokines collectively serve critical roles in the immune system, controlling lymphocyte development, growth, differentiation, and survival. In this review, we have focused primarily on IL-2 and IL-21, clarifying ways in which they regulate the immune response physiologically as well as how they can be utilized and manipulated to modulate the immune system in disease settings. Novel approaches, including the generation of new variants of IL-2 such as an IL-2 superkine or IL-2 partial agonists or the “stabilization” of IL-2 with anti-IL-2 antibodies with effects on binding specificity, show promise for modulating the actions of IL-2 and potentially IL-15 to therapeutic benefit.
Acknowledgements
We thank Mr. Dalton Hermans for assistance with figure preparation.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Michael Caligiuri, The Ohio State University, Columbus, Ohio, USA
Thomas Malek, Department of Microbiology and Immunology, Miller School of Medicine, University of Miami, Miami, FL, 33101, USA
Cecile King, Immunology Division, Garvan Institute of Medical Research, Darlinghurst, New South Wales, Australia
Funding Statement
This work was supported by the Division of Intramural Research, National Heart, Lung, and Blood Institute. D.G. was supported by the National Institutes of Health Medical Research Scholars Program.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; referees: 3 approved]
References
- 1. Rochman Y, Spolski R, Leonard WJ: New insights into the regulation of T cells by γ c family cytokines. Nat Rev Immunol. 2009;9(7):480–90. 10.1038/nri2580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Noguchi M, Yi H, Rosenblatt HM, et al. : Interleukin-2 receptor gamma chain mutation results in X-linked severe combined immunodeficiency in humans. Cell. 1993;73(1):147–57. [DOI] [PubMed] [Google Scholar]
- 3. Fischer A, Hacein-Bey-Abina S, Cavazzana-Calvo M: Gene therapy for primary adaptive immune deficiencies. J Allergy Clin Immunol. 2011;127(6):1356–9. 10.1016/j.jaci.2011.04.030 [DOI] [PubMed] [Google Scholar]
- 4. Leonard WJ: Cytokines and immunodeficiency diseases. Nat Rev Immunol. 2001;1(3):200–8. 10.1038/35105066 [DOI] [PubMed] [Google Scholar]
- 5. Noguchi M, Nakamura Y, Russell SM, et al. : Interleukin-2 receptor gamma chain: a functional component of the interleukin-7 receptor. Science. 1993;262(5141):1877–80. 10.1126/science.8266077 [DOI] [PubMed] [Google Scholar]
- 6. Russell SM, Keegan AD, Harada N, et al. : Interleukin-2 receptor gamma chain: a functional component of the interleukin-4 receptor. Science. 1993;262(5141):1880–3. 10.1126/science.8266078 [DOI] [PubMed] [Google Scholar]
- 7. Kondo M, Takeshita T, Ishii N, et al. : Sharing of the interleukin-2 (IL-2) receptor gamma chain between receptors for IL-2 and IL-4. Science. 1993;262(5141):1874–7. 10.1126/science.8266076 [DOI] [PubMed] [Google Scholar]
- 8. Kondo M, Takeshita T, Higuchi M, et al. : Functional participation of the IL-2 receptor gamma chain in IL-7 receptor complexes. Science. 1994;263(5121):1453–4. 10.1126/science.8128231 [DOI] [PubMed] [Google Scholar]
- 9. Ozaki K, Spolski R, Feng CG, et al. : A critical role for IL-21 in regulating immunoglobulin production. Science. 2002;298(5598):1630–4. 10.1126/science.1077002 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 10. Leonard WJ: The molecular basis of X-linked severe combined immunodeficiency: defective cytokine receptor signaling. Annu Rev Med. 1996;47:229–39. 10.1146/annurev.med.47.1.229 [DOI] [PubMed] [Google Scholar]
- 11. Leonard WJ, O'Shea JJ: Jaks and STATs: biological implications. Annu Rev Immunol. 1998;16:293–322. 10.1146/annurev.immunol.16.1.293 [DOI] [PubMed] [Google Scholar]
- 12. Russell SM, Johnston JA, Noguchi M, et al. : Interaction of IL-2R beta and gamma c chains with Jak1 and Jak3: implications for XSCID and XCID. Science. 1994;266(5187):1042–5. 10.1126/science.7973658 [DOI] [PubMed] [Google Scholar]
- 13. Macchi P, Villa A, Giliani S, et al. : Mutations of Jak-3 gene in patients with autosomal severe combined immune deficiency (SCID). Nature. 1995;377(6544):65–8. 10.1038/377065a0 [DOI] [PubMed] [Google Scholar]
- 14. Russell SM, Tayebi N, Nakajima H, et al. : Mutation of Jak3 in a patient with SCID: essential role of Jak3 in lymphoid development. Science. 1995;270(5237):797–800. 10.1126/science.270.5237.797 [DOI] [PubMed] [Google Scholar]
- 15. Puel A, Ziegler SF, Buckley RH, et al. : Defective IL7R expression in T -B +NK + severe combined immunodeficiency. Nat Genet. 1998;20(4):394–7. 10.1038/3877 [DOI] [PubMed] [Google Scholar]
- 16. Al-Shami A, Spolski R, Kelly J, et al. : A role for thymic stromal lymphopoietin in CD4 + T cell development. J Exp Med. 2004;200(2):159–68. 10.1084/jem.20031975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lin JX, Migone TS, Tsang M, et al. : The role of shared receptor motifs and common Stat proteins in the generation of cytokine pleiotropy and redundancy by IL-2, IL-4, IL-7, IL-13, and IL-15. Immunity. 1995;2(4):331–9. 10.1016/1074-7613(95)90141-8 [DOI] [PubMed] [Google Scholar]
- 18. Liao W, Lin J, Leonard WJ: Interleukin-2 at the crossroads of effector responses, tolerance, and immunotherapy. Immunity. 2013;38(1):13–25. 10.1016/j.immuni.2013.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liao W, Lin J, Wang L, et al. : Modulation of cytokine receptors by IL-2 broadly regulates differentiation into helper T cell lineages. Nat Immunol. 2011;12(6):551–9. 10.1038/ni.2030 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 20. Cote-Sierra J, Foucras G, Guo L, et al. : Interleukin 2 plays a central role in Th2 differentiation. Proc Natl Acad Sci U S A. 2004;101(11):3880–5. 10.1073/pnas.0400339101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liao W, Schones DE, Oh J, et al. : Priming for T helper type 2 differentiation by interleukin 2-mediated induction of interleukin 4 receptor alpha-chain expression. Nat Immunol. 2008;9(11):1288–96. 10.1038/ni.1656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liao W, Spolski R, Li P, et al. : Opposing actions of IL-2 and IL-21 on Th9 differentiation correlate with their differential regulation of BCL6 expression. Proc Natl Acad Sci U S A. 2014;111(9):3508–13. 10.1073/pnas.1301138111 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 23. Laurence A, Tato CM, Davidson TS, et al. : Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26(3):371–81. 10.1016/j.immuni.2007.02.009 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 24. Rosenberg SA: IL-2: the first effective immunotherapy for human cancer. J Immunol. 2014;192(12):5451–8. 10.4049/jimmunol.1490019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hou J, Schindler U, Henzel WJ, et al. : An interleukin-4-induced transcription factor: IL-4 Stat. Science. 1994;265(5179):1701–6. 10.1126/science.8085155 [DOI] [PubMed] [Google Scholar]
- 26. Quelle FW, Shimoda K, Thierfelder W, et al. : Cloning of murine Stat6 and human Stat6, Stat proteins that are tyrosine phosphorylated in responses to IL-4 and IL-3 but are not required for mitogenesis. Mol Cell Biol. 1995;15(6):3336–43. 10.1128/MCB.15.6.3336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rolling C, Treton D, Pellegrini S, et al. : IL4 and IL13 receptors share the gamma c chain and activate STAT6, STAT3 and STAT5 proteins in normal human B cells. FEBS Lett. 1996;393(1):53–6. 10.1016/0014-5793(96)00835-6 [DOI] [PubMed] [Google Scholar]
- 28. Paul WE: History of interleukin-4. Cytokine. 2015;75(1):3–7. 10.1016/j.cyto.2015.01.038 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 29. Aman MJ, Tayebi N, Obiri NI, et al. : cDNA cloning and characterization of the human interleukin 13 receptor alpha chain. J Biol Chem. 1996;271(46):29265–70. 10.1074/jbc.271.46.29265 [DOI] [PubMed] [Google Scholar]
- 30. Demoulin JB, van Roost E, Stevens M, et al. : Distinct roles for STAT1, STAT3, and STAT5 in differentiation gene induction and apoptosis inhibition by interleukin-9. J Biol Chem. 1999;274(36):25855–61. 10.1074/jbc.274.36.25855 [DOI] [PubMed] [Google Scholar]
- 31. Rathmell JC, Farkash EA, Gao W, et al. : IL-7 enhances the survival and maintains the size of naive T cells. J Immunol. 2001;167(12):6869–76. 10.4049/jimmunol.167.12.6869 [DOI] [PubMed] [Google Scholar]
- 32. Opferman JT, Letai A, Beard C, et al. : Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature. 2003;426(6967):671–6. 10.1038/nature02067 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 33. Sprent J, Surh CD: Normal T cell homeostasis: the conversion of naive cells into memory-phenotype cells. Nat Immunol. 2011;12(6):478–84. 10.1038/ni.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fry TJ, Mackall CL: Interleukin-7: from bench to clinic. Blood. 2002;99(11):3892–904. 10.1182/blood.V99.11.3892 [DOI] [PubMed] [Google Scholar]
- 35. Melén E, Gullstén H, Zucchelli M, et al. : Sex specific protective effects of interleukin-9 receptor haplotypes on childhood wheezing and sensitisation. J Med Genet. 2004;41(12):e123. 10.1136/jmg.2004.023135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lu Y, Hong S, Li H, et al. : Th9 cells promote antitumor immune responses in vivo. J Clin Invest. 2012;122(11):4160–71. 10.1172/JCI65459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Purwar R, Schlapbach C, Xiao S, et al. : Robust tumor immunity to melanoma mediated by interleukin-9-producing T cells. Nat Med. 2012;18(8):1248–53. 10.1038/nm.2856 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 38. Waldmann TA: The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol. 2006;6(8):595–601. 10.1038/nri1901 [DOI] [PubMed] [Google Scholar]
- 39. Lodolce JP, Boone DL, Chai S, et al. : IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9(5):669–76. 10.1016/S1074-7613(00)80664-0 [DOI] [PubMed] [Google Scholar]
- 40. Kennedy MK, Glaccum M, Brown SN, et al. : Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med. 2000;191(5):771–80. 10.1084/jem.191.5.771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ozaki K, Spolski R, Ettinger R, et al. : Regulation of B cell differentiation and plasma cell generation by IL-21, a novel inducer of Blimp-1 and Bcl-6. J Immunol. 2004;173(9):5361–71. 10.4049/jimmunol.173.9.5361 [DOI] [PubMed] [Google Scholar]
- 42. Ettinger R, Sims GP, Fairhurst AM, et al. : IL-21 induces differentiation of human naive and memory B cells into antibody-secreting plasma cells. J Immunol. 2005;175(12):7867–79. 10.4049/jimmunol.175.12.7867 [DOI] [PubMed] [Google Scholar]
- 43. Zeng R, Spolski R, Finkelstein SE, et al. : Synergy of IL-21 and IL-15 in regulating CD8 + T cell expansion and function. J Exp Med. 2005;201(1):139–48. 10.1084/jem.20041057 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 44. Vogelzang A, McGuire HM, Yu D, et al. : A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity. 2008;29(1):127–37. 10.1016/j.immuni.2008.06.001 [DOI] [PubMed] [Google Scholar]
- 45. Korn T, Bettelli E, Gao W, et al. : IL-21 initiates an alternative pathway to induce proinflammatory T H17 cells. Nature. 2007;448(7152):484–7. 10.1038/nature05970 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 46. Nurieva R, Yang XO, Martinez G, et al. : Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448(7152):480–3. 10.1038/nature05969 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 47. Zhou L, Ivanov II, Spolski R, et al. : IL-6 programs T H-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8(9):967–74. 10.1038/ni1488 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 48. Spolski R, Leonard WJ: Interleukin-21: a double-edged sword with therapeutic potential. Nat Rev Drug Discov. 2014;13(5):379–95. 10.1038/nrd4296 [DOI] [PubMed] [Google Scholar]
- 49. Mehra P, Wells AD: Long-Range Transcriptional Control of the Il2 Gene by an Intergenic Enhancer. Mol Cell Biol. 2015;35(22):3880–91. 10.1128/MCB.00592-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Park JH, Choi Y, Song MJ, et al. : Dynamic Long-Range Chromatin Interaction Controls Expression of IL-21 in CD4 + T Cells. J Immunol. 2016;196(10):4378–89. 10.4049/jimmunol.1500636 [DOI] [PubMed] [Google Scholar]
- 51. Hinrichs CS, Spolski R, Paulos CM, et al. : IL-2 and IL-21 confer opposing differentiation programs to CD8 + T cells for adoptive immunotherapy. Blood. 2008;111(11):5326–33. 10.1182/blood-2007-09-113050 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 52. Ross SH, Rollings C, Anderson KE, et al. : Phosphoproteomic Analyses of Interleukin 2 Signaling Reveal Integrated JAK Kinase-Dependent and -Independent Networks in CD8 + T Cells. Immunity. 2016;45(3):685–700. 10.1016/j.immuni.2016.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 53. Huynh A, DuPage M, Priyadharshini B, et al. : Control of PI(3) kinase in Treg cells maintains homeostasis and lineage stability. Nat Immunol. 2015;16(2):188–96. 10.1038/ni.3077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Oyler-Yaniv A, Oyler-Yaniv J, Whitlock BM, et al. : A Tunable Diffusion-Consumption Mechanism of Cytokine Propagation Enables Plasticity in Cell-to-Cell Communication in the Immune System. Immunity. 2017;46(4):609–20. 10.1016/j.immuni.2017.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 55. Marks-Konczalik J, Dubois S, Losi JM, et al. : IL-2-induced activation-induced cell death is inhibited in IL-15 transgenic mice. Proc Natl Acad Sci U S A. 2000;97(21):11445–50. 10.1073/pnas.200363097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Waldmann TA: The shared and contrasting roles of IL2 and IL15 in the life and death of normal and neoplastic lymphocytes: implications for cancer therapy. Cancer Immunol Res. 2015;3(3):219–27. 10.1158/2326-6066.CIR-15-0009 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 57. Su EW, Moore CJ, Suriano S, et al. : IL-2Rα mediates temporal regulation of IL-2 signaling and enhances immunotherapy. Sci Transl Med. 2015;7(311):311ra170. 10.1126/scitranslmed.aac8155 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 58. Hurton LV, Singh H, Najjar AM, et al. : Tethered IL-15 augments antitumor activity and promotes a stem-cell memory subset in tumor-specific T cells. Proc Natl Acad Sci U S A. 2016;113(48):E7788–E7797. 10.1073/pnas.1610544113 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 59. Arce Vargas F, Furness AJS, Solomon I, et al. : Fc-Optimized Anti-CD25 Depletes Tumor-Infiltrating Regulatory T Cells and Synergizes with PD-1 Blockade to Eradicate Established Tumors. Immunity. 2017;46(4):577–86. 10.1016/j.immuni.2017.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 60. Golgher D, Jones E, Powrie F, et al. : Depletion of CD25 + regulatory cells uncovers immune responses to shared murine tumor rejection antigens. Eur J Immunol. 2002;32(11):3267–75. [DOI] [PubMed] [Google Scholar]
- 61. Jones E, Dahm-Vicker M, Simon AK, et al. : Depletion of CD25 + regulatory cells results in suppression of melanoma growth and induction of autoreactivity in mice. Cancer Immun. 2002;2:1. [PubMed] [Google Scholar]
- 62. Kalia V, Sarkar S, Subramaniam S, et al. : Prolonged interleukin-2Ralpha expression on virus-specific CD8 + T cells favors terminal-effector differentiation in vivo. Immunity. 2010;32(1):91–103. 10.1016/j.immuni.2009.11.010 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 63. Pipkin ME, Sacks JA, Cruz-Guilloty F, et al. : Interleukin-2 and inflammation induce distinct transcriptional programs that promote the differentiation of effector cytolytic T cells. Immunity. 2010;32(1):79–90. 10.1016/j.immuni.2009.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 64. Rajagopalan A, Berezhnoy A, Schrand B, et al. : Aptamer-Targeted Attenuation of IL-2 Signaling in CD8 + T Cells Enhances Antitumor Immunity. Mol Ther. 2017;25(1):54–61. 10.1016/j.ymthe.2016.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 65. Arenas-Ramirez N, Zou C, Popp S, et al. : Improved cancer immunotherapy by a CD25-mimobody conferring selectivity to human interleukin-2. Sci Transl Med. 2016;8(367):367ra166. 10.1126/scitranslmed.aag3187 [DOI] [PubMed] [Google Scholar]
- 66. Spangler JB, Tomala J, Luca VC, et al. : Antibodies to Interleukin-2 Elicit Selective T Cell Subset Potentiation through Distinct Conformational Mechanisms. Immunity. 2015;42(5):815–25. 10.1016/j.immuni.2015.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 67. Sun Y, Peng S, Yang A, et al. : Coinjection of IL2 DNA enhances E7-specific antitumor immunity elicited by intravaginal therapeutic HPV DNA vaccination with electroporation. Gene Ther. 2017;24(7):408–15. 10.1038/gt.2017.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Levin AM, Bates DL, Ring AM, et al. : Exploiting a natural conformational switch to engineer an interleukin-2 'superkine'. Nature. 2012;484(7395):529–33. 10.1038/nature10975 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 69. Mitra S, Ring AM, Amarnath S, et al. : Interleukin-2 activity can be fine tuned with engineered receptor signaling clamps. Immunity. 2015;42(5):826–38. 10.1016/j.immuni.2015.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Klatzmann D, Abbas AK: The promise of low-dose interleukin-2 therapy for autoimmune and inflammatory diseases. Nat Rev Immunol. 2015;15(5):283–94. 10.1038/nri3823 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 71. He J, Zhang X, Wei Y, et al. : Low-dose interleukin-2 treatment selectively modulates CD4 + T cell subsets in patients with systemic lupus erythematosus. Nat Med. 2016;22(9):991–3. 10.1038/nm.4148 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 72. Koreth J, Matsuoka K, Kim HT, et al. : Interleukin-2 and regulatory T cells in graft-versus-host disease. N Engl J Med. 2011;365(22):2055–2066. 10.1056/NEJMoa1108188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Asano T, Meguri Y, Yoshioka T, et al. : PD-1 modulates regulatory T-cell homeostasis during low-dose interleukin-2 therapy. Blood. 2017;129(15):2186–97. 10.1182/blood-2016-09-741629 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 74. Lüthje K, Kallies A, Shimohakamada Y, et al. : The development and fate of follicular helper T cells defined by an IL-21 reporter mouse. Nat Immunol. 2012;13(5):491–8. 10.1038/ni.2261 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 75. Reinhardt RL, Liang H, Locksley RM: Cytokine-secreting follicular T cells shape the antibody repertoire. Nat Immunol. 2009;10(4):385–93. 10.1038/ni.1715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Weinstein JS, Herman EI, Lainez B, et al. : T FH cells progressively differentiate to regulate the germinal center response. Nat Immunol. 2016;17(10):1197–205. 10.1038/ni.3554 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 77. Jones L, Ho WQ, Ying S, et al. : A subpopulation of high IL-21-producing CD4 + T cells in Peyer's Patches is induced by the microbiota and regulates germinal centers. Sci Rep. 2016;6: 30784. 10.1038/srep30784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ballesteros-Tato A, Randall TD, Lund FE, et al. : T Follicular Helper Cell Plasticity Shapes Pathogenic T Helper 2 Cell-Mediated Immunity to Inhaled House Dust Mite. Immunity. 2016;44(2):259–73. 10.1016/j.immuni.2015.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 79. Pallikkuth S, Parmigiani A, Silva SY, et al. : Impaired peripheral blood T-follicular helper cell function in HIV-infected nonresponders to the 2009 H1N1/09 vaccine. Blood. 2012;120(5):985–93. 10.1182/blood-2011-12-396648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Rao DA, Gurish MF, Marshall JL, et al. : Pathologically expanded peripheral T helper cell subset drives B cells in rheumatoid arthritis. Nature. 2017;542(7639):110–4. 10.1038/nature20810 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 81. Linterman MA, Pierson W, Lee SK, et al. : Foxp3 + follicular regulatory T cells control the germinal center response. Nat Med. 2011;17(8):975–82. 10.1038/nm.2425 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 82. Chung Y, Tanaka S, Chu F, et al. : Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat Med. 2011;17(8):983–8. 10.1038/nm.2426 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 83. Wollenberg I, Agua-Doce A, Hernández A, et al. : Regulation of the germinal center reaction by Foxp3 + follicular regulatory T cells. J Immunol. 2011;187(9):4553–60. 10.4049/jimmunol.1101328 [DOI] [PubMed] [Google Scholar]
- 84. Sage PT, Ron-Harel N, Juneja VR, et al. : Suppression by T FR cells leads to durable and selective inhibition of B cell effector function. Nat Immunol. 2016;17(12):1436–46. 10.1038/ni.3578 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 85. Ballesteros-Tato A, León B, Graf BA, et al. : Interleukin-2 inhibits germinal center formation by limiting T follicular helper cell differentiation. Immunity. 2012;36(5):847–56. 10.1016/j.immuni.2012.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 86. Li J, Lu E, Yi T, et al. : EBI2 augments Tfh cell fate by promoting interaction with IL-2-quenching dendritic cells. Nature. 2016;533(7601):110–4. 10.1038/nature17947 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 87. Ray JP, Staron MM, Shyer JA, et al. : The Interleukin-2-mTORc1 Kinase Axis Defines the Signaling, Differentiation, and Metabolism of T Helper 1 and Follicular B Helper T Cells. Immunity. 2015;43(4):690–702. 10.1016/j.immuni.2015.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Fontenot JD, Rasmussen JP, Gavin MA, et al. : A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6(11):1142–51. 10.1038/ni1263 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 89. León B, Bradley JE, Lund FE, et al. : FoxP3 + regulatory T cells promote influenza-specific Tfh responses by controlling IL-2 availability. Nat Commun. 2014;5: 3495. 10.1038/ncomms4495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Schmitz I, Schneider C, Fröhlich A, et al. : IL-21 restricts virus-driven Treg cell expansion in chronic LCMV infection. PLoS Pathog. 2013;9(5):e1003362. 10.1371/journal.ppat.1003362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Attridge K, Wang CJ, Wardzinski L, et al. : IL-21 inhibits T cell IL-2 production and impairs Treg homeostasis. Blood. 2012;119(20):4656–64. 10.1182/blood-2011-10-388546 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 92. Jandl C, Liu SM, Cañete PF, et al. : IL-21 restricts T follicular regulatory T cell proliferation through Bcl-6 mediated inhibition of responsiveness to IL-2. Nat Commun. 2017;8: 14647. 10.1038/ncomms14647 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 93. Kaplan MH, Hufford MM, Olson MR: The development and in vivo function of T helper 9 cells. Nat Rev Immunol. 2015;15(5):295–307. 10.1038/nri3824 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 94. Schmitt E, Germann T, Goedert S, et al. : IL-9 production of naive CD4 + T cells depends on IL-2, is synergistically enhanced by a combination of TGF-beta and IL-4, and is inhibited by IFN-gamma. J Immunol. 1994;153(9):3989–96. [PubMed] [Google Scholar]
- 95. Bassil R, Orent W, Olah M, et al. : BCL6 controls Th9 cell development by repressing Il9 transcription. J Immunol. 2014;193(1):198–207. 10.4049/jimmunol.1303184 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 96. Gomez-Rodriguez J, Meylan F, Handon R, et al. : Itk is required for Th9 differentiation via TCR-mediated induction of IL-2 and IRF4. Nat Commun. 2016;7: 10857. 10.1038/ncomms10857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Ulrich BJ, Verdan FF, McKenzie AN, et al. : STAT3 Activation Impairs the Stability of Th9 Cells. J Immunol. 2017;198(6):2302–9. 10.4049/jimmunol.1601624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Végran F, Berger H, Boidot R, et al. : The transcription factor IRF1 dictates the IL-21-dependent anticancer functions of T H9 cells. Nat Immunol. 2014;15(8):758–66. 10.1038/ni.2925 [DOI] [PubMed] [Google Scholar]
- 99. Moretti S, Renga G, Oikonomou V, et al. : A mast cell-ILC2-Th9 pathway promotes lung inflammation in cystic fibrosis. Nat Commun. 2017;8: 14017. 10.1038/ncomms14017 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 100. Wan CK, Andraski AB, Spolski R, et al. : Opposing roles of STAT1 and STAT3 in IL-21 function in CD4 + T cells. Proc Natl Acad Sci U S A. 2015;112(30):9394–9. 10.1073/pnas.1511711112 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 101. Lin JX, Li P, Liu D, et al. : Critical Role of STAT5 transcription factor tetramerization for cytokine responses and normal immune function. Immunity. 2012;36(4):586–99. 10.1016/j.immuni.2012.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation